Investigating the Effect of Biomaterials Such as Poly-(l-Lactic Acid) Particles on Collagen Synthesis In Vitro: Method Is Matter
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Nanoparticle Preparation and Characterization
2.3. Cell Viability Assay
2.4. PLLA Treatment on Fibroblast and Macrophage Single Cultures
2.5. PLLA Treatment on Fibroblast/Macrophage Co-Culture
2.6. Collagen Assay
2.7. Statistics Analysis
3. Results
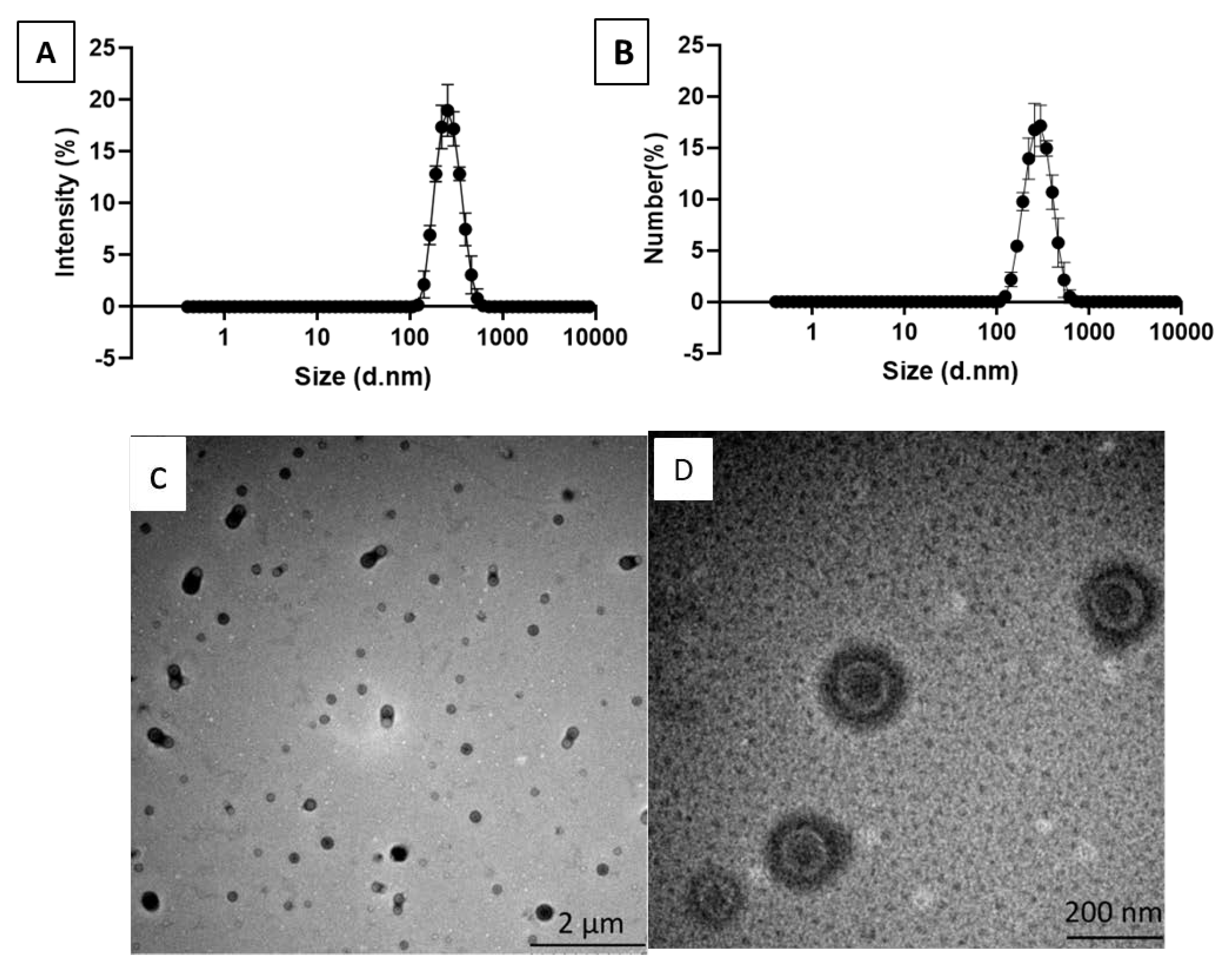
3.1. PLLA Nanoparticle Characterization
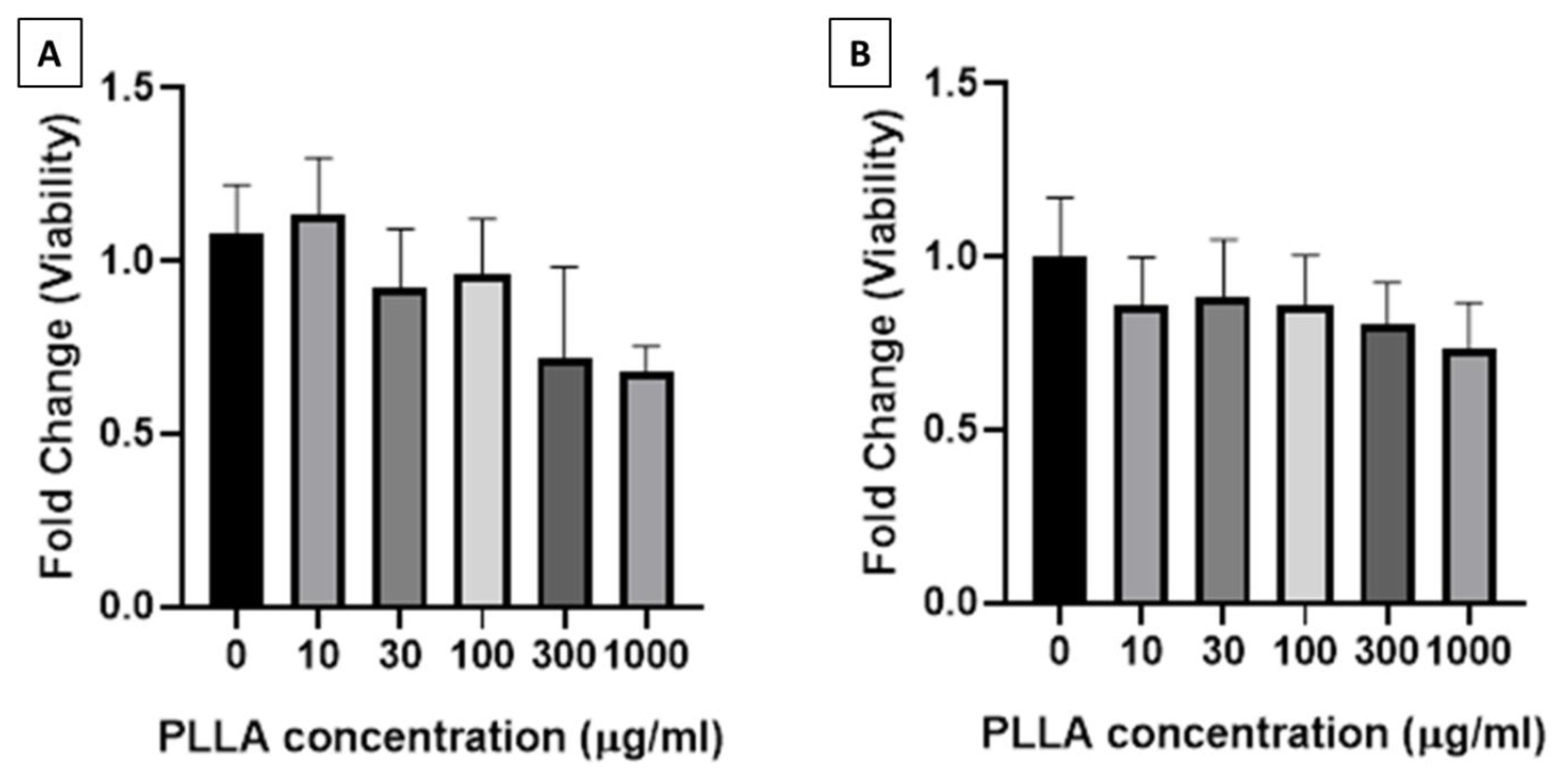
3.2. Viability of Macrophages and Fibroblasts Treated with PLLA Nanoparticles
3.3. Collagen Synthesis Stimulated by PLLA Nanoparticles
4. Discussion
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Xiao, L.; Wang, B.; Yang, G.; Gauthier, M. Poly (lactic acid)-based biomaterials: Synthesis, modification and applications. Biomed. Sci. Eng. Technol. 2012, 11, 247–282. [Google Scholar]
- Burgess, C.M.; Quiroga, R.M. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV-associated facial lipoatrophy. J. Am. Acad. Dermatol. 2005, 52, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Danny, V.; Ute, B. Facial enhancement and the European experience with Sculptra™(poly-l-lactic acid). J. Drugs Dermatol. 2004, 3, 542–547. [Google Scholar]
- Lacombe, V. Sculptra: A stimulatory filler. Facial Plastic Surg. 2009, 25, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.M.; Redbord, K.P.; Hanke, C.W. Treatment of HIV lipoatrophy and lipoatrophy of aging with poly-L-lactic acid: A prospective 3-year follow-up study. J. Am. Acad. Dermatol. 2008, 59, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Marc-Antoine, V.; Camille, A.-O.; Jade, G.; Elisabeth, L.; Michelle, P.; Hélène, S.; Raymond, B.; Philippe, K.; Dominique, C.; Christine, K. Polylactic acid implants (New-Fill)® to correct facial lipoatrophy in HIV-infected patients: Results of the open-label study VEGA. Aids 2003, 17, 2471–2477. [Google Scholar]
- Lowe, N.J. Optimizing poly-l-lactic acid use. J. Cosmet. Laser Ther. 2008, 10, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Schulman, M.R.; Lipper, J.; Skolnik, R.A. Correction of Chest Wall Deformity After Implant-Based Breast Reconstruction Using poly-l-Lactic Acid (Sculptra). Breast J. 2008, 14, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, F.; Lawrence, M.B.; David, J.G.; Miles, H.G.; Lorenc, Z.P. Physiochemical Characteristics of Poly-L-Lactic Acid (PLLA). Aesthet. Surg. J. 2018, 38, 13–17. [Google Scholar]
- Vleggaar, D. Facial volumetric correction with injectable poly-L-lactic acid. Dermatol. Surg. 2005, 31, 1511–1517; discussion 1517–1518. [Google Scholar] [CrossRef] [PubMed]
- Molly, S.; Rebecca, E.; Antoanella, C.; Arianne, S.K. Late-onset granuloma formation after poly-l-lactic acid injection. JAAD Case Rep. 2016, 2, 54–56. [Google Scholar]
- Kim, S.-A.; Kim, H.-S.; Jung, J.-W.; Suh, S.-I.; Ryoo, Y.-W. Poly-L-Lactic Acid Increases Collagen Gene Expression and Synthesis in Cultured Dermal Fibroblast (Hs68) Through the p38 MAPK Pathway. Ann. Dermatol. 2019, 31, 97–100. [Google Scholar] [CrossRef]
- David, G.; Adriana, G.; Andrea, V.; Elizabeth, D.-K. Single-arm study for the characterization of human tissue response to injectable poly-L-lactic acid. Dermatol. Surg. 2013, 39, 915–922. [Google Scholar]
- Natalia, N.; Irina, G.L.; Kendrick, H.; Virginia, L.; Kerill, P.; Zachary, A.C.; Michael, P.G.; Nevins, W.T.; Sergei, P.A. Macrophages produce TGF-β-induced (β-ig-h3) following ingestion of apoptotic cells and regulate MMP14 levels and collagen turnover in fibroblasts. J. Immunol. 2008, 180, 5036–5044. [Google Scholar]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef]
- Nadège, G.; Hervé, H.; Juliette, V.; Nicolas, T.; Marc, P.; Saadia, K.-R.; Elias, F. Surface coating mediates the toxicity of polymeric nanoparticles towards human-like macrophages. Int. J. Pharm. 2015, 482, 75–83. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ray, S.; Ta, H.T. Investigating the Effect of Biomaterials Such as Poly-(l-Lactic Acid) Particles on Collagen Synthesis In Vitro: Method Is Matter. J. Funct. Biomater. 2020, 11, 51. https://doi.org/10.3390/jfb11030051
Ray S, Ta HT. Investigating the Effect of Biomaterials Such as Poly-(l-Lactic Acid) Particles on Collagen Synthesis In Vitro: Method Is Matter. Journal of Functional Biomaterials. 2020; 11(3):51. https://doi.org/10.3390/jfb11030051
Chicago/Turabian StyleRay, Subarna, and Hang T. Ta. 2020. "Investigating the Effect of Biomaterials Such as Poly-(l-Lactic Acid) Particles on Collagen Synthesis In Vitro: Method Is Matter" Journal of Functional Biomaterials 11, no. 3: 51. https://doi.org/10.3390/jfb11030051
APA StyleRay, S., & Ta, H. T. (2020). Investigating the Effect of Biomaterials Such as Poly-(l-Lactic Acid) Particles on Collagen Synthesis In Vitro: Method Is Matter. Journal of Functional Biomaterials, 11(3), 51. https://doi.org/10.3390/jfb11030051





