Effect of Modification with Helium Atmospheric-Pressure Plasma and Deep-Ultraviolet Light on Adhesive Shear Strength of Fiber-Reinforced Poly(ether-ether-ketone) Polymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Surface Modification
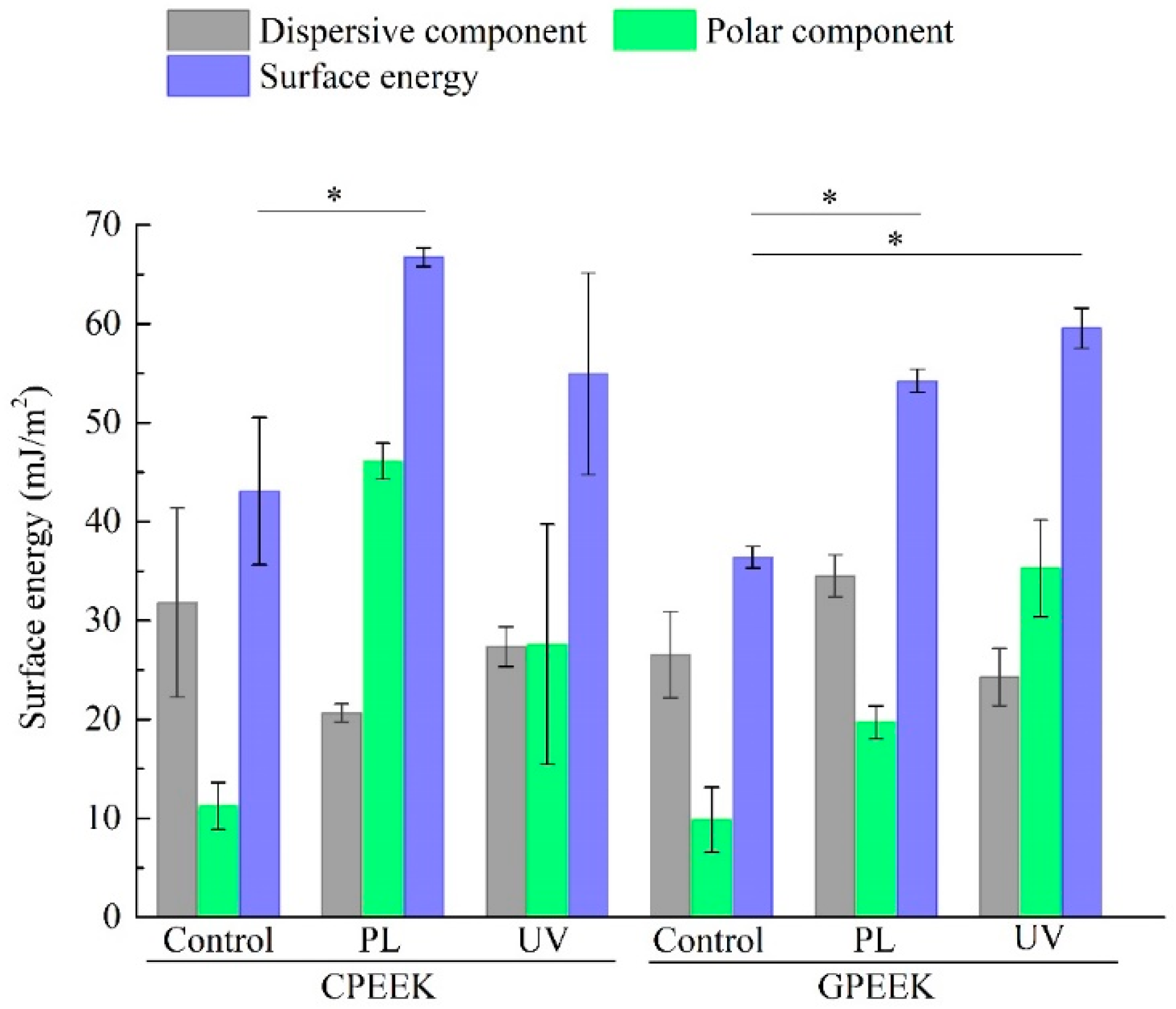
2.3. Measurement of Contact Angle and Evaluation of Surface Energy
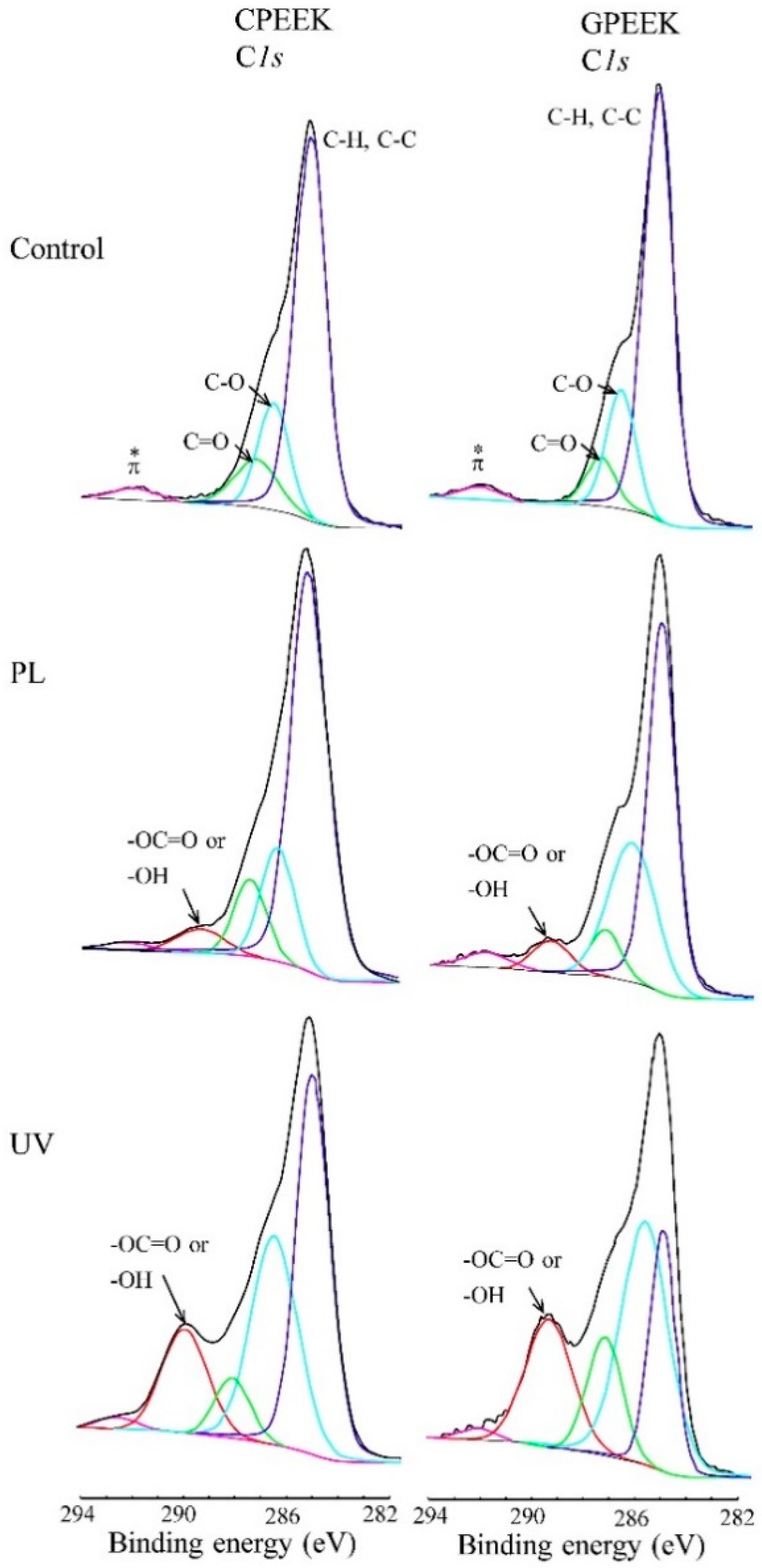
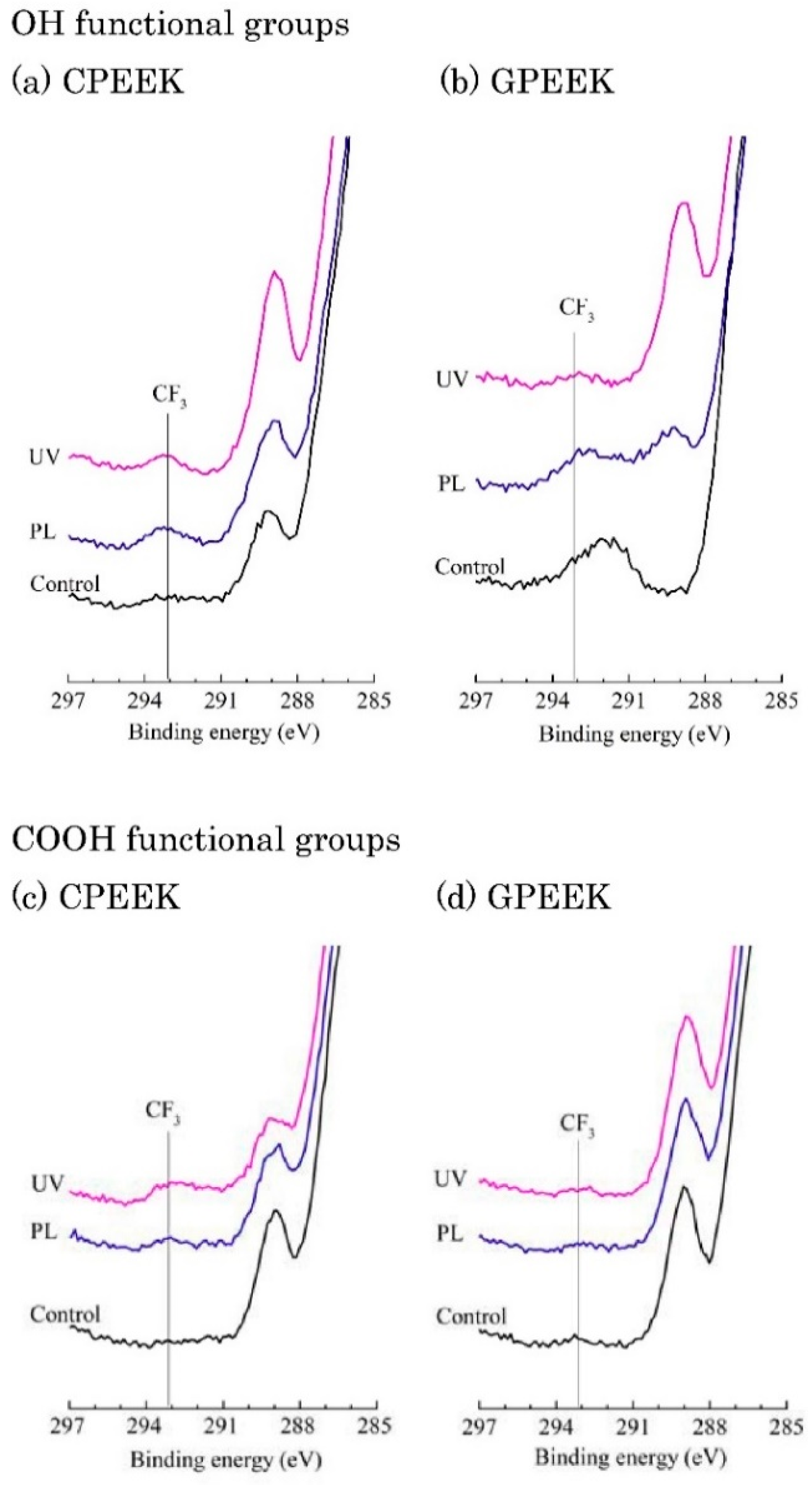
2.4. Surface Analysis
2.5. Bonding Procedure
2.6. Compressive Shear Bond Strength Test
2.7. Observation of Fractured Surfaces
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Eichberger, M.; Uhrenbacher, J.; Wimmer, T.; Edelhoff, D.; Schmidlin, P.R. Three-unit reinforced Polyetheretherketone composite FDPs: Influence of fabrication method on load-bearing capacity and failure types. Dent. Mater. J. 2015, 34, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, K.; Huang, Z.M.; Ramakrishna, S.; Satknanantham, K.; Hamada, H. Feasibility of knitted carbon/PEEK composites for orthopedic bone plates. Biomaterials 2004, 25, 3877–3885. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of Polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Sandler, J.; Werner, P.; Shaffer, M.S.; Demchuk, V.; Altstadt, V.; Windle, A.H. Carbon-nanofibre- reinforced poly(ether ether ketone) composites. Compos. Part A 2002, 33, 1033–1039. [Google Scholar] [CrossRef]
- Tsuka, H.; Morita, K.; Kato, K.; Kawano, H.; Abekura, H.; Tsuga, K. Evaluation of shear bond strength between PEEK and resin-based luting material. J. Oral. Biosci. 2017, 59, 231–236. [Google Scholar] [CrossRef]
- Lümkemann, N.; Strickstrock, M.; Eichberger, M.; Zylla, I.M.; Stawarczyk, B. Impact of air-abrasion pressure and adhesive systems on bonding parameters for polyetheretherketone dental restorations. Int. J. Adhes. Adhes. 2018, 80, 30–38. [Google Scholar] [CrossRef]
- Schmidlin, P.R.; Stawarczyk, B.; Wieland, M.; Attin, T.; Hammerle, C.H.F.; Fischer, J. Effect of different surface pre-treatments and luting materials on shear strength to PEEK. Dent. Mater. 2010, 26, 553–559. [Google Scholar] [CrossRef]
- Hallmann, L.; Mehl, A.; Sereno, N.; Hammerle, C.H.F. The improvement of adhesive properties of PEEK through different pre-treatments. Appl. Surf. Sci. 2012, 258, 7213–7218. [Google Scholar] [CrossRef]
- Zhou, L.; Qian, Y.; Zhu, Y.; Liu, H.; Gan, K.; Guo, J. The effect of different surface treatments on the bond strength of PEEK composite materials. Dent Mater. 2014, 30, e209–e215. [Google Scholar] [CrossRef]
- Sproesser, O.; Schmidlin, P.R.; Uhrenbacher, J.; Eichberger, M.; Roos, M.; Stawarczyk, B. Work of adhesion between resin composite cements and PEEK as a function of etching duration with sulfuric acid and its correlation with bond strength values. Int. J. Adhes. Adhes. 2014, 54, 184–190. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Jordan, P.; Schmidlin, P.R.; Roos, M.; Eichberger, M.; Gernet, W.; Keul, C. PEEK surface treatment effects on tensile bond strength to veneering resins. J Prosthet. Dent. 2014, 112, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Beuer, F.; Wimmer, T.; Jahn, D.; Sener, B.; Roos, M.; Schmidlin, P.R. Polyetheretherketone- A suitable material for fixed denture prostheses? J. Biomed. Mater. Res. Part B 2013, 101, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Jones, I.; Salamat-Zadeh, F.; Watts, J.F. Laser surface modification of poly(etheretherketone) to enhance surface free energy, wettability and adhesion. Int. J. Adhes. Adhes. 2015, 62, 69–77. [Google Scholar] [CrossRef]
- Riveiro, A.; Soto, R.; Comesana, R.; Boutinguiza, M.; Val, J.D.; Quintero, F.; Lusquinos, F.; Pou, J. Laser surface modification of PEEK. Appl. Surf. Sci. 2012, 258, 9437–9442. [Google Scholar] [CrossRef]
- Laurens, P.; Sadras, B.; Decobert, F.; Arefi-Khonsari, F.; Amouroux, J. Enhancement of the adhesive bonding properties of PEEK by excimer laser treatment. Int. J. Adhes. Adhes. 1998, 18, 19–27. [Google Scholar] [CrossRef]
- Mathieson, I.; Bradley, R.H. Improved adhesion to polymers by UV/ozone surface oxidation. Int. J. Adhes. Adhes. 1996, 16, 29–31. [Google Scholar] [CrossRef]
- Comyn, J.; Mascia, L.; Xia, G.; Parker, B.M. Plasma-treatment of Polyetheretherketone (PEEK) for adhesive bonding. Int. J. Adhes. Adhes. 1996, 16, 97–104. [Google Scholar] [CrossRef]
- Zhang, S.; Awaja, F.; James, N.; Mckenzie, D.R.; Ruys, A.J. Autohesion of plasma modified semi-crystallinc PEEK: Comparative study of argon nitrogen and oxygen treatments. Colloids Surf. A 2011, 374, 88–95. [Google Scholar] [CrossRef]
- Iqbal, H.M.S.; Bhowmik, S.; Benedictus, R. Surface modification of high performance polymers by atmospheric pressure plasma and failure mechanism of adhesive bonded joints. Int. J. Adhes. Adhes. 2010, 30, 418–424. [Google Scholar] [CrossRef]
- Ha, S.W.; Hauert, R.; Ernst, K.H.; Wintermantel, E. Surface analysis of chemically-etched and plasma-modified polyetheretherketone(PEEK) for biomedical applications. Surf. Coat. Technol. 1997, 96, 293–299. [Google Scholar] [CrossRef]
- Jha, S.; Bhowmik, S.; Bhatnagar, N.; Bhattacharya, N.K.; Deka, U.; Iqbal, H.M.S.; Benedictus, R. Experimental investigation into the effect of adhesion properties of PEEK modified by atmospheric pressure plasma and low pressure plasma. J. Appl. Polym. Sci. 2010, 118, 173–179. [Google Scholar] [CrossRef]
- Inagaki, N.; Tasaka, S.; Horiuchi, T.; Suyama, R. Surface modification of Poly(aryl ether ether ketone) film by remote oxygen plasma. J. Appl. Polym. Sci. 1998, 68, 271–279. [Google Scholar] [CrossRef]
- Langley, L.A.; Villanueva, D.E.; Fairbrother, D.H. Quantification of surface oxides on carbonaceous materials. Chem. Mater. 2006, 18, 169–178. [Google Scholar] [CrossRef]
- Ameen, A.P.; Ward, R.J.; Short, R.D.; Beamson, G.; Briggs, D. A high-resolution X-ray photoelectron spectroscopy study of trifluoroacetic anhydride labelling of hydroxyl groups: Demonstration of the beta shift due to –OC(O)CF3. Polymer 1993, 34, 1795–1799. [Google Scholar] [CrossRef]
- Gonzalez, E., II; Barankin, M.D.; Guschl, P.C.; Hicks, R.F. Ring opening of aromatic polymers by remote atomospheric-pressure plasm. IEEE Trans. Plasma Sci. 2009, 37, 823–831. [Google Scholar] [CrossRef]
- Legan, R.W. Ultraviolet light takes on CPI role. Chem. Eng. J. 1982, 89, 95–100. [Google Scholar]
- Niu, Y.F.; Yang, Y.; Li, T.Y.; Yao, J.W. Effects of irradiation and condensation on poly(ether-ether-ketone)/carbon fiber composites from nano- to macro-scale. High Perform. Polym. 2018, 30, 230–238. [Google Scholar] [CrossRef]


| Mechanical and Physical Properties | PMMA | PEEK | CPEEK | GPEEK |
|---|---|---|---|---|
| Specific gravity | 1.18 | 1.31 | 1.41 | 1.51 |
| Tensile strength (MPa) | 55–76 | 110 | 131 | 97 |
| Tensile modulus of elasticity (GPa) | 2.4–3.4 | 4.3 | 7.6 | 6.9 |
| Tensile elongation (at break) (%) | 2 | 40 | 5 | 2 |
| Flexural strength (MPa) | 83–117 | 172 | 178 | 159 |
| Flexural modulus of elasticity (Gpa) | 2.4–3.4 | 4.1 | 8.6 | 6.9 |
| Shear strength (MPa) | - | 55 | 103 | 97 |
| Water absorption immersion 24 h (% by wt) | 0.3 | 0.1 | 0.06 | 0.1 |
| Liquid | Modifications | Specimens | |
|---|---|---|---|
| CPEEK | GPEEK | ||
| Mean (SD) | Mean (SD) | ||
| Distilled water | Control | 88.6 (0.9) | 74.4 (3.0) |
| PL | 37.2 (2.6) | 37.3 (4.2) | |
| UV | 23.8 (2.0) | 51.4 (1.6) | |
| Formamide | Control | 55.3 (2.8) | 55.1 (1.2) |
| PL | 11.2 (0.8) | 19.4 (0.6) | |
| UV | 8.6 (0.7) | 21.8 (3.6) | |
| Specimen | Control | PL | UV |
|---|---|---|---|
| CPEEK | 0.20 | 0.44 | 0.61 |
| GPEEK | 0.26 | 0.38 | 0.61 |
| Binding Energy (eV) | References | |||||
|---|---|---|---|---|---|---|
| Control | PL | UV | [19] | [21] | [23] | |
| C-H/C-C | 285.1 | 285.0 | 285.0 | 285 | 285.0 | 285.0 |
| C-O-C | 286.5 | 286.3 | 286.3 | 286.4 | 286.3 | 286.4 |
| C=O | 287.3 | 287.3 | 287.3 | 288 | 287.4 | 287.2 |
| COOH or COH | 289.0 | 289.0 | 289.2 | 289 | 288.7 | |
| π-π * | 292.0 | 291.7 | 291.5 | 291.79 | 291.7 | 291.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okawa, S.; Taka, N.; Aoyagi, Y. Effect of Modification with Helium Atmospheric-Pressure Plasma and Deep-Ultraviolet Light on Adhesive Shear Strength of Fiber-Reinforced Poly(ether-ether-ketone) Polymer. J. Funct. Biomater. 2020, 11, 27. https://doi.org/10.3390/jfb11020027
Okawa S, Taka N, Aoyagi Y. Effect of Modification with Helium Atmospheric-Pressure Plasma and Deep-Ultraviolet Light on Adhesive Shear Strength of Fiber-Reinforced Poly(ether-ether-ketone) Polymer. Journal of Functional Biomaterials. 2020; 11(2):27. https://doi.org/10.3390/jfb11020027
Chicago/Turabian StyleOkawa, Seigo, Norimasa Taka, and Yujin Aoyagi. 2020. "Effect of Modification with Helium Atmospheric-Pressure Plasma and Deep-Ultraviolet Light on Adhesive Shear Strength of Fiber-Reinforced Poly(ether-ether-ketone) Polymer" Journal of Functional Biomaterials 11, no. 2: 27. https://doi.org/10.3390/jfb11020027
APA StyleOkawa, S., Taka, N., & Aoyagi, Y. (2020). Effect of Modification with Helium Atmospheric-Pressure Plasma and Deep-Ultraviolet Light on Adhesive Shear Strength of Fiber-Reinforced Poly(ether-ether-ketone) Polymer. Journal of Functional Biomaterials, 11(2), 27. https://doi.org/10.3390/jfb11020027




