The Regenerative Applicability of Bioactive Glass and Beta-Tricalcium Phosphate in Bone Tissue Engineering: A Transformation Perspective
Abstract
:1. Introduction
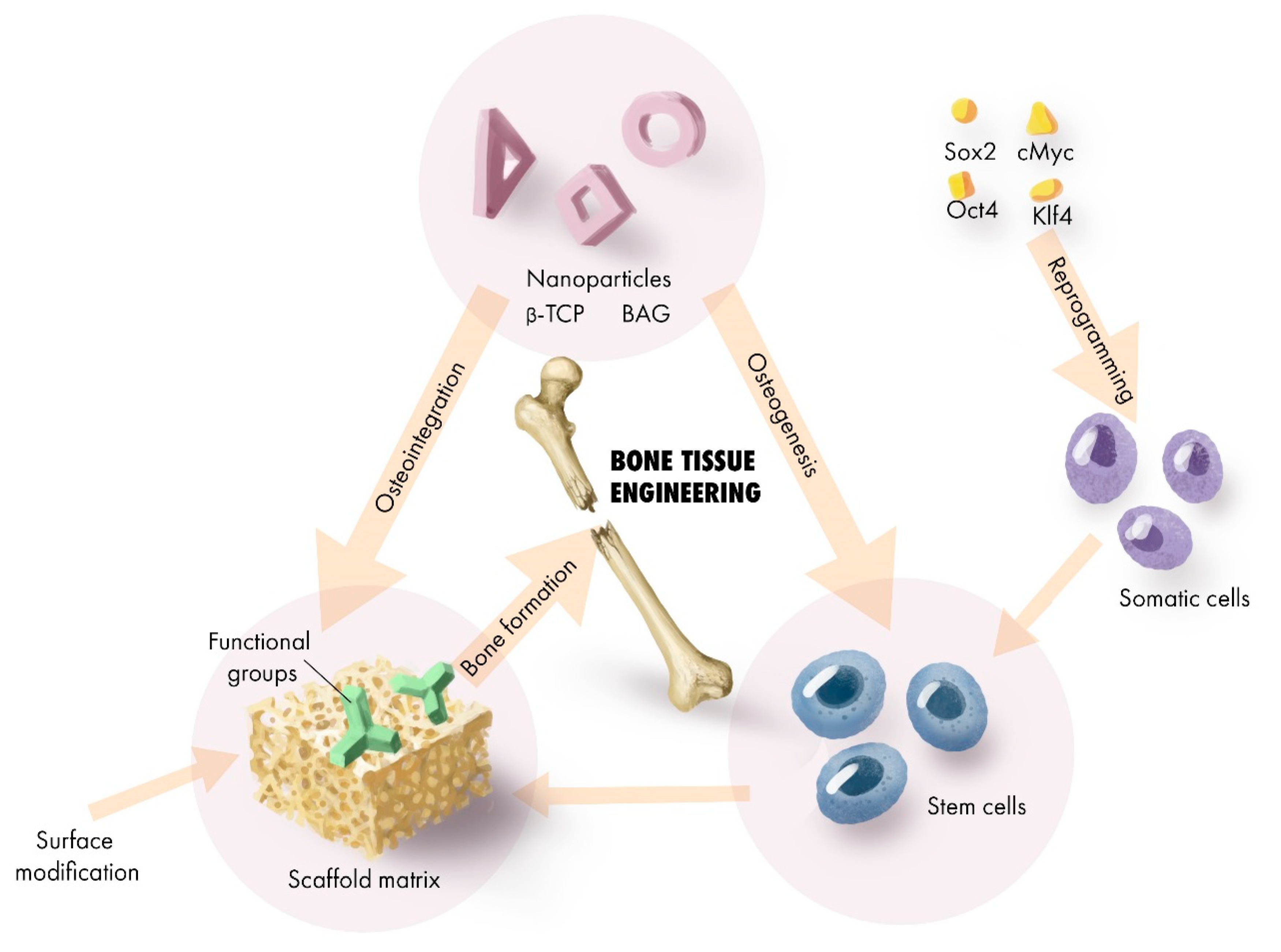
2. Scaffold Transformation for Regenerative Application
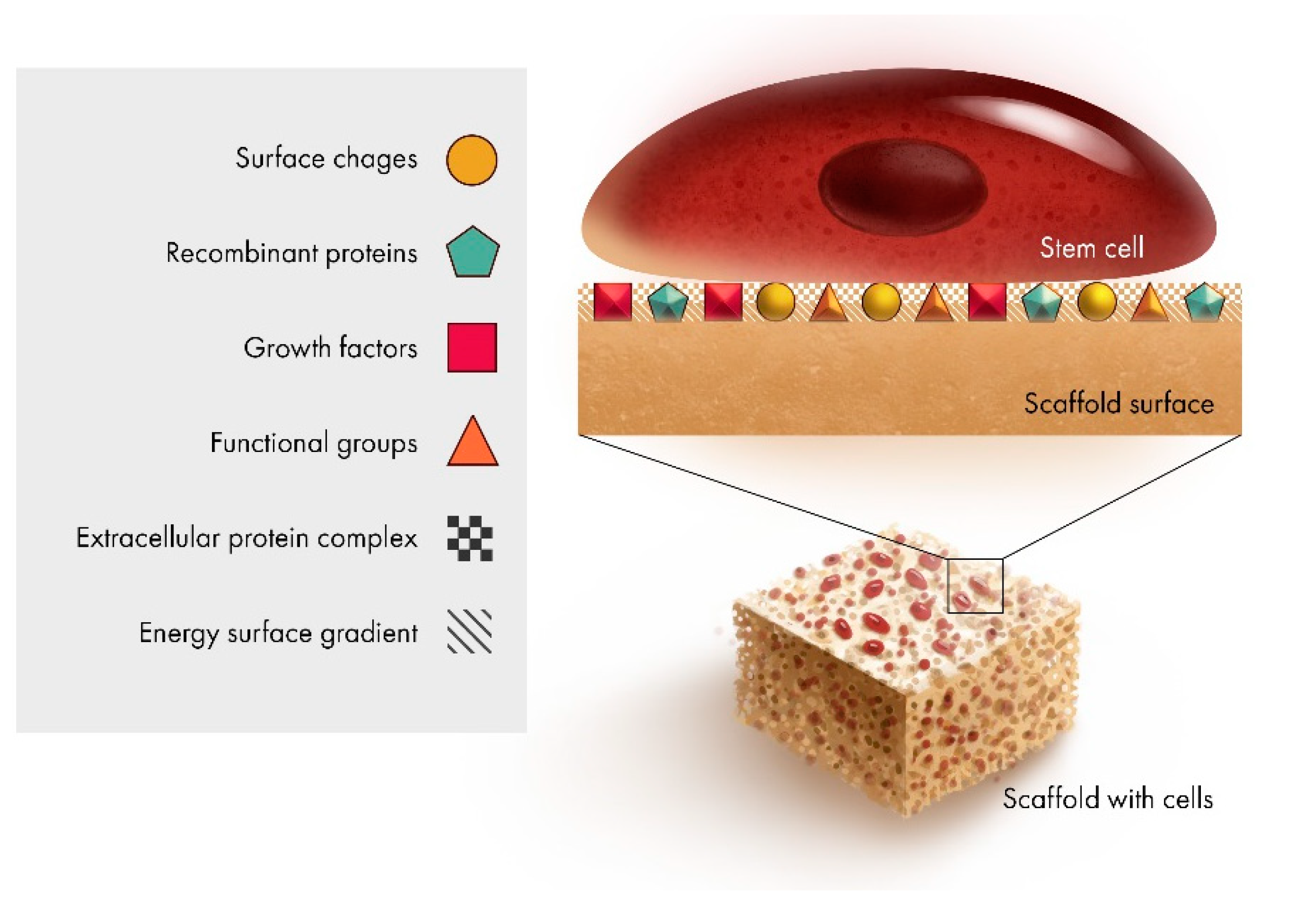
2.1. Scaffold Interface
2.2. Surface Modification
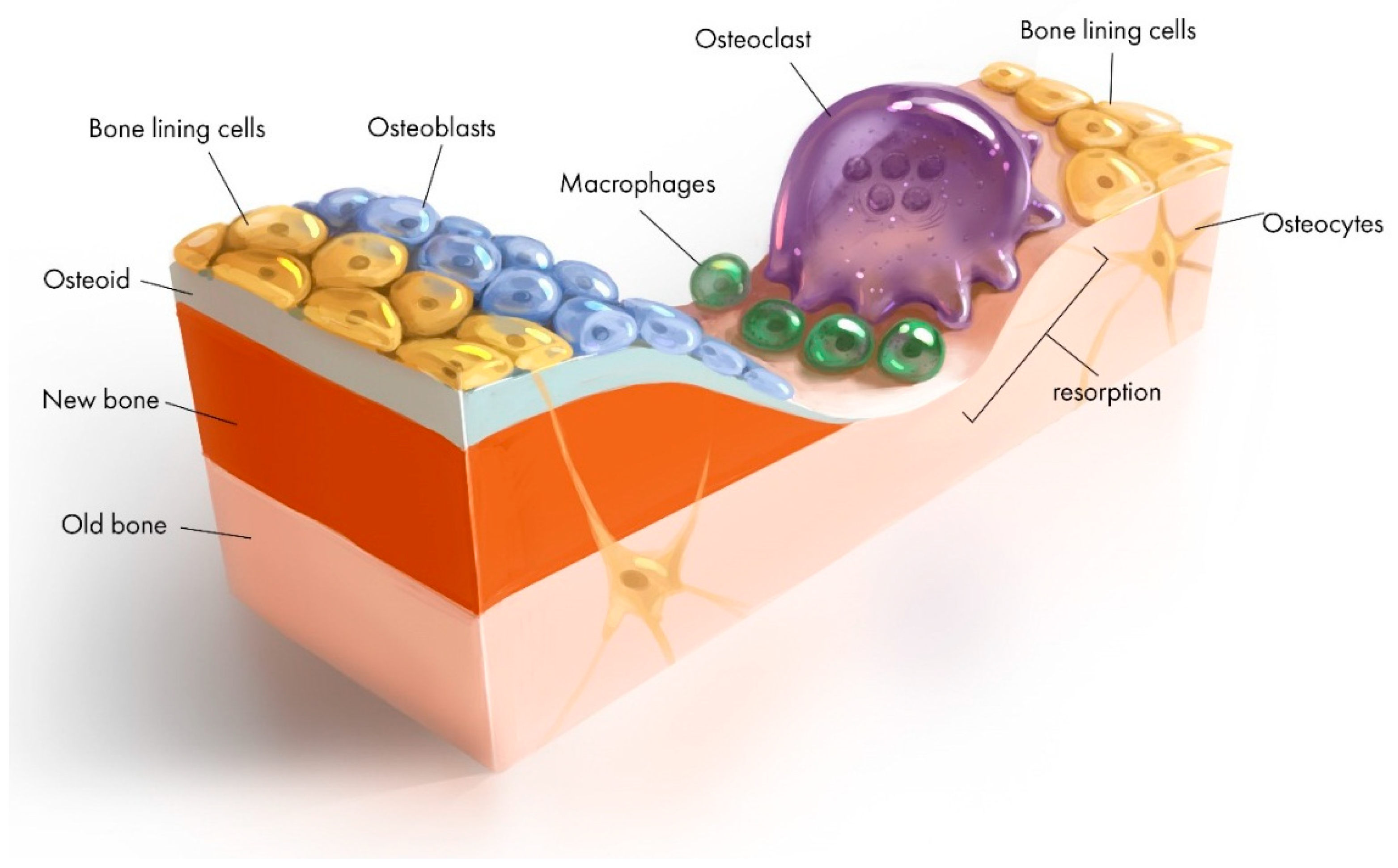
2.3. Cells
2.4. Micro-Architecture
2.5. Nano-Architecture
2.6. Dopants
3. Application of Regenerative Scaffold for BTE and or Regeneration
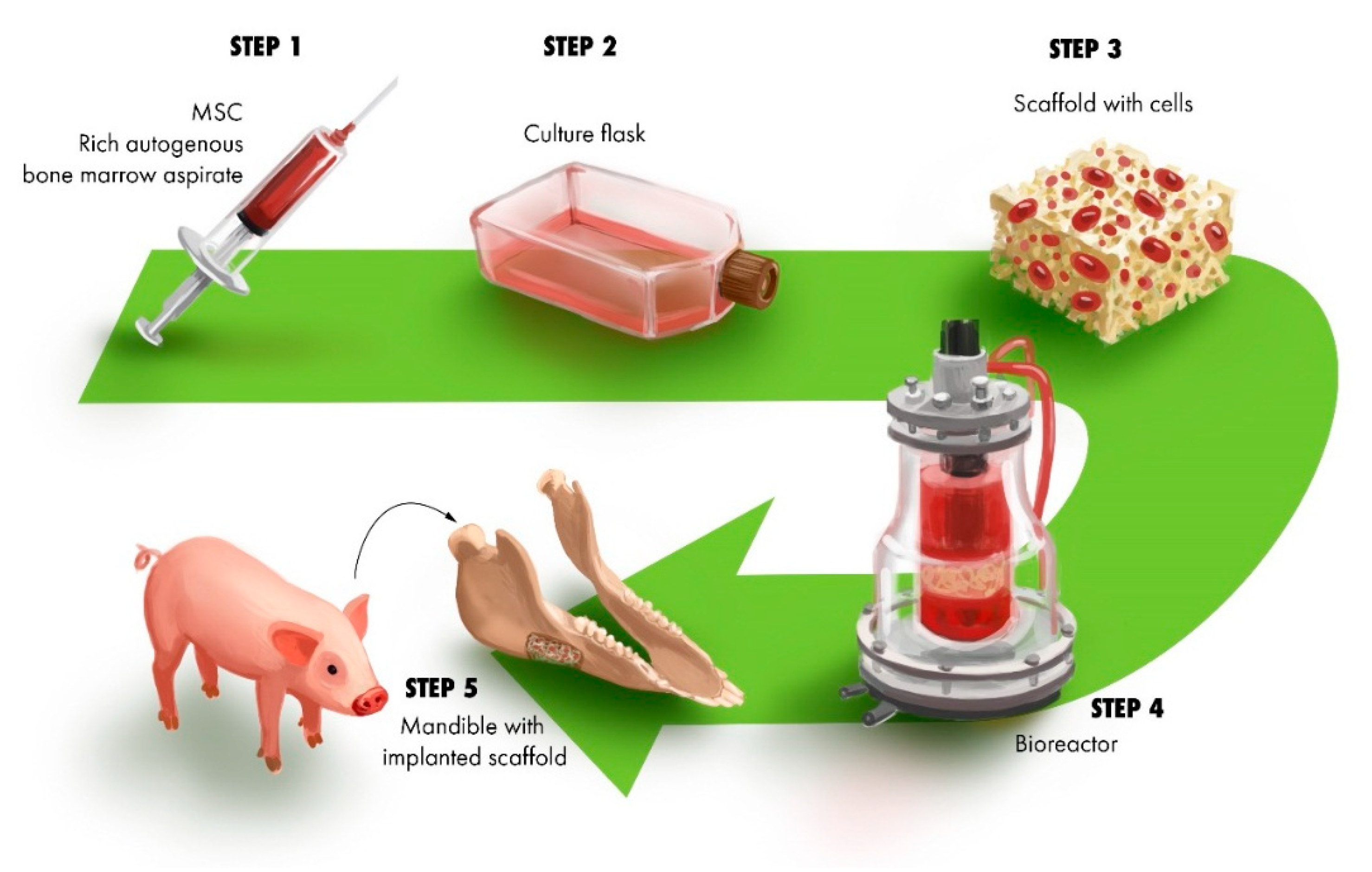
3.1. Construct for Reconstruction of Segmental Defects
3.2. Delivery
3.3. Reinforcement of Stability in Guided Bone Regeneration (GBR) Membrane
3.4. Reinforcement of Mechanical Stability
4. Summary and Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gomes, M.E.; Rodrigues, M.T.; Domingues, R.M.; Reis, R.L. Tissue engineering and regenerative medicine: New trends and directions—A year in review. Tissue Eng. Part B. Rev. 2017, 23, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Rose, F.R.; Oreffo, R.O. Bone tissue engineering: Hope vs hype. Biochem. Biophys. Res. Commun. 2002, 292, 1–7. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Dekker, R.; De Bruijn, J.; Van Den Brink, I.; Bovell, Y.; Layrolle, P.; Van Blitterswijk, C. Bone tissue engineering on calcium phosphate-coated titanium plates utilizing cultured rat bone marrow cells: A preliminary study. J. Mater. Sci. Mater. Med. 1998, 9, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Kohli, N.; Ho, S.; Brown, S.J.; Sawadkar, P.; Sharma, V.; Snow, M.; García-Gareta, E. Bone remodelling in vitro: Where are we headed?:-A review on the current understanding of physiological bone remodelling and inflammation and the strategies for testing biomaterials in vitro. Bone 2018, 110, 38–46. [Google Scholar] [CrossRef]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Bioactive glass scaffolds for bone tissue engineering: State of the art and future perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 1245–1256. [Google Scholar] [CrossRef]
- Abdollahi, S.; Ma, A.C.C.; Cerruti, M. Surface transformations of Bioglass 45S5 during scaffold synthesis for bone tissue engineering. Langmuir 2013, 29, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Peng, J.; Xu, Y.; Chang, J.; Li, H. Bioglass activated skin tissue engineering constructs for wound healing. ACS. Appl. Mater. Interfaces 2015, 8, 703–715. [Google Scholar] [CrossRef]
- Jones, J.R.; Brauer, D.S.; Hupa, L.; Greenspan, D.C. Bioglass and bioactive glasses and their impact on healthcare. Int. J. Appl. Glass Sci. 2016, 7, 423–434. [Google Scholar] [CrossRef]
- Naseri, S.; Lepry, W.C.; Nazhat, S.N. Bioactive glasses in wound healing: Hope or hype? J. Mater. Chem. B 2017, 5, 6167–6174. [Google Scholar] [CrossRef]
- Tang, Z.; Li, X.; Tan, Y.; Fan, H.; Zhang, X. The material and biological characteristics of osteoinductive calcium phosphate ceramics. Regen. Biomater. 2017, 5, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hunziker, E.; Randall, N.; De Groot, K.; Layrolle, P. Proteins incorporated into biomimetically prepared calcium phosphate coatings modulate their mechanical strength and dissolution rate. Biomaterials 2003, 24, 65–70. [Google Scholar] [CrossRef]
- Sharpe, J.; Sammons, R.; Marquis, P. Effect of pH on protein adsorption to hydroxyapatite and tricalcium phosphate ceramics. Biomaterials 1997, 18, 471–476. [Google Scholar] [CrossRef]
- El-Ghannam, A.; Ducheyne, P.; Shapiro, I. Effect of serum proteins on osteoblast adhesion to surface-modified bioactive glass and hydroxyapatite. J. Orthop. Res. 1999, 17, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Rouahi, M.; Champion, E.; Gallet, O.; Jada, A.; Anselme, K. Physico-chemical characteristics and protein adsorption potential of hydroxyapatite particles: Influence on in vitro biocompatibility of ceramics after sintering. Colloids. Surf. B Biointerfaces 2006, 47, 10–19. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Dalas, E. The effect of acidic amino acids on hydroxyapatite crystallization. J. Cryst. Growth 2000, 217, 410–415. [Google Scholar] [CrossRef]
- Bar-Yosef Ofir, P.; Govrin-Lippman, R.; Garti, N.; Füredi-Milhofer, H. The influence of polyelectrolytes on the formation and phase transformation of amorphous calcium phosphate. Cryst. Growth Des. 2004, 4, 177–183. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Adsorption of proteins and calcium phosphate materials bioactivity. Biomaterials 2002, 23, 2817–2823. [Google Scholar] [CrossRef]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317. [Google Scholar]
- Vasin, S.L.; Rosanova, I.B.; Sevastianov, V.I. The role of proteins in the nucleation and formation of calcium-containing deposits on biomaterial surfaces. J. Biomed. Mater. Res. 1998, 39, 491–497. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yamamoto, M.; Tabata, Y. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and β-tricalcium phosphate. Biomaterials 2005, 26, 4856–4865. [Google Scholar] [CrossRef]
- Thomas, A. Preparation and Characterization of Gelatin-Bioactive Glass Ceramic Scaffolds for Bone Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2019, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bothe, F.; Lotz, B.; Seebach, E.; Fischer, J.; Hesse, E.; Diederichs, S.; Richter, W. Stimulation of calvarial bone healing with human bone marrow stromal cells versus inhibition with adipose-tissue stromal cells on nanostructured β-TCP-collagen. Acta Biomater. 2018, 76, 135–145. [Google Scholar] [CrossRef]
- Huang, W.; Day, D.E.; Kittiratanapiboon, K.; Rahaman, M.N. Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J. Mater. Sci. Mater. Med. 2006, 17, 583–596. [Google Scholar] [CrossRef]
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef]
- Zhang, L.; Ke, X.; Lin, L.; Xiao, J.; Yang, X.; Wang, J.; Yang, G.; Xu, S.; Gou, Z.; Shi, Z. Systematic evaluation of the osteogenic capacity of low-melting bioactive glass-reinforced 45S5 Bioglass porous scaffolds in rabbit femoral defects. Biomed. Mater. 2017, 12, 035010. [Google Scholar] [CrossRef] [PubMed]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Liu, B.; Lun, D.x. Current application of β -tricalcium phosphate composites in orthopaedics. Orthop. Surg. 2012, 4, 139–144. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, H.; Liu, Y.; Fan, B.; Li, X.; Xiao, X.; Lan, P.; Li, M.; Geng, L.; Liu, D. Beta-tricalcium phosphate granules improve osteogenesis in vitro and establish innovative osteo-regenerators for bone tissue engineering in vivo. Sci. Rep. 2016, 6, 23367. [Google Scholar] [CrossRef] [PubMed]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Baheiraei, N.; Nourani, M.R.; Mortazavi, S.M.J.; Movahedin, M.; Eyni, H.; Bagheri, F.; Norahan, M.H. Development of a bioactive porous collagen/β-tricalcium phosphate bone graft assisting rapid vascularization for bone tissue engineering applications. J. Biomed. Mater. Res. A 2018, 106, 73–85. [Google Scholar] [CrossRef]
- Roohani-Esfahani, S.-I.; Newman, P.; Zreiqat, H. Design and fabrication of 3D printed scaffolds with a mechanical strength comparable to cortical bone to repair large bone defects. Sci. Rep. 2016, 6, 19468. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Sergeeva, N.; Britaev, T.; Komlev, V.; Sviridova, I.; Kirsanova, V.; Akhmedova, S.; Dgebuadze, P.Y.; Teterina, A.Y.; Kuvshinova, E. Some Physical, Chemical, and Biological Parameters of Samples of Scleractinium Coral Aquaculture Skeleton Used for Reconstruction/Engineering of the Bone Tissue. Bull. Exp. Biol. Med. 2015, 159, 494–497. [Google Scholar] [CrossRef]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Shor, L.; Güçeri, S.; Wen, X.; Gandhi, M.; Sun, W. Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro. Biomaterials 2007, 28, 5291–5297. [Google Scholar] [CrossRef] [PubMed]
- Erol, M.; Mouriňo, V.; Newby, P.; Chatzistavrou, X.; Roether, J.; Hupa, L.; Boccaccini, A.R. Copper-releasing, boron-containing bioactive glass-based scaffolds coated with alginate for bone tissue engineering. Acta Biomater. 2012, 8, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.H.; Pollack, S.R.; Ducheyne, P. 45S5 bioactive glass surface charge variations and the formation of a surface calcium phosphate layer in a solution containing fibronectin. J. Biomed. Mater. Res. 2001, 54, 454–461. [Google Scholar] [CrossRef]
- Lee, M.H.; Ducheyne, P.; Lynch, L.; Boettiger, D.; Composto, R.J. Effect of biomaterial surface properties on fibronectin–α5β1 integrin interaction and cellular attachment. Biomaterials 2006, 27, 1907–1916. [Google Scholar] [CrossRef]
- Di Maggio, N.; Martella, E.; Frismantiene, A.; Resink, T.J.; Schreiner, S.; Lucarelli, E.; Jaquiery, C.; Schaefer, D.J.; Martin, I.; Scherberich, A. Extracellular matrix and α 5 β 1 integrin signaling control the maintenance of bone formation capacity by human adipose-derived stromal cells. Sci. Rep. 2017, 7, 44398. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.; Farina, M.; Soares, G.; Anselme, K. Surface energy of hydroxyapatite and β-tricalcium phosphate ceramics driving serum protein adsorption and osteoblast adhesion. J. Mater. Sci. Mater. Med. 2008, 19, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Meng, S.; Huang, Y.; Xu, M.; He, Y.; Lin, H.; Han, J.; Chai, Y.; Wei, Y.; Deng, X. Electrospun Gelatin/-TCP Composite Nanofibers Enhance Osteogenic Differentiation of BMSCs and In Vivo Bone Formation by Activating Ca2. Stem Cells Int. 2015, 2015, 507154. [Google Scholar] [CrossRef] [PubMed]
- Valliant, E.M.; Jones, J.R. Softening bioactive glass for bone regeneration: Sol–gel hybrid materials. Soft Matter 2011, 7, 5083–5095. [Google Scholar] [CrossRef]
- Ertel, S.I.; Ratner, B.D.; Horbett, T.A. The adsorption and elutability of albumin, IgG, and fibronectin on radiofrequency plasma deposited polystyrene. J. Colloid. Interface Sci. 1991, 147, 433–442. [Google Scholar] [CrossRef]
- Intranuovo, F.; Gristina, R.; Brun, F.; Mohammadi, S.; Ceccone, G.; Sardella, E.; Rossi, F.; Tromba, G.; Favia, P. Plasma Modification of PCL Porous Scaffolds Fabricated by Solvent-Casting/Particulate-Leaching for Tissue Engineering. Plasma Process. Polym. 2014, 11, 184–195. [Google Scholar] [CrossRef]
- Domingos, M.; Intranuovo, F.; Gloria, A.; Gristina, R.; Ambrosio, L.; Bártolo, P.; Favia, P. Improved osteoblast cell affinity on plasma-modified 3-D extruded PCL scaffolds. Acta Biomater. 2013, 9, 5997–6005. [Google Scholar] [CrossRef]
- Sankar, D.; Shalumon, K.; Chennazhi, K.; Menon, D.; Jayakumar, R. Surface plasma treatment of poly (caprolactone) micro, nano, and multiscale fibrous scaffolds for enhanced osteoconductivity. Tissue Eng. Part A 2014, 20, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.-S.; Jung, S.-C.; Kook, M.-S.; Kim, B.-H. In vitro study of 3D PLGA/n-HAp/β-TCP composite scaffolds with etched oxygen plasma surface modification in bone tissue engineering. Appl. Surf. Sci. 2016, 388, 321–330. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Q.; Bachhuka, A.; Vasilev, K. Surface modification by allylamine plasma polymerization promotes osteogenic differentiation of human adipose-derived stem cells. ACS Appl. Mater. Interfaces 2014, 6, 9733–9741. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weng, L.Q.; Wu, Z.Z.; Wang, C.B. The Properties of Polyetheretherketone Biocomposite Reinforced by Surface-Modified Nano-Hydroxyapatite. Adv. Mater. Res. 2015, 1096, 214–218. [Google Scholar] [CrossRef]
- Heule, M.; Rezwan, K.; Cavalli, L.; Gauckler, L.J. A miniaturized enzyme reactor based on hierarchically shaped porous ceramic microstruts. Adv. Mater. 2003, 15, 1191–1194. [Google Scholar] [CrossRef]
- Lin, C.C.; Ki, C.S.; Shih, H. Thiol–norbornene photoclick hydrogels for tissue engineering applications. J. Appl. Polym. Sci. 2015, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Porté-Durrieu, M.-C.; Guillemot, F.; Pallu, S.; Labrugère, C.; Brouillaud, B.; Bareille, R.; Amédée, J.; Barthe, N.; Dard, M.; Baquey, C. Cyclo-(DfKRG) peptide grafting onto Ti–6Al–4V: Physical characterization and interest towards human osteoprogenitor cells adhesion. Biomaterials 2004, 25, 4837–4846. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, D.; Li, N.; Wen, J.; Jiang, X.; Liu, C.; Li, Y. Functionalized mesoporous bioactive glass scaffolds for enhanced bone tissue regeneration. Sci. Rep. 2016, 6, 19361. [Google Scholar] [CrossRef]
- Curran, J.M.; Chen, R.; Hunt, J.A. The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials 2006, 27, 4783–4793. [Google Scholar] [CrossRef]
- Curran, J.; Chen, R.; Hunt, J. Controlling the phenotype and function of mesenchymal stem cells in vitro by adhesion to silane-modified clean glass surfaces. Biomaterials 2005, 26, 7057–7067. [Google Scholar] [CrossRef]
- Phillips, J.E.; Petrie, T.A.; Creighton, F.P.; García, A.J. Human mesenchymal stem cell differentiation on self-assembled monolayers presenting different surface chemistries. Acta Biomater. 2010, 6, 12–20. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Shu, Y.; Wu, Z.; Cao, W.; Huang, W. Amino-functionalized mesoporous bioactive glass for drug delivery. Biomed. Mater. 2017, 12, 025017. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, X.; Xie, C.; Zhuang, H.; Zhou, S.; Weng, J. Hydroxyapatite nucleation and growth mechanism on electrospun fibers functionalized with different chemical groups and their combinations. Biomaterials 2010, 31, 4620–4629. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res. Part A 2003, 66, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Tzoneva, R.; Faucheux, N.; Groth, T. Wettability of substrata controls cell–substrate and cell–cell adhesions. BBA. Gen. Subj. 2007, 1770, 1538–1547. [Google Scholar] [CrossRef]
- Sperling, C.; Fischer, M.; Maitz, M.F.; Werner, C. Blood coagulation on biomaterials requires the combination of distinct activation processes. Biomaterials 2009, 30, 4447–4456. [Google Scholar] [CrossRef] [PubMed]
- Roohani-Esfahani, S.; Nouri-Khorasani, S.; Lu, Z.; Appleyard, R.; Zreiqat, H. Effects of bioactive glass nanoparticles on the mechanical and biological behavior of composite coated scaffolds. Acta Biomater. 2011, 7, 1307–1318. [Google Scholar] [CrossRef]
- Shu, X.-l.; Shi, Q.-s.; Feng, J.; Yang, Y.-h.; Zhou, G.; Li, W.-r. Poly (γ-glutamic acid)/beta-TCP nanocomposites via in situ copolymerization: Preparation and characterization. J. Biomater. Appl. 2016, 31, 102–111. [Google Scholar] [CrossRef]
- Yusa, K.; Yamamoto, O.; Iino, M.; Takano, H.; Fukuda, M.; Qiao, Z.; Sugiyama, T. Eluted zinc ions stimulate osteoblast differentiation and mineralization in human dental pulp stem cells for bone tissue engineering. Arch. Oral Biol. 2016, 71, 162–169. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, B.; Hu, K.; Cao, C.; Man, Y.; Wang, P. Deriving osteogenic cells from induced pluripotent stem cells for bone tissue engineering. Tissue Eng. Part B Rev. 2017, 23, 1–8. [Google Scholar] [CrossRef]
- Mali, P.; Cheng, L. Concise review: Human cell engineering: Cellular reprogramming and genome editing. Stem Cells 2012, 30, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, C.-H.; Moon, J.-I.; Chung, Y.-G.; Chang, M.-Y.; Han, B.-S.; Ko, S.; Yang, E.; Cha, K.Y.; Lanza, R. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009, 4, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, Y.; Ke, X.; Xie, Y.; Zhu, H.; Crawford, R.; Xiao, Y. Bioactive mesopore-glass microspheres with controllable protein-delivery properties by biomimetic surface modification. J. Biomed. Mater. Res. Part A 2010, 95, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, E.; Marquis, M.; Chretien, I.; Faucheux, N. Differentiation of preosteoblasts using a delivery system with BMPs and bioactive glass microspheres. J. Mater. Sci. Mater. Med. 2007, 18, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- de Wild, M.; Zimmermann, S.; Rüegg, J.; Schumacher, R.; Fleischmann, T.; Ghayor, C.; Weber, F.E. Influence of Microarchitecture on Osteoconduction and Mechanics of Porous Titanium Scaffolds Generated by Selective Laser Melting. 3D Print. Addit. Manuf. 2016, 3, 142–151. [Google Scholar] [CrossRef]
- Kapat, K.; Srivas, P.K.; Rameshbabu, A.P.; Maity, P.P.; Jana, S.; Dutta, J.; Majumdar, P.; Chakrabarti, D.; Dhara, S. Influence of Porosity and Pore-Size Distribution in Ti6Al4 V Foam on Physicomechanical Properties, Osteogenesis, and Quantitative Validation of Bone Ingrowth by Micro-Computed Tomography. ACS Appl. Mater. Interfaces 2017, 9, 39235–39248. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Nieto, A.; Vallet-Regí, M. Hydroxyapatite/β-tricalcium phosphate/agarose macroporous scaffolds for bone tissue engineering. Chem. Eng. J. 2008, 137, 62–71. [Google Scholar] [CrossRef]
- Nandi, S.K.; Fielding, G.; Banerjee, D.; Bandyopadhyay, A.; Bose, S. 3D-printed β-TCP bone tissue engineering scaffolds: Effects of chemistry on in vivo biological properties in a rabbit tibia model. J. Mater. Res. 2018, 33, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Konopnicki, S.; Sharaf, B.; Resnick, C.; Patenaude, A.; Pogal-Sussman, T.; Hwang, K.-G.; Abukawa, H.; Troulis, M.J. Tissue-engineered bone with 3-dimensionally printed β-tricalcium phosphate and polycaprolactone scaffolds and early implantation: An in vivo pilot study in a porcine mandible model. J. Oral Maxillofac. Surg. 2015, 73, 1016.e1–1016.e11. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and 8nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Fan, J.P.; Kalia, P.; Di Silvio, L.; Huang, J. In vitro response of human osteoblasts to multi-step sol–gel derived bioactive glass nanoparticles for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 36, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Maçon, A.L.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Wang, X.; Song, G.; Gu, Z.; Yang, Z. Structure and properties of PLLA/β-TCP nanocomposite scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2015, 26, 34. [Google Scholar] [CrossRef] [PubMed]
- Fielding, G.; Bose, S. SiO2 and ZnO dopants in three-dimensionally printed tricalcium phosphate bone tissue engineering scaffolds enhance osteogenesis and angiogenesis in vivo. Acta Biomater. 2013, 9, 9137–9148. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Petersen, J.; Fielding, G.; Banerjee, S.; Bose, S. ZnO, SiO2, and SrO doping in resorbable tricalcium phosphates: Influence on strength degradation, mechanical properties, and in vitro bone–Cell material interactions. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2203–2212. [Google Scholar] [CrossRef]
- Philippart, A.; Gómez-Cerezo, N.; Arcos, D.; Salinas, A.J.; Boccardi, E.; Vallet-Regi, M.; Boccaccini, A.R. Novel ion-doped mesoporous glasses for bone tissue engineering: Study of their structural characteristics influenced by the presence of phosphorous oxide. J. Non-Cryst. Solids 2017, 455, 90–97. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S.; Banerjee, S.S.; Davies, N.M.; Bandyopadhyay, A. Understanding in vivo response and mechanical property variation in MgO, SrO and SiO2 doped β-TCP. Bone 2011, 48, 1282–1290. [Google Scholar] [CrossRef]
- Yuan, J.; Cui, L.; Zhang, W.J.; Liu, W.; Cao, Y. Repair of canine mandibular bone defects with bone marrow stromal cells and porous β-tricalcium phosphate. Biomaterials 2007, 28, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Ioku, K.; Yonezawa, I.; Minagi, H.; Kawachi, G.; Gonda, Y.; Murayama, H.; Shibata, Y.; Minami, S.; Kamihira, S. The effect of the microstructure of β-tricalcium phosphate on the metabolism of subsequently formed bone tissue. Biomaterials 2007, 28, 2612–2621. [Google Scholar] [CrossRef]
- Stewart, R.; Goldstein, J.; Eberhardt, A.; Chu, T.-M.G.; Gilbert, S. Increasing vascularity to improve healing of a segmental defect of the rat femur. J. Orthop. Trauma 2011, 25, 472. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Gilbert, S.R.; Wang, Y.; Cao, X.; Shen, X.; Ramaswamy, G.; Jacobsen, K.A.; Alaql, Z.S.; Eberhardt, A.W.; Gerstenfeld, L.C. Activation of the hypoxia-inducible factor-1α pathway accelerates bone regeneration. Proc. Natl. Acad. Sci. USA 2008, 105, 686–691. [Google Scholar] [CrossRef]
- Yin, J.; Gong, G.; Sun, C.; Yin, Z.; Zhu, C.; Wang, B.; Hu, Q.; Zhu, Y.; Liu, X. Angiopoietin 2 promotes angiogenesis in tissue-engineered bone and improves repair of bone defects by inducing autophagy. Biomed. Pharmacother. 2018, 105, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Szivek, J.A.; Gonzales, D.A.; Wojtanowski, A.M.; Martinez, M.A.; Smith, J.L. Mesenchymal stem cell seeded, biomimetic 3D printed scaffolds induce complete bridging of femoral critical sized defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 242–252. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Q.; Shores, L.S.; Aly, A.; Ramakrishnan, M.; Kim, G.H.; Lu, Q.; Su, L.; Elisseeff, J.H. Use of a chondroitin sulfate bioadhesive to enhance integration of bioglass particles for repairing critical-size bone defects. J. Biomed. Mater. Res. A 2015, 103, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Lin, S.; Yue, S.; Lee, P.; Hanna, J.V.; Smith, M.E.; Newport, R.J. Bioactive glass scaffolds for bone regeneration and their hierarchical characterisation. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1373–1387. [Google Scholar] [CrossRef]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef]
- Munukka, E.; Leppäranta, O.; Korkeamäki, M.; Vaahtio, M.; Peltola, T.; Zhang, D.; Hupa, L.; Ylänen, H.; Salonen, J.I.; Viljanen, M.K. Bactericidal effects of bioactive glasses on clinically important aerobic bacteria. J. Mater. Sci. Mater. Med. 2008, 19, 27–32. [Google Scholar] [CrossRef]
- Seidenstuecker, M.; Mrestani, Y.; Neubert, R.H.; Bernstein, A.; Mayr, H.O. Release kinetics and antibacterial efficacy of microporous β-TCP coatings. J. Nanomater. 2013, 2013, 13. [Google Scholar] [CrossRef]
- Liu, S.; Fan, C.; Jin, F.; Zhao, L.; Dai, K.; Lu, J. Preparation and Antibacterial Activities of Porous Silver-doped β-Tricalcium Phosphate Bioceramics. Int. J. Appl. Ceram. Technol. 2015, 12, 294–299. [Google Scholar] [CrossRef]
- Piccirillo, C.; Pullar, R.; Tobaldi, D.; Castro, P.L.; Pintado, M.E. Silver-containing calcium phosphate materials of marine origin with antibacterial activity. Ceram. Int. 2015, 41, 10152–10159. [Google Scholar] [CrossRef]
- Labay, C.; Buxadera-Palomero, J.; Avilés, M.; Canal, C.; Ginebra, M. Modulation of release kinetics by plasma polymerization of ampicillin-loaded β-TCP ceramics. J. Phys. D Appl. Phys. 2016, 49, 304004. [Google Scholar] [CrossRef]
- Nazemi, Z.; Mehdikhani-Nahrkhalaji, M.; Nazarpak, M.H.; Staji, H. The Antibacterial Activity Evaluation of Sol–Gel Prepared Zn-Doped Biphasic Calcium Phosphate Nanopowders. Adv. Sci. Eng. Med. 2015, 7, 995–1002. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Hurley, L.A.; Stinchfield, F.E.; Bassett, C.A.L.; Lyon, W.H. The Role of Soft Tissues in Osteogenesis: An Experimental Study of Canine Spine Fusions. J. Bone Jt. Surg. Am. 1959, 41, 1243–1266. [Google Scholar] [CrossRef]
- Shim, J.-H.; Won, J.-Y.; Sung, S.-J.; Lim, D.-H.; Yun, W.-S.; Jeon, Y.-C.; Huh, J.-B. Comparative efficacies of a 3D-printed PCL/PLGA/β-TCP membrane and a titanium membrane for guided bone regeneration in beagle dogs. Polymers 2015, 7, 2061–2077. [Google Scholar] [CrossRef]
- Rodrigues, J.R.; Alves, N.M.; Mano, J.F. Biomimetic polysaccharide/bioactive glass nanoparticles multilayer membranes for guided tissue regeneration. RSC Adv. 2016, 6, 75988–75999. [Google Scholar] [CrossRef]
- Shim, J.-H.; Yoon, M.-C.; Jeong, C.-M.; Jang, J.; Jeong, S.-I.; Cho, D.-W.; Huh, J.-B. Efficacy of rhBMP-2 loaded PCL/PLGA/β-TCP guided bone regeneration membrane fabricated by 3D printing technology for reconstruction of calvaria defects in rabbit. Biomed. Mater. 2014, 9, 065006. [Google Scholar] [CrossRef]
- Won, J.; Park, C.; Bae, J.; Ahn, G.; Kim, C.; Lim, D.; Cho, D.; Yun, W.; Shim, J.; Huh, J. Evaluation of 3D printed PCL/PLGA/β-TCP versus collagen membranes for guided bone regeneration in a beagle implant model. Biomed. Mater. 2016, 11, 055013. [Google Scholar] [CrossRef]
- Smeets, R.; Knabe, C.; Kolk, A.; Rheinnecker, M.; Gröbe, A.; Heiland, M.; Zehbe, R.; Sachse, M.; Große-Siestrup, C.; Wöltje, M. Novel silk protein barrier membranes for guided bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2603–2611. [Google Scholar] [CrossRef]
- Dahlin, C.; Obrecht, M.; Dard, M.; Donos, N. Bone tissue modelling and remodelling following guided bone regeneration in combination with biphasic calcium phosphate materials presenting different microporosity. Clin. Oral Implant. Res. 2015, 26, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Noritake, K.; Kuroda, S.; Nyan, M.; Atsuzawa, Y.; Uo, M.; Ohya, K.; KASUGAI, S. Use of a gelatin hydrogel membrane containing β-tricalcium phosphate for guided bone regeneration enhances rapid bone formation. Dent. Mater. J. 2014, 33, 674–680. [Google Scholar] [CrossRef]
- Al-Askar, M.; Javed, F.; Al-Hezaimi, K.; Al-Hamdan, K.S.; Ramalingam, S.; Aldahmash, A.; Nooh, N.; Al-Rasheed, A. Guided Bone Regeneration in Standardized Calvarial Defects in Rats Using Bio-Oss and β-Tricalcium Phosphate with Adjunct Platelet-Derived Growth Factor Therapy: A Real-Time In Vivo Microcomputed Tomographic, Biomechanical, and Histologic Analysis. Int. J. Periodontics Restor. Dent. 2016, 36, s61–s73. [Google Scholar] [CrossRef]
- Fernandes, J.S.; Gentile, P.; Martins, M.; Neves, N.M.; Miller, C.; Crawford, A.; Pires, R.A.; Hatton, P.; Reis, R.L. Reinforcement of poly-l-lactic acid electrospun membranes with strontium borosilicate bioactive glasses for bone tissue engineering. Acta Biomater. 2016, 44, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ding, Y.; Yu, S.; Yao, Q.; Boccaccini, A.R. Multifunctional chitosan-45S5 bioactive glass-poly (3-hydroxybutyrate-co-3-hydroxyvalerate) microsphere composite membranes for guided tissue/bone regeneration. ACS Appl. Mater. Interfaces 2015, 7, 20845–20854. [Google Scholar] [CrossRef] [PubMed]
| Material | Method | Form | Activity Test | Observations | Ref. |
|---|---|---|---|---|---|
| Ag/β-TCP | Doping | Nanoparticles Pore interconnectivity with even distribution of macropores. | L929 cells; S. epidermidis and S. aureus | Significant inhibition of bacteria and no toxic effect to fibroblast cells | [101] |
| PEG/β-TCP | Plasma polymerization | Disc | S. aureus | Exhibited a controlled release profile of antimicrobial drug and showed astrong bactericidal effect | [103] |
| Zn/β-TCP | Sol-gel | Nanoparticles Nanoparticle size (10-500nm) | S.aureus, E. coli, S. typhi | Zn-β-TCP (3.2wt% of Zn) showed the most antibacterial activity | [104] |
| Material | Fabrication | Model/Defect | Time Points | Results | Ref. |
|---|---|---|---|---|---|
| rhBMP-2/PCL/PLGA/β-TCP | 3D printing | Calvaria, rabbit | 8 weeks | Bone turn over significantly higher than control group (p < 0.05). Bone to implant contact ratio significantly higher (p < 0.05). Full or partial absorption of implant observed. | [111] |
| PCL/PLGA/β-TCP/rhBMP-2 | 3D printing | Lower Jaw, Beagle Dog | 4 to 8 weeks | The stability of the membrane was maintained at 4 weeks (post-implantation). Complete healing; new bone deposition observed. Significant increase in bone formation from 4 to 8 weeks. No inflammatory reaction. | [109] |
| PCL/PLGA/β-TCP | 3D printing | Extracted premolars; mandibular alveolar ridge; Beagle Dog | 8 weeks | Higher levels of new bone area and bone implant contact, compared to the control (collagen membrane). Remaining biomaterial was much higher compared to control. Results were insignificant to each other. | [112] |
| Modified Silk/β-TCP | Casting, particle deposition | Rabbit; Calvaria | 5 and 10 weeks | Control (collagen membrane) Rate of resorption higher in control compared to membrane. Silk/β-TCP highly support bone formation and restoration of microarchitecture of defect compared to the control. Less statistical difference from histomorphometric data between the two (p < 0.05). | [113] |
| β-TCP/HA granules | N/A | Minipig; Lower premolar | 3 and 8 weeks | Group with higher percentage of β-TCP (90%) showed more mineralized bone. | [114] |
| Gelatin/β-TCP | Freeze-dried/Cross-linking | Calvaria, Rat | 2, 4, 8 weeks | Bone volume was higher in Gelatin/β-TCP membrane compared to control. Absorption was greater in collagen compared to Gelatin/β-TCP. No significant difference in bone volume between collagen membrane and Gelatin/β-TCP. | [115] |
| Bio-Oss/β-TCP/rhPDGF | N/A | Calvaria; Rat | 2, 4, 6, 8, 10 weeks | Significant increase in bone mineral density. A 30% reduction in mean volume of remnant bone particles. | [116] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lowe, B.; Ottensmeyer, M.P.; Xu, C.; He, Y.; Ye, Q.; Troulis, M.J. The Regenerative Applicability of Bioactive Glass and Beta-Tricalcium Phosphate in Bone Tissue Engineering: A Transformation Perspective. J. Funct. Biomater. 2019, 10, 16. https://doi.org/10.3390/jfb10010016
Lowe B, Ottensmeyer MP, Xu C, He Y, Ye Q, Troulis MJ. The Regenerative Applicability of Bioactive Glass and Beta-Tricalcium Phosphate in Bone Tissue Engineering: A Transformation Perspective. Journal of Functional Biomaterials. 2019; 10(1):16. https://doi.org/10.3390/jfb10010016
Chicago/Turabian StyleLowe, Baboucarr, Mark P. Ottensmeyer, Chun Xu, Yan He, Qingsong Ye, and Maria J. Troulis. 2019. "The Regenerative Applicability of Bioactive Glass and Beta-Tricalcium Phosphate in Bone Tissue Engineering: A Transformation Perspective" Journal of Functional Biomaterials 10, no. 1: 16. https://doi.org/10.3390/jfb10010016
APA StyleLowe, B., Ottensmeyer, M. P., Xu, C., He, Y., Ye, Q., & Troulis, M. J. (2019). The Regenerative Applicability of Bioactive Glass and Beta-Tricalcium Phosphate in Bone Tissue Engineering: A Transformation Perspective. Journal of Functional Biomaterials, 10(1), 16. https://doi.org/10.3390/jfb10010016






