Objective Assessment of Cognition for Detecting Subjective Cognitive Decline Is More Accurate than Subjective Estimations: The Role of Trait Affect
Abstract
1. Introduction
Aim and Hypotheses of the Study
2. Materials and Methods
2.1. Participants
2.2. Instruments
2.2.1. The Positive and Negative Affect Schedule (PANAS: Trait Version)
2.2.2. Multifactorial Memory Questionnaire (MMQ): Ability Scale
2.2.3. R4Alz-pc Index
2.3. Procedure
2.4. Ethics
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Limitations of the Study
4.2. Future Research Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Albert, Marilyn S., Steven T. DeKosky, Dennis Dickson, Bruno Dubois, Howard H. Feldman, Nick C. Fox, Anthony Gamst, David M. Holtzman, William J. Jagust, Ronald C. Petersen, and et al. 2011. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 7: 270–79. [Google Scholar] [CrossRef]
- Aschwanden, Damaris, Matthias Kliegel, and Mathias Allemand. 2018. Cognitive complaints mediate the effect of cognition on emotional stability across 12 years in old age. Psychology and Aging 33: 425–38. [Google Scholar] [CrossRef] [PubMed]
- Bampa, Grigoria, Magdalini Tsolaki, Despina Moraitou, Panagiota Metallidou, Elvira Masoura, Maria Mintziviri, Konstantinos Paparis, Dorothea Tsourou, Georgia Papantoniou, Maria Sofologi, and et al. 2023. Metacognitive differences in amnestic mild cognitive impairment and healthy cognition: A cross-sectional study employing online measures. Journal of Intelligence 11: 184. [Google Scholar] [CrossRef] [PubMed]
- Bentler, P. M. 2020. EQS 6.4 for Windows: Structural Equation Modeling Program [Computer software]. München: Multivariate Software, Inc. [Google Scholar]
- Buratti, Sandra, Carl Martin Allwood, and Sabina Kleitman. 2013. First- and second-order metacognitive judgments of semantic memory reports: The influence of personality traits and cognitive styles. Metacognition Learning 8: 79–102. [Google Scholar] [CrossRef]
- Burmester, Bridget, Janet Leathem, and Paul Merrick. 2016. Subjective cognitive complaints and objective cognitive function in aging: A systematic review and meta-analysis of recent cross-sectional findings. Neuropsychology Review 26: 376–93. [Google Scholar] [CrossRef]
- Carstensen, Laura L., and Joseph A. Mikels. 2005. At the intersection of emotion and cognition: Aging and the positivity effect. Current Directions in Psychological Science 14: 117–21. [Google Scholar] [CrossRef]
- Colvin, Leigh E., Matteo Malgaroli, Silvia Chapman, Anna MacKay-Brandt, and Stephanie Cosentino. 2018. Mood and Personality Characteristics are Associated with Metamemory Knowledge Accuracy in a Community-Based Cohort of Older Adults. Journal of the International Neuropsychological Society 24: 498–510. [Google Scholar] [CrossRef]
- Costa, Amy N., Lauren M. Nowakowski, Christina S. McCrae, Nelson Cowan, and Ashley F. Curtis. 2023. Discrepancies in objective and subjective cognition in middle-aged and older adults: Does personality matter? Gerontology and Geriatric Medicine 9: 1–11. [Google Scholar] [CrossRef]
- Crumley, Jessica J., Cinnamon A. Stetler, and Michelle Horhota. 2014. Examining the relationship between subjective and objective memory performance in older adults: A meta-analysis. Psychology and Aging 29: 250–63. [Google Scholar] [CrossRef]
- Diamond, Adele. 2013. Executive functions. Annual Review of Psychology 64: 135–68. [Google Scholar] [CrossRef]
- Drabo, Emmanuel F., Jennifer L. Wolff, Linda C. Chyr, Julie Zissimopoulos, and Bryan Lau. 2025. Subjective Cognitive Impairment (SCI) and Future Dementia Risk in the National Health and Aging Trends Study (NHATS) During 2012–2019. Journal of Aging and Health 37 Suppl. 3–4: 76S–90S. [Google Scholar] [CrossRef]
- Dubois, Bruno, Christine A. F. von Arnim, Nerida Burnie, Sasha Bozeat, and Jeffrey Cummings. 2023. Biomarkers in Alzheimer’s disease: Role in early and differential diagnosis and recognition of atypical variants. Alzheimer’s Research & Therapy 15: 175. [Google Scholar] [CrossRef]
- Dubois, Bruno, Harald Hampel, Howard H. Feldman, Philip Scheltens, Paul Aisen, Sandrine Andrieu, Hovagim Bakardjian, Habib Benali, Lars Bertram, Kaj Blennow, and et al. 2016. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s & Dementia 12: 292–323. [Google Scholar] [CrossRef]
- Efklides, Anastasia. 2011. Interactions of Metacognition with Motivation and Affect in Self-Regulated Learning: The MASRL Model. Educational Psychologist 46: 6–25. [Google Scholar] [CrossRef]
- Emmanouilidou, Eudokia., Despina Moraitou, and Nikoleta Frantzi. 2023. Subjective Cognitive Decline and/or Objective Cognitive Decline in middle-aged adults? Examining the relationship between Cognitive Complaints and the Function of the Episodic Buffer. Scientific Yearbook of the Department of Early Childhood Education, University of Ioannina 16: 92–113. [Google Scholar]
- Engle, Randall W., and Michael J. Kane. 2003. Executive attention, working memory capacity, and a two-factor theory of cognitive control. Psychology of Learning and Motivation 44: 145–99. [Google Scholar] [CrossRef]
- Faul, Franz, Edgar Erdfelder, Albert-Georg Lang, and Axel Buchner. 2007. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods 39: 175–91. [Google Scholar] [CrossRef]
- Flavell, John H. 1979. Metacognition and cognitive monitoring: A new area of cognitive—Developmental inquiry. American Psychologist 34: 906–11. [Google Scholar] [CrossRef]
- Harrington, Michael G., Jiarong Chiang, Janice M. Pogoda, Megan Gomez, Kris Thomas, Sarah DeBoard Marion, Karen J. Miller, Prabha Siddarth, Xinyao Yi, Feimeng Zhou, and et al. 2013. Executive function changes before memory in preclinical Alzheimer’s pathology: A prospective, cross-sectional, case control study. PLoS ONE 8: e79378. [Google Scholar] [CrossRef]
- Hill, Nikki L., Jacqueline Mogle, Rachel Wion, Elizabeth Munoz, Nicole DePasquale, Andrea M. Yevchak, and Jeanine M. Parisi. 2016. Subjective Cognitive Impairment and Affective Symptoms: A Systematic Review. The Gerontologist 56: e109–e127. [Google Scholar] [CrossRef]
- Huff, Mark J., David A. Balota, Andrew J. Aschenbrenner, Janet M. Duchek, Anne M. Fagan, David M. Holtzman, Tammie L.S. Benzinger, and John C. Morris. 2015. P2-085: Task-Switching Errors Show Sensitivity to Preclinical Alzheimer’s Disease Biomarkers. Alzheimer’s & Dementia 11: P516. [Google Scholar] [CrossRef]
- Hülür, Gizem, Christopher Hertzog, Ann M. Pearman, and Denis Gerstorf. 2015. Correlates and moderators of change in subjective memory and memory performance: Findings from the Health and Retirement Study. Gerontology 61: 232–40. [Google Scholar] [CrossRef]
- Jack, Clifford R., David A. Bennett, Kaj Blennow, Maria C. Carrillo, Billy Dunn, Samantha Budd Haeberlein, David M. Holtzman, William Jagust, Frank Jessen, Jason Karlawish, and et al. 2018. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s & Dementia 14: 535–62. [Google Scholar] [CrossRef]
- Jenkins, Amy, Jeremy Tree, and Andrea Tales. 2021. Distinct profile differences in subjective cognitive decline in the general public are associated with metacognition, negative affective symptoms, neuroticism, stress, and poor quality of life. Journal of Alzheimer’s Disease 80: 1231–42. [Google Scholar] [CrossRef] [PubMed]
- Jessen, Frank, Rebecca E. Amariglio, Martin van Boxtel, Monique Breteler, Mathieu Ceccaldi, Gaël Chételat, Bruno Dubois, Carole Dufouil, Kathryn A. Ellis, Wiesje M. van der Flier, and et al. 2014. Subjective Cognitive Decline Initiative (SCD-I) Working Group. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia 10: 844–52. [Google Scholar] [CrossRef]
- Jessen, Frank, Rebecca E. Amariglio, Rachel F. Buckley, Wiesje M. van der Flier, Ying Han, José Luis Molinuevo, Laura Rabin, Dorene M. Rentz, Octavio Rodriguez-Gomez, Andrew J. Saykin, and et al. 2020. The characterisation of subjective cognitive decline. The Lancet Neurology 19: 271–78. [Google Scholar] [CrossRef]
- Johns, Erin K., Natalie A. Phillips, Sylvie Belleville, Diane Goupil, Lennie Babins, Nora Kelner, Bernadette Ska, Brigitte Gilbert, Fadi Massoud, Chloé de Boysson, and et al. 2012. The profile of executive functioning in amnestic mild cognitive impairment: Disproportionate deficits in inhibitory control. Journal of the International Neuropsychological Society 18: 541–55. [Google Scholar] [CrossRef]
- Könen, Tanja, and Julia Karbach. 2018. Self-reported cognitive failures in everyday life: A closer look at their relation to personality and cognitive performance. Assessment 27: 982–95. [Google Scholar] [CrossRef]
- Kuhn, Elizabeth, Audrey Perrotin, Clémence Tomadesso, Claire André, Siya Sherif, Alexandre Bejanin, Edelweiss Touron, Brigitte Landeau, Florence Mezenge, Denis Vivien, and et al. 2021. Subjective cognitive decline: Opposite links to neurodegeneration across the Alzheimer’s continuum. Brain Communications 3: fcab199. [Google Scholar] [CrossRef]
- Lange, Stefanie, and Heinz-Martin Süß. 2014. Measuring slips and lapses when they occur—Ambulatory assessment in application to cognitive failures. Consciousness and Cognition 24: 1–11. [Google Scholar] [CrossRef]
- Lavie, Nilli, Aleksandra Hirst, Jan W. de Fockert, and Essi Viding. 2004. Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General 133: 339–54. [Google Scholar] [CrossRef]
- Lenehan, Megan Elizabeth, Shannon Zofia Klekociuk, and Mathew James Summers. 2012. Absence of a relationship between subjective memory complaint and objective memory impairment in mild cognitive impairment (MCI): Is it time to abandon subjective memory complaint as an MCI diagnostic criterion? International Psychogeriatrics 24: 1505–14. [Google Scholar] [CrossRef]
- López-Higes, Ramón, Susana Rubio-Valdehita, David López-Sanz, Sara M. Fernandes, Pedro F. S. Rodrigues, and María Luisa Delgado-Losada. 2025. Cognitive performance among older adults with subjective cognitive decline. Geriatrics 10: 39. [Google Scholar] [CrossRef] [PubMed]
- Mather, Mara, and Laura L. Carstensen. 2005. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences 9: 496–502. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, Niklas, Philip S. Insel, Rachel Nosheny, Duygu Tosun, John Q. Trojanowski, Leslie M. Shaw, Clifford R. Jack, Michael C. Donohue, and Michael W. Weiner. 2014. Emerging β-amyloid pathology and accelerated cortical atrophy. JAMA Neurology 71: 725–34. [Google Scholar] [CrossRef]
- Minett, Thaís Soares Cianciarullo, Rosimeire Vieira Da Silva, Karin Zazo Ortiz, and Paulo Henrique Ferreira Bertolucci. 2008. Subjective memory complaints in an elderly sample: A cross-sectional study. International Journal of Geriatric Psychiatry 23: 49–54. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Akira, Naomi P. Friedman, Michael J. Emerson, Alexander H. Witzki, Amy Howerter, and Tor D. Wager. 2000. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology 41: 49–100. [Google Scholar] [CrossRef]
- Moraitou, Despina, and Anastasia Efklides. 2009. The Blank in the Mind Questionnaire (BIMQ). European Journal of Psychological Assessment 25: 115–22. [Google Scholar] [CrossRef]
- Mulligan, Bryce P., Colette M. Smart, and Jordan I. Ali. 2016. Relationship of subjective and objective performance indicators in subjective cognitive decline. Psychology & Neuroscience 9: 362–78. [Google Scholar] [CrossRef]
- Pantsiou, Krystallia, Ourania Sfakianaki, Vasileios Papaliagkas, Dimitra Savvoulidou, Vassiliki Costa, Georgia Papantoniou, and Despina Moraitou. 2018. Inhibitory control, task/rule switching, and cognitive planning in vascular dementia: Are there any differences from vascular aging? Frontiers in Aging Neuroscience 10: 330. [Google Scholar] [CrossRef]
- Petersen, R. C., B. Caracciolo, C. Brayne, S. Gauthier, V. Jelic, and L. Fratiglioni. 2014. Mild cognitive impairment: A concept in evolution. Journal of Internal Medicine 275: 214–28. [Google Scholar] [CrossRef]
- Pérez-Cordón, Alba, Gemma Monté-Rubio, Angela Sanabria, Octavio Rodriguez-Gomez, Sergi Valero, Carla Abdelnour, Marta Marquié, Ana Espinosa, Gemma Ortega, Isabel Hernandez, and et al. 2020. Subtle executive deficits are associated with higher brain amyloid burden and lower cortical volume in subjective cognitive decline: The FACEHBI cohort. Scientific Reports 10: 17721. [Google Scholar] [CrossRef]
- Poptsi, Eleni. 2024. Development of a Cognitive Control Assessment Battery in Aging: Adaptation of the Reaction Measurement System “REMEDES”. Ph.D. Dissertation, Aristotle University of Thessaloniki, IKEE Institutional Repository, Thessaloniki, Greece. [Google Scholar]
- Poptsi, Eleni, Despina Moraitou, Emmanouil Tsardoulias, Andreas L. Symeonidis, and Magda Tsolaki. 2024. Subjective Cognitive Impairment Can Be Detected from the Decline of Complex Cognition: Findings from the Examination of Remedes 4 Alzheimer’s (R4Alz) Structural Validity. Brain Sciences 14: 548. [Google Scholar] [CrossRef]
- Poptsi, Eleni, Despina Moraitou, Emmanouil Tsardoulias, Andreas L. Symeonidis, Vasileios Papaliagkas, and Magdalini Tsolaki. 2023. R4Alz-Revised: A Tool Able to Strongly Discriminate ‘Subjective Cognitive Decline’ from Healthy Cognition and ‘Minor Neurocognitive Disorder’. Diagnostics 13: 338. [Google Scholar] [CrossRef] [PubMed]
- Poptsi, Eleni, Despina Moraitou, Emmanouil Tsardoulias, Andreas L. Symeonidisd, and Magda Tsolaki. 2020. Is the Discrimination of Subjective Cognitive Decline from Cognitively Healthy Adulthood and Mild Cognitive Impairment Possible? A Pilot Study Utilizing the R4Alz Battery. Journal of Alzheimer’s Disease 77: 715–32. [Google Scholar] [CrossRef]
- Poptsi, Eleni, Emmanouil Tsardoulias, Despina Moraitou, Andreas L. Symeonidis, and Magda Tsolaki. 2019. REMEDES for Alzheimer-R4Alz Battery: Design and Development of a New Tool of Cognitive Control Assessment for the Diagnosis of Minor and Major Neurocognitive Disorders. Journal of Alzheimer’s Disease 72: 783–801. [Google Scholar] [CrossRef] [PubMed]
- Rabi, Rahel, Brandon P. Vasquez, Claude Alain, Lynn Hasher, Sylvie Belleville, and Nicole D. Anderson. 2020. Inhibitory control deficits in individuals with amnestic mild cognitive impairment: A meta-analysis. Neuropsychology Review 30: 97–125. [Google Scholar] [CrossRef] [PubMed]
- Rabin, Laura A., Colette M. Smart, and Rebecca E. Amariglio. 2017. Subjective cognitive decline in preclinical Alzheimer’s disease. Annu. Rev. Clin. Psychol. 13: 369–396. [Google Scholar] [CrossRef]
- Rao, Akshata, Abhijith R. Rao, Bhawana Painkra, Sakthi Kiruthika, Sunil Kumar, Meenal Thakral, Swati Bajpai, Shodhan Aithal, Aparajit Ballav Dey, Prasun Chatterjee, and et al. 2025. Cognitive assessment across normal aging and subjective cognitive decline using PGIMS and Stroop color-word test: A cross-sectional study. Archives of Gerontology and Geriatrics Plus 2: 100133. [Google Scholar] [CrossRef]
- Ready, Rebecca E., Brian R. Ott, Janet Grace, and Deborah A. Cahn-Weiner. 2003. Apathy and executive dysfunction in mild cognitive impairment and Alzheimer disease. The American Journal of Geriatric Psychiatry 11: 222–28. [Google Scholar] [CrossRef]
- Reid, Louise M., and Alasdair M.J. MacLullich. 2006. Subjective memory complaints and cognitive impairment in older people. Dementia and Geriatric Cognitive Disorders 22: 471–85. [Google Scholar] [CrossRef]
- Scheltens, Philip, Bart De Strooper, Miia Kivipelto, Henne Holstege, Gael Chételat, Charlotte E Teunissen, Jeffrey Cummings, and Wiesje M van der Flier. 2021. Alzheimer’s disease. The Lancet 397: 1577–90. [Google Scholar] [CrossRef]
- Schlosser, Marco, Harriet Demnitz-King, Tim Whitfield, Miranka Wirth, and Natalie L. Marchant. 2020. Repetitive negative thinking is associated with subjective cognitive decline in older adults: A cross-sectional study. BMC Psychiatry 20: 500. [Google Scholar] [CrossRef]
- Si, Tong, Guoqiang Xing, and Ying Han. 2020. Subjective Cognitive Decline and Related Cognitive Deficits. Frontiers in Neurology 11: 247. [Google Scholar] [CrossRef]
- Snitz, Beth E., Lisa A. Weissfeld, Ann D. Cohen, Oscar L. Lopez, Robert D. Nebes, Howard J. Aizenstein, Eric McDade, Julie C. Price, Chester A. Mathis, William E. Klunk, and et al. 2015. Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. American Journal of Geriatric Psychiatry 23: 985–93. [Google Scholar] [CrossRef] [PubMed]
- Sperling, Reisa A., Paul S. Aisen, Laurel A. Beckett, David A. Bennett, Suzanne Craft, Anne M. Fagan, Takeshi Iwatsubo, Clifford R. Jack, Jeffrey Kaye, Thomas J. Montine, and et al. 2011. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 7: 280–92. [Google Scholar] [CrossRef]
- Sutin, Angelina R., Damaris Aschwanden, Yannick Stephan, and Antonio Terracciano. 2020. Five-Factor Model personality traits and subjective cognitive failures. Personality and Individual Differences 150: 109741. [Google Scholar] [CrossRef] [PubMed]
- Troyer, Angela K., and Jill B. Rich. 2002. Psychometric properties of a new metamemory questionnaire for older adults. Journals of Gerontology: Psychological Sciences 57: 19–27. [Google Scholar] [CrossRef]
- Tsentidou, Glykeria, Despina Moraitou, Magdalini Tsolaki, Elvira Masoura, and Vasileios Papaliagkas. 2022. Trajectories of Cognitive Impairment in Adults Bearing Vascular Risk Factors, with or without Diagnosis of Mild Cognitive Impairment: Findings from a Longitudinal Study Assessing Executive Functions, Memory, and Social Cognition. Diagnostics 12: 3017. [Google Scholar] [CrossRef] [PubMed]
- Valech, Natalia, Adrià Tort-Merino, Nina Coll-Padrós, Jaume Olives, María León, Lorena Rami, and José Luis Molinuevo. 2017. Executive and Language Subjective Cognitive Decline Complaints Discriminate Preclinical Alzheimer’s Disease from Normal Aging. Journal of Alzheimer’s Disease 61: 689–703. [Google Scholar] [CrossRef]
- Verfaillie, Sander C. J., Tessa Timmers, Rosalinde E. R. Slot, Chris W. J. van der Weijden, Linda M. P. Wesselman, Niels D. Prins, Sietske A. M. Sikkes, Maqsood Yaqub, Annemiek Dols, Adriaan A. Lammertsma, and et al. 2019. Amyloid-β load is related to worries, but not to severity of cognitive complaints in individuals with subjective cognitive decline: The SCIENCe project. Alzheimer’s Research & Therapy 11: 90. [Google Scholar] [CrossRef]
- Vlassenko, Andrei G., Mark A. Mintun, Chengjie Xiong, Yvette I. Sheline, Alison M. Goate, Tammie L. S. Benzinger, and John C. Morris. 2011. Amyloid-Beta Plaque Growth in Cognitively Normal Adults: Longitudinal [11C] Pisburgh Compound B Data. Annals of Neurology 70: 857–61. [Google Scholar] [CrossRef]
- Watson, David, and Lori McKee Walker. 1996. The long-term stability and predictive validity of trait measures of affect. Journal of Personality and Social Psychology 70: 567–77. [Google Scholar] [CrossRef] [PubMed]
- Watson, David, Lee Anna Clark, and Auke Tellegen. 1988. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology 54: 1063–70. [Google Scholar] [CrossRef] [PubMed]
- Webster-Cordero, Felipe, and Lydia Giménez-Llort. 2022. The Challenge of Subjective Cognitive Complaints and Executive Functions in Middle-Aged Adults as a Preclinical Stage of Dementia: A Systematic Review. Geriatrics 7, 30. [Google Scholar] [CrossRef]
- Williams, Paula G., Yana Suchy, and Matthew L. Kraybill. 2010. Five-factor model personality traits and executive functioning among older adults. Journal of Research in Personality 44: 485–91. [Google Scholar] [CrossRef]
- Wolfsgruber, Steffen, Luca Kleineidam, Jannis Guski, Alexandra Polcher, Ingo Frommann, Sandra Roeske, Eike Jakob Spruth, Christiana Franke, Josef Priller, Ingo Kilimann, and et al. 2020. Minor Neuropsychological Deficits in Patients with Subjective Cognitive Decline. Neurology 95: e1134–e1143. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. 1997. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 8 July 2025).
- Zlatar, Zvinka Z, Martha Muniz, Douglas Galasko, and David P. Salmon. 2018. Subjective cognitive decline correlates with depression symptoms and not with concurrent objective cognition in a clinic-based sample of older adults. Journal of Gerontology: Series B, Psychological Sciences and Social Sciences 73: 1198–202. [Google Scholar] [CrossRef]
| Variable | n | % | M | SD | Range |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 70 | 66.7 | |||
| Male | 35 | 33.3 | |||
| Age (years) | 59.25 | 5.21 | 45–74 |
| 1 | |
|---|---|
| 18. You misplace something that you put away a few days ago. | 0.774 |
| 13. You forget to pass on a message. | 0.696 |
| 5. You leave something behind when you meant to bring it with you. | 0.695 |
| 20. You forget details about a recent conversation. | 0.663 |
| 8. You forget to run an errand. | 0.659 |
| 19. You forget to buy something you intended to buy. | 0.656 |
| 15. You forget a birthday or anniversary that you used to know well. | 0.637 |
| 7. You forget what you were just about to do; for example, walk into a room and forget what you went there to do. | 0.632 |
| 2. You misplace something you use daily, like your keys or glasses | 0.626 |
| 14. You forget what you were going to say in conversation | 0.609 |
| 6. You forget an appointment. | 0.605 |
| 10. You have trouble remembering details from a newspaper or magazine article you read earlier that day. | 0.592 |
| 1. You forget to pay a bill on time. | 0.550 |
| 9. You have difficulty coming up with a specific word that you want. | 0.547 |
| 17. You retell a story or joke to the same person because you forgot that you had already told him or her. | 0.545 |
| 16. You forget a telephone number you use frequently. | 0.513 |
| 3. You have trouble remembering a telephone number you just looked up. | 0.479 |
| 11. You forget to take your medication. | 0.478 |
| 12. You cannot recall the name of someone you have known for some time. | 0.447 |
| 4. You cannot recall the name of someone you just met. | 0.433 |
| Variable | Μ | SD | Range |
|---|---|---|---|
| MMQ-Ability | 54.11 | 10.47 | 22–79 |
| Negative Trait Affect | 21.97 | 6.67 | 10–39 |
| Positive Trait Affect | 34.79 | 7.04 | 19–49 |
| R4Alz-pc | 1.30 | 0.82 | 0.07–4.73 |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| 1. MMQ-Ability | 1 | |||
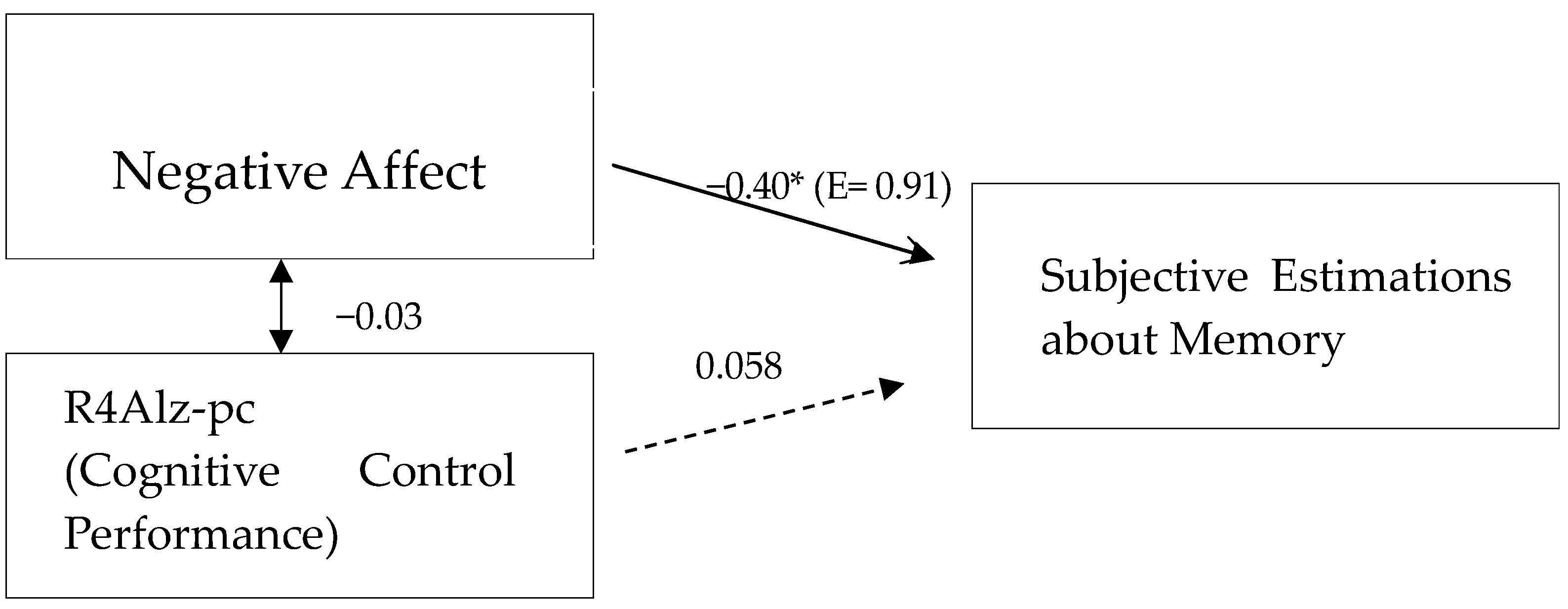
| 2. Negative Trait Affect | −0.40 ** | 1 | ||
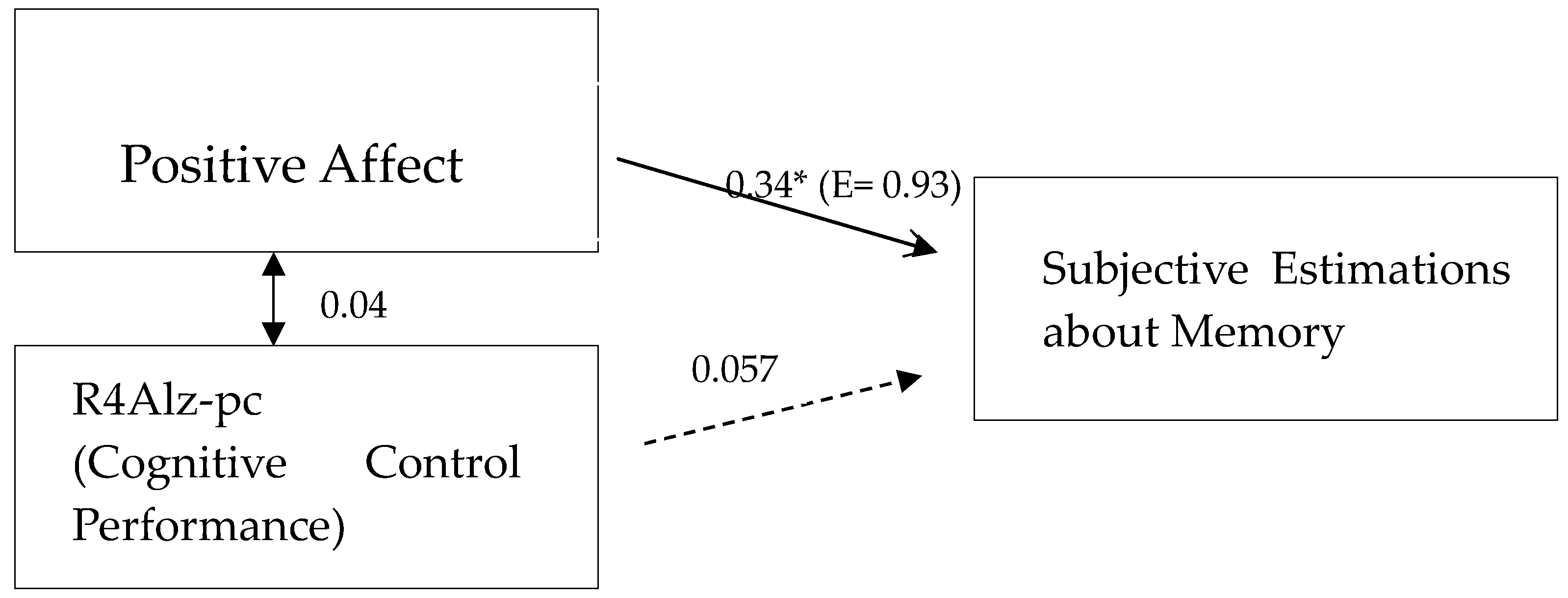
| 3. Positive Trait Affect | 0.34 ** | −0.35 ** | 1 | |
| 4. R4Alz-pcAD | 0.07 | −0.03 | 0.04 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frantzi, N.; Moraitou, D.; Emmanouilidou, E.; Poptsi, E.; Tsardoulias, E.; Symeonidis, A.L.; Papantoniou, G.; Sofologi, M.; Masoura, E.; Tsentidou, G.; et al. Objective Assessment of Cognition for Detecting Subjective Cognitive Decline Is More Accurate than Subjective Estimations: The Role of Trait Affect. J. Intell. 2025, 13, 118. https://doi.org/10.3390/jintelligence13090118
Frantzi N, Moraitou D, Emmanouilidou E, Poptsi E, Tsardoulias E, Symeonidis AL, Papantoniou G, Sofologi M, Masoura E, Tsentidou G, et al. Objective Assessment of Cognition for Detecting Subjective Cognitive Decline Is More Accurate than Subjective Estimations: The Role of Trait Affect. Journal of Intelligence. 2025; 13(9):118. https://doi.org/10.3390/jintelligence13090118
Chicago/Turabian StyleFrantzi, Nikoleta, Despina Moraitou, Eudokia Emmanouilidou, Eleni Poptsi, Emmanouil Tsardoulias, Andreas L. Symeonidis, Georgia Papantoniou, Maria Sofologi, Elvira Masoura, Glykeria Tsentidou, and et al. 2025. "Objective Assessment of Cognition for Detecting Subjective Cognitive Decline Is More Accurate than Subjective Estimations: The Role of Trait Affect" Journal of Intelligence 13, no. 9: 118. https://doi.org/10.3390/jintelligence13090118
APA StyleFrantzi, N., Moraitou, D., Emmanouilidou, E., Poptsi, E., Tsardoulias, E., Symeonidis, A. L., Papantoniou, G., Sofologi, M., Masoura, E., Tsentidou, G., Katsouri, I.-G., & Tsolaki, M. (2025). Objective Assessment of Cognition for Detecting Subjective Cognitive Decline Is More Accurate than Subjective Estimations: The Role of Trait Affect. Journal of Intelligence, 13(9), 118. https://doi.org/10.3390/jintelligence13090118










