Temporal Synchrony in Bodily Interaction Enhances the Aha! Experience: Evidence for an Implicit Metacognitive Predictive Processing Mechanism
Abstract
1. Introduction
1.1. Problem Awareness: Can the Aha! Experience Be Explained by the Metacognitive Predictive Processing Model?
1.1.1. From Representational Change to Metacognitive Predictive Processing: A Theoretical Shift in Understanding Aha! Experiences
1.1.2. The Predictive Processing Model as a Hierarchically Nested System: Theoretical Advantages for Explaining Aha! Experiences
1.1.3. The Challenge of Insight: How Does the Body Influence the Aha! Experience Through Implicit Metacognitive Predictive Processing?
1.2. How Bodily Interaction Influences the Aha! Experience: The Experimental Potential of the Mirror Game Paradigm
1.2.1. The Mirror Game as a Canonical Experimental Paradigm
1.2.2. The Experimental Potential of the Mirror Game Paradigm for Investigating How Bodily Interaction Influences the Aha! Experience
1.3. A Novel Experimental Paradigm of Metacognitive Predictive Processing for Investigating the Mechanism Linking Bodily Interaction and Insight
2. Experimental Procedure
2.1. Participants
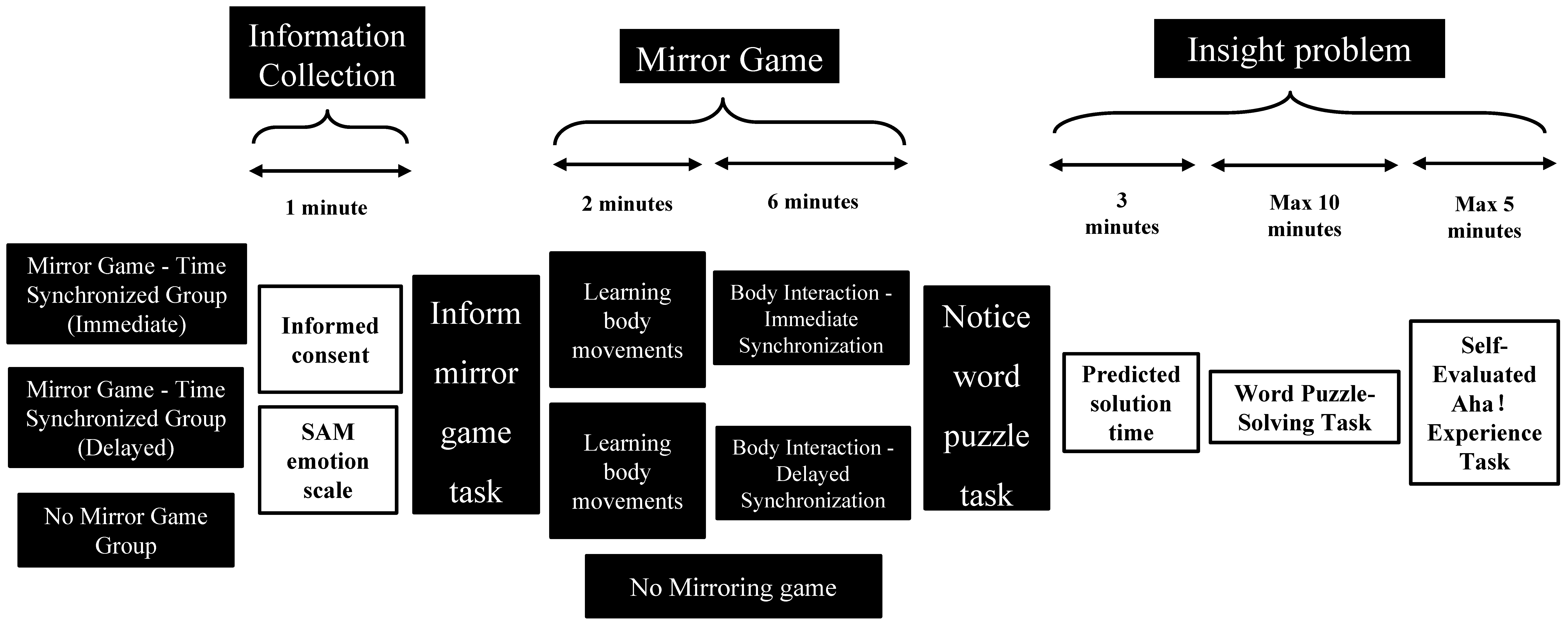
2.2. Experimental Design and Procedure
2.2.1. Information Collection Stage (Approximately 1 Min)
- (1)
- Informed Consent Form:
- (2)
- Self-Assessment Manikin (SAM) Scale:
2.2.2. Mirror Game Stage
- (3)
- Learning Phase (approximately 2 min):
- (4)
- Formal Experimental Phase (6 min):
2.2.3. Insight Problem Stage
- (1)
- Predicting the “Solution Time” (3 min):
- (2)
- Solving the Riddles (10 min):
- (3)
- Self-Assessment of “Aha! Experience” (about 5 min):
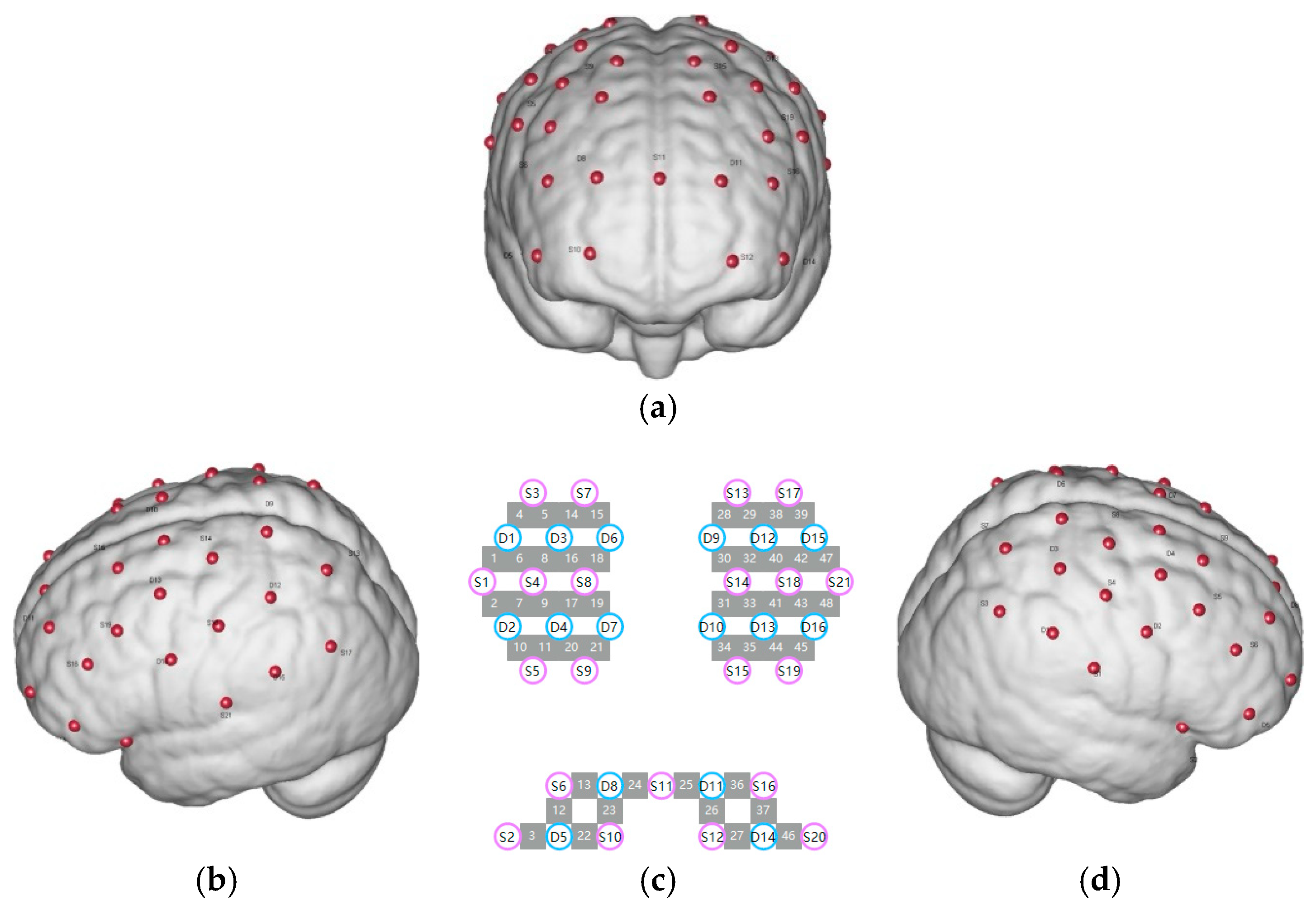
2.3. fNIRS Data Collection
2.3.1. Experimental Equipment
2.3.2. Identification of Regions of Interest (ROI)
- (1)
- Are Aha! Experiences and Metacognitive Temporal Prediction Errors Related to the SCAN Network?
- (2)
- Are Aha! Experiences and Metacognitive Processes Related to the DAN, RN, and VAN Networks?
- (1)
- The Somato-Cognitive Action Network (SCAN) (M1 and SMA): Channels 19, 20, 21, 22, 29, 30, 31, 32, 34, 35, 38, 46, 48.
- (2)
- The Dorsal Attention Network (DAN) (FEF): Channels 27, 28, 39, 40.
- (3)
- The Ventral Attention Network (VAN) (DLPFC): Channels 3, 8, 13, 14, 17, 18, 23, 24, 25, 26.
- (4)
- The Reward Network (RN) (FPC): Channels 4, 5, 6, 7, 15, 16.
3. Experimental Results
3.1. Behavioral Data Statistical Analysis
3.1.1. Primary Variables: Predictive Processing Performance in Aha! Experiences
- (1)
- Predicted Solution Time: 2 × 3 Weighted Chi-Square Test
- (2)
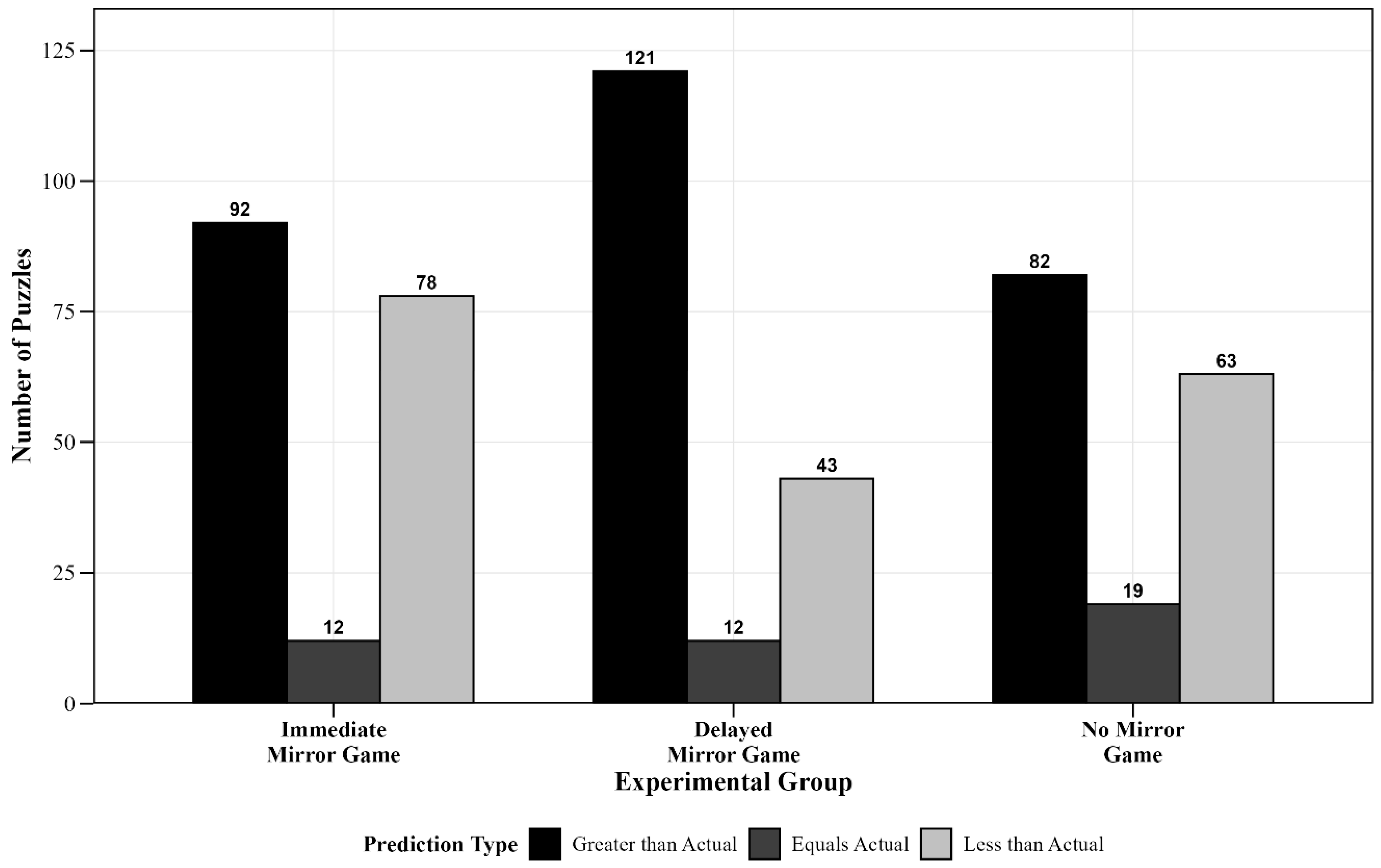
- Time Prediction Error: 2 × 3 Weighted Chi-Square Test
- (3)
- Aha! Experience Intensity: One-Way ANOVA and Bayesian estimation
3.1.2. Secondary Dependent Variables: Auxiliary Indicators of Aha! Experience Predictive Processing Performance
- (1)
- Number of Solved Riddles, Correct Answers, and Aha! Experience Count: One-Way ANOVA
- (2)
- Five Dimensions of Aha! Experience (Pleasure, Suddenness, Surprise, Confidence, and Impasse): One-Way ANOVA
3.1.3. Additional Variables: Emotions
- (1)
- Emotion (Pleasure, Arousal, and Control): One-Way ANOVA
3.2. Statistical Analysis of fNIRS Data
3.2.1. Preprocessing
- (1)
- Pruning Channels (hmrR_PruneChannels):
- (2)
- Converting Intensity to Optical Density (hmrR_Intensity2OD):
- (3)
- Motion Artifact Correction (hmrR_MotionArtifactByChannel):
- (4)
- Spline Motion Correction (hmrR_MotionCorrectSpline):
- (5)
- Bandpass Filtering (hmrR_BandpassFilt):
- (6)
- Converting Optical Density to Concentration (hmrR_OD2Conc):
- (7)
- CBSI Motion Correction (Cbsi_Motion_Correction):
3.2.2. Low-Frequency Amplitude
- (1)
- Predicted Solution Time: Significant differences among the three groups.
3.2.3. Channel Functional Connectivity
- (1)
- Word Puzzle-Solving Task
- (2)
- Self-Evaluated Aha! Experience Task
- (3)
- Channel Functional Connectivity Similarity
3.2.4. Brain Network Functional Connectivity
- (1)
- Mirror Game Learning Stage
- (2)
- Word Puzzle-Solving Task
- (3)
- Self-Evaluated Aha! Experience Task
4. Discussion
4.1. Behavioral Results: Temporal Synchronization in Bodily Interaction Influences the Aha! Experience via Metacognitive Predictive Processing
4.2. Low-Frequency Amplitude: Aha! Experience and the SCAN Network
4.3. Channel Functional Connectivity: Ch16 in the Reward Circuit Plays a Key Role
4.4. Brain Network Functional Connectivity: The Role of RN, DAN, and VAN in Metacognitive Predictive Processing
5. General Conclusions and Future Directions
5.1. Theoretical Contributions: From Bodily Interaction to Insight—An Innovative Pathway Through Implicit Metacognitive Predictive Processing
5.1.1. Theoretical Innovation: Proposal of the “Implicit Metacognitive Predictive Processing” Mechanism
5.1.2. Methodological Innovation: Establishing a Three-Phase Paradigm for Metacognitive Predictive Processing
5.2. Empirical Limitations: Constraints and Areas for Refinement
5.3. Practical Implications: Cross-Disciplinary Applications and Future Potential
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abu-Akel, A., and Simone Shamay-Tsoory. 2011. Neuroanatomical and Neurochemical Bases of Theory of Mind. Neuropsychologia 49: 2971–84. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, Rakefet, and Valerie A. Thompson. 2017. Meta-reasoning: Monitoring and Control of Thinking and Reasoning. Trends in Cognitive Sciences 21: 607–17. [Google Scholar] [CrossRef]
- Bader, Felix, and Martin Wiener. 2021. Awareness of Errors and Feedback in Human Time Estimation. Learning & Memory 28: 171–77. [Google Scholar]
- Becker, Miriam, Xinyue Wang, and Roberto Cabeza. 2024. Surprise!—Clarifying the Link between Insight and Prediction Error. Psychonomic Bulletin & Review 31: 2714–23. [Google Scholar]
- Becker, Miriam, Yifan Yu, and Roberto Cabeza. 2023. The Influence of Insight on Risky Decision Making and Nucleus Accumbens Activation. Scientific Reports 13: 17159. [Google Scholar] [CrossRef] [PubMed]
- Bitu, Fabienne, Béatrice Galinon-Mélénec, and María Molina. 2021. Digital Touchscreens as a Media for Creativity during Adolescence. L’Année Psychologique 121: 489–518. [Google Scholar] [CrossRef]
- Blakemore, Sarah-Jayne, Daniel M. Wolpert, and Chris D. Frith. 2002. Abnormalities in the Awareness of Action. Trends in Cognitive Sciences 6: 237–42. [Google Scholar] [CrossRef]
- Burgess, Paul W., Sam J. Gilbert, and Irene Dumontheil. 2007. Function and Localization within Rostral Prefrontal Cortex (Area 10). Philosophical Transactions of the Royal Society B: Biological Sciences 362: 887–99. [Google Scholar] [CrossRef]
- Clark, Andy. 2013. Whatever Next? Predictive Brains, Situated Agents, and the Future of Cognitive Science. Behavioral and Brain Sciences 36: 181–204. [Google Scholar] [CrossRef]
- Corbetta, Maurizio, and Gordon L. Shulman. 2002. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nature Reviews Neuroscience 3: 201–15. [Google Scholar] [CrossRef]
- Corlett, Philip R., Jordan A. Mollick, and Hedy Kober. 2022. Meta-Analysis of Human Prediction Error for Incentives, Perception, Cognition, and Action. Neuropsychopharmacology 47: 1339–49. [Google Scholar] [CrossRef] [PubMed]
- Danek, Amory H. 2023. The Phenomenology of Insight: The Aha! Experience. In The Routledge International Handbook of Creative Cognition. Edited by Linden J. Ball and Frédéric Vallée-Tourangeau. London: Routledge, pp. 308–31. [Google Scholar]
- Danek, Amory H., and Jennifer Wiley. 2017. What about False Insights? Deconstructing the Aha! Experience along Its Multiple Dimensions for Correct and Incorrect Solutions Separately. Frontiers in Psychology 7: 2077. [Google Scholar] [CrossRef] [PubMed]
- Danek, Amory H., Thomas Fraps, Andreas von Müller, Benedikt Grothe, and Michael Öllinger. 2014. It’s a Kind of Magic—What Self-Reports Can Reveal about the Phenomenology of Insight Problem Solving. Frontiers in Psychology 5: 1408. [Google Scholar] [CrossRef] [PubMed]
- Den Ouden, Hanneke E. M., Peter Kok, and Floris P. de Lange. 2012. How Prediction Errors Shape Perception, Attention, and Motivation. Frontiers in Psychology 3: 548. [Google Scholar] [CrossRef]
- Dienes, Zoltan, and Josef Perner. 2002. The Metacognitive Implications of the Implicit-Explicit Distinction. In Individual Differences in Conscious Experience. Boston: Springer, pp. 171–89. [Google Scholar]
- Di Paolo, Ezequiel, and Hanne De Jaegher. 2012. The Interactive Brain Hypothesis. Frontiers in Human Neuroscience 6: 163. [Google Scholar] [CrossRef]
- Dubey, Rachit, Mark K. Ho, Himabindu Mehta, and Thomas L. Griffiths. 2021. Aha! Moments Correspond to Metacognitive Prediction Errors. PsyArXiv. [Google Scholar] [CrossRef]
- Duncker, Karl. 1945. On Problem-Solving. Psychological Monographs 58: 1–113. [Google Scholar] [CrossRef]
- Faivre, Nathan, Laurène Vuillaume, Fosco Bernasconi, Roy Salomon, Olaf Blanke, and Axel Cleeremans. 2020. Sensorimotor conflicts alter metacognitive and action monitoring. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior 124: 224–34. [Google Scholar] [CrossRef]
- Farrant, Kristyn, and Lucina Q. Uddin. 2015. Asymmetric Development of Dorsal and Ventral Attention Networks in the Human Brain. Developmental Cognitive Neuroscience 12: 165–74. [Google Scholar] [CrossRef]
- Flavell, John H. 1979. Metacognition and Cognitive Monitoring: A New Area of Cognitive–Developmental Inquiry. American Psychologist 34: 906–11. [Google Scholar] [CrossRef]
- Fox, Michael D., Maurizio Corbetta, Abraham Z. Snyder, Jessica L. Vincent, and Marcus E. Raichle. 2006. Spontaneous Neuronal Activity Distinguishes Human Dorsal and Ventral Attention Systems. Proceedings of the National Academy of Sciences 103: 10046–51. [Google Scholar] [CrossRef]
- Friston, Karl. 2010. The Free-Energy Principle: A Unified Brain Theory? Nature Reviews Neuroscience 11: 127–38. [Google Scholar] [CrossRef]
- Friston, Karl J., Thomas Parr, and Boudewijn de Vries. 2017. The Graphical Brain: Belief Propagation and Active Inference. Network Neuroscience 1: 381–414. [Google Scholar] [CrossRef]
- Frith, Christopher D. 2012. The Role of Metacognition in Human Social Interactions. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 2213–23. [Google Scholar] [CrossRef]
- Gallagher, Shaun. 2005. How the Body Shapes the Mind. Oxford: Oxford University Press. [Google Scholar]
- Gawronski, Bertram, and Galen V. Bodenhausen. 2006. Associative and Propositional Processes in Evaluation: An Integrative Review of Implicit and Explicit Attitude Change. Psychological Bulletin 132: 692–731. [Google Scholar] [CrossRef] [PubMed]
- Gick, Mary L., and Robert S. Lockhart. 1995. Cognitive and Affective Components of Insight. In The Nature of Insight. Edited by Robert J. Sternberg and Janet E. Davidson. Cambridge, MA: MIT Press, pp. 197–228. [Google Scholar]
- Gilbert, Sam J., Sarah Spengler, Jon S. Simons, Jonathan D. Steele, Stephen M. Lawrie, Chris D. Frith, and Paul W. Burgess. 2006. Functional Specialization within Rostral Prefrontal Cortex (Area 10): A Meta-Analysis. Journal of Cognitive Neuroscience 18: 932–48. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Evan M., Riley J. Chauvin, Alexander N. Van, Anish Rajesh, Ashley Nielsen, Daniel J. Newbold, Colleen J. Lynch, Nicholas A. Seider, Sarah R. Krimmel, Katherine M. Scheidter, and et al. 2023. A Somato-Cognitive Action Network Alternates with Effector Regions in Motor Cortex. Nature 617: 351–59. [Google Scholar] [CrossRef]
- Gueugnon, Mathieu, Rémy N. Salesse, Antoine Coste, Zengyuan Zhao, Benoît G. Bardy, and Ludovic Marin. 2016. The Acquisition of Socio-Motor Improvisation in the Mirror Game. Human Movement Science 46: 1–13. [Google Scholar] [CrossRef]
- Haber, Suzanne N., and Brian Knutson. 2010. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology 35: 4–26. [Google Scholar] [CrossRef]
- Hart, Yulia, Lior Noy, Ravit Feniger-Schaal, Avraham E. Mayo, and Uri Alon. 2014. Individuality and Togetherness in Joint Improvised Motion. PLoS ONE 9: e87213. [Google Scholar] [CrossRef]
- Häfner, Michael. 2013. When Body and Mind Are Talking: Interoception Moderates Embodied Cognition. Experimental Psychology 60: 255–59. [Google Scholar] [CrossRef]
- Herbert, Beate M., and Olga Pollatos. 2012. The Body in the Mind: On the Relationship between Interoception and Embodiment. Topics in Cognitive Science 4: 692–704. [Google Scholar] [CrossRef]
- Herold, Florian, Philipp Wiegel, Felix Scholkmann, and Notger G. Müller. 2018. Applications of Functional Near-Infrared Spectroscopy (fNIRS) Neuroimaging in Exercise–Cognition Science: A Systematic, Methodology-Focused Review. Journal of Clinical Medicine 7: 466. [Google Scholar] [CrossRef]
- Keefer, Lucas A., Mark J. Landau, Daniel Sullivan, and Zachary K. Rothschild. 2014. Embodied Metaphor and Abstract Problem Solving: Testing a Metaphoric Fit Hypothesis in the Health Domain. Journal of Experimental Social Psychology 55: 12–20. [Google Scholar] [CrossRef]
- Kizilirmak, Jasmin M., Helge Thuerich, Katja Folta-Schoofs, Björn H. Schott, and Andreas Richardson-Klavehn. 2016. Neural Correlates of Learning from Induced Insight: A Case for Reward-Based Episodic Encoding. Frontiers in Psychology 7: 1693. [Google Scholar] [CrossRef]
- Knoblich, Günther, Stellan Ohlsson, Helmut Haider, and Dirk Rhenius. 1999. Constraint Relaxation and Chunk Decomposition in Insight Problem Solving. Journal of Experimental Psychology: Learning, Memory, and Cognition 25: 1534–55. [Google Scholar] [CrossRef]
- Kringelbach, Morten L. 2005. The Human Orbitofrontal Cortex: Linking Reward to Hedonic Experience. Nature Reviews Neuroscience 6: 691–702. [Google Scholar] [CrossRef]
- Laukkonen, Ruben E., Daniel J. Ingledew, Hilary J. Grimmer, Jonathan W. Schooler, and Jason M. Tangen. 2021. Getting a grip on insight: Real-time and embodied Aha experiences predict correct solutions. Cognition & Emotion 35: 918–35. [Google Scholar]
- Luo, Jin. 2004. Neural Correlates of Insight. Acta Psychologica Sinica 36: 219–34. (In Chinese). [Google Scholar]
- Luo, Jin, Hiroshi Niki, and Günther Knoblich. 2006. Perceptual Contributions to Problem Solving: Chunk Decomposition of Chinese Characters. Brain Research Bulletin 70: 430–43. [Google Scholar] [CrossRef]
- Martinez, Michael E. 2006. What Is Metacognition? Phi Delta Kappan 87: 696–99. [Google Scholar] [CrossRef]
- Matsuda, Sho, Hiroshi Matsumoto, Toshihiko Furubayashi, Ryota Hanajima, Shinji Tsuji, Yoshikazu Ugawa, and Yasuo Terao. 2015. The 3-Second Rule in Hereditary Pure Cerebellar Ataxia: A Synchronized Tapping Study. PLoS ONE 10: e0118592. [Google Scholar] [CrossRef] [PubMed]
- Moroshkina, Natalia V., Ekaterina I. Pavliuchik, Andrei V. Ammalainen, Vladimir A. Gershkovich, and Olga V. Lvova. 2024. The Aha! Experience Is Associated with a Drop in the Perceived Difficulty of the Problem. Frontiers in Psychology 15: 1314531. [Google Scholar] [CrossRef]
- Noy, Lior, Erez Dekel, and Uri Alon. 2011. The Mirror Game as a Paradigm for Studying the Dynamics of Two People Improvising Motion Together. Proceedings of the National Academy of Sciences 108: 20947–52. [Google Scholar] [CrossRef] [PubMed]
- Noy, Lior, Neta Levit-Binun, and Yulia Golland. 2015. Being in the Zone: Physiological Markers of Togetherness in Joint Improvisation. Frontiers in Human Neuroscience 9: 187. [Google Scholar] [CrossRef]
- Ogawa, Takeshi, Toshinori Aihara, Takahiro Shimokawa, and Osamu Yamashita. 2018. Large-Scale Brain Network Associated with Creative Insight: Combined Voxel-Based Morphometry and Resting-State Functional Connectivity Analyses. Scientific Reports 8: 6477. [Google Scholar] [CrossRef]
- Oh, Yejin, Connor Chesebrough, Brent Erickson, Fei Zhang, and John Kounios. 2020. An Insight-Related Neural Reward Signal. NeuroImage 214: 116757. [Google Scholar] [CrossRef]
- Ohlsson, Stellan. 2011. Deep Learning: How the Mind Overrides Experience. Cambridge: Cambridge University Press. [Google Scholar]
- Petty, Richard E., and Pablo Briñol. 2006. A Metacognitive Approach to ‘Implicit’ and ‘Explicit’ Evaluations: Comment on Gawronski and Bodenhausen. Psychological Bulletin 132: 740–44. [Google Scholar] [CrossRef]
- Pöppel, Ernst. 2004. Lost in Time: A Historical Frame, Elementary Processing Units and the 3-Second Window. Acta Neurobiologiae Experimentalis 64: 295–301. [Google Scholar] [CrossRef]
- Qiu, Jiang, Yuejia Luo, Zhenzhen Wu, and Qinglin Zhang. 2006. A Further Study of the ERP Effects of ‘Insight’ in a Riddle Guessing Task. Acta Psychologica Sinica 38: 507–14. (In Chinese). [Google Scholar]
- Sengupta, Biswa, Arturo Tozzi, Gert K. Cooray, Peter K. Douglas, and Karl J. Friston. 2016. Towards a Neuronal Gauge Theory. PLoS Biology 14: e1002400. [Google Scholar] [CrossRef]
- Skaar, Øyvind Odd, and Rolf Reber. 2020. The Phenomenology of Aha-Experiences. Motivation Science 6: 49–60. [Google Scholar] [CrossRef]
- Skulmowski, Alexander, and Gunter Daniel Rey. 2017. Bodily Effort Enhances Learning and Metacognition: Investigating the Relation Between Physical Effort and Cognition Using Dual-Process Models of Embodiment. Advances in Cognitive Psychology 13: 3–10. [Google Scholar] [CrossRef]
- Skvortsova, Varvara, Stefano Palminteri, and Mathias Pessiglione. 2014. Learning to Minimize Efforts versus Maximizing Rewards: Computational Principles and Neural Correlates. Journal of Neuroscience 34: 15621–30. [Google Scholar] [CrossRef]
- Sternberg, Robert J., and Janet E. Davidson. 1995. The Nature of Insight. Cambridge, MA: MIT Press. [Google Scholar]
- Stuyck, Helena, Bart Aben, Axel Cleeremans, and Evy Van den Bussche. 2021. The Aha! Moment: Is Insight a Different Form of Problem Solving? Consciousness and Cognition 90: 103055. [Google Scholar] [CrossRef]
- Sutton, Richard S., and Andrew G. Barto. 2018. Reinforcement Learning: An Introduction. Cambridge, MA: MIT Press. [Google Scholar]
- Tamber-Rosenau, Benjamin J., Christina L. Asplund, and René Marois. 2018. Functional Dissociation of the Inferior Frontal Junction from the Dorsal Attention Network in Top-Down Attentional Control. Journal of Neurophysiology 120: 2498–512. [Google Scholar] [CrossRef]
- Tik, Marek, Robert Sladky, Caroline D. B. Luft, David Willinger, Alexander Hoffmann, Michael J. Banissy, Joydeep Bhattacharya, and Christian Windischberger. 2018. Ultra-High-Field fMRI Insights on Insight: Neural Correlates of the Aha!-Moment. Human Brain Mapping 39: 3241–52. [Google Scholar] [CrossRef]
- Topolinski, Sascha, and Rolf Reber. 2010. Gaining Insight into the ‘Aha’ Experience. Current Directions in Psychological Science 19: 402–5. [Google Scholar] [CrossRef]
- Valtulina, Julia, and Alwin De Rooij. 2019. The Predictive Creative Mind: A First Look at Spontaneous Predictions and Evaluations during Idea Generation. Frontiers in Psychology 10: 481691. [Google Scholar] [CrossRef]
- Varela, Francisco J., Evan Thompson, and Eleanor Rosch. 1991. The Embodied Mind: Cognitive Science and Human Experience. Cambridge, MA: MIT Press. [Google Scholar]
- Ye, Haosheng. 2011. Embodied Cognition: A Consideration from Theoretical Psychology. Acta Psychologica Sinica 43: 589–98. (In Chinese). [Google Scholar]

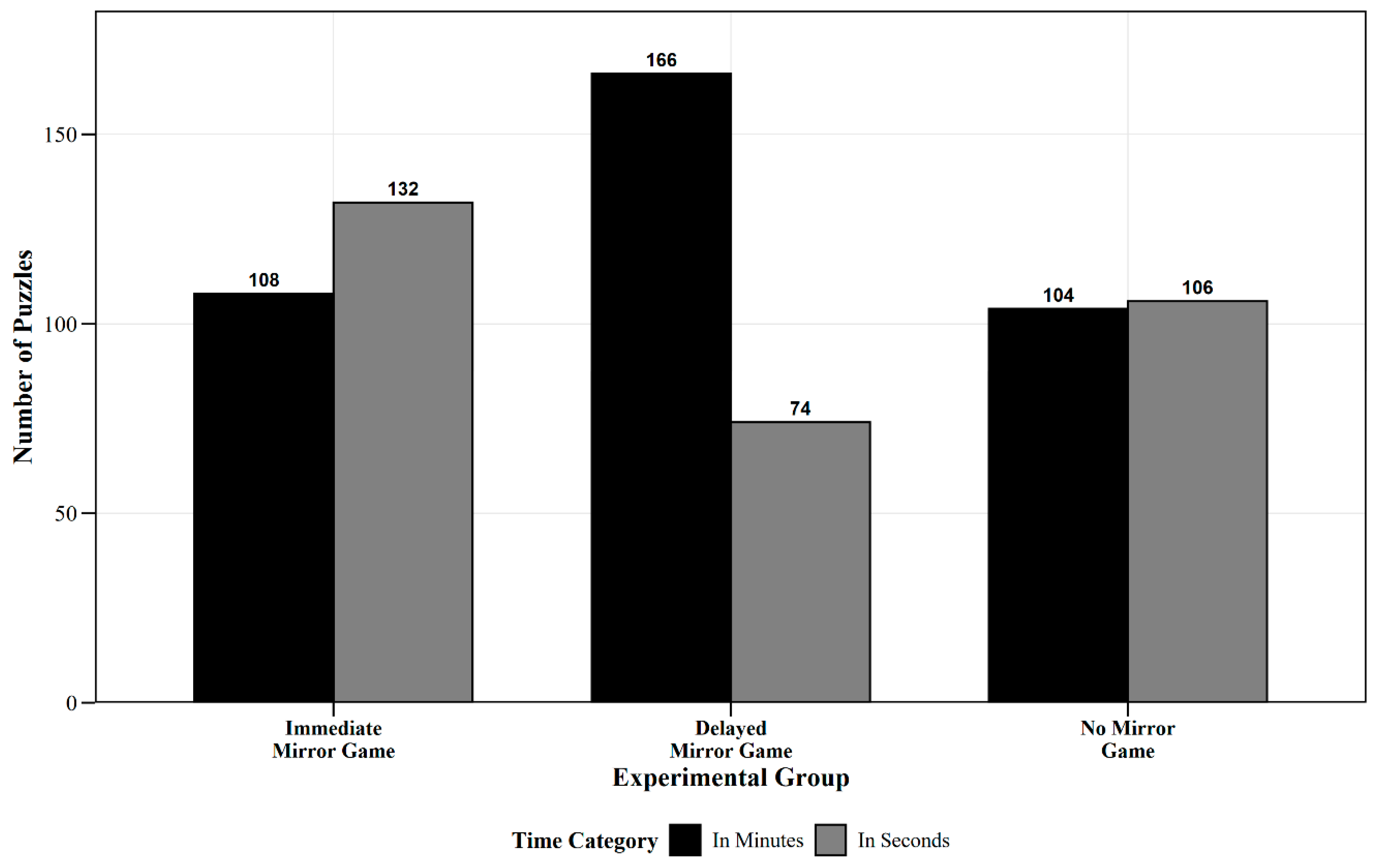


| Experimental Group | Total | In Seconds | In Minutes | Chi-Square Test | |
|---|---|---|---|---|---|
| n | n (%) | n (%) | χ2 | p | |
| Immediate Mirror Game Group | 240 | 132 (55%) | 108 (45%) | 32.15 | <0.001 |
| Delayed Mirror Game Group | 240 | 74 (30.8%) | 166 (69.2%) | ||
| No Mirror Game Group | 210 | 106 (50.5%) | 104 (49.5%) | ||
| Experimental Group | Total | Prediction Greater than Actual | Prediction Equals Actual | Prediction Less than Actual | Chi-Square Test | |
|---|---|---|---|---|---|---|
| n | n (%) | n (%) | n (%) | χ2 | p | |
| Immediate Mirror Game Group | 182 | 92 (50.5%) | 12 (6.6%) | 78 (42.9%) | 19.56 | <0.001 |
| Delayed Mirror Game Group | 176 | 121 (68.8%) | 12 (6.8%) | 43 (24.4%) | ||
| No Mirror Game Group | 164 | 82 (50%) | 19 (11.6%) | 63 (38.4%) | ||
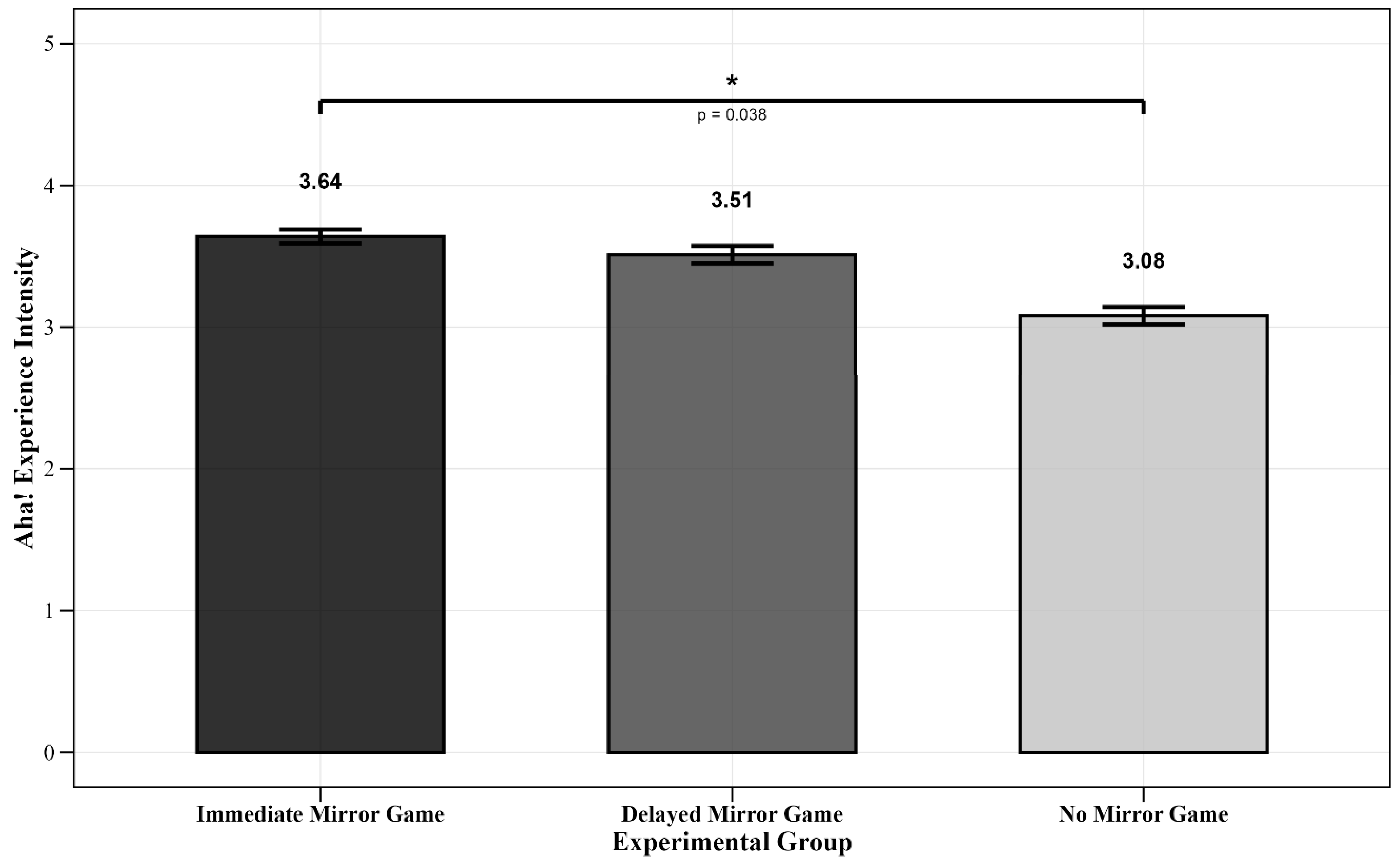
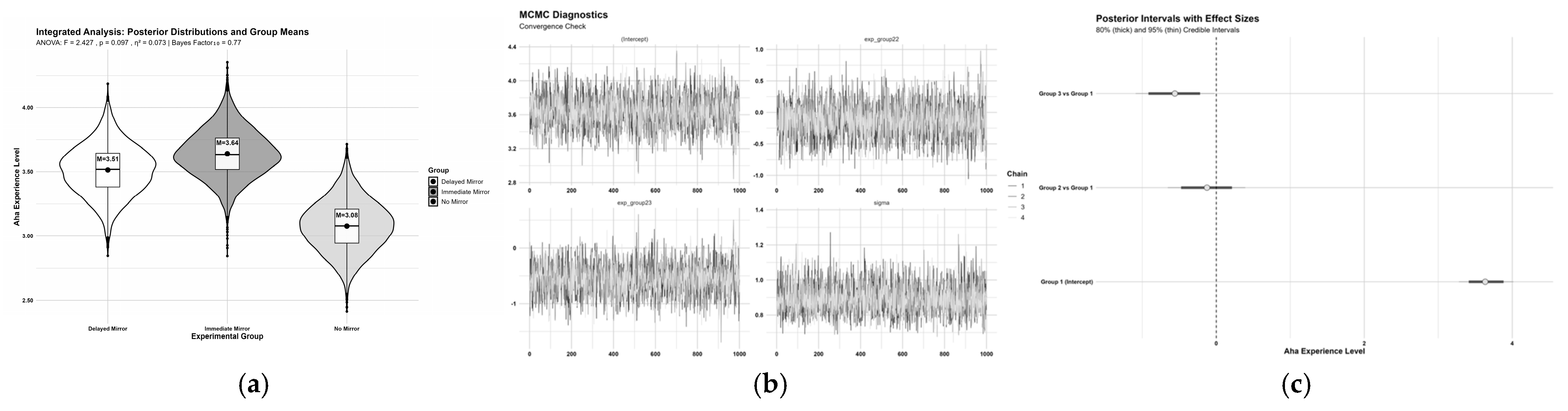
| ANOVA | Experimental Group | M (SD) | F-Test | Multiple Comparisons | ||
| F | p | ηp2 | ||||
| Immediate Mirror Game Group | 3.64 (0.77) | F(2, 62) = 2.47 | 0.097 | 0.07 | Immediate Mirror Game Group > No Mirror Game Group (p = 0.038) | |
| Delayed Mirror Game Group | 3.51 (0.97) | |||||
| No Mirror Game Group | 3.08 (0.90) | |||||
| BAYES | Experimental Group Comparison | t(df), p-Value | Cohen’s d [95% CI] | BF10 | Bayesian Evidence | Posterior Probability |
| Immediate vs. Delayed | t(38) = 0.49, p = 1.000 | 0.15 [−0.46, 0.76] | 0.33 | Strong evidence for H0 | 68.3% (Immediate > Delayed) | |
| Immediate vs. No Mirror | t(40) = 2.22, p = 0.096 | 0.68 [0.05, 1.30] | 2.12 | Weak evidence for H1 | 98.0% (Immediate > No Mirror) | |
| Delayed vs. No Mirror | t(40) = 1.50, p = 0.421 | 0.46 [−0.17, 1.10] | 0.74 | Moderate evidence for H0 | 94.3% (Delayed > No Mirror) | |
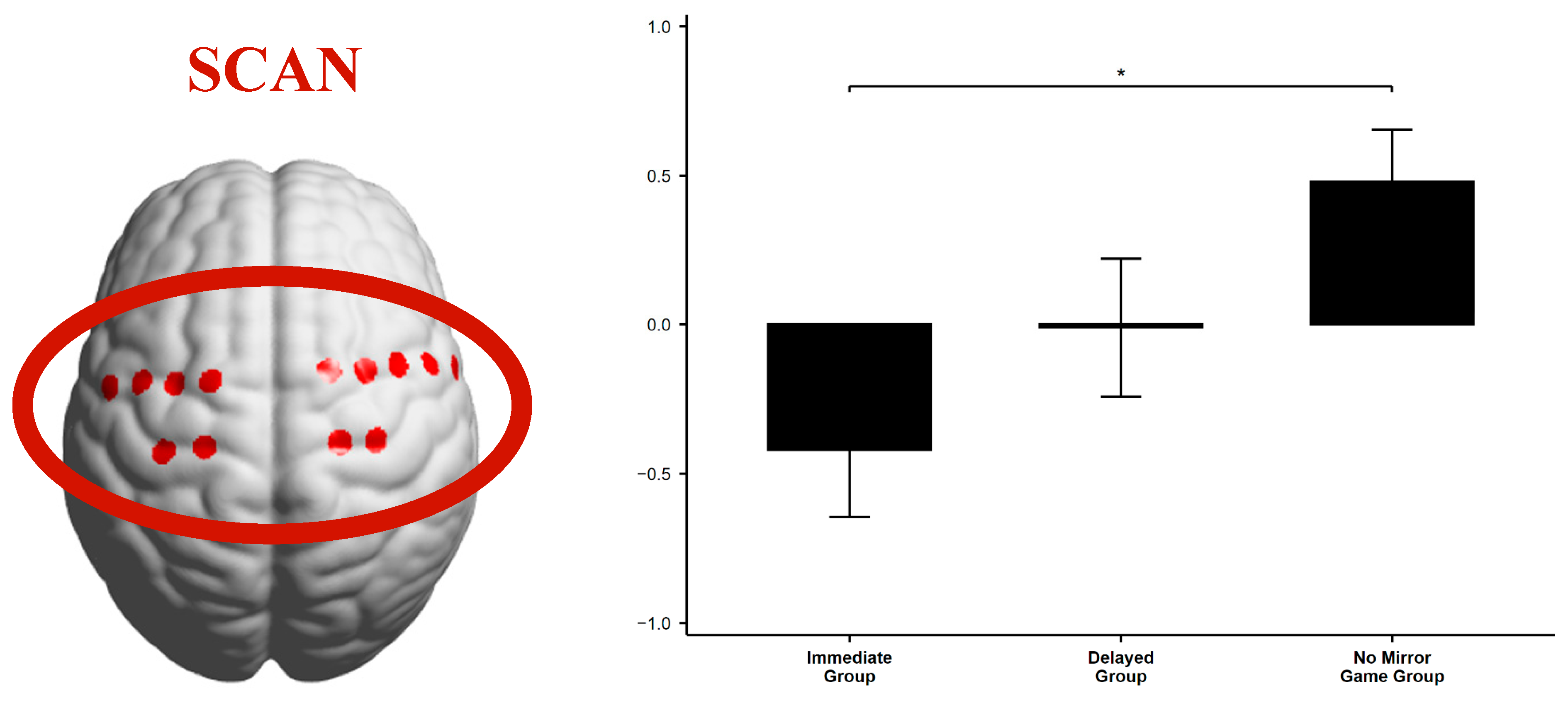
| Brain Network | Experimental Group | Descriptive Statistics | F-Test | ||||
|---|---|---|---|---|---|---|---|
| M | SD | N | F (df1, df2) | p | ηp2 | ||
| SCAN | Immediate Group | −0.42 | 1.03 | 21 | F(2, 56) = 4.50 | 0.015 | 0.14 |
| Delayed Group | −0.01 | 1.01 | 19 | ||||
| No Mirror Game Group | 0.48 | 0.76 | 19 | ||||
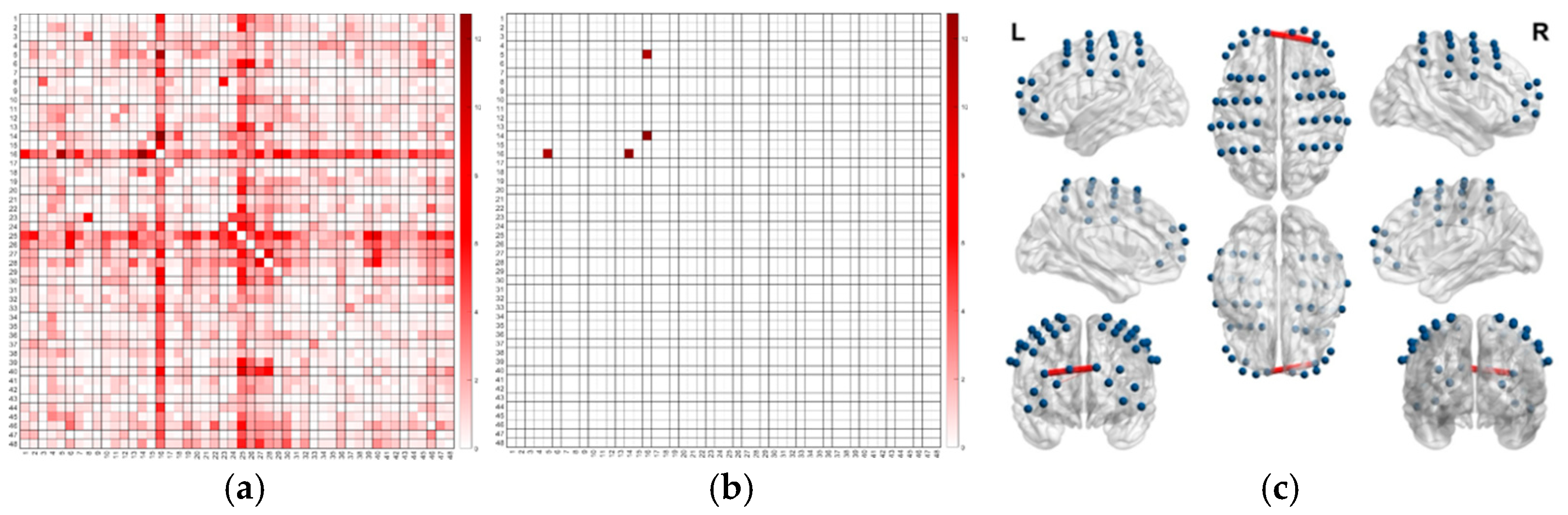
| Channel Functional Connectivity | Experiment Group | Descriptive Statistics | F-Test | ||||
|---|---|---|---|---|---|---|---|
| M | SD | N | F (df1, df2) | p | ηp2 | ||
| Ch16 and Ch4 | Immediate | 0.57 | 0.23 | 21 | F(2, 56) = 13.80 | <0.001 | 0.33 |
| Delayed | 0.59 | 0.21 | 19 | ||||
| No Mirror | 0.24 | 0.26 | 19 | ||||
| Ch16 and Ch5 | Immediate | 0.74 | 0.19 | 21 | F(2, 56) = 25.90 | <0.001 | 0.481 |
| Delayed | 0.80 | 0.26 | 19 | ||||
| No Mirror | 0.28 | 0.28 | 19 | ||||
| Ch16 and Ch6 | Immediate | 0.53 | 0.23 | 21 | F(2, 56) = 11.84 | <0.001 | 0.297 |
| Delayed | 0.62 | 0.24 | 19 | ||||
| No Mirror | 0.25 | 0.26 | 19 | ||||
| Ch16 and Ch7 | Immediate | 0.76 | 0.22 | 21 | F(2, 56) = 12.27 | <0.001 | 0.305 |
| Delayed | 0.86 | 0.23 | 19 | ||||
| No Mirror | 0.44 | 0.36 | 19 | ||||
| Ch16 and Ch13 | Immediate | 0.52 | 0.23 | 21 | F(2, 56) = 10.30 | <0.001 | 0.269 |
| Delayed | 0.59 | 0.23 | 19 | ||||
| No Mirror | 0.27 | 0.24 | 19 | ||||
| Ch16 and Ch14 | Immediate | 0.62 | 0.33 | 21 | F(2, 56) = 11.32 | <0.001 | 0.288 |
| Delayed | 0.78 | 0.26 | 19 | ||||
| No Mirror | 0.34 | 0.26 | 19 | ||||
| Ch16 and Ch15 | Immediate | 0.76 | 0.40 | 21 | F(2, 56) = 16.39 | <0.001 | 0.369 |
| Delayed | 0.90 | 0.24 | 19 | ||||
| No Mirror | 0.34 | 0.27 | 19 | ||||
| Ch16 and Ch23 | Immediate | 0.46 | 0.31 | 21 | F(2, 56) = 11.80 | <0.001 | 0.296 |
| Delayed | 0.52 | 0.26 | 19 | ||||
| No Mirror | 0.13 | 0.22 | 19 | ||||
| Ch16 and Ch27 | Immediate | 0.52 | 0.30 | 21 | F(2, 56) = 11.71 | <0.001 | 0.295 |
| Delayed | 0.53 | 0.22 | 19 | ||||
| No Mirror | 0.18 | 0.23 | 19 | ||||
| Ch16 and Ch40 | Immediate | 0.57 | 0.26 | 21 | F(2, 56) = 18.92 | <0.001 | 0.403 |
| Delayed | 0.53 | 0.23 | 19 | ||||
| No Mirror | 0.17 | 0.15 | 19 | ||||
| Ch24 and Ch25 | Immediate | 0.62 | 0.30 | 21 | F(2, 56) = 11.63 | <0.001 | 0.293 |
| Delayed | 0.70 | 0.25 | 19 | ||||
| No Mirror | 0.31 | 0.24 | 19 | ||||
| Channel Functional Connectivity | Experiment Group | Descriptive Statistics | F-Test | ||||
|---|---|---|---|---|---|---|---|
| M | SD | N | F (df1, df2) | p | ηp2 | ||
| Ch16 and Ch5 | Immediate | 0.60 | 0.32 | 21 | F(2, 56) = 11.41 | <0.001 | 0.290 |
| Delayed | 0.75 | 0.39 | 19 | ||||
| No Mirror | 0.24 | 0.30 | 19 | ||||
| Ch16 and Ch14 | Immediate | 0.52 | 0.36 | 21 | F(2, 56) = 12.74 | <0.001 | 0.313 |
| Delayed | 0.74 | 0.45 | 19 | ||||
| No Mirror | 0.14 | 0.27 | 19 | ||||
| Task 1 | Task 2 | Experimental Group | Channel Functional Connectivity Similarity |
|---|---|---|---|
| Formal Experiment Stage of the Mirror Game | Predicted Solution Time | Immediate Group | 0.72 |
| Delayed Group | 0.75 | ||
| Solved Word Puzzle Task | Immediate Group | 0.80 | |
| Delayed Group | 0.87 | ||
| Self-Evaluated Aha! Experience | Immediate Group | 0.73 | |
| Delayed Group | 0.80 |
| Brain Network Functional Connectivity | Experiment Group | M | SD | N | t(df) | p | Cohen’s d |
|---|---|---|---|---|---|---|---|
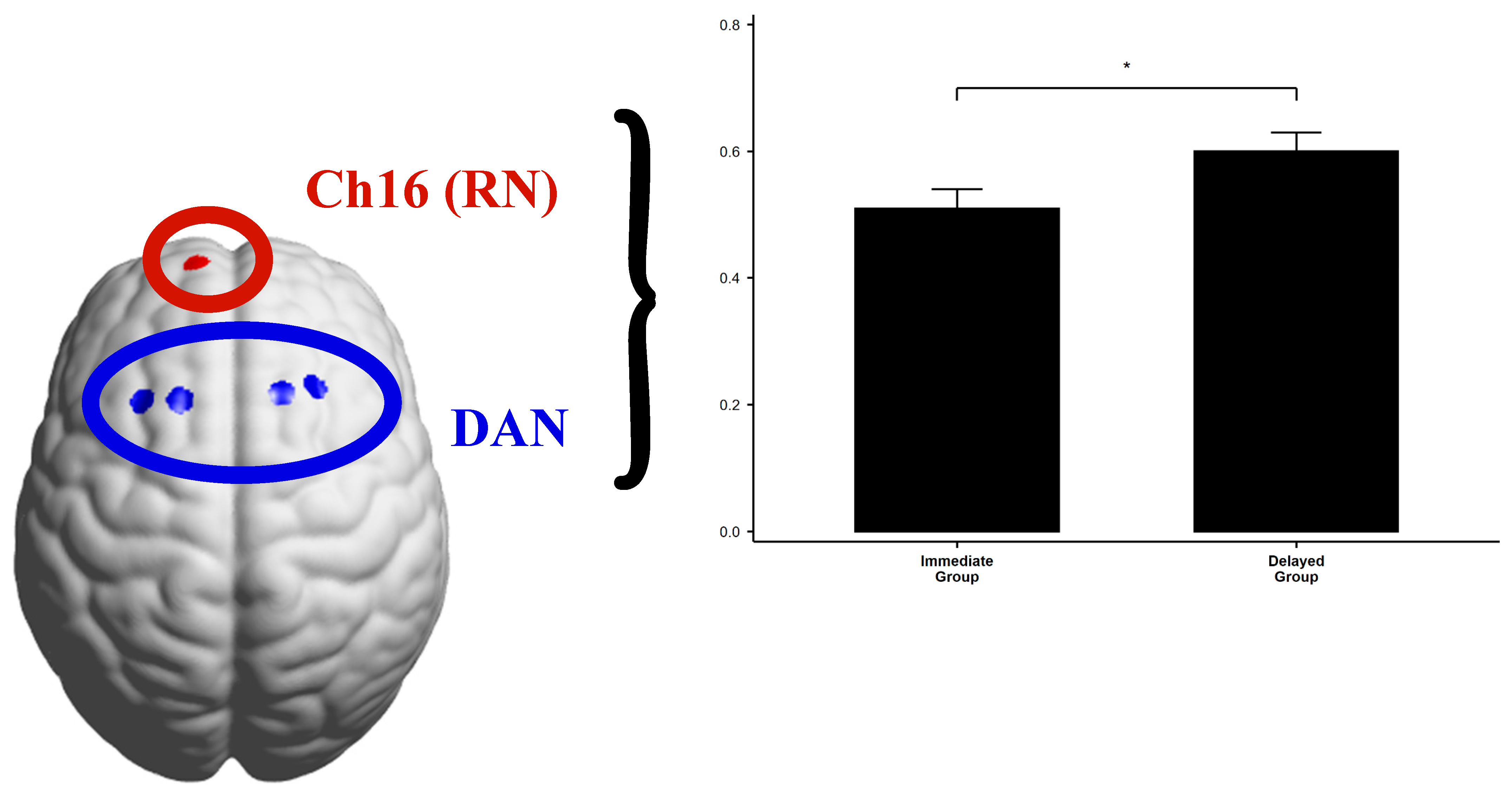
| DAN and Ch16 | Immediate Group | 0.51 | 0.14 | 21 | t(38) = −2.20 | 0.034 | −0.696 |
| Delayed Group | 0.60 | 0.13 | 19 |
| Brain Network Functional Connectivity | Experiment Group | M | SD | F (df1, df2) | p | ηp2 |
|---|---|---|---|---|---|---|
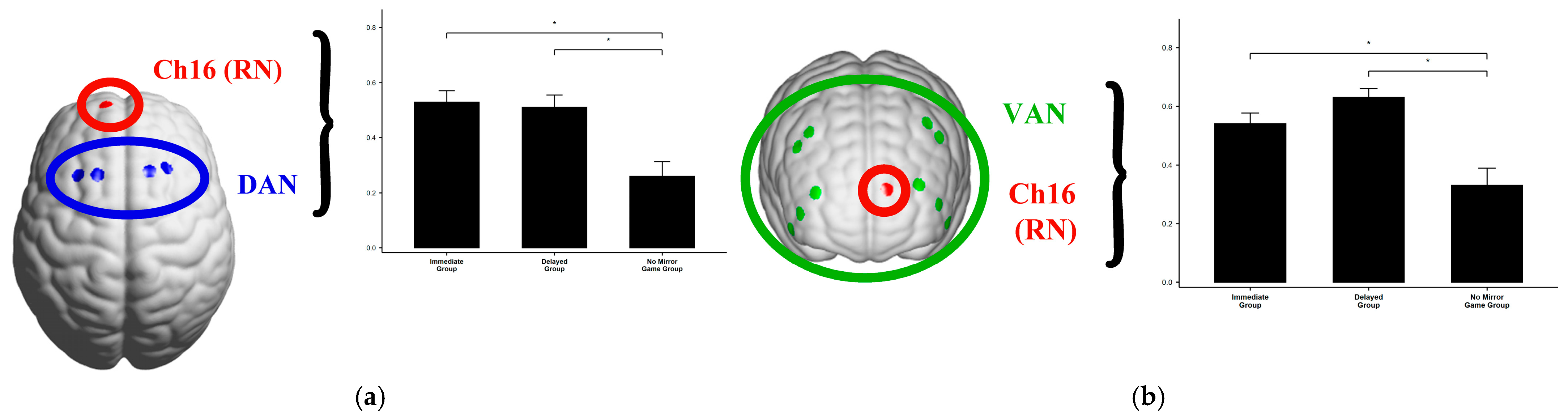
| DAN and Ch16 | Immediate | 0.53 | 0.19 | F(2, 56) = 10.75 | <0.001 | 0.278 |
| Delayed | 0.51 | 0.20 | ||||
| No Mirror | 0.26 | 0.23 | ||||
| VAN and Ch16 | Immediate | 0.54 | 0.17 | F(2, 56) = 12.61 | <0.001 | 0.310 |
| Delayed | 0.63 | 0.13 | ||||
| No Mirror | 0.33 | 0.26 |
| Brain Network Functional Connectivity | Experiment Group | M | SD | F (df1, df2) | p | ηp2 |
|---|---|---|---|---|---|---|
| DAN and Ch16 | Immediate | 0.49 | 0.26 | F(2, 56) = 4.97 | 0.010 | 0.151 |
| Delayed | 0.53 | 0.26 | ||||
| No Mirror | 0.28 | 0.27 | ||||
| VAN and Ch16 | Immediate | 0.53 | 0.25 | F(2, 56) = 7.07 | 0.002 | 0.202 |
| Delayed | 0.59 | 0.27 | ||||
| No Mirror | 0.27 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Ye, H. Temporal Synchrony in Bodily Interaction Enhances the Aha! Experience: Evidence for an Implicit Metacognitive Predictive Processing Mechanism. J. Intell. 2025, 13, 83. https://doi.org/10.3390/jintelligence13070083
Su J, Ye H. Temporal Synchrony in Bodily Interaction Enhances the Aha! Experience: Evidence for an Implicit Metacognitive Predictive Processing Mechanism. Journal of Intelligence. 2025; 13(7):83. https://doi.org/10.3390/jintelligence13070083
Chicago/Turabian StyleSu, Jiajia, and Haosheng Ye. 2025. "Temporal Synchrony in Bodily Interaction Enhances the Aha! Experience: Evidence for an Implicit Metacognitive Predictive Processing Mechanism" Journal of Intelligence 13, no. 7: 83. https://doi.org/10.3390/jintelligence13070083
APA StyleSu, J., & Ye, H. (2025). Temporal Synchrony in Bodily Interaction Enhances the Aha! Experience: Evidence for an Implicit Metacognitive Predictive Processing Mechanism. Journal of Intelligence, 13(7), 83. https://doi.org/10.3390/jintelligence13070083





