Abstract
Mild cognitive impairment (MCI) is associated with deficits in decision-making, which is of utmost importance for daily functioning. Despite evidence of declined decision-making abilities, research on decision-making interventions for MCI is scarce. As metacognition seems to play an important role in decision-making, the present study’s aim was to examine whether a metacognitive strategy training can improve MCI patients’ decision-making abilities. Older adults—patients of a day care center, diagnosed with amnestic MCI (n = 55) were randomly allocated in two groups, which were matched in gender, age and educational level. Τhe experimental group (n = 27, 18 women, mean age = 70.63, mean years of education = 13.44) received the metacognitive strategy training in parallel with the cognitive and physical training programs of the day care center, and the active control group (n = 28, 21 women, mean age = 70.86, mean years of education = 13.71) received only the cognitive and physical training of the center. The metacognitive strategy training included three online meeting sessions that took place once per week. The basis of the intervention was using analytical thinking, by answering four metacognitive-strategic questions, to make decisions about everyday situations. To examine the efficacy of the training, the ability to make decisions about everyday decision-making situations and the ability to apply decision rules were measured. Both groups participated in a pre-test session and a post-test session, while the experimental group also participated in a follow-up session, one month after the post-test session. The results showed that the experimental group improved its ability to decide, based on analytical thinking, about economic and healthcare-related everyday decision-making situations after they received the metacognitive strategy training. This improvement was maintained one month later. However, the ability to apply decision rules, which requires high cognitive effort, did not improve. In conclusion, it is important that some aspects of the analytical decision-making ability of amnestic MCI patients were improved due to the present metacognitive intervention.
1. Introduction
Mild cognitive impairment (MCI) is considered a stage between normal cognitive function and dementia (Albert et al. 2011; Petersen 2004). More precisely, the cognitive decline is greater than expected for the patient’s age and educational level. One or more cognitive functions are affected, including memory, attention, executive functions, language, and visuospatial skills. Older adults with a diagnosis of MCI, even those that revert to normal cognition, have a high risk of developing dementia (Roberts et al. 2014).
There are two main subtypes of MCI. Non-amnestic MCI is characterized by impairment in one or more non-memory domains, but preserved memory (Albert et al. 2011; Petersen 2004). The other subtype of MCI, which is the most common one (Peters et al. 2006), and which has been mostly considered a prodromal stage of Alzheimer’s disease (Petersen 2004), is amnestic MCI (aMCI). Single-domain aMCI patients present deficits only in memory, while multiple-domain aMCI patients present deficits not only in memory, but also in one or more non-memory cognitive functions (Petersen 2004).
In MCI, criteria for a diagnosis of dementia are not met, because cognitive decline is not extensive, and daily functioning is not significantly affected (Albert et al. 2011; Petersen 2004). However, although MCI diagnosis requires that independence in daily functioning is preserved (Albert et al. 2011; Petersen 2004), difficulties are observed in more complex daily tasks, concerning financial issues (Fernandes et al. 2021) and health issues such as capacity to consent to medical treatment (Okonkwo et al. 2008).
Decision-making is an essential ability of a person to live independently and successfully (Mather 2006; Salthouse 2012). Decision-making is the process of choosing between at least two competing options by analysing the costs and benefits of each option and estimating its future consequences (da Mata et al. 2011). There are two classic behavioral task types to assess decision-making ability. In decision under ambiguity tasks explicit information about the outcomes and probabilities of their occurrence is not offered and should be learned through experience (Bechara et al. 1997), while in decision under risk tasks this information is provided and should be used to evaluate options (Brand et al. 2006).
Two different decision-making processes (or systems) described by dual process theories (Epstein 1994; Evans 2008; Kahneman 2003) are thought to be engaged during decision-making under risk and under ambiguity (Schiebener and Brand 2015; Sinclair et al. 2022). Decision under ambiguity tasks require an experiential mode of thinking (or intuitive system or system 1), which is unconscious, automatic, effortless, and rapid, based on intuition and past experiences. On the contrary, decision under risk tasks require an analytical mode of thinking (or deliberative system or system 2), which is conscious, cognitively effortful, and slow, based on reasoning and analysis of information.
MCI patients present deficits in both experiential and analytical processes. They show difficulties in the Iowa Gambling Task, which is a decision under ambiguity task (Bayard et al. 2014, 2015; Zamarian et al. 2011), as well as in the Probability-Associated Gambling Task (Zamarian et al. 2011), the Game of Dice Task (Sinclair et al. 2022; Zhang et al. 2022), the Game of Dice Task-Double (Pertl et al. 2015), which are laboratory computerized decision under risk tasks, and in a decision under risk task about real-life health-related situations (Pertl et al. 2017). To our knowledge, there is only one decision-making intervention study targeting MCI patients. Burgio et al. (2018) found that cognitive training on number processing and executive functions improved MCI patients’ performance on a decision-making under risk task as well as on a health-related ratio processing task.
1.1. Metacognition and Decision-Making Interventions
A theoretical model about decision-making that has practical implications for decision-making interventions is the Integrated Judgment and Decision-Making Model (IJDM; Dansereau et al. 2013). According to this model, there is a metacognitive system that monitors and controls the analytical processes, the experiential processes and the processes of a wisdom/expertise system which consists of schemas formulated by analytical and experiential processes. Dansereau et al. (2013) proposed that decision-making interventions should focus on analytically created schemas, which are steps to analyse problems (analytical mode of thinking) and to monitor and control the decision-making process (metacognition). If these schematic structures are repeatedly applied to make decisions, they are internalized as a part of the wisdom/expertise system and ultimately, they are activated automatically every time a decision situation emerges.
A metacognitive perspective in decision-making training was also adopted by Batha and Carroll (2007). University students improved their decision-making performance after receiving a “metacognitive strategy instruction”. This instruction was based on the Cardelle-Elawar (1995) metacognitive instruction model, which consists of four steps. Translation refers to understanding the problem, while integration focuses on gathering and organizing the necessary information (Batha and Carroll 2007; Cardelle-Elawar 1995). Then, solution planning and monitoring emphasizes finding an appropriate strategy to solve the problem and monitoring its application. Finally, solution execution is reaching a decision and then checking for errors or missed information.
The IJDM (Dansereau et al. 2013) and Batha and Carroll’s (2007) instruction were utilized by Rosi et al. (2019a) to create a “metacognitive-strategy decision-making training” for older adults. During training, participants practiced in answering a series of metacognitive-strategic questions to make decisions about hypothetical real-life situations. Additionally, they practiced in choosing the decision rule (Payne et al. 1993) that would be most suitable to apply in specific decision-making situations. This training enhanced older adults’ analytical mode of thinking in everyday decision-making contexts and their ability to apply decision rules.
1.2. The Present Study
Based on evidence of impaired analytical decision-making in MCI and on metacognitive approaches for improving decision-making, the aim of the present study was to examine whether the addition of a metacognitive strategy training can improve the decision-making abilities of older adult patients diagnosed with aMCI and attending cognitive and physical intervention programs in a day care center for Alzheimer’s Disease.
The hypothesis was that aMCI patients’ decision-making ability in everyday contexts and their ability to apply decision rules will improve directly after the metacognitive training, in comparison to before such a training, and that this improvement will be maintained or increased one month after the metacognitive training is finished. In addition, aMCI patients’ decision-making ability in everyday contexts and their ability to apply decision rules will improve more after their participation in metacognitive training, in comparison to aMCI patients that attend only the classic intervention programs (cognitive and physical interventions) offered by the day care center.
2. Methods
2.1. Study Design and Procedure
The study followed an experimental design (Figure 1). The experimental group (EG), which was participating in cognitive and/or physical intervention programs as patients—visitors of a day care center for Alzheimer’s disease, received an additional metacognitive strategy decision-making training, which consisted of three sessions, one each week for three consecutive weeks. The active control group (CG) of aMCI patients was also attending the same cognitive and physical intervention programs offered by the day care center. There was a pre-testing and a post-testing session for both groups one week before and after the metacognitive intervention in the EG respectively. There also was a follow-up session only for the EG, one month after the post-testing session. During all testing sessions, the ability to make decisions in everyday decision-making situations and the ability to apply decision rules was assessed.
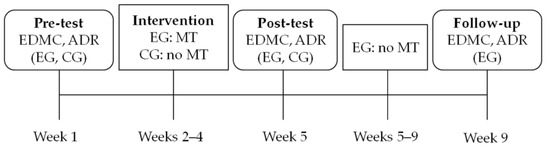
Figure 1.
Study design. Note: EDMC = Everyday Decision-Making Competence task; ADR = Applying Decision Rules task; EG = Experimental Group; CG = Active Control Group; MT = Metacognitive Strategy Training; It must be noted that all aMCI patients of the EG and the CG were attending the classic interventions offered by the day care center (cognitive and physical training) during all these weeks.
The first researcher conducted all the metacognitive strategy training sessions via a video conferencing program. Each testing session lasted 40 min to one and a half hours, depending on the participant, and each training session lasted one hour to one hour and a half. The EG took all sessions in groups of four to seven people, with a few exceptions that took some sessions alone or in groups of two, due to unexpected circumstances (e.g., illness). Most of the participants of the CG took both testing sessions one by one.
During all testing sessions, the measures were administered using a Google form. Participants had to complete firstly the appropriate version of the Everyday Decision-Making Competence task (Rosi et al. 2019a; see below for a description), and then the appropriate version of the Applying Decision Rules task (Bruine de Bruin et al. 2007; see below for a description). There was no time limit for the completion of the form. The researcher provided explanations about the instructions of the measures, when she was asked, and help with technical difficulties. It should be noted that all measures and all decision-making problems that were used during training were previously pilot-tested.
2.2. Participants and Ethical Standards
The sample comprised 55 older adults with aMCI that were recruited from the Day Care Centre “Saint Helen” of the Alzheimer Hellas (DCCAH) via phone calls or while they were attending online physical intervention programs or cognitive training programs. Based on a power analysis that was conducted, using G*Power (Faul et al. 2007), a total sample size of 34 participants was recommended to detect an effect size of η2 = 0.25, with an alpha of 0.05 and to achieve a power of 0.80.
Inclusion criterion was a diagnosis of single-domain or multiple-domain aMCI. The diagnosis was given at most 10 months before the pre-test of the present study, by a consensus of specialized health professionals of the DCCAH, considered experts in neurocognitive disorders. A neurological examination, a neuropsychological and neuropsychiatric assessment, neuroimaging (computed tomography or magnetic resonance imaging), and blood tests were considered for diagnosis.
The criteria for the diagnosis of MCI were: (a) diagnosis of Minor Neurocognitive Disorders according to DSM-5 (American Psychiatric Association 2013), (b) Mini-Mental State Examination (Folstein et al. 1975; Fountoulakis et al. 2000) total score ≥ 24, (c) stage 3 of the disease according to Global Deterioration Scale (Reisberg et al. 1982), and (d) 1.5 standard deviation below the normal mean according to age and education, in at least one cognitive domain according to the utilized neuropsychological tests. In addition, the Montreal Cognitive Assessment Scale (Poptsi et al. 2019; Nasreddine et al. 2005) was used to assess the general cognitive status, and the Functional Cognitive Assessment Scale (Kounti et al. 2006) to assess the ability to organize and execute six different activities of daily living. Standardized tests for the assessment of general cognitive and functional abilities, memory capacity, language abilities, executive functions, and attention were used as well. The entirety of the neuropsychological tests included in the battery is presented in detail in Tsolaki et al. (2017).
Exclusion criteria were a psychiatric illness or an untreated affective disorder (Major Depression/General Anxiety Disorder). Thus, the Geriatric Depression Scale (Fountoulakis et al. 1999; Yesavage et al. 1982) and the Beck Depression Inventory (Beck et al. 1961), the Short Anxiety Screening Test (Grammatikopoulos et al. 2010; Sinoff et al. 1999) and the Beck Anxiety Inventory (Beck et al. 1988) were used to exclude affective disorders and the Neuropsychiatric Inventory (Cummings et al. 1994; Politis et al. 2004) to exclude neuropsychiatric symptoms.
The EG consisted of 27 participants (18 women and 9 men) aged 63–79 years (M = 70.63, SD = 4.47). Twenty-three were given a diagnosis of multiple-domain aMCI and four a diagnosis of single-domain aMCI. Their years of education ranged from 6 to 20 (M = 13.44, SD = 3.95) and the years they have been attending programs at the DCCAH ranged from 1 to 12 (M = 4.11, SD = 3.69). One participant was receiving cholinesterase inhibitors, six were receiving antidepressants and five anxiolytics.
The CG included 28 participants (21 women and 7 men) aged 62–80 years (M = 70.86, SD = 4.67). Twenty-five were given a diagnosis of multiple-domain aMCI and three a diagnosis of single-domain aMCI. Their years of education ranged from 6 to 21 (M = 13.71, SD = 3.71) and the years they have been attending programs at the DCCAH ranged from 1 to 13 (M = 4.50, SD = 3.47). Two were receiving cholinesterase inhibitors, six antidepressants and four were receiving anxiolytics.
Participants were assigned randomly to the two groups. The two groups did not differ significantly in gender [χ2(1, 55) = 0.463, p = .496], subtype of aMCI [χ2(1, 55) = 0.208, p = .648], years of age [F(1, 53) = 0.034, p = .854], years of education [F(1, 53) = 0.068, p = .795] and years at the DCCAH [F(1, 53) = 0.162, p = .689].
Participants were informed about the aim and the procedure of the study both orally and via an informative email. In addition, since demographic data, which are considered personal data, were collected, the General Data Protection Regulation, which is the European Union law that exists since 25 May 2018 was applied. According to the law, the use of sensitive personal data is allowed only for research reasons. So, participants were also informed that their data would be kept confidential. Therefore, participants gave informed consent, agreeing that their participation was voluntary and that they could withdraw at any time, without providing a reason and without cost. The protocol of the study was approved by the Scientific and Ethics Committee of Alzheimer Hellas (Scientific Committee Approved Meeting Number: 82/19-10-2022) and followed the principles outlined in the Helsinki Declaration.
2.3. Measures
2.3.1. Everyday Decision-Making Competence Τask
The Everyday Decision-Making Competence task (EDMC; Rosi et al. 2019a) was used to assess the decision-making ability in everyday situations. It consists of 12 decision-making problems about daily (four problems, e.g., decide from which supermarket to buy groceries), economic (four problems, e.g., decide which insurance policy to buy for a car) and healthcare (four problems, e.g., decide which therapy is best to treat hypothyroidism) scenarios (see Table 1). Half are analytical-based, which means that they require effortful analytical processing, such as doing mathematical calculations. The rest are experiential-based and present two conflicting options. One option is based on base-rate information provided by a reliable source of information, thus engaging analytical thinking. The other option is based on information about a single case or a personal experience and thus is chosen if experiential processing is preferred over reliance on base-rate evidence. The participants had to choose from a set of four possible answers [“Certainly (option A/B)”, “Probably (option A/B)”], so that scores ranged from 1 (indicating the disadvantageous or the experiential decision for the analytical-based and the experiential-based problems respectively) to 4 (indicating the advantageous or the analytical decision for the analytical-based and the experiential-based problems respectively). Total scores were computed for each of the two types of problems and for each of the three types of scenarios. The task has two versions that were translated in Greek. The follow-up version was created by changing some superficial information of the post-test version (i.e., names, objects and numbers).

Table 1.
Pre-Test Everyday Decision-Making Competence Τask Items Examples.
2.3.2. Applying Decision Rules Τask
The Applying Decision Rules task (ADR), which is a subtest of the Adult Decision-Making Competence battery (Bruine de Bruin et al. 2007), consists of 10 problems that evaluate the ability to correctly apply decision rules of varying complexity. For each problem, five electronic products with numeric ratings of their features (e.g., picture quality) are presented in a table. Participants must select one or more products by applying the decision rule that is described each time. Decision rules were elimination by aspects, satisficing, lexicographic, and equal weights (Payne et al. 1993; for a short description of each decision rule see the following section). The task’s final score was computed as the mean of correct answers. The task was translated in Greek and three versions were created, one for each time of assessment. The differences between them were superficial (i.e., computers, televisions and mobile phones on pre-test, post-test and follow-up assessment respectively, and names).
2.4. Intervention
During the first training session, the researcher stressed the difference between experiential and analytical thinking (e.g., Kahneman 2003) during daily, economic, and healthcare decision-making. Four metacognitive-strategic questions (see Table 2) which are a simplified version of Batha and Carroll (2007) “metacognitive strategy instructions”, were introduced as an “analytically created schema” (Dansereau et al. 2013) to promote metacognitive and analytical thinking. These questions are the core of the present intervention as they were answered every time a decision problem was analyzed. During this session participants answered the questions to analyze two daily (one analytical-based and one experiential-based) and two healthcare (one analytical-based and one experiential-based) decision-making problems of the pre-test version of the EDMC (Rosi et al. 2019a, see Table 1), as well as similar problems that participants were bringing up from their everyday life. Emphasis was given to the second metacognitive-strategic question, i.e., collecting and organizing all necessary information (see Table 2).

Table 2.
Metacognitive-Strategic Questions.
During the second training session, at first, a review of the previous session’s content was made, and participants practiced on answering the metacognitive-strategic questions to analyze two economic problems (one analytical-based and one experiential-based) of the pre-test version of the EDMC (Rosi et al. 2019a). During the rest of the session, the emphasis was given on the third metacognitive-strategic question. Specifically, four examples of everyday decision-making situations that provided instructions leading participants to apply a specific decision rule were presented (see Table 3). After each example was analyzed by applying the metacognitive-strategic questions, the researcher explained the relevant decision rule and then a discussion was made about some possible situations each rule could be applied in.

Table 3.
Examples of Decision Rules Problems.
The four decision rules used refer to heuristics defined as methods that reduce the cognitive effort associated with decision-making (Payne et al. 1993; Shah and Oppenheimer 2008). Satisficing decision rule is choosing the first in order alternative that meets the predefined cutoff values for all features. Lexicographic decision rule is selecting the alternative with the highest value on the most important feature and then on the second most important feature if there is a tie and so on. Elimination-by-aspects decision rule refers to choosing the alternative that meets a cutoff value predefined for the most important feature and then for the second most important feature if there is a tie and so on. Finally, equal weights decision rule refers to choosing the alternative with the highest total value computed by summing the values of all features of the alternative (see Table 3).
At the beginning of the third session, at first, a review about the previous sessions’ content was made. Then, participants answered the metacognitive-strategic questions for four examples of everyday decision-making situations which provided instructions that were leading them to apply a specific decision rule. The examples were more complex than the ones analyzed in the second session, because they had more than one characteristic of the possible choices to take into account. Finally, two analytical-based (one daily and one healthcare) and two empirical-based (one economic and one healthcare) decision-making problems of the pre-test version of the EDMC (Rosi et al. 2019a) were analyzed using the metacognitive-strategic questions.
2.5. Statistical Analyses
The data analysis was conducted in SPSS version 29. After computation of the EDMC mean total scores (experiential-based, analytical-based, daily, economic and healthcare scores) and the ADR scores (see Table 4), repeated measures ANOVAs were conducted to compare the performance of the EG in the three times of assessment. Subsequently, mixed-design 2 × 2 ANOVAs (representing pre-test and post-test measurements × two groups, EG and CG) were conducted to examine the main and the interaction effects of group and time of assessment on performance. In a third step, given that the CG had higher performance in the pre-tests, compared to the EG, we proceeded to ANCOVAs, using as independent variable the group (EG, CG), as dependent variable the performance in each task in the post-test measurement, and the pre-test measurement as the covariate variable. Only the statistically significant results of the ANCOVAs will be mentioned. Partial eta-squared (ηp2) was used for the estimation of the effect size. Finally, to control for multiple testing, a Bonferroni correction was applied, i.e., significant p = .5/6 = .008.

Table 4.
Mean and Standard Deviation of the EDMC and ADR Scores as a Function of Group and Time.
3. Results
3.1. Experimental Group: The Effects of Time of Assessment (Pre-, Post-Test, and Follow Up) on the Performance
A significant effect of the time of assessment was found on both EDMC analytical-based scores, F(2, 52) = 10.254, p < .001, ηp2 = 0.28, and EDMC experiential-based scores, F(2, 52) = 14.299, p < .001, ηp2 = 0.36. Specifically, both analytical-based scores, I-J = −0.364, p = .005, and experiential-based scores, I-J = −0.648, p < .001, increased between the pre-test assessment and the post-test assessment. In addition, a significant increase of both analytical-based scores, I-J = −0.414, p < .001, and experiential-based scores, I-J = −0.716, p < .001, was found between the pre-test assessment and the follow-up assessment. However, there was no significant increase of either score types between the post-test assessment and the follow-up assessment.
A significant effect of the time of assessment was also found on the EDMC daily scores, F(2, 52) = 20.321, p < .001, ηp2 = 0.44 and economic scores, F(2, 52) = 9.830, p < .001, ηp2 = 0.27, as well as a same trend as regards the time of assessment and healthcare scores, F(2, 52) = 4.098, p = .022, ηp2 = 0.14. Specifically, between the pre-test assessment and the post-test assessment, there was a significant increase of daily scores, I-J = −0.639, p < .001, and economic scores, I-J = −0.537, p < .001, but not of healthcare scores. Furthermore, between the pre-test assessment and the follow-up assessment, a significant increase of daily scores, I-J = −0.750, p < .001, economic scores, I-J = −0.519, p = .008, as well as a trend of increase of healthcare scores, I-J = −0.426, p < .033, was found. However, there was no significant increase of none of the score types between the post-test assessment and the follow-up assessment. The effect of time of assessment on ADR scores was not significant (see Table 4, Figure 2).
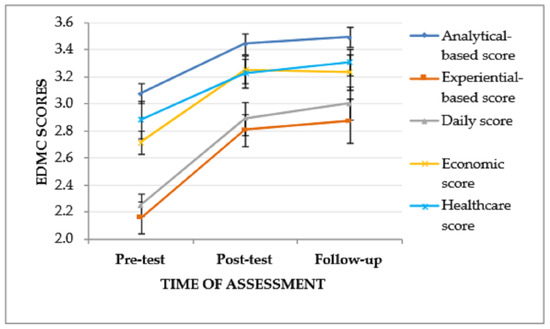
Figure 2.
EDMC scores of the experimental group in the three times of assessment. Error bars represent standard errors of the means.
3.2. Comparison of the Experimental Group and the Active Control Group: The Effects of Group and Time of Assessment (Pre- and Post-Test) on Performance
Mixed-design ANOVA revealed a significant interaction effect (time of assessment × group) on EDMC analytical-based scores, F(1, 53) = 8.702, p = .005, ηp2 = 0.141, suggesting that the pattern of change in analytical-based scores from the pre-test to the post-test assessment differed between the two groups. Specifically, as depicted in Figure 3, the EG, although it performed lower than the CG at the pre-test assessment, it showed improved post-test performance and outperformed the CG, while the CG showed slightly decreased post-test performance. The main effects of the time of assessment, F(1, 53) = 2.779, p = .101, and the group, F(1, 53) = 0.616, p = .436, were not significant.
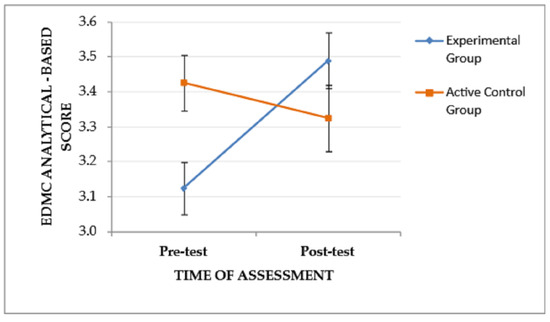
Figure 3.
The effect of the group and the time of assessment on the EDMC analytical-based score. Error bars represent standard errors of the means.
A similar trend (time of assessment × group), F(1, 53) = 4.445, p = .04, ηp2 = 0.077, and a significant main effect of the time of assessment, F(1, 53) = 25.787, p < .001, ηp2 = 0.327, were found on EDMC experiential-based scores, indicating that the pattern of change in the experiential-based scores from the pre-test to the post-test assessment tends to vary between the two groups. Specifically, as depicted in Figure 4, the EG, although it performed lower than the CG at the pre-test assessment, showed improved post-test performance and outperformed the CG. The main effect of the group was not significant, F(1, 53) = 0.000, p = .995. Regarding the main effect of the time of assessment, Bonferroni pairwise comparisons showed improved post-test EDMC experiential-based scores for both groups (EG and CG), I-J = −0.458, p < .001. The subsequent ANCOVA revealed that there was a trend of the covariate (pre-test EDMC experiential-based score) to affect the post-test EDMC experiential-based score, F(1, 52) = 5.012, p = .029, ηp2 = 0.088. When this trend was taken into account, only a trend of difference between the post-test EDMC experiential-based score of the two groups remained, F(2, 52) = 3.399, p = .041, ηp2 = 0.116.
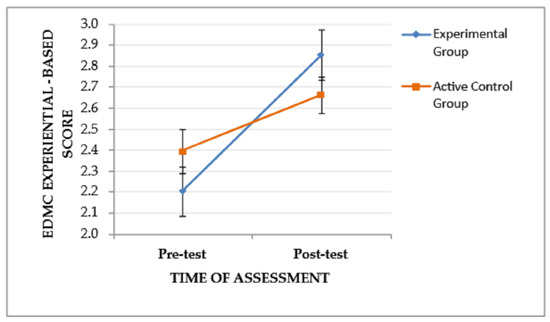
Figure 4.
The effect of the group and the time of assessment on the EDMC experiential-based score. Error bars represent standard errors of the means.
A significant main effect of the time of assessment was found on EDMC daily scores, F(1, 53) = 32.132, p < .001, ηp2 = 0.377, indicating an improvement from the pre-test to the post-test assessment for both groups. The main effect of the group, F(1, 53) = 1.42, p = .239, and the interaction effect (time of assessment × group), F(1, 53) = 3.014, p = .088, were not significant. Regarding the main effect of the time of assessment, Bonferroni pairwise comparisons showed improved post-test EDMC daily scores for both groups (EG and CG), I-J = −0.489, p < .001.
A significant interaction effect (time of assessment × group), F(1, 53) = 14.178, p < .001, ηp2 = 0.211, and a trend of the time of assessment to affect performance, F(1, 53) = 4.435, p = .04, ηp2 = 0.077, were found on EDMC economic scores, indicating that the pattern of change in experiential-based scores from the pre-test to the post-test assessment varied between the two groups. Specifically, as depicted in Figure 5, the EG, although it performed lower than the CG at the pre-test assessment, showed improved post-test performance and outperformed the CG. The main effect of the group was not significant, F(1, 53) = 2.189, p = .145.
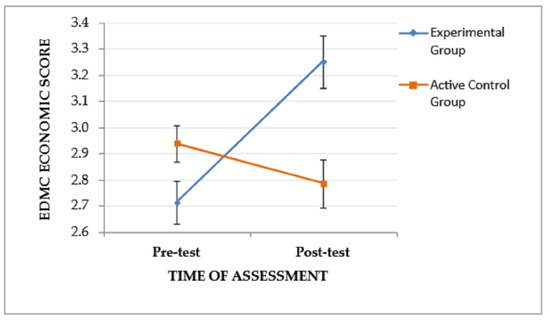
Figure 5.
The effect of the group and the time of assessment on the EDMC economic score. Error bars represent standard errors of the means.
The main effect of the time of assessment, F(1, 53) = 2.865, p = .096, the main effect of the group, F(1, 53) = 0.795, p = .376, and the interaction effect (time of assessment × group), F(1, 53) = 1.37, p = .247, on the EDMC healthcare scores were not significant.
The main effect of the time of assessment, F(1, 53) = 3.838, p = .055, the main effect of the group, F(1, 53) = 1.622, p = .208, and the interaction effect (time of assessment × group), F(1, 53) = 0.017, p = .898, on the ADR scores were also not significant.
4. Discussion
The purpose of the present study was to assess the effectiveness of a metacognitive strategy training on aMCI patients’ decision-making abilities. According to the hypothesis of the study, aMCI patients’ ability to make decisions about everyday situations and their ability to apply decision rules would improve directly after the metacognitive intervention and this improvement would be maintained or increased one month later. Furthermore, aMCI patients’ decision-making ability in everyday contexts and their ability to apply decision rules would improve after training in comparison to an active control group of aMCI patients. The hypothesis was partially confirmed.
4.1. Decision-Making Ability in Everyday Situations
Results showed that aMCI patients’ performance on analytical-based and experiential-based problems of the EDMC, was increased after the intervention, in comparison to before the intervention. Importantly, these benefits were maintained one month after the post-test assessment. In addition, the effectiveness of the metacognitive intervention was also found when the two groups were compared. The findings indicate that aMCI patients learned to rely more on analytical thinking while making decisions not only about analytical-based problems that require the analysis of the problem’s structure and thus increased cognitive effort, but also about experiential-based problems that require rejection of experience-based information and reliance on base-rate information (Rosi et al. 2019a). In line with the IJDM (Dansereau et al. 2013), the present metacognitive training enhanced the use of the analytical system during decision-making, because of the repeated application of an “analytically created schema”. This schema was internalized as a part of the wisdom/expertise system and participants could use it consciously or unconsciously to make advantageous decisions. In addition, these results are in agreement with Batha and Carroll (2007), who found an improvement in decision-making ability of university students after a “metacognitive strategy instruction”. Finally, like in the present study, Rosi et al. (2019a) combined IJDM (Dansereau et al. 2013) and Batha and Carroll (2007) instruction and created a “metacognitive-strategy decision-making training” that improved decision-making abilities of older adults.
Analytical decision-making requires executive functions and working memory capacity to categorize information, evaluate options and monitor the application of strategies (e.g., Brand et al. 2006; Schiebener et al. 2014; Schiebener and Brand 2015). However, aMCI patients present deficits in executive functions and working memory (Klekociuk and Summers 2014). Therefore, it is unsurprising that MCI patients make more disadvantageous decisions than healthy peers in risk situations, which require analytical thinking (Schiebener and Brand 2015) and which have been associated with executive functions and ratio processing abilities (Pertl et al. 2015). Even though cognitive deficits affect analytical decision-making, in the present study aMCI patients’ analytical decision-making ability has improved. Thus, it can be assumed that the metacognitive system can compensate for the effect of cognitive deficits in analytical thinking. Specifically, during the present intervention, participants’ metacognitive system was improved by obtaining declarative knowledge about decision-making process (the analytically created schema and the decision rules). Additionally, their ability to regulate their thinking process (metacognitive control) while making decisions was enhanced by using the analytically created schema.
In regard to the performance on the economic decision-making scenarios, the results indicated that it improved after the metacognitive intervention, in comparison to before the intervention and in comparison to the CG of aMCI patients. This could be explained by the fact that more economic decision-making problems were discussed during the intervention. The majority of the problems participants were bringing up from their everyday life, as well as the decision-making problems that provided instructions to apply a decision rule, were mostly economic.
Additionally, as far as the performance on the EDMC healthcare scenarios is concerned, it was not improved after the metacognitive intervention in comparison to before the intervention and in comparison to the CG. Older adults with an aMCI diagnosis face a plethora of health-related decision-making situations in their everyday life. Therefore, according to IJDM (Dansereau et al. 2013), it is possible that participants had already formed schemas about decision-making in healthcare situations, by accumulating similar decision-making experiences. As this kind of schemas is “more resistant to change” (Dansereau et al. 2013, p. 278), training effects were not observed immediately after the intervention. However, an increased performance on healthcare scenarios was found on the follow-up assessment in comparison to the pre-test assessment of the EG, which means that participants were making more advantageous decisions about health-related issues a month after the post-test. This probably indicates that participants abandoned their previous schemas, only after further repeated application of the “analytically created schema”, that they were taught during the intervention, in their daily life after the completion of the intervention.
Finally, aMCI patient’s decision-making ability about daily scenarios was improved after the intervention in comparison to before the intervention, but not in comparison to the CG. Thus, it cannot be stated with certainty that it was the metacognitive intervention that caused this improvement. Probably participants had already formed schemas about decision-making in daily situations which were effective and thus they were maintained even after the intervention.
It should be noted that participants of the present study were attending cognitive training and/or physical intervention programs at the same time period that they were participating in the present study, as visitors of DCCAH. In the future, metacognitive decision-making interventions should be applied in MCI patients that do not attend other programs- if ethically possible, so that any improvement in decision-making abilities due to this specific type of intervention will be measured in its real magnitude.
4.2. Ability to Apply Decision Rules
Concerning performance on the ADR (Bruine de Bruin et al. 2007), results did not show a significant improvement after the intervention and in comparison to the CG. Like the EDMC (Rosi et al. 2019a), the ADR (Bruine de Bruin et al. 2007) is a task that requires analytical thinking, since comparisons of the values, information integration, and suppression of irrelevant or no longer relevant stimuli are needed (Del Missier et al. 2010). However, in comparison to the EDMC, the ADR is more cognitively effortful, as it consists of five possible alternatives and an arithmetic value for each one of five features of each alternative. Moreover, some items of the task require the application of two or three decision rules in a predefined order. So, the ADR places high demands on fluid cognitive abilities (Bruine de Bruin et al. 2007, 2012), working memory (Del Missier et al. 2012, 2013, 2017; Rosi et al. 2019b) and the inhibition dimension of executive functioning (Del Missier et al. 2010, 2012). Therefore, aMCI patients did not improve in this task, probably because the practice on applying decision rules was not adequate and not with material as complex as the ADR, or because their cognitive deficits played more vital role than metacognition, while applying decision rules. However, as participants of the present study were already receiving cognitive training in the DCCAH, the effectiveness of an intervention targeting more intensely the cognitive functions that seem to influence the ability to apply decision rules should probably be more extensively studied in the future.
4.3. Neuropsychology of Decision-Making
According to a review by Gleichgerrcht et al. (2010), there is a complex neural network that supports decision-making. The main brain areas of this network are the orbitofrontal cortex and the ventromedial prefrontal cortex (stimulus encoding system), the anterior cingulate cortex and the lateral prefrontal and parietal cortices (action selection system), the basal ganglia, the amygdala and the insula (expected reward system). Vaidya and Fellows (2017) have also highlighted the significant role of frontal lobes in the identification of the decision options, and the construction of the options’ values (ventromedial and dorsomedial frontal lobe). In addition, anodal transcranial direct current stimulation to the right dorsolateral prefrontal cortex (DLPFC) has been associated with an increase in analytical judgment and decision-making (Edgcumbe et al. 2019), and with creative problem-solving, by promoting convergent analytical thinking (Guo et al. 2023). Finally, analytical decision-making under risk performance has been associated with dorsolateral and ventromedial prefrontal cortices’ activity (Gleichgerrcht et al. 2010).
Most of the above mentioned brain areas seem to be negatively affected in MCI. In particular, MCI patients present changes in the orbitofrontal cortex (e.g., Fan et al. 2008), the anterior cingulated cortex (e.g., Borsa et al. 2018), the DLPFC (e.g., Liang et al. 2011), the parietal lobe (e.g., Jacobs et al. 2012), the basal ganglia, the amygdala (e.g., Xiong et al. 2021) and the insula (e.g., Fan et al. 2008). However, some of the brain areas involved in decision-making are probably less impaired than others in aMCI. So, maybe the decision-making ability in everyday situations was improved, because it is based on the less impaired brain areas’ activity, which was probably enhanced during the present intervention. Also, it can be assumed that the ability to apply decision rules was not improved, because the brain areas that are involved in this decision-making ability are significantly impaired in aMCI. It should be noted that financial capacity in aMCI has been predicted by the volume of the angular gyrus (Griffith et al. 2010a) and that medical decision-making capacity in aMCI patients has been associated with posterior cortical brain metabolism of N-acetylaspartate/Creatine (Griffith et al. 2010b).
4.4. Limitations and Future Research
This study has limitations that future research should address. Firstly, the EDMC is not as complex as real-life decision-making situations can be, because all necessary information is given in the problem description, there are only two options every time and no time limit. Thus, future research could use additional tasks that resemble real-life decision-making situations even more. In addition, the short duration of the intervention (only 3 sessions) and the relatively small time period between the post-test assessment and the follow-up assessment should be taken into account in future studies. Finally, longitudinal designs, neurophysiological measures and other types of MCI should be considered in future research.
5. Conclusions
In conclusion, as MCI is associated with deficits in analytical decision-making (e.g., Sinclair et al. 2022), an ability highly relevant to daily functioning (Mather 2006), the present study’s aim was to examine the efficacy of a metacognitive strategy training on aMCI patients analytical decision-making abilities. Significant improvement was observed in the ability to think analytically while making decisions about everyday situations, economic and healthcare-related, but not in the ability to apply decision rules, which is a cognitively demanding analytical ability. The present study is an important step in examining decision-making interventions for aMCI.
Author Contributions
Conceptualization, F.A.P. and D.M.; methodology, F.A.P., D.M. and M.T.; formal analysis, F.A.P. and D.M.; investigation, F.A.P.; resources, E.P. and M.T.; data curation, F.A.P.; writing—original draft preparation, F.A.P.; writing—review and editing, F.A.P., D.M., M.S. and G.K.; visualization, D.M. and M.T.; supervision, D.M., G.P. and V.P.; project administration, F.A.P. and D.M.; funding acquisition, D.M., G.P., M.S., V.P., G.K., E.P. and M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Scientific and Ethics Committee of the Greek Association of Alzheimer’s Disease and Related Disorders (protocol code 82, approved on 19 October 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available upon duly justified request.
Acknowledgments
The authors thank Alessia Rosi for providing the “Everyday Decision-Making Competence Τask” and useful material for the intervention, and Vasiliki Boza for translating the task in Greek.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Albert, Marilyn S., Steven T. DeKosky, Dennis Dickson, Bruno Dubois, Howard H. Feldman, Nick C. Fox, Anthony Gamst, David M. Holtzmani, William J. Jagust, Ronald C. Petersen, and et al. 2011. The Diagnosis of Mild Cognitive Impairment Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s and Dementia 7: 270–79. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. 2013. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Publishing. [Google Scholar] [CrossRef]
- Batha, Kate, and Marie Carroll. 2007. Metacognitive Training Aids Decision Making. Australian Journal of Psychology 59: 64–69. [Google Scholar] [CrossRef]
- Bayard, Sophie, Jean-Pierre Jacus, Stéphane Raffard, and Marie-Christine Gely-Nargeot. 2014. Apathy and Emotion-Based Decision-Making in Amnesic Mild Cognitive Impairment and Alzheimer’s Disease. Behavioural Neurology 2014: 231469. [Google Scholar] [CrossRef]
- Bayard, Sophie, Jean-Pierre Jacus, Stéphane Raffard, and Marie-Christine Gély-Nargeot. 2015. Conscious Knowledge and Decision Making under Ambiguity in Mild Cognitive Impairment and Alzheimer Disease. Alzheimer Disease and Associated Disorders 29: 357–59. [Google Scholar] [CrossRef]
- Bechara, Antoine, Hanna Damasio, Daniel Tranel, and Antonio R. Damasio. 1997. Deciding Advantageously before Knowing the Advantageous Strategy. Science 275: 1293–95. Available online: http://www.jstor.org/stable/2892390 (accessed on 12 June 2023). [CrossRef] [PubMed]
- Beck, Aaron T., Charlotte Hammerbeck Ward, M. Mendelson, J. Mock, and J. Erbaugh. 1961. An Inventory for Measuring Depression. Archives of General Psychiatry 4: 561–71. [Google Scholar] [CrossRef]
- Beck, Aaron T., Norman Epstein, Gary Brown, and Robert A. Steer. 1988. An Inventory for Measuring Clinical Anxiety: Psychometric Properties. Journal of Consulting and Clinical Psychology 56: 893–97. [Google Scholar] [CrossRef]
- Borsa, Virginia M., Pasquale A. Della Rosa, Eleonora Catricalà, Matteo Canini, Antonella Iadanza, Andrea Falini, Jubin Abutalebi, and Sandro Iannaccone. 2018. Interference and Conflict Monitoring in Individuals with Amnestic Mild Cognitive Impairment: A Structural Study of the Anterior Cingulate Cortex. Journal of Neuropsychology 12: 23–40. [Google Scholar] [CrossRef]
- Brand, Matthias, Kirsten Labudda, and Hans J. Markowitsch. 2006. Neuropsychological Correlates of Decision-Making in Ambiguous and Risky Situations. Neural Networks 19: 1266–76. [Google Scholar] [CrossRef]
- Bruine de Bruin, Wändi, Andrew M. Parker, and Baruch Fischhoff. 2007. Individual Differences in Adult Decision-Making Competence. Journal of Personality and Social Psychology 92: 938–56. [Google Scholar] [CrossRef]
- Bruine de Bruin, Wändi, Andrew M. Parker, and Baruch Fischhoff. 2012. Explaining Adult Age Differences in Decision-Making Competence. Journal of Behavioral Decision Making 25: 352–60. [Google Scholar] [CrossRef]
- Burgio, Francesca, Margarete Delazer, Francesca Meneghello, Marie-Theres Pertl, Carlo Semenza, and Laura Zamarian. 2018. Cognitive Training Improves Ratio Processing and Decision Making in Patients with Mild Cognitive Impairment. Journal of Alzheimer’s Disease 64: 1213–26. [Google Scholar] [CrossRef]
- Cardelle-Elawar, Maria. 1995. Effects of Metacognitive Instruction on Low Achievers in Mathematics Problems. Teaching and Teacher Education 11: 81–95. [Google Scholar] [CrossRef]
- Cummings, Jeffrey L., Michael Mega, Katherine Gray, Susan Rosenberg-Thompson, Daniela Anne Carusi, and Jeffrey Gornbein. 1994. The Neuropsychiatric Inventory: Comprehensive Assessmenat of Psychopathology in Dementia. Neurology 44: 2308. [Google Scholar] [CrossRef] [PubMed]
- da Mata, Fernanda Gomes, Fernando Silva Neves, Guilherme Menezes Lage, Paulo Henrique Paiva de Moraes, Paulo Mattos, Daniel Fuentes, Humberto Corrêa, and Leandro Malloy-Diniz. 2011. Neuropsychological Assessment of the Decision Making Process in Children and Adolescents: An Integrative Review of the Literature. Archives of Clinical Psychiatry (São Paulo) 38: 106–15. [Google Scholar] [CrossRef]
- Dansereau, Donald F., Danica K. Knight, and Patrick M. Flynn. 2013. Improving Adolescent Judgment and Decision Making. Professional Psychology: Research and Practice 44: 274–82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Del Missier, Fabio, Patrik Hansson, Andrew M. Parker, Wändi Bruine de Bruin, Lars Göran Nilsson, and Timo Mäntylä. 2017. Unraveling the Aging Skein: Disentangling Sensory and Cognitive Predictors of Age-Related Differences in Decision Making. Journal of Behavioral Decision Making 30: 123–39. [Google Scholar] [CrossRef]
- Del Missier, Fabio, Timo Mäntylä, and Wändi Bruine de Bruin. 2010. Executive Functions in Decision Making: An Individual Differences Approach. Thinking and Reasoning 16: 69–97. [Google Scholar] [CrossRef]
- Del Missier, Fabio, Timo Mäntylä, and Wändi Bruine de Bruin. 2012. Decision-Making Competence, Executive Functioning, and General Cognitive Abilities. Journal of Behavioral Decision Making 25: 331–51. [Google Scholar] [CrossRef]
- Del Missier, Fabio, Timo Mäntylä, Patrik Hansson, Wändi Bruine De Bruin, Andrew M. Parker, and Lars Göran Nilsson. 2013. The Multifold Relationship between Memory and Decision Making: An Individual-Differences Study. Journal of Experimental Psychology: Learning Memory and Cognition 39: 1344–64. [Google Scholar] [CrossRef]
- Edgcumbe, Daniel R., Volker Thoma, Davide Rivolta, Michael A. Nitsche, and Cynthia H.Y. Fu. 2019. Anodal Transcranial Direct Current Stimulation over the Right Dorsolateral Prefrontal Cortex Enhances Reflective Judgment and Decision-Making. Brain Stimulation 12: 652–58. [Google Scholar] [CrossRef]
- Epstein, Seymour. 1994. Integration of the Cognitive and the Psychodynamic Unconscious. American Psychologist 49: 709–24. [Google Scholar] [CrossRef] [PubMed]
- Evans, Jonathan St. B. T. 2008. Dual-Processing Accounts of Reasoning, Judgment, and Social Cognition. Annual Review of Psychology 59: 255–78. [Google Scholar] [CrossRef] [PubMed]
- Fan, Yong, Nematollah Batmanghelich, Chris M. Clark, and Christos Davatzikos. 2008. Spatial Patterns of Brain Atrophy in MCI Patients, Identified via High-Dimensional Pattern Classification, Predict Subsequent Cognitive Decline. NeuroImage 39: 1731–43. [Google Scholar] [CrossRef]
- Faul, Franz, Edgar Erdfelder, Albert-Georg Lang, and Axel Buchner. 2007. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behavior Research Methods 39: 175–91. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Carina, Inês Macedo, Fernando Barbosa, and João Marques-Teixeira. 2021. Economic Decision-Making in the Continuum between Healthy Aging and Alzheimer’s Disease: A Systematic Review of 20 Years of Research. Neuroscience and Biobehavioral Reviews 131: 1243–63. [Google Scholar] [CrossRef] [PubMed]
- Folstein, Marshal F., Susan E. Folstein, and Paul R. McHugh. 1975. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 12: 189–98. [Google Scholar] [CrossRef]
- Fountoulakis, Konstantinos N., Magdalini Tsolaki, Apostolos Iacovides, Jerome Yesavage, Ruth O’Hara, Aristides Kazis, and C. Ierodiakonou. 1999. The Validation of the Short Form of the Geriatric Depression Scale (GDS) in Greece. Aging Clinical and Experimental Research 11: 367–72. [Google Scholar] [CrossRef]
- Fountoulakis, Konstantinos N., Magdalini Tsolaki, Helen Chantzi, and Aristides Kazis. 2000. Mini Mental State Examination (MMSE): A Validation Study in Greece. American Journal of Alzheimer’s Disease and Other Dementias 15: 342–45. [Google Scholar] [CrossRef]
- Gleichgerrcht, Ezequiel, Agustín Ibáñez, María Roca, Teresa Torralva, and Facundo Manes. 2010. Decision-Making Cognition in Neurodegenerative Diseases. Nature Reviews Neurology 6: 611–23. [Google Scholar] [CrossRef]
- Grammatikopoulos, Ilias A., Gary Sinoff, Athanasios Alegakis, Dimitrios Kounalakis, Maria Antonopoulou, and Christos Lionis. 2010. The Short Anxiety Screening Test in Greek: Translation and Validation. Annals of General Psychiatry 9: 1. [Google Scholar] [CrossRef]
- Griffith, H. Randall, Christopher C. Stewart, Luke E. Stoeckel, Ozioma C. Okonkwo, Jan A. Den Hollander, Roy C. Martin, Katherine Belue, Jacquelynn N. Copeland, Lindy E. Harrell, John C. Brockington, and et al. 2010a. Magnetic Resonance Imaging Volume of the Angular Gyri Predicts Financial Skill Deficits in People with Amnestic Mild Cognitive Impairment. Journal of the American Geriatrics Society 58: 265–74. [Google Scholar] [CrossRef] [PubMed]
- Griffith, H. Randall, Ozioma C. Okonkwo, Jan A. Den Hollander, Katherine Belue, Jacqueline Copeland, Lindy E. Harrell, John C. Brockington, David G. Clark, and Daniel C. Marson. 2010b. Brain Metabolic Correlates of Decision Making in Amnestic Mild Cognitive Impairment. Aging, Neuropsychology, and Cognition 17: 492–504. [Google Scholar] [CrossRef] [PubMed]
- Guo, Jiayue, Jiani Luo, Yi An, and Tiansheng Xia. 2023. TDCS Anodal Stimulation of the Right Dorsolateral Prefrontal Cortex Improves Creative Performance in Real-World Problem Solving. Brain Sciences 13: 449. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, Heidi I. L., Martin P. J. Van Boxtel, Jelle Jolles, Frans R. J. Verhey, and Harry B. M. Uylings. 2012. Parietal Cortex Matters in Alzheimer’s Disease: An Overview of Structural, Functional and Metabolic Findings. Neuroscience & Biobehavioral Reviews 36: 297–309. [Google Scholar] [CrossRef]
- Kahneman, Daniel. 2003. A Perspective on Judgment and Choice: Mapping Bounded Rationality. American Psychologist 58: 697–720. [Google Scholar] [CrossRef]
- Klekociuk, Shannon Zofia, and Mathew James Summers. 2014. Exploring the Validity of Mild Cognitive Impairment (MCI) Subtypes: Multiple-Domain Amnestic MCI Is the Only Identifiable Subtype at Longitudinal Follow-Up. Journal of Clinical and Experimental Neuropsychology 36: 290–301. [Google Scholar] [CrossRef]
- Kounti, Fotini, Magdalini Tsolaki, and Grigoris Kiosseoglou. 2006. Functional Cognitive Assessment Scale (FUCAS): A New Scale to Assess Executive Cognitive Function in Daily Life Activities in Patients with Dementia and Mild Cognitive Impairment. Human Psychopharmacology: Clinical and Experimental 21: 305–11. [Google Scholar] [CrossRef]
- Liang, Peipeng, Zhiqun Wang, Yanhui Yang, Xiuqin Jia, and Kuncheng Li. 2011. Functional Disconnection and Compensation in Mild Cognitive Impairment: Evidence from DLPFC Connectivity Using Resting-State FMRI. PLoS ONE 6: e22153. [Google Scholar] [CrossRef]
- Mather, Mara. 2006. A Review of Decision-Making Processes: Weighing the Risks and Benefits of Aging. In When I’m 64; Edited by Laura L. Carstensen and Christine R. Hartel. Washington, DC: The National Academies Press. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK83778/ (accessed on 10 May 2023).
- Nasreddine, Ziad S., Natalie A. Phillips, Valerie Bedirian, Simon Charbonneau, Victor Whitehead, Isabelle Collin, Jeffrey L. Cummings, and Howard Chertkow. 2005. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. Journal of the American Geriatrics Society 53: 695–99. [Google Scholar] [CrossRef]
- Okonkwo, Ozioma C., H. Randall Griffith, J. N. Copeland, K. Belue, S. Lanza, E. Y. Zamrini, L. E. Harrell, J. C. Brockington, D. Clark, R. Raman, and et al. 2008. Medical Decision-Making Capacity in Mild Cognitive Impairment: A 3-Year Longitudinal Study. Neurology 71: 1474–80. [Google Scholar] [CrossRef] [PubMed]
- Payne, John W., James R. Bettman, and Eric J. Johnson. 1993. The Adaptive Decision Maker. Cambridge: Cambridge University Press. [Google Scholar] [CrossRef]
- Pertl, Marie-Theres, Thomas Benke, Laura Zamarian, and Margarete Delazer. 2015. Decision Making and Ratio Processing in Patients with Mild Cognitive Impairment. Journal of Alzheimer’s Disease 48: 765–79. [Google Scholar] [CrossRef]
- Pertl, Marie-Theres, Thomas Benke, Laura Zamarian, and Margarete Delazer. 2017. Effects of Healthy Aging and Mild Cognitive Impairment on a Real-Life Decision-Making Task. Journal of Alzheimer’s Disease 58: 1077–87. [Google Scholar] [CrossRef]
- Peters, Ellen, Daniel Västfjäll, Paul Slovic, C. K. Mertz, Ketti Mazzocco, and Stephan Dickert. 2006. Numeracy and Decision Making. Psychological Science 17: 407–13. [Google Scholar] [CrossRef] [PubMed]
- Petersen, Ronald C. 2004. Mild Cognitive Impairment as a Diagnostic Entity. Journal of Internal Medicine 256: 183–94. [Google Scholar] [CrossRef] [PubMed]
- Politis, Antonios, Lawrence D. Mayer, Maria Passa, Antonis Maillis, and Constantine G. Lyketsos. 2004. Validity and Reliability of the Newly Translated Hellenic Neuropsychiatric Inventory (H-NPI) Applied to Greek Outpatients with Alzheimer’s Disease: A Study of Disturbing Behaviors among Referrals to a Memory Clinic. International Journal of Geriatric Psychiatry 19: 203–8. [Google Scholar] [CrossRef] [PubMed]
- Poptsi, Eleni, Despina Moraitou, Marina Eleftheriou, Fotini Kounti-Zafeiropoulou, Chrysa Papasozomenou, Christina Agogiatou, Evaggelia Bakoglidou, Georgia Batsila, Despina Liapi, Nefeli Markou, and et al. 2019. Normative Data for the Montreal Cognitive Assessment in Greek Older Adults with Subjective Cognitive Decline, Mild Cognitive Impairment and Dementia. Journal of Geriatric Psychiatry and Neurology 32: 265–74. [Google Scholar] [CrossRef]
- Reisberg, Barry, Steven H. Ferris, Mony J. De Leon, and Thomas Crook. 1982. The Global Deterioration Scale for Assessment of Primary Degenerative Dementia. The American Journal of Psychiatry 139: 1136–39. [Google Scholar] [CrossRef]
- Roberts, Rosebud O., David S. Knopman, Michelle M. Mielke, Ruth H. Cha, V. Shane Pankratz, Teresa J. H. Christianson, Yonas E. Geda, Bradley F. Boeve, Robert J. Ivnik, Eric G. Tangalos, and et al. 2014. Higher Risk of Progression to Dementia in Mild Cognitive Impairment Cases Who Revert to Normal. Neurology 82: 317–25. [Google Scholar] [CrossRef]
- Rosi, Alessia, Tomaso Vecchi, and Elena Cavallini. 2019a. Metacognitive-Strategy Training Promotes Decision-Making Ability in Older Adults. Open Psychology 1: 200–14. [Google Scholar] [CrossRef]
- Rosi, Alessia, Wändi Bruine de Bruin, Fabio Del Missier, Elena Cavallini, and Riccardo Russo. 2019b. Decision-Making Competence in Younger and Older Adults: Which Cognitive Abilities Contribute to the Application of Decision Rules? Aging, Neuropsychology, and Cognition 26: 174–89. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, Timothy. 2012. Consequences of Age-Related Cognitive Declines. Annual Review of Psychology 63: 201–26. [Google Scholar] [CrossRef]
- Schiebener, Johannes, and Matthias Brand. 2015. Decision Making Under Objective Risk Conditions–a Review of Cognitive and Emotional Correlates, Strategies, Feedback Processing, and External Influences. Neuropsychology Review 25: 171–98. [Google Scholar] [CrossRef] [PubMed]
- Schiebener, Johannes, Elisa Wegmann, Bettina Gathmann, Christian Laier, Mirko Pawlikowski, and Matthias Brand. 2014. Among Three Different Executive Functions, General Executive Control Ability Is a Key Predictor of Decision Making under Objective Risk. Frontiers in Psychology 5: 1386. [Google Scholar] [CrossRef] [PubMed]
- Shah, Anuj K., and Daniel M. Oppenheimer. 2008. Heuristics Made Easy: An Effort-Reduction Framework. Psychological Bulletin 134: 207–22. [Google Scholar] [CrossRef]
- Sinclair, Craig, Ranmalee Eramudugolla, Nicolas Cherbuin, Moyra E. Mortby, and Kaarin J. Anstey. 2022. The Impact of Mild Cognitive Impairment on Decision-Making under Explicit Risk Conditions: Evidence from the Personality and Total Health (PATH) Through Life Longitudinal Study. Journal of the International Neuropsychological Society 29: 594–604. [Google Scholar] [CrossRef]
- Sinoff, Gary, Liora Ore, David Zlotogorsky, and Ada Tamir. 1999. Short Anxiety Screening Test—A Brief Instrument for Detecting Anxiety in the Elderly. International Journal of Geriatric Psychiatry 14: 1062–71. [Google Scholar] [CrossRef]
- Tsolaki, Magdalini, Eleni Poptsi, Chrisitna Aggogiatou, Nefeli Markou, and Stavros Zafeiropoulos. 2017. Computer-Based Cognitive Training Versus Paper and Pencil Training: Which Is More Effective? A Randomized Controlled Trial in People with Mild Cognitive Impairment. JSM Alzheimer’s Dis Related Dementia 4: 1032. [Google Scholar]
- Vaidya, Avinash R., and Lesley K. Fellows. 2017. The Neuropsychology of Decision-Making: A View from the Frontal Lobes. In Decision Neuroscience: An Integrative Perspective. Edited by Jean-Claude Dreher and Leon Tremblay. Cambridge: Elsevier Academic Press. [Google Scholar] [CrossRef]
- Xiong, Guanxing, Zhe She, Jun Zhao, and Hanqi Zhang. 2021. Transcranial Direct Current Stimulation over the Right Dorsolateral Prefrontal Cortex Has Distinct Effects on Choices Involving Risk and Ambiguity. Behavioural Brain Research 400: 113044. [Google Scholar] [CrossRef]
- Yesavage, Jerome A., T. L. Brink, Terence L. Rose, Owen Lum, Virginia Huang, Michael Adey, and Von Otto Leirer. 1982. Development and Validation of a Geriatric Depression Screening Scale: A Preliminary Report. Journal of Psychiatric Research 17: 37–49. [Google Scholar] [CrossRef]
- Zamarian, Laura, Elisabeth M. Weiss, and Margarete Delazer. 2011. The Impact of Mild Cognitive Impairment on Decision Making in Two Gambling Tasks. Journals of Gerontology-Series B Psychological Sciences and Social Sciences 66: 23–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Ying, Jing Wang, Tingting Sun, Luchun Wang, Tao Li, Huizi Li, Yaonan Zheng, Zili Fan, Ming Zhang, Lihui Tu, and et al. 2022. Decision-Making Profiles and Their Associations with Cognitive Performance in Mild Cognitive Impairment. Journal of Alzheimer’s Disease 87: 1215–27. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).