The ANTI-Vea-UGR Platform: A Free Online Resource to Measure Attentional Networks (Alertness, Orienting, and Executive Control) Functioning and Executive/Arousal Vigilance
Abstract
:1. Attentional Networks and the ANTI-Vea
1.1. What Is the ANTI-Vea Task?
1.2. ANTI-Vea Relevance: Dissociation between Executive and Arousal Vigilance
1.3. A Summary of the ANTI-Vea Design
1.4. ANTI-Vea Indexes
2. Reliability of the ANTI-Vea Measures
3. Online Version: The ANTI-Vea-UGR Platform
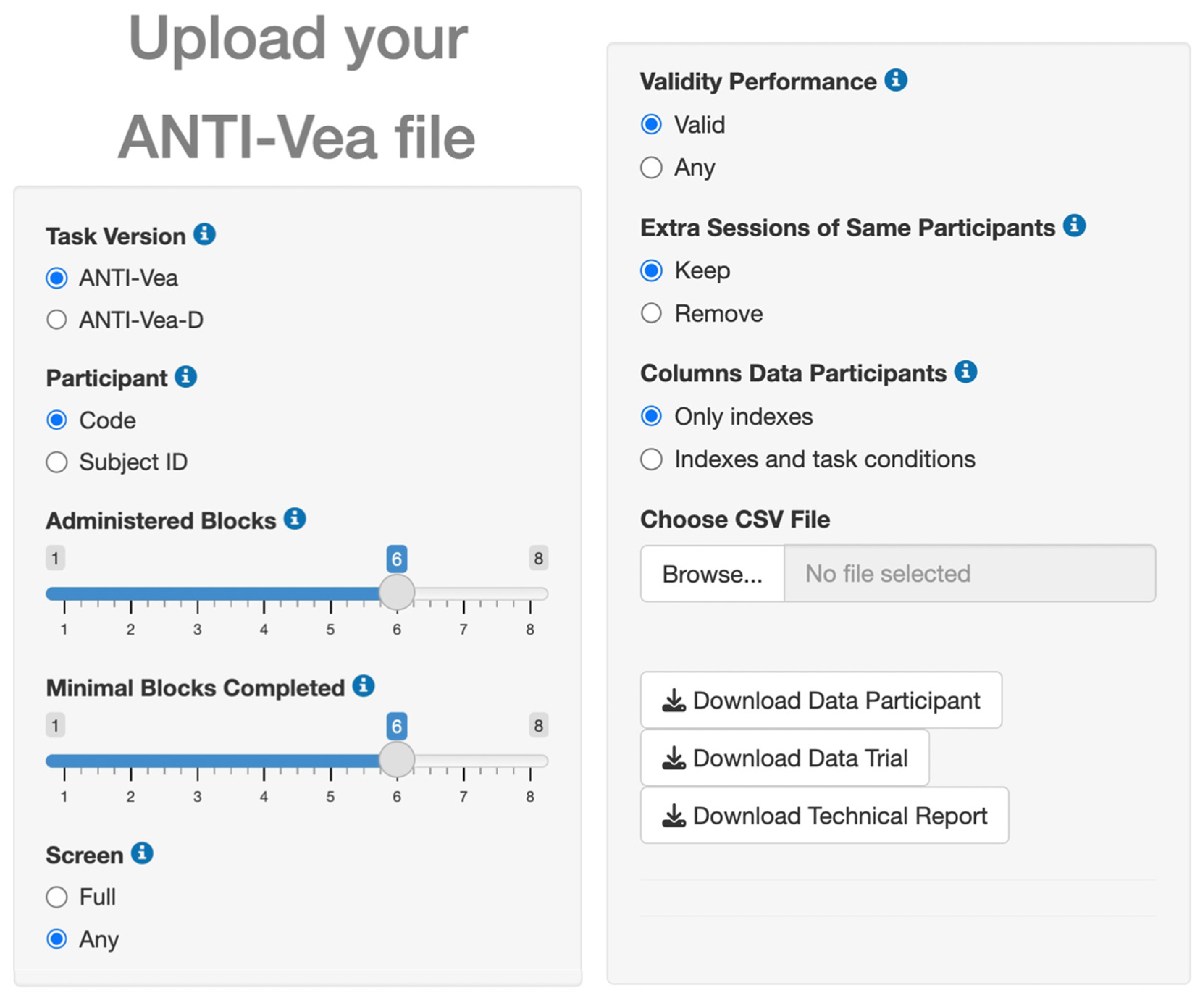
3.1. Summary of Features and Options
3.2. Data Collection, Protection, and Download
4. Analyzing Data: Scripts and Tools for Analysis of Data
5. Discussion: Summary of Published Research with ANTI-Vea
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
| 1 | Technically, a decrement in sensitivity across the blocks of the ANTI-Vea has been also observed. However, compared to the change in response bias, that effect is substantially lower and likely due to a floor effect in FAs (Luna et al. 2021a) or other artifacts (Román-Caballero et al. 2023). |
| 2 | In order for the system to assign the same Subject ID to two or more different sessions, the codes for Participant, Name, Experiment, and Group must match (in older versions, the access cookies also need to match). Therefore, in studies where participants perform multiple sessions of the task, it is recommended that the experimenter distinguish their participants by using the Participant Code instead of the Subject ID. |
References
- Almeida de Souza, Rafael, Aydamari Faria-Jr, and Raymond M. Klein. 2021. On the Origins and Evolution of the Attention Network Tests. Neuroscience & Biobehavioral Reviews 126: 560–72. [Google Scholar] [CrossRef]
- Al-Shargie, Fares, Usman Tariq, Hasan Mir, Hamad Alawar, Fabio Babiloni, and Hasan Al-Nashash. 2019. Vigilance Decrement and Enhancement Techniques: A Review. Brain Sciences 9: 178. [Google Scholar] [CrossRef] [PubMed]
- Basner, Mathias, Daniel Mollicone, and David F. Dinges. 2011. Validity and Sensitivity of a Brief Psychomotor Vigilance Test (PVT-B) to Total and Partial Sleep Deprivation. Acta Astronautica 69: 949–59. [Google Scholar] [CrossRef] [PubMed]
- Callejas, Alicia, Juan Lupiáñez, and Pío Tudela. 2004. The Three Attentional Networks: On Their Independence and Interactions. Brain and Cognition 54: 225–27. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, Maria, Andrea Marotta, Diana Martella, Elisa Volpari, Francesca Agostini, Francesca Favieri, Giuseppe Forte, Monica Rea, Rosa Ferri, Vito Giordano, and et al. 2022. Assessing the Three Attentional Networks in Children from Three to Six Years: A Child-Friendly Version of the Attentional Network Test for Interaction. Behavior Research Methods 54: 1403–15. [Google Scholar] [CrossRef]
- Cásedas, Luis, Ausiàs Cebolla, and Juan Lupiáñez. 2022. Individual Differences in Dispositional Mindfulness Predict Attentional Networks and Vigilance Performance. Mindfulness 13: 967–81. [Google Scholar] [CrossRef]
- Claypoole, Victoria L., Alexis R. Neigel, Nicholas W. Fraulini, Gabriella M. Hancock, and James L. Szalma. 2018. Can Vigilance Tasks Be Administered Online? A Replication and Discussion. Journal of Experimental Psychology: Human Perception and Performance 44: 1348–55. [Google Scholar] [CrossRef]
- Coll-Martín, Tao, Hugo Carretero-Dios, and Juan Lupiáñez. 2021. Attentional Networks, Vigilance, and Distraction as a Function of Attention-deficit/Hyperactivity Disorder Symptoms in an Adult Community Sample. British Journal of Psychology 112: 1053–79. [Google Scholar] [CrossRef]
- Coll-Martín, Tao, Hugo Carretero-Dios, and Juan Lupiáñez. 2023. Attention-Deficit/Hyperactivity Disorder Symptoms as Function of Arousal and Executive Vigilance: Testing the Halperin and Schulz’s Neurodevelopmental Model in an Adult Community Sample. Available online: https://psyarxiv.com/jzk7b/ (accessed on 13 June 2023).
- Corbetta, Maurizio. 1998. Frontoparietal Cortical Networks for Directing Attention and the Eye to Visual Locations: Identical, Independent, or Overlapping Neural Systems? Proceedings of the National Academy of Sciences of the United States of America 95: 831–38. [Google Scholar] [CrossRef]
- Dinges, David F., and John W. Powell. 1985. Microcomputer Analyses of Performance on a Portable, Simple Visual RT Task during Sustained Operations. Behavior Research Methods, Instruments, & Computers 17: 652–55. [Google Scholar] [CrossRef]
- Doran, Scott M., Hans P. A. Van Dongen, and David F. Dinges. 2001. Sustained Attention Performance during Sleep Deprivation: Evidence of State Instability. Archives Italiennes de Biologie 139: 253–67. [Google Scholar] [PubMed]
- Eriksen, Barbara A., and Charles W. Eriksen. 1974. Effects of Noise Letters upon the Identification of a Target Letter in a Nonsearch Task. Perception & Psychophysics 16: 143–49. [Google Scholar] [CrossRef]
- Fan, Jin, Bruce D. McCandliss, Tobias Sommer, Amir Raz, and Michael I. Posner. 2002. Testing the Efficiency and Independence of Attentional Networks. Journal of Cognitive Neuroscience 14: 340–47. [Google Scholar] [CrossRef] [PubMed]
- Feltmate, Brett B. T., Austin J. Hurst, and Raymond M. Klein. 2020. Effects of Fatigue on Attention and Vigilance as Measured with a Modified Attention Network Test. Experimental Brain Research 238: 2507–19. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Duque, Diego, and Michael I. Posner. 2001. Brain Imaging of Attentional Networks in Normal and Pathological States. Journal of Clinical and Experimental Neuropsychology 23: 74–93. [Google Scholar] [CrossRef] [PubMed]
- Fortenbaugh, Francesca C., Joseph Degutis, Laura Germine, Jeremy B. Wilmer, Mallory Grosso, Kathryn Russo, and Michael Esterman. 2015. Sustained Attention across the Life Span in a Sample of 10,000: Dissociating Ability and Strategy. Psychological Science 26: 1497–510. [Google Scholar] [CrossRef]
- Germine, Laura, Ken Nakayama, Bradley C. Duchaine, Christopher F. Chabris, Garga Chatterjee, and Jeremy B. Wilmer. 2012. Is the Web as Good as the Lab? Comparable Performance from Web and Lab in Cognitive/Perceptual Experiments. Psychonomic Bulletin & Review 19: 847–57. [Google Scholar] [CrossRef]
- Hemmerich, Klara, Juan Lupiáñez, Fernando G Luna, and Elisa Martín-Arévalo. 2023. The Mitigation of the Executive Vigilance Decrement via HD-TDCS over the Right Posterior Parietal Cortex and Its Association with Neural Oscillations. Cerebral Cortex 33: 6761–71. [Google Scholar] [CrossRef]
- Huertas, Florentino, Rafael Ballester, Honorato José Gines, Abdel Karim Hamidi, Consuelo Moratal, and Juan Lupiáñez. 2019. Relative Age Effect in the Sport Environment. Role of Physical Fitness and Cognitive Function in Youth Soccer Players. International Journal of Environmental Research and Public Health 16: 2837. [Google Scholar] [CrossRef]
- Ishigami, Yoko, and Raymond M. Klein. 2010. Repeated Measurement of the Components of Attention Using Two Versions of the Attention Network Test (ANT): Stability, Isolability, Robustness, and Reliability. Journal of Neuroscience Methods 190: 117–28. [Google Scholar] [CrossRef]
- Johnston, Samantha-Kaye, Neville W. Hennessey, and Suze Leitão. 2019. Determinants of Assessing Efficiency within Auditory Attention Networks. The Journal of General Psychology 146: 134–69. [Google Scholar] [CrossRef]
- Lamond, Nicole, Sarah M. Jay, Jillian Dorrian, Sally A. Ferguson, Gregory D. Roach, and Drew Dawson. 2008. The Sensitivity of a Palm-Based Psychomotor Vigilance Task to Severe Sleep Loss. Behavior Research Methods 40: 347–52. [Google Scholar] [CrossRef] [PubMed]
- Loh, Sylvia, Nicole Lamond, Jill Dorrian, Gregory Roach, and Drew Dawson. 2004. The Validity of Psychomotor Vigilance Tasks of Less than 10-Minute Duration. Behavior Research Methods, Instruments, & Computers 36: 339–46. [Google Scholar] [CrossRef]
- Luna, Fernando Gabriel, Julián Marino, Javier Roca, and Juan Lupiáñez. 2018. Executive and Arousal Vigilance Decrement in the Context of the Attentional Networks: The ANTI-Vea Task. Journal of Neuroscience Methods 306: 77–87. [Google Scholar] [CrossRef]
- Luna, Fernando Gabriel, Javier Roca, Elisa Martín-Arévalo, and Juan Lupiáñez. 2021a. Measuring Attention and Vigilance in the Laboratory vs. Online: The Split-Half Reliability of the ANTI-Vea. Behavior Research Methods 53: 1124–47. [Google Scholar] [CrossRef]
- Luna, Fernando Gabriel, Pablo Barttfeld, Elisa Martín-Arévalo, and Juan Lupiáñez. 2021b. The ANTI-Vea Task: Analyzing the Executive and Arousal Vigilance Decrements While Measuring the Three Attentional Networks. Psicológica 42: 1–26. [Google Scholar] [CrossRef]
- Luna, Fernando Gabriel, Miriam Tortajada, Elisa Martín-Arévalo, Fabiano Botta, and Juan Lupiáñez. 2022. A Vigilance Decrement Comes along with an Executive Control Decrement: Testing the Resource-Control Theory. Psychonomic Bulletin & Review 29: 1831–43. [Google Scholar] [CrossRef]
- Mackworth, Norman H. 1948. The Breakdown of Vigilance during Prolonged Visual Search. Quarterly Journal of Experimental Psychology 1: 6–21. [Google Scholar] [CrossRef]
- Mackworth, Norman H. 1950. Researches on the Measurement of Human Performance. Journal of the American Medical Association 144: 1526. [Google Scholar] [CrossRef]
- MacLeod, Jeffrey W., Michael A. Lawrence, Meghan M. McConnell, Gail A. Eskes, Raymond M. Klein, and David I. Shore. 2010. Appraising the ANT: Psychometric and Theoretical Considerations of the Attention Network Test. Neuropsychology 24: 637–51. [Google Scholar] [CrossRef]
- Petersen, Steven E., and Michael I. Posner. 2012. The Attention System of the Human Brain: 20 Years After. Annual Review of Neuroscience 35: 73–89. [Google Scholar] [CrossRef] [PubMed]
- Posner, Michael I. 1980. Orienting of Attention. The Quarterly Journal of Experimental Psychology 32: 3–25. [Google Scholar] [CrossRef] [PubMed]
- Posner, Michael I. 1994. Attention: The Mechanisms of Consciousness. Proceedings of the National Academy of Sciences of the United States of America 91: 7398–403. [Google Scholar] [CrossRef] [PubMed]
- Posner, Michael I., and Stanislas Dehaene. 1994. Attentional Networks. Trends in Neurosciences 17: 75–79. [Google Scholar] [CrossRef] [PubMed]
- Posner, Michael I., and Steven E. Petersen. 1990. The Attention System of The Human Brain. Annual Reviews of Neuroscience 13: 25–42. [Google Scholar] [CrossRef]
- Roberts, Katherine L., A. Quentin Summerfield, and Deborah A. Hall. 2006. Presentation Modality Influences Behavioral Measures of Alerting, Orienting, and Executive Control. Journal of the International Neuropsychological Society 12: 485–92. [Google Scholar] [CrossRef]
- Robertson, Ian H., Tom Manly, Jackie Andrade, Bart T. Baddeley, and Jenny Yiend. 1997. ‘Oops!’: Performance Correlates of Everyday Attentional Failures in Traumatic Brain Injured and Normal Subjects. Neuropsychologia 35: 747–58. [Google Scholar] [CrossRef]
- Roca, Javier, Cándida Castro, María Fernanda López-Ramón, and Juan Lupiáñez. 2011. Measuring Vigilance While Assessing the Functioning of the Three Attentional Networks: The ANTI-Vigilance Task. Journal of Neuroscience Methods 198: 312–24. [Google Scholar] [CrossRef]
- Roca, Javier, Pedro García-Fernández, Cándida Castro, and Juan Lupiáñez. 2018. The Moderating Effects of Vigilance on Other Components of Attentional Functioning. Journal of Neuroscience Methods 308: 151–61. [Google Scholar] [CrossRef]
- Román-Caballero, Rafael, Elisa Martín-Arévalo, and Juan Lupiáñez. 2021. Attentional Networks Functioning and Vigilance in Expert Musicians and Non-Musicians. Psychological Research 85: 1121–35. [Google Scholar] [CrossRef]
- Román-Caballero, Rafael, Elisa Martín-Arévalo, and Juan Lupiáñez. 2023. Changes in Response Criterion and Lapse Rate as General Mechanisms of Vigilance Decrement: Commentary on McCarley and Yamani (2021). Psychological Science 34: 132–36. [Google Scholar] [CrossRef]
- Rueda, M. Rosario, Jin Fan, Bruce D. McCandliss, Jessica D. Halparin, Dana B. Gruber, Lisha Pappert Lercari, and Michael I. Posner. 2004. Development of Attentional Networks in Childhood. Neuropsychologia 42: 1029–40. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, Avi, Orrie Dan, and Yair Bar-Haim. 2011. Online Assessment of Sustained Attention Following Sleep Restriction. Sleep Medicine 12: 257–61. [Google Scholar] [CrossRef]
- Sanchis, Carlos, Esther Blasco, Fernando Gabriel Luna, and Juan Lupiáñez. 2020. Effects of Caffeine Intake and Exercise Intensity on Executive and Arousal Vigilance. Scientific Reports 10: 8393. [Google Scholar] [CrossRef] [PubMed]
- See, Judi E., Steven R. Howe, Joel S. Warm, and William N. Dember. 1995. Meta-Analysis of the Sensitivity Decrement in Vigilance. Psychological Bulletin 117: 230–49. [Google Scholar] [CrossRef]
- Thomson, David R., Derek Besner, and Daniel Smilek. 2016. A Critical Examination of the Evidence for Sensitivity Loss in Modern Vigilance Tasks. Psychological Review 123: 70–83. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, Trayambak, Anju L. Singh, and Indramani L. Singh. 2009. Task Demand and Workload: Effects on Vigilance Performance and Stress. Journal of the Indian Academy of Applied Psychology 35: 265–75. [Google Scholar]


| Domain | Index | Description | Observed Results (In-Lab/Online Version) M (SD) * |
|---|---|---|---|
| Attentional networks (ANTI) | Overall RT | Mean correct RT across all ANTI trials. | 629 ms (98)/652 ms (98) |
| Overall errors | Percentage of errors across all ANTI trials. | 6.10% (4.74)/5.95% (4.36) | |
| Alerting RT | RT difference between No Tone and Tone conditions in trials with no cue. | 40 ms (26)/37 ms (43) | |
| Alerting errors | Error difference between No Tone and Tone conditions in trials with no cue. | 2.42% (4.79)/1.46% (4.75) | |
| Orienting RT | RT difference between Invalid and Valid conditions. | 40 ms (27)/46 ms (27) | |
| Orienting errors | Error difference between Invalid and Valid conditions. | −0.07% (3.76)/0.44% (3.98) | |
| Congruency RT | RT difference between Incongruent and Congruent conditions. | 43 ms (27)/41 ms (33) | |
| Congruency errors | Error difference between Incongruent and Congruent conditions. | 0.81% (4.70)/0.36% (3.88) | |
| Executive vigilance (EV) | Hits | Percentage of times the displacement of the central arrow is correctly detected by pressing the spacebar. Synonymous with 1 minus omission errors or misses. | 73.24% (17.34)/78.87% (14.04) |
| Hits slope | Linear slope of hits over blocks, which tends to decrease. | −1.89% (3.64)/−1.93% (3.61) | |
| False alarms | Percentage of times the spacebar is pressed when there is no substantial displacement of the central arrow. Synonymous with commission errors. | 6.35% (5.80)/6.88% (6.02) | |
| False alarms slope | Linear slope of false alarms over blocks, which tends to decrease. | −0.27% (0.94)/−0.23% (1.23) | |
| Arousal vigilance (AV) | Mean RT | Average time to stop the red down counter. | 491 ms (62)/509 ms (85) |
| Mean RT slope | Linear slope of mean RT over blocks, which tends to increase. | 4 ms (11)/5 ms (14) | |
| SD RT | Response speed variability to stop the red down counter. | 90 ms (39)/83 ms (32) | |
| SD RT slope | Linear slope of SD RT over blocks, which tends to increase. | 4 ms (11)/6 ms (13) | |
| Lapses | Percentage of times with an excessively large (RT > 600 ms) or no response to the red down counter. | 11.35% (14.57)/13.19% (17.53) | |
| Lapses slope | Linear slope of lapses over blocks, which tends to increase. | 1.47% (3.32)/1.67% (3.73) |
| Task Index | In-Lab Reliability (rSB) | Online Reliability (rSB) | ||
|---|---|---|---|---|
| Luna et al. (2021a) | Coll-Martín et al. (2021) | Luna et al. (2021a) | Cásedas et al. (2022) | |
| N | 314 | 113 | 303 | 219 |
| Attentional networks | ||||
| Overall RT | .99 | .99 | .99 | .99 |
| Overall errors | .92 | .91 | .89 | .91 |
| Alerting RT | .22 | .47 | .36 | .45 |
| Alerting errors | .18 | .51 | .11 | .24 |
| Orienting RT | .31 | .36 | .30 | .40 |
| Orienting errors | .60 | .26 | .28 | .22 |
| Congruency RT | .67 | .66 | .68 | .64 |
| Congruency errors | .66 | .60 | .52 | .51 |
| Executive vigilance | ||||
| Hits | .94 | .94 | .92 | .91 |
| Hits slope | .27 | .58 | ||
| False alarms | .85 | .85 | .79 | .78 |
| False alarms slope | .40 | .21 | ||
| Arousal vigilance | ||||
| Mean RT | .98 | .97 | .99 | .96 |
| Mean RT slope | .75 | .65 | ||
| SD RT | .84 | .88 | .76 | .71 |
| SD RT slope | .54 | .65 | ||
| Lapses | .96 | .96 | .98 | .96 |
| Lapses slope | .78 | .81 | ||
| Setting Parameter (Parameter = Default Value) | Description and Setting Values |
|---|---|
| lang = en | Language of instructions: “de” for German, “en” for English, “es” for Spanish, “fr” for French, “it” for Italian, and “pl” for Polish. |
| type = ANTI_VEA | Specific task to be performed: ANTI_VEA, ANTI, SART, PVT, SART-PVT, ANTI-Only, SART-Only-Go, SART-Only-NoGo, PVT-Only, ANTI-Vea-D. |
| pc = 1234 | Participant code; only numbers allowed here. Any combination of digits is fine. If this parameter is not specified, the task does not start. |
| exp = Power_ANTI-Vea | The name of your experiment. |
| gr = Exp | The name of the experimental group, in case there is one. |
| no = 2 | Noise: this parameter refers to the random variability of the spatial position of the arrows (1–6); the default value is 2, keep it if you are not interested in this manipulation. |
| dif = 2 | Difficulty: this parameter manipulates the perceptual salience of the target and therefore affects EV. It refers to the spatial distance of the central arrow in relation to the adjacent arrows; 1 (most difficult) to 5 (less difficult) values are allowed; the default value is 2, keep it if you are not interested in this manipulation. |
| st = 200 | Target display duration: integers from 0 to 1700 ms are accepted values, 200 ms being the value in the standard version of the task. |
| dP = false | This value should be set to “true” if you want participants to do the whole practice blocks before the experimental blocks, and to “false” if you want them to go straight to the experimental blocks, with just a reminder of the instructions. This feature is useful when collecting data from several sessions in within-subjects designs. |
| B = 6 | Number of experimental blocks (1–8); the value can be set to 0 if you want the participants to only run the practice, with no experimental block; 6 is the number of blocks by default. |
| probes = 0 | This parameter refers to the number of thought probes (TP) used to measure mind wandering. Depending on the value given to this variable TPs are presented 4, 8, or 12 times per block. By default, the standard version of the task does not include any thought probes. Leave this parameter at 0 to run the standard version of the task, without thought probes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coll-Martín, T.; Román-Caballero, R.; Martínez-Caballero, M.d.R.; Martín-Sánchez, P.d.C.; Trujillo, L.; Cásedas, L.; Castellanos, M.C.; Hemmerich, K.; Manini, G.; Aguirre, M.J.; et al. The ANTI-Vea-UGR Platform: A Free Online Resource to Measure Attentional Networks (Alertness, Orienting, and Executive Control) Functioning and Executive/Arousal Vigilance. J. Intell. 2023, 11, 181. https://doi.org/10.3390/jintelligence11090181
Coll-Martín T, Román-Caballero R, Martínez-Caballero MdR, Martín-Sánchez PdC, Trujillo L, Cásedas L, Castellanos MC, Hemmerich K, Manini G, Aguirre MJ, et al. The ANTI-Vea-UGR Platform: A Free Online Resource to Measure Attentional Networks (Alertness, Orienting, and Executive Control) Functioning and Executive/Arousal Vigilance. Journal of Intelligence. 2023; 11(9):181. https://doi.org/10.3390/jintelligence11090181
Chicago/Turabian StyleColl-Martín, Tao, Rafael Román-Caballero, María del Rocío Martínez-Caballero, Paulina del Carmen Martín-Sánchez, Laura Trujillo, Luis Cásedas, M. Concepción Castellanos, Klara Hemmerich, Greta Manini, María Julieta Aguirre, and et al. 2023. "The ANTI-Vea-UGR Platform: A Free Online Resource to Measure Attentional Networks (Alertness, Orienting, and Executive Control) Functioning and Executive/Arousal Vigilance" Journal of Intelligence 11, no. 9: 181. https://doi.org/10.3390/jintelligence11090181
APA StyleColl-Martín, T., Román-Caballero, R., Martínez-Caballero, M. d. R., Martín-Sánchez, P. d. C., Trujillo, L., Cásedas, L., Castellanos, M. C., Hemmerich, K., Manini, G., Aguirre, M. J., Botta, F., Marotta, A., Martín-Arévalo, E., Luna, F. G., & Lupiáñez, J. (2023). The ANTI-Vea-UGR Platform: A Free Online Resource to Measure Attentional Networks (Alertness, Orienting, and Executive Control) Functioning and Executive/Arousal Vigilance. Journal of Intelligence, 11(9), 181. https://doi.org/10.3390/jintelligence11090181





