Correlation Effects in Trimeric Acylphloroglucinols
Abstract
1. Introduction
2. Computational Details
3. Results
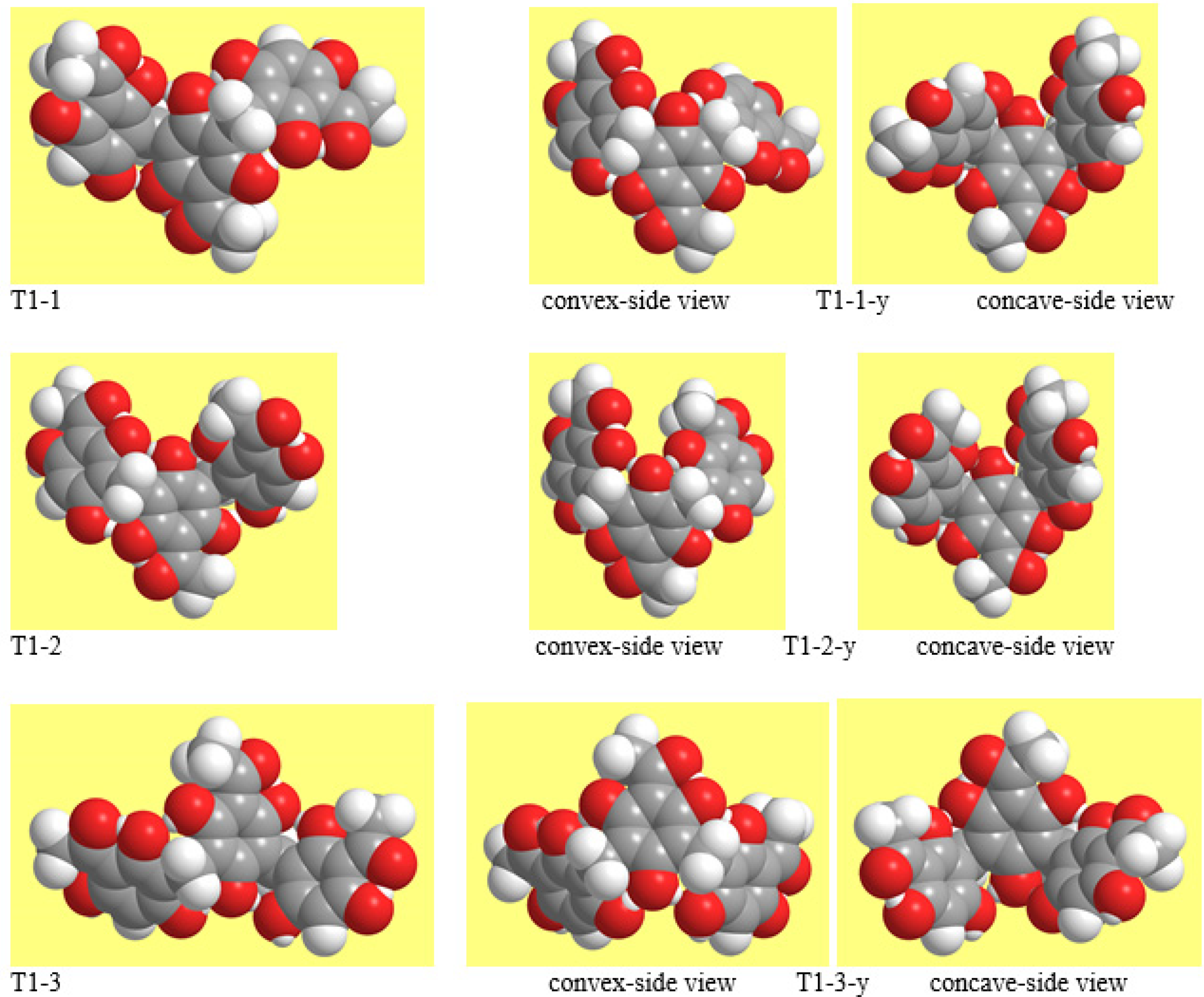
3.1. Selection and Concise Naming of Calculated T-ACPL Molecules
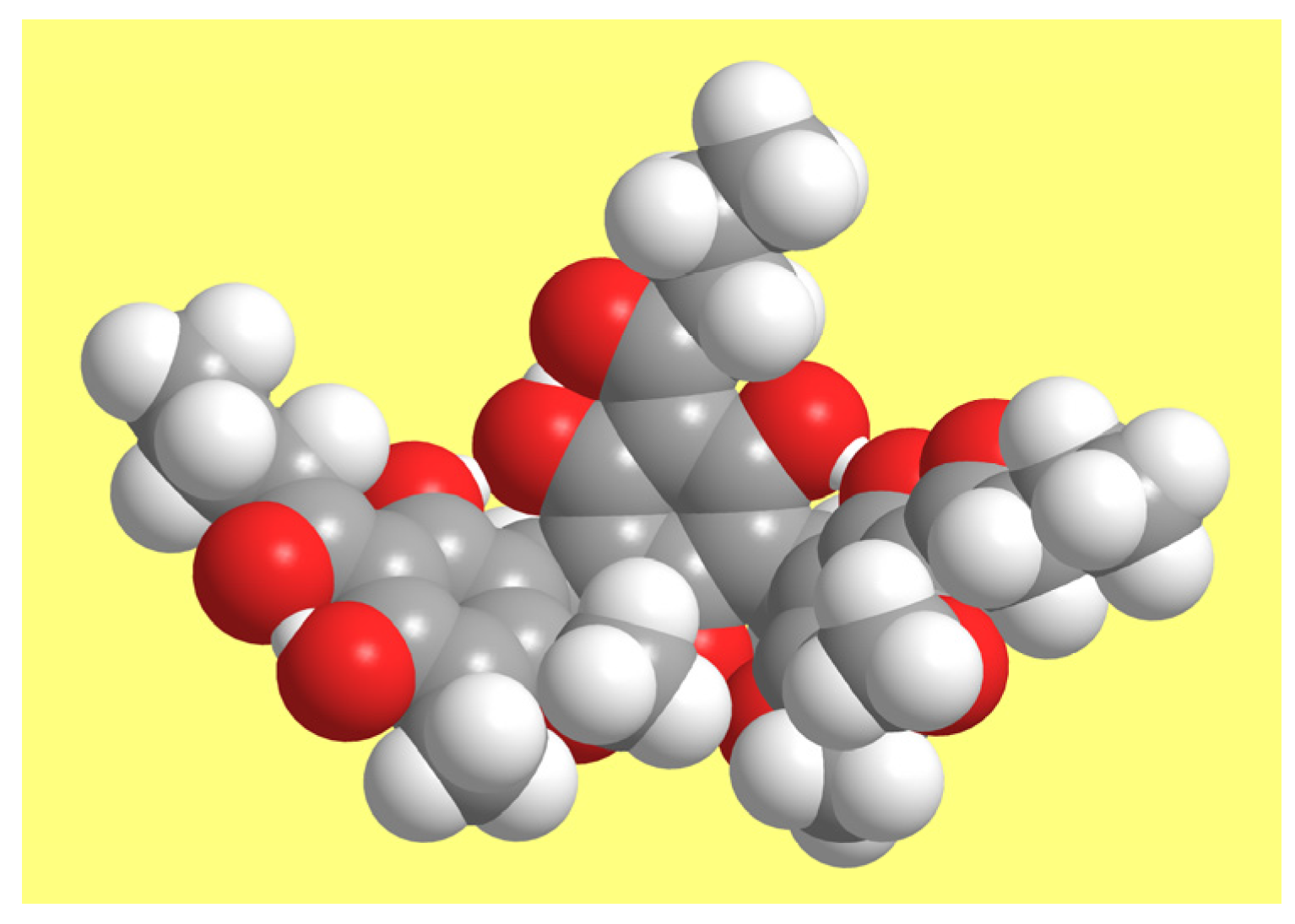
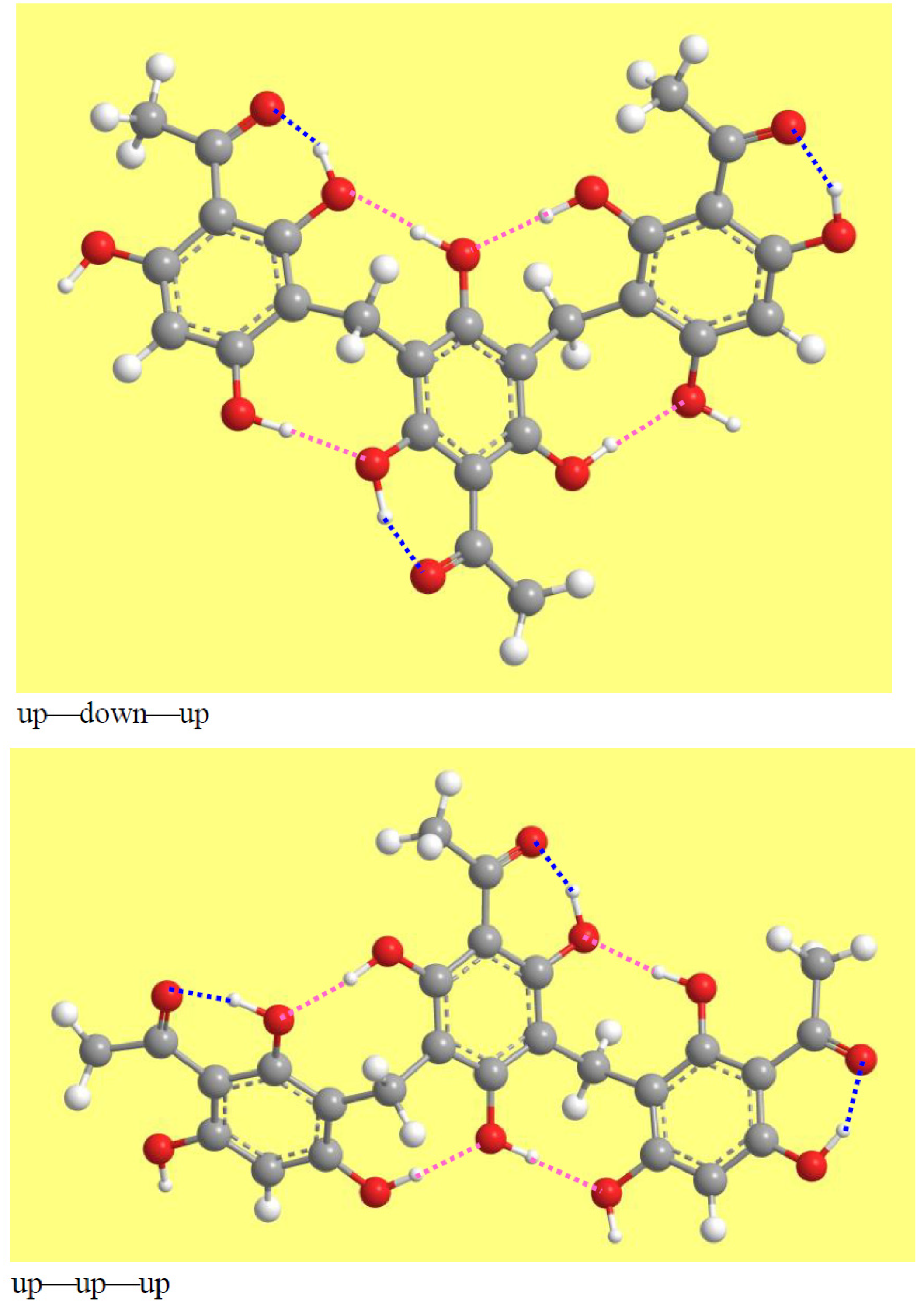
3.2. Conformers’ Geometries and Their Concise Naming
3.3. Conformers’ Geometry Preferences and Energetics
3.3.1. General Features
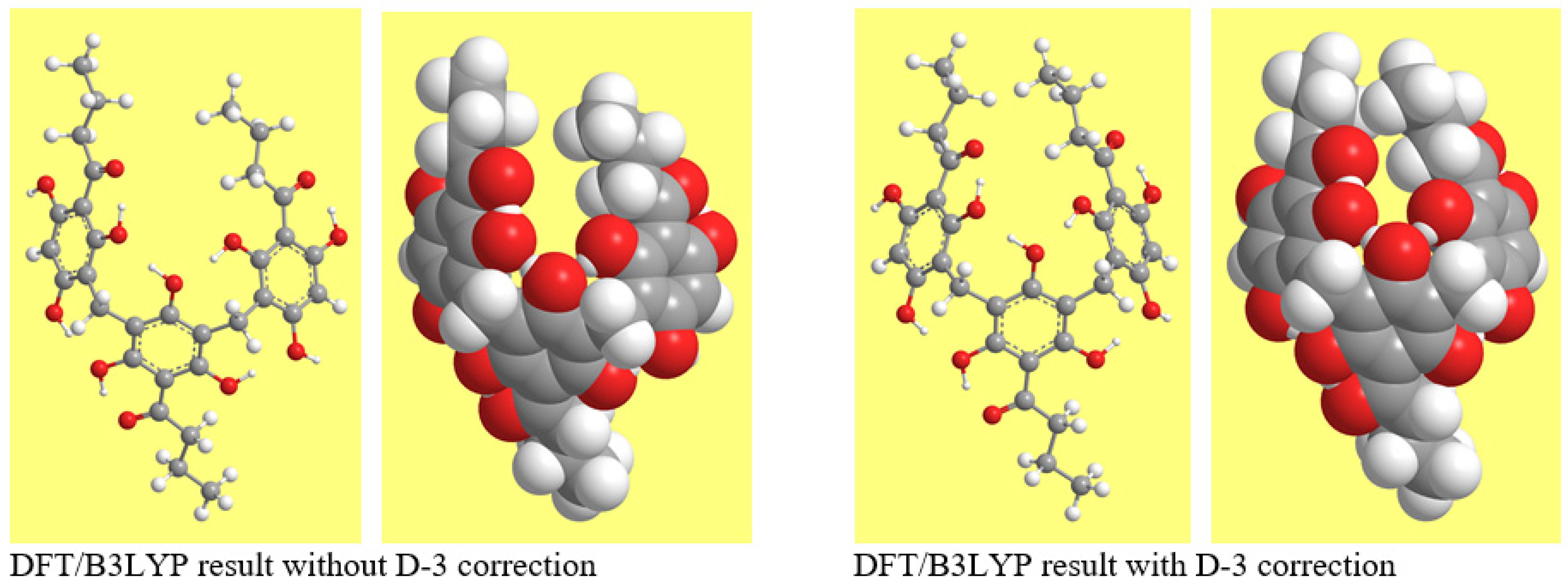
3.3.2. Effects of Grimme’s Correction on Geometries and Energetics
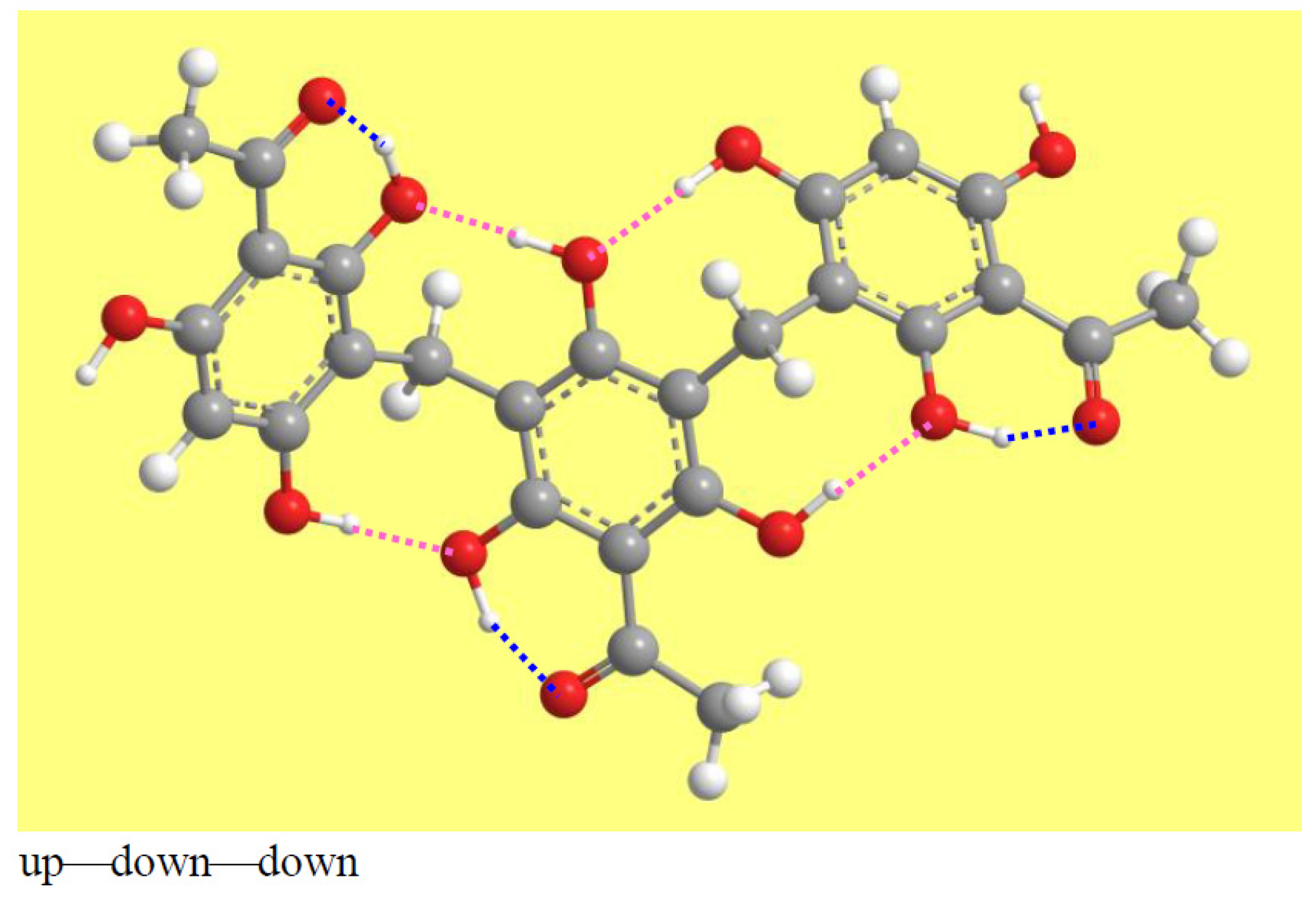
3.4. Characteristics of the Intramolecular Hydrogen Bonds (IHBs)
3.4.1. General Features of the IHBs in Trimeric Acylphloroglucinols
3.4.2. Effects of Grimme’s Correction on the Characteristics of the IHBs
3.5. Other Computable Molecular Properties
3.6. Indications from a Limited Number of MP2 Results
4. Discussion and Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef] [PubMed]
- Verotta, L. Are acylphloroglucinols lead structures for the treatment of degenerative diseases? Phytochem. Rev. 2002, 1, 389–407. [Google Scholar] [CrossRef]
- Kusumaningsih, T.; Prasetyo, W.E.; Wibowo, F.R.; Firdaus, M. Toward an efficient and eco-friendly route for the synthesis of dimeric 2,4-diacetyl phloroglucinol and its potential as a SARS-CoV-2 main protease antagonist: Insight from in silico studies. New J. Chem. 2021, 45, 7830–7843. [Google Scholar] [CrossRef]
- Bushelyev, S.N.; Stepanov, N.F. Elektronnaya Struktura y Biologhicheskaya Aktivnost Molecul; Khimiya, Snanye: Moscow, Russia, 1989. [Google Scholar]
- Mammino, L.; Kabanda, M.M. Model structures for the study of acylated phloroglucinols and computational study of the caespitate molecule. J. Mol. Struct. (Theochem) 2007, 805, 39–52. [Google Scholar] [CrossRef]
- Mammino, L.; Kabanda, M.M. A study of the intramolecular hydrogen bond in acylphloroglucinols. J. Mol. Struct. (Theochem) 2009, 901, 210–219. [Google Scholar] [CrossRef]
- Kabanda, M.M.; Mammino, L. The conformational preferences of acylphloroglucinols—A promising class of biologically active compounds. Int. J. Quantum Chem. 2012, 112, 3691–3702. [Google Scholar] [CrossRef]
- Mammino, L.; Kabanda, M.M. Computational study of the patterns of weaker intramolecular hydrogen bonds stabilizing acylphloroglucinols. Int. J. Quantum Chem. 2012, 112, 2650–2658. [Google Scholar] [CrossRef]
- Mammino, L.; Kabanda, M.M. The role of additional O–H···O intramolecular hydrogen bonds for acylphloroglucinols’ conformational preferences in vacuo and in solution. Mol. Simul. 2013, 39, 1–13. [Google Scholar] [CrossRef]
- Mammino, L.; Kabanda, M.M. Adducts of acylphloroglucinols with explicit water molecules: Similarities and differences across a sufficiently representative number of structures. Int. J. Quantum Chem. 2010, 110, 2378–2390. [Google Scholar] [CrossRef]
- Mammino, L. Intramolecular hydrogen bonding patterns, conformational preferences and molecular properties of dimeric acylphloroglucinols: An ab initio and DFT study. J. Mol. Struct. 2019, 1176, 488–500. [Google Scholar] [CrossRef]
- Rudyk, R.; Molina, M.A.A.; Yurquina, A.; Go’mez, M.I.; Blanco, S.E.; Ferretti, F.H. A theoretical and experimental study on the structure and dipole moment of phloroglucinol in ethanol. J. Mol. Struct. (Theochem) 2004, 673, 231–238. [Google Scholar] [CrossRef]
- Hernandez, E.G.; Garza, J. Reactivity sites in dopamine depend on its intramolecular hydrogen bond. J. Mex. Chem. Soc. 2017, 61, 222–228. [Google Scholar]
- Fersht, A.R. The hydrogen bond in molecular recognition. Trend. Biochem. Sci. 1987, 12, 301–304. [Google Scholar] [CrossRef]
- Desiraju, G.R. Chemistry Beyond the Molecule. Nature 2001, 412, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Seto, L.O.N.; Cai, Y.; Leinala, E.K.; Borisova, S.N.; Palcic, M.N.; Evans, S.V. The influence of an intramolecular hydrogen bod in a differential recognition of inhibitory acceptor analogs by human ABO(H) blood group A and B glycosyltransferases. J. Biol. Chem. 2003, 49, 4191–4195. [Google Scholar]
- Sanchez, G. Introduction to “Intramolecular Hydrogen Bonding 2018”. Molecules 2019, 24, 2858. [Google Scholar] [CrossRef] [PubMed]
- Yunta, M.J.R. It is important to compute intramolecular hydrogen bonding in drug design? Am. J. Model. Optim. 2017, 5, 24–57. [Google Scholar] [CrossRef]
- Reenu, V. Evaluating the role of electron-correlation in the external prediction of the toxicity of nitrobenzenes towards Tetrahymena pyriformis. New J. Chem. 2016, 40, 2343–2353. [Google Scholar]
- Grimme, S.; Antony, J.; Schwabe, T.; Mück-Lichtenfeld, C. Density functional theory with dispersion corrections for supramolecular structures, aggregates, and complexes of (bio)organic molecules. Org. Biomol. Chem. 2007, 5, 741–758. [Google Scholar] [CrossRef]
- Thirman, J.; Head-Gordon, M. Electrostatic domination of the effect of electron correlation in intermolecular interactions. J. Phys. Chem. Lett. 2014, 5, 1380–1385. [Google Scholar] [CrossRef]
- Mcdonagh, J.; Silva, A.F.; Vincent, M.; Popelier, P. Quantifying electron correlation of the chemical bond. J. Phys. Chem. Lett. 2017, 8, 1937–1942. [Google Scholar] [CrossRef]
- Hongo, K.; Cuong, N.T.; Maezono, R. The importance of electron correlation on stacking interaction of adenine-thymine base-pair step in B-DNA: A quantum Monte Carlo study. J. Chem. Theory Comput. 2013, 12, 1081–1086. [Google Scholar] [CrossRef]
- Vos, R.J.; Hendriks, R.; van Duijneveldt, F.B. SCF, MP2, and CEPA-1 calculations on the OH ‥ O hydrogen bonded complexes (H2O)2 and (H2O-H2CO). J. Comp. Chem. 1990, 11, 1–18. [Google Scholar] [CrossRef]
- Mó, O.; Yáñez, M.; Elguero, J. Cooperative (nonpairwise) effects in water trimers: An ab initio molecular orbital study. J. Chem. Phys. 1992, 97, 6628–6638. [Google Scholar] [CrossRef]
- Ferrari, A.M.; Garrone, E.; Ugliengo, P. Ab initio study of the gas-phase equilibrium between (H2O) 4 and (H2O) 8. Chem. Phys. Lett. 1993, 212, 644–648. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1998, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Becke, A.D. Density functional thermochemistry III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Alagona, G.; Ghio, C. Competitive H-bonds in vacuo and in aqueous solution for N-protonated adrenaline and its monohydrated complexes. J. Mol. Struct. (Theochem) 2007, 811, 223–240. [Google Scholar] [CrossRef]
- Mammino, L. Stacking interactions in cavity-containing molecular structures built from acylphloroglucinols: A computational study. Mol. Phys. 2020, 119, e1800852. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, T.; Grimme, S. Double-hybrid density functionals with long-range dispersion corrections: Higher accuracy and extended applicability. Phys. Chem. Chem. Phys. 2007, 9, 3397–3406. [Google Scholar] [CrossRef] [PubMed]
- Neese, F.; Schwabe, T.; Grimme, S. Analytic derivatives for perturbatively corrected “double hybrid” density functionals: Theory, implementation, and applications. J. Chem. Phys. 2007, 126, 124115. [Google Scholar] [CrossRef]
- Grimme, S. Density functional theory with London dispersion corrections. Wiley Interdiscip. Rev. WIREs Comput. Mol. Sci. 2011, 1, 211–228. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Hujo, W.; Grimme, S. Comparison of the performance of dispersion-corrected density functional theory for weak hydrogen bonds. Phys. Chem. Chem. Phys. 2011, 13, 13942–13950. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Goerigk, L.; Fink, R.F. Spin-component-scaled electron correlation methods. WIREs Comput. Mol. Sci. 2012, 2, 886–906. [Google Scholar] [CrossRef]
- Grimme, S. Supramolecular binding thermodynamics by dispersion-corrected density functional theory. Chem. Eur. J. 2012, 18, 9955–9964. [Google Scholar] [CrossRef]
- Grimme, S.; Steinmetz, M. Effects of London dispersion correction in density functional theory on the structures of organic molecules in the gas phase. Phys. Chem. Chem. Phys. 2013, 15, 16031–16042. [Google Scholar] [CrossRef]
- Ehrlich, S.; Moellmann, J.; Grimme, S. Dispersion-corrected density functional theory for aromatic interactions in complex systems. Acc. Chem. Res. 2013, 46, 916–926. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Brandenburg, J.G.; Bannwarth, C. Dispersion-corrected mean-field electronic structure methods. Chem. Rev. 2016, 116, 5105–5154. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- GaussView 4.1; Gaussian Inc.: Pittsburgh, PA, USA, 2006.
- Chem3D; Version 8.0.3; Chemoffice, Cambridge Software: St Neots, UK, 2003.
- Allouche, A.R. Gabedit. 2017. Available online: http://gabedit.sourceforge.net/ (accessed on 10 October 2021).
- Penttila, A.; Sundman, J. The structures of filixic acid. Acta Chem. Scand. 1963, 17, 191–198. [Google Scholar] [CrossRef]
- Penttila, A.; Sundman, J. The chemistry of Dryopteris acylphloroglucinols. J. Pharm. Pharmacol. 1970, 22, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Hisada, S.; Shiraishi, K.; Inagaki, I. Phloroglucinol derivatives of Dryoptehs Dicklensi and some related ferns. Phytochemistry 1972, 11, 2881–2882. [Google Scholar] [CrossRef]
- Hisada, S.; Inoue, O.; Inagaki, I. Isolation of flavaspidic acid-PB from Dryopteris sieboldii. Phytochemistry 1973, 12, 1493–1494. [Google Scholar] [CrossRef]
- Hisada, S.; Inoue, O.; Inagaki, I. Phloroglucinol derivatives of Dryopteris Szeboldzi. Phytochemistry 1973, 12, 2055. [Google Scholar] [CrossRef]
- Lounasmaa, M.; Widén, C.J.; Huhtikangas, A. Phloroglucinol derivatives of Hagenia abyssinica. II. The structure determination of kosotoxin and protokosin. Acta Chem. Scand. 1974, 28, 1200–1208. [Google Scholar] [CrossRef][Green Version]
- Puri, H.S.; Widén, C.J.; Lounasmaa, M. Phloroglucinol derivatives in Dryopterzs chrysocoma. Phytochemistry 1976, 15, 343–344. [Google Scholar] [CrossRef]
- Coskun, M.; Sakushima, T.A.; Nishibe, S.; Hisada, S.; Tanker, N. A phloroglucinol derivative of Dryopteris Abbreviata. Phytochemistry 1982, 21, 1453–1454. [Google Scholar] [CrossRef]
- Patama, T.T.; Widen, C.J. Phloroglucinol derivatives from Dryopteris fusco-atra and d. hawaizensis. Phytochemistry 1991, 30, 3305–3310. [Google Scholar] [CrossRef]
- Woldemariam, T.Z.; Fell, A.F.; Linley, P.A.; Bibby, M.C.; Phillips, R.M. Evaluation of the anti-tumour action and acute toxicity of kosins from Hagenia abyssinica. Pharm. Biomed. Anal. 1992, 10, 555–560. [Google Scholar] [CrossRef]
- Widen, K.J.; Fraser-Jenkins, C.; Reichstein, T.; Gibby, M.; Sarvela, J. Phloroglucinol derivatives in Driopteris sect Fibrillosae and related taxa (Pteridophyta, Dryopteridaceae). Ann. Bot. Fenn. 1996, 33, 69–100. [Google Scholar]
- Katekhaye, S.D.; Shinde, P.B.; Laddha, K.S. Isolation and HPLC method development for filixic acid PBP from Dryopteris filix-mas. Int. J. Phytopharm. 2011, 1, 1–7. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, Q.; Liu, L.; Chen, Z.; Zeng, W.; Lei, H.; Zhang, Y. Compounds from Dryopteris Fragrans (L.) Schott with Cytotoxic Activity. Molecules 2014, 19, 3345–3355. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, P.; Liang, P.; Hu, X. Development of a liquid chromatography-mass spectrometry method for determination of agrimol B in rat plasma: Application to preclinical pharmacokinetics. Biomed. Chromatogr. 2015, 29, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, Z.; Li, C.; Jia, W.; Wang, H.; Wang, C. Phytochemical constituents and biological activities of plants from the genus Dryopteris. Chem. Biodivers. 2015, 12, 1131–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Q.; Zhang, Y.; Shen, C.; Wang, Z.; Wu, Q.; Zhang, Y.; Li, S.; Qiao, Y. Agrimol B suppresses adipogenesis through modulation of SIRT1-PPAR gamma signal pathway. Biochem. Biophys. Res. Commun. 2016, 477, 454–460. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Y.; Fu, S.; Peng, B.; Bao, L.; Zhang, Y.; Hu, J.; Zeng, Z.; Geng, D.; Gao, Z. Anti-Influenza Virus (H5N1) Activity Screening on the Phloroglucinols from Rhizomes of Dryopteris crassirhizoma. Molecules 2017, 22, 431. [Google Scholar] [CrossRef] [PubMed]
- Adamu, M.; Mukandiwa, L.; Awouafack, M.D.; Ahmed, A.S.; Eloff, J.N.; Naidoo, V. Ultrastructure changes induced by the phloroglucinol derivative agrimol G isolated from Leucosidea sericea in Haemonchus contortus. Exp. Parasitol. 2019, 207, 107780. [Google Scholar] [CrossRef]
- Garcia-Bustos, J.F.; Sleebs, B.E.; Gasser, R.B. An appraisal of natural products active against parasitic nematodes of animals. Parasites Vectors 2019, 12, 306. [Google Scholar] [CrossRef]
- Yim, N.; Lee, J.; Lee, B.H.; Li, W.; Ma, J.Y. Antiplatelet activity of acylphloroglucinol derivatives isolated from Dryopteris crassirhizoma. Molecules 2019, 24, 2212. [Google Scholar] [CrossRef] [PubMed]
- SHnit, U.S.T.; Ding, R.; Bi, L.; Xie, C.; Yao, M.; de Souza, P.; Xu, L.; Li, Z.; Dong, Q. Agrimol B present in Agrimonia pilosa Ledeb impedes cell cycle progression of cancer cells through G0 state arrest. Biomed. Pharmacother. 2021, 141, 111795. [Google Scholar] [CrossRef]
- Mammino, L. Bowl-shaped structures from acylphloroglucinols: An ab initio and DFT study. Mol. Phys. 2017, 115, 2254–2266. [Google Scholar] [CrossRef]
- Mammino, L. Five- and six-member bowl-shaped structures from acylphloroglucinols: An ab initio and DFT study. J. Mol. Model. 2020, 26, 13. [Google Scholar] [CrossRef] [PubMed]
- Mammino, L. Computational chemistry for green design in chemistry and pharmacy: Building awareness in the classroom. Sustain. Chem. Pharm. 2020, 18, 100283. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystalstructure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef] [PubMed]
- de la Paz, M.L.; Ellis, G.; Pérez, M.; Perkins, J.; Jiménez-Barbero, J.; Vicent, C. Carbohydrate hydrogen-bonding cooperativity—Intramolecular hydrogen bonds and their cooperative effect on intermolecular processes—Binding to a Hydrogen-bond acceptor molecule. Eur. J. Org. Chem. 2002, 5, 840–855. [Google Scholar] [CrossRef]
- Deshmukh, M.M.; Bartolotti, L.J.; Gadre, S.R. Intramolecular Hydrogen bonding and cooperative interactions in carbohydrates via the molecular tailoring approach. J. Chem. Phys. A 2008, 112, 312–321. [Google Scholar] [CrossRef]
- Parra, R.D.; Gong, B.; Zeng, X.C. Energetics and cooperativity in three-center hydrogen bonding interactions. II. Intramolecular hydrogen bonding systems. J. Chem. Phys. 2001, 115, 6036–6041. [Google Scholar] [CrossRef]
- Xing, B.; Yu, C.W.; Chow, K.H.; Ho, P.L.; Fu, D.; Xu, B. Hydrophobic interaction and hydrogen bonding cooperatively confer a vancomycin hydrogel: A potential candidate for biomaterials. J. Am. Chem. Soc. 2002, 124, 14846–14847. [Google Scholar] [CrossRef] [PubMed]
- Dashnau, J.L.; Sharp, K.A.; Vanderkooi, J.M. Carbohydrate intramolecular hydrogen bonding cooperativity and its effect on water structure. J. Phys. Chem. B 2005, 109, 24152–24159. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, S.; Trievel, R.C. Carbon-oxygen hydrogen bonding in biological structure and function. J. Biol. Chem. 2012, 287, 41576–41582. [Google Scholar] [CrossRef] [PubMed]
- Gilli, G.; Bellucci, F.; Ferretti, V.; Bertolasi, V. Evidence for resonance-assisted hydrogen bonding from crystal-structure correlations on the enol form of the beta-diketone fragment. J. Am. Chem. Soc. 1989, 111, 1023–1028. [Google Scholar] [CrossRef]
- Bertolasi, V.; Gilli, P.; Ferretti, V.; Gilli, G. Evidence for resonance-assisted hydrogen bonding. 2. Intercorrelation between crystal structure and spectroscopic parameters in eight intramolecularly hydrogen bonded 1, 3-diaryl-1, 3-propanedione enols. J. Am. Chem. Soc. 1991, 113, 4917–4925. [Google Scholar] [CrossRef]
- Guevara-Vela, J.M.; Gallegos, M.; Valentin-Rodriguez, M.A.; Costales, A.; Rocha-Rinza, T.; Pendás, A.M. On the relationships between hydrogen bond strength and the formation energy in resonance-assisted hydrogen bonds. Molecules 2021, 26, 4196. [Google Scholar] [CrossRef]
- Grosch, A.A.; van der Lubbe, S.C.C.; Guerra, C.F. Nature of intramolecular resonance assisted hydrogen bonding in malonalehyde and its saturated analogue. J. Phys. Chem. A 2018, 122, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.L.; Kar, T.; Scheiner, S. Fundamental properties of the CH···O interaction: Is it a true hydrogen bond? J. Am. Chem. Soc. 1999, 121, 9411–9422. [Google Scholar] [CrossRef]
- Desiraju, G.R. The C-H···O hydrogen bond: Structural implications and supramolecular design. Acc. Chem. Res. 1996, 29, 441–449. [Google Scholar] [CrossRef]
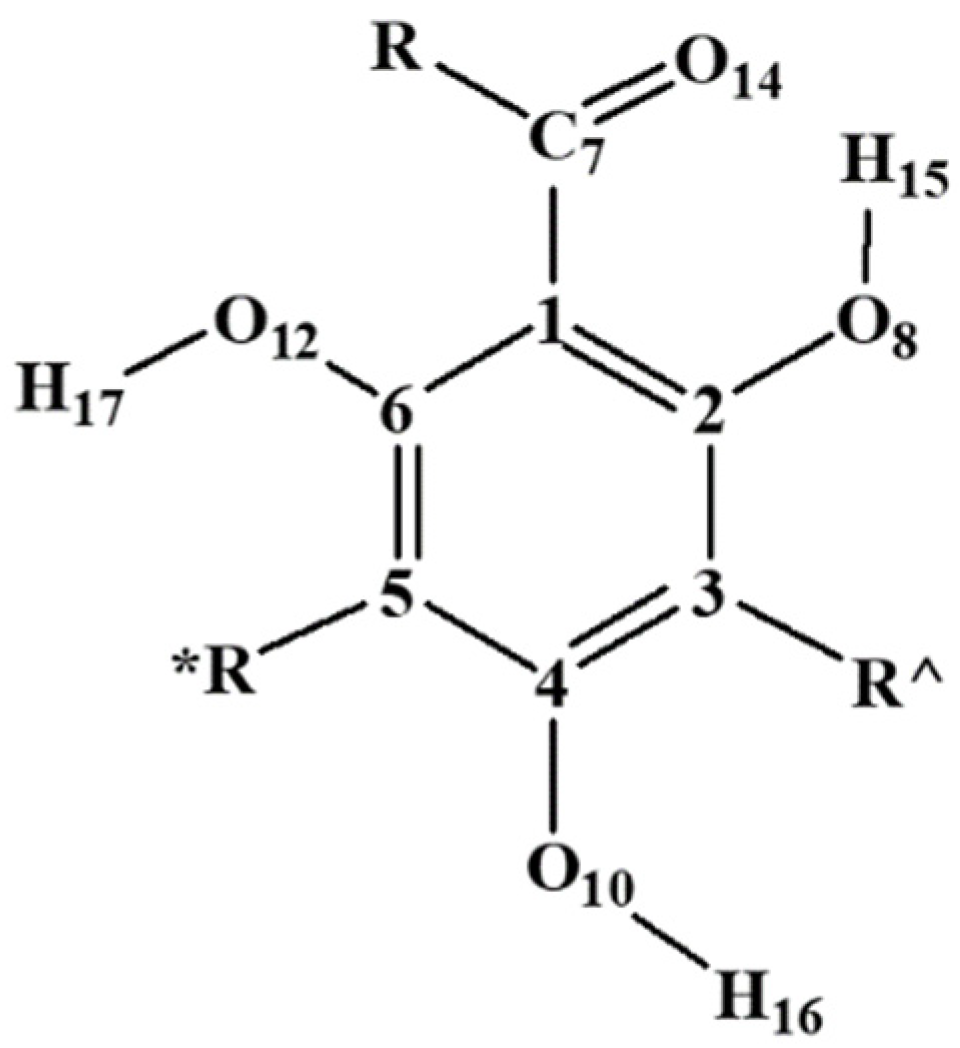
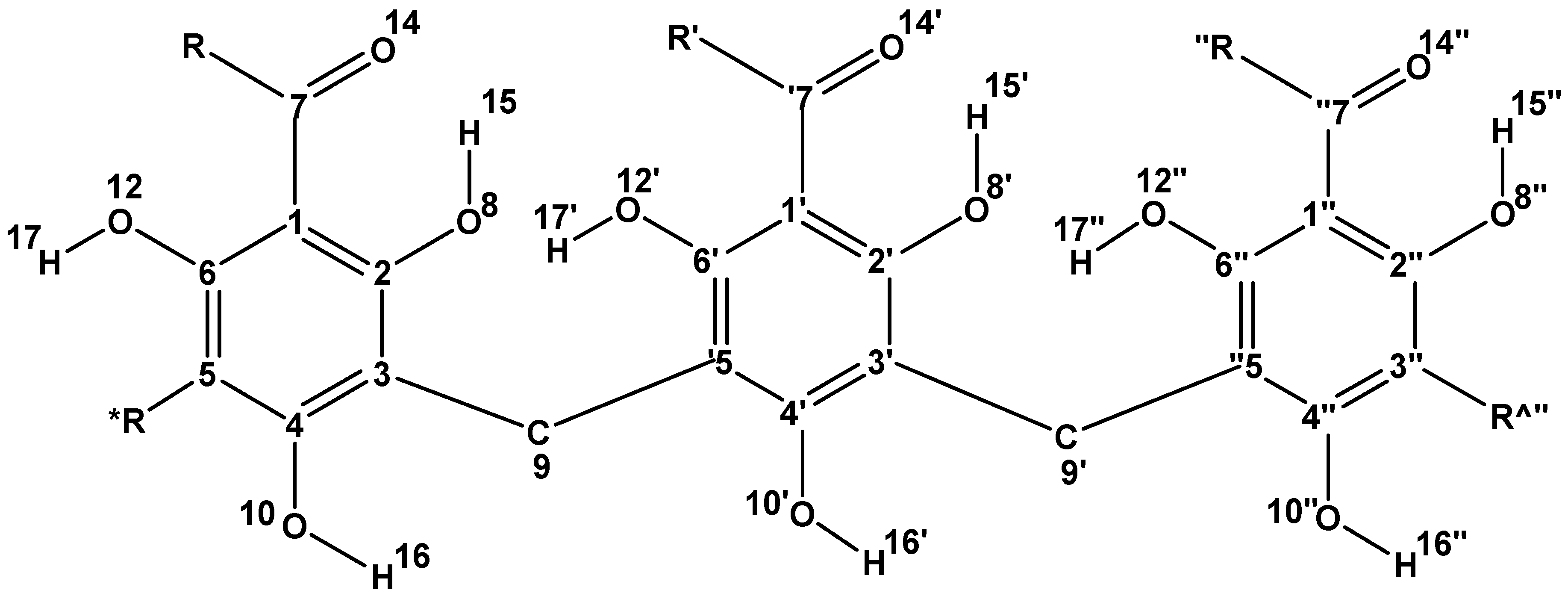
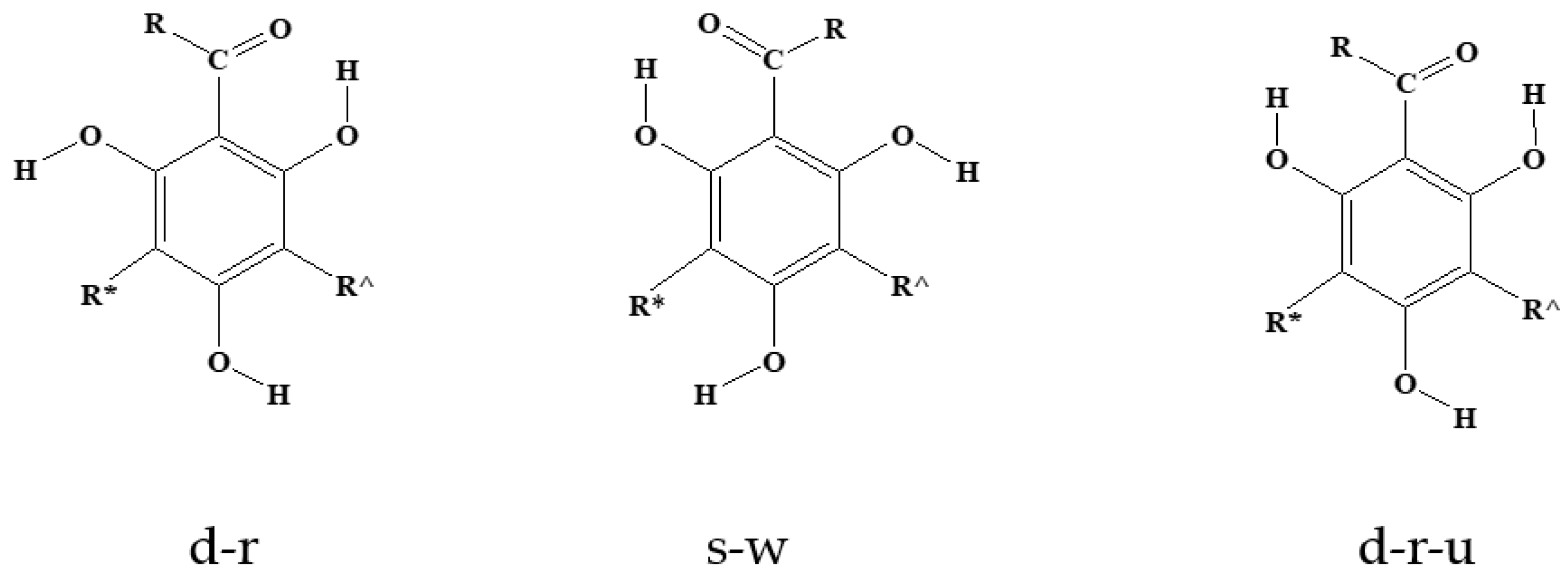
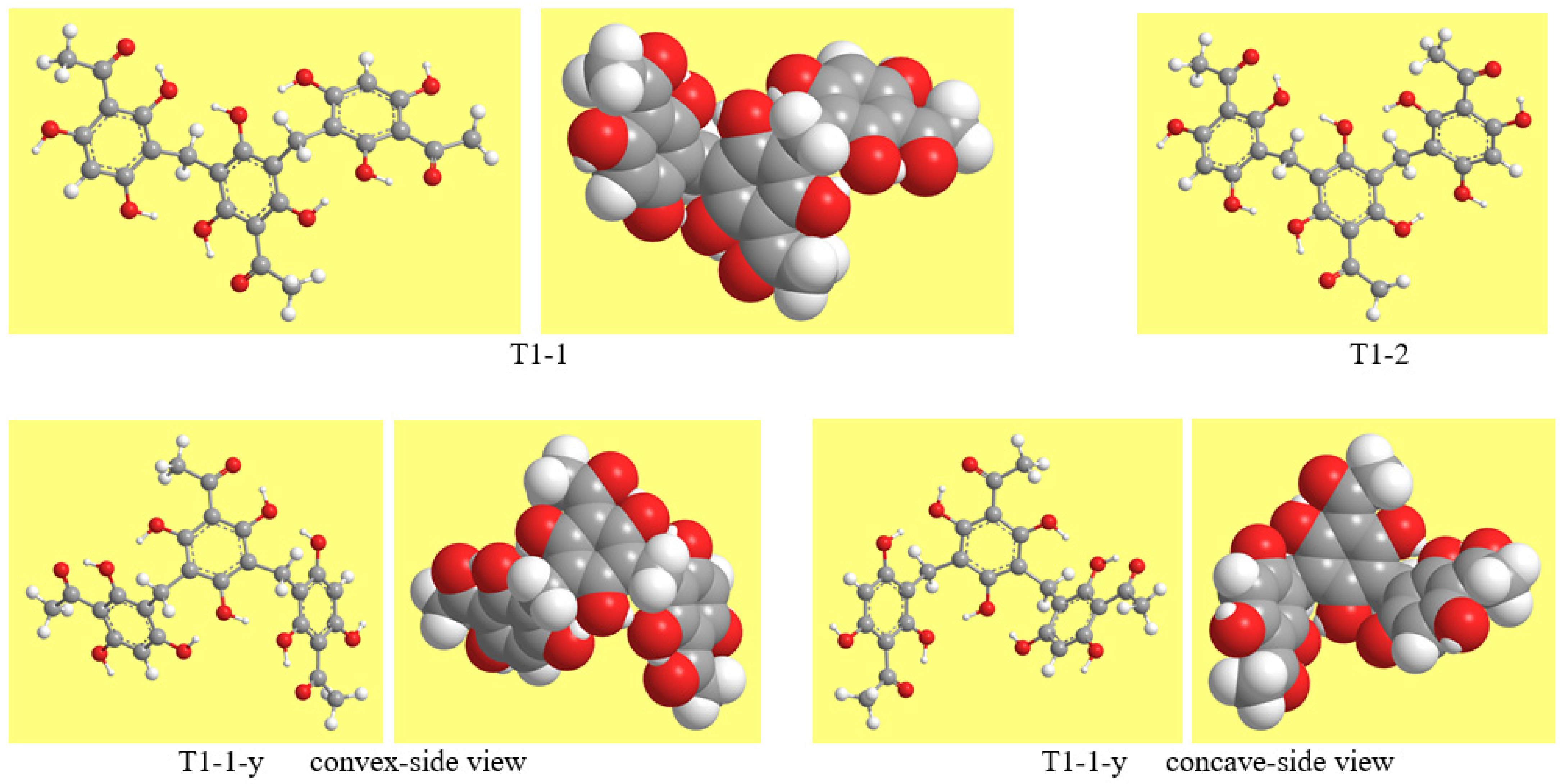
| Symbol | R, R′ and R″ |
|---|---|
| T1 | R = R′ = R″ = CH3 |
| T2 | R = R″ = CH3, R′ = CH2CH2CH3 |
| T3 | R = R″ = CH3, R′ = CH(CH3)CH2CH3 |
| T4 | R = CH2CH3, R′ = CH2CH2CH3, R″ = CH3 |
| T5 | R = R″ = CH2CH3, R′ = CH2CH2CH3 |
| T6 | R = R″ = CH2CH2CH3, R′ = CH3 |
| T7 | R = R′ = CH2CH2CH3, R″ = CH3 |
| T8 | R = R′ = CH2CH2CH3, R″ = CH2CH3 |
| T9 | R = R′ = R″ = CH2CH2CH3 |
| T10 | R = R′ = R″ = CH(CH3)2 |
| T11 | R = CH(CH3)2, R′ = CH(CH3)CH2CH3, R″ = CH3 |
| T12 | R = CH2CH2CH2CH3, R′ = R″ = CH2CH2CH3 |
| T13 | R = CH(CH3)CH2CH3, R′ = R″= CH2CH2CH3 |
| T14 | R = CH(CH3)CH2CH3, R′ = R″= CH(CH3)2 |
| Acronyms Denoting the Calculated Trimeric Acylphloroglucinols | ||
|---|---|---|
| T1 | T8-KT2-M5,5,3″ | T10 |
| T2-KT2,6″-M5,5,3″,3″ | T8-KT6″-M5,3″,3″ | T10-ET6,2″ |
| T3-ET6,2″ | T8-KT2,6″-M5,5,3″,3″ | T10-M5,3″-ET6,2″ |
| T3-M5,3″-ET6,2″ | T9 | T10-M5,3″-ET6,2″,6″ |
| T4-KT2,6″-M5,5,3″,3″ | T9-M5,5′ | T10-KT2,6″-M5,5,3″,3″ |
| T5-KT6″-M3″,3″-ET4 | T9-ET6,2″ | T11-ET6,2″ |
| T5-KT2,6″-M5,5,3″,3″ | T9-M5,3″-ET6,2″ | T11-M5,3″-ET6,2″ |
| T6-M5,3″-ET6,4″ | T9-M5,3″-ET6,4″ | T12-KT2-M5,5,3″ |
| T7 | T9-KT6″-M5,3″,3″ | T12-KT6″-M5,3″,3″ |
| T7-ET6,2″ | T9-KT6″-M3″,3″-ET4 | T13-ET6,2″ |
| T7-M5,3″-ET6,2″ | T9-KT6″-M5,3″,3″-ET6 | T13-M5,3″-ET6,2″ |
| T7-KT6″-M5,3″,3″ | T9-KT2,6″-M5,5,3″,3″ | T14-ET6,2″ |
| T7-KT2,6″-M5,5,3″,3″ | T14-M5,3″-ET6,2″ | |
| Molecule | Energy Difference Range (kcal/mol) | |
|---|---|---|
| Outstretched Geometry | Half-Bowl Geometry | |
| T1 | 47.89–48.09 | 48.24–50.33 |
| T2-KT2,6″-M5,5,3″,3″ | 65.49 | 66.05 |
| T3-ET6,2″ | 59.73 | 60.37 |
| T3-M5,3″-ET6,2″ | 66.95 | 67.31 |
| T4-KT2,6″-M5,5,3″,3″ | 67.50 | 68.06 |
| T5-KT2,6″-M5,5,3″,3″ | 69.51 | 70.21 |
| T5-KT6″-M3″,3″-ET4 | 65.22 | 65.76 |
| T6-M5,3″-ET6,4″ | 67.43 | 67.77–69.54 |
| T7 | 55.68–55.81 | 55.96–57.45 |
| T7-ET6,2″ | 59.93 | 60.23 |
| T7-M5,3″-ET6,2″ | 67.15 | 67.62 |
| T7-KT6″-M5,3″,3″ | 64.83–65.05 | 65.27–67.48 |
| T7-KT2,6″-M5,5,3″,3″ | 69.36 | 69.89 |
| T8-KT6″-M5,3″,3″ | 66.84–67.08 | 67.33–70.52 |
| T8-KT2-M5,5,3″ | 66.89–68.27 | 67.29–71.20 |
| T8-KT2,6″-M5,5,3″,3″ | 71.38 | 72.09 |
| T9 | 59.49–59.67 | 59.77–62.44 |
| T9-M5,3″ | 64.21–64.38 | 64.61–67.28 |
| T9-ET6,2″ | 63.80 | 64.12 |
| T9-M5,3″-ET6,2″ | 71.10 | 71.57 |
| T9-M5,3″-ET6,4″ | 71.31 | 71.64–73.42 |
| T9-KT6″-M5,3″,3″ | 68.70–68.95 | 69.15–71.83 |
| T9-KT6″-M3″,3″-ET4 | 68.91 | 69.45 |
| T9-KT6″-M5,3″,3″-ET6 | 72.16 | 72.65 |
| T9-KT2,6″-M5,5,3″,3″ | 73.24 | 74.59 |
| T10 | 62.98–63.42 | 63.25–65.07 |
| T10-ET6,2″ | 67.54 | 67.81 |
| T10-M5,3″-ET6,2″ | 74.62 | 75.10 |
| T10-M5,3″-ET6,2″,6″ | 79.16 | 79.41 |
| T10-KT2,6″-M5,5,3″,3″ | 78.13 | 77.30 |
| T11-ET6,2″ | 64.65 | 64.98 |
| T11-M5,3″-ET6,2″ | 71.59 | 72.64 |
| T12-KT2-M5,5,3″ | 70.46–70.71 | 70.95–75.27 |
| T12-KT6″-M5,3″,3″ | 70.46–70.73 | 70.94–74.29 |
| T13-ET6,2″ | 67.23 | 67.56 |
| T13-M5,3″-ET6,2″ | 74.86 | 75.35 |
| T14-ET6,2″ | 70.11 | 70.21 |
| T14-M5,3″-ET6,2″ | 77.13 | 77.31–77.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mammino, L. Correlation Effects in Trimeric Acylphloroglucinols. Computation 2021, 9, 121. https://doi.org/10.3390/computation9110121
Mammino L. Correlation Effects in Trimeric Acylphloroglucinols. Computation. 2021; 9(11):121. https://doi.org/10.3390/computation9110121
Chicago/Turabian StyleMammino, Liliana. 2021. "Correlation Effects in Trimeric Acylphloroglucinols" Computation 9, no. 11: 121. https://doi.org/10.3390/computation9110121
APA StyleMammino, L. (2021). Correlation Effects in Trimeric Acylphloroglucinols. Computation, 9(11), 121. https://doi.org/10.3390/computation9110121





