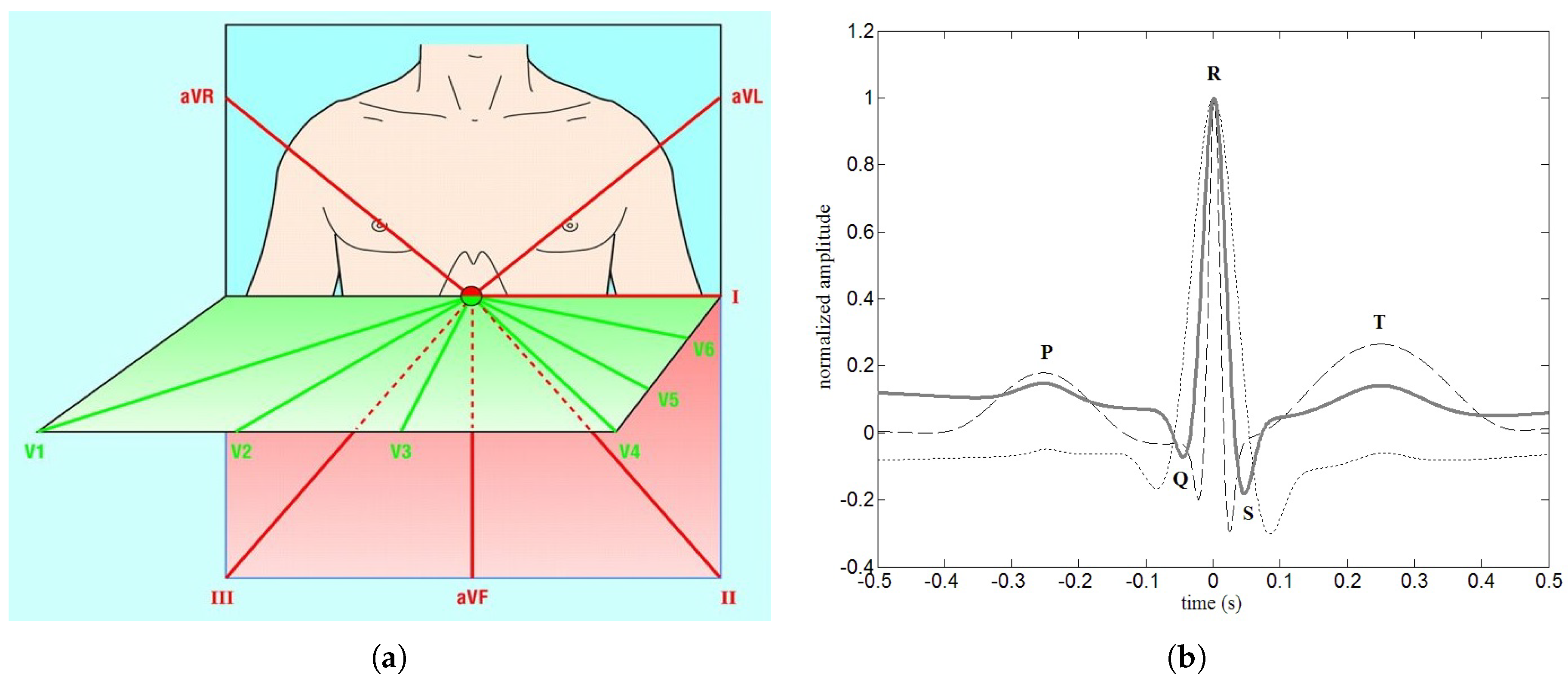
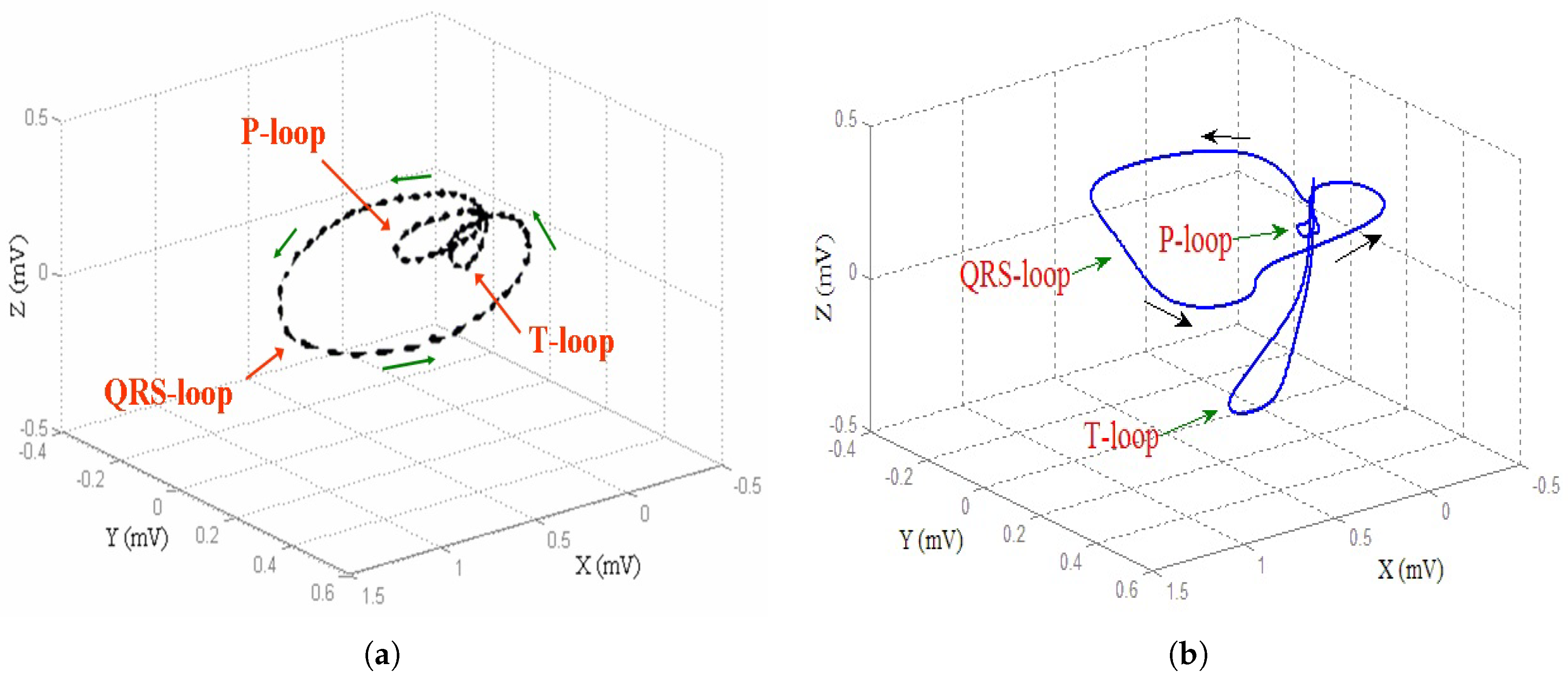
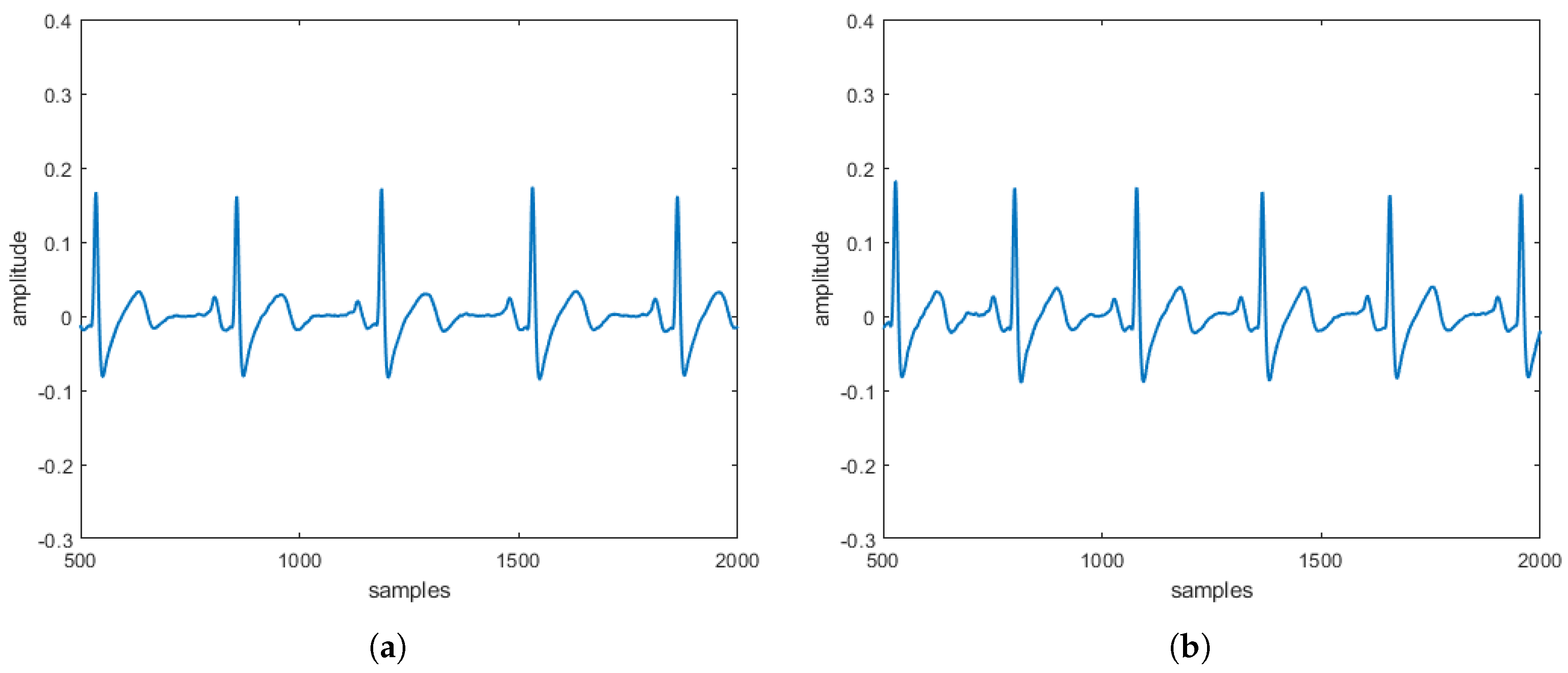
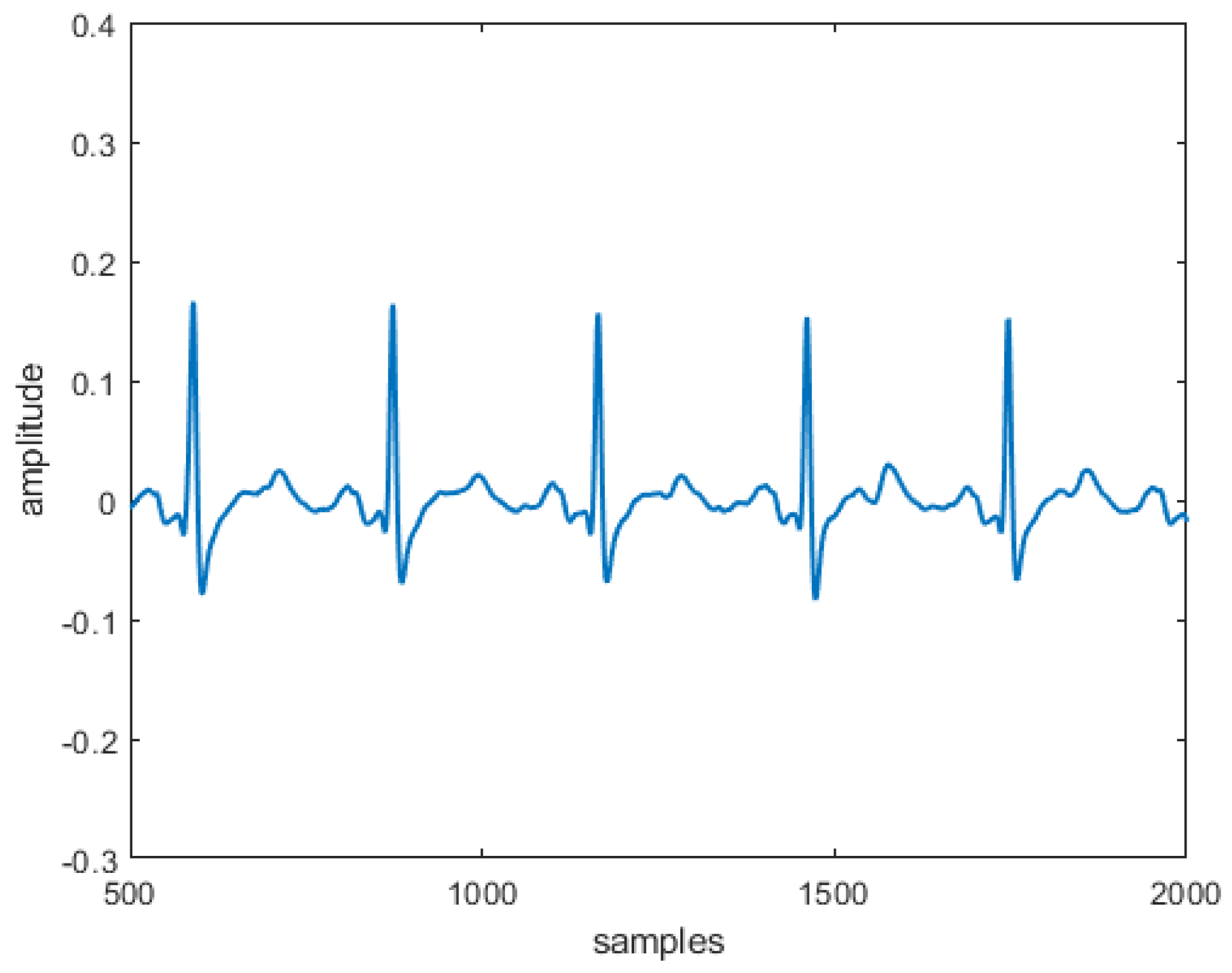
Ever since Einthoven [
1,
2] discovered the possibility of obtaining an electrocardiogram (
) from cardiac activity, cardiologists have been able to take advantage of this effective and efficient tool for diagnosing cardiac disorders [
1,
3,
4]. Specifically, an
is a particular recording of the electrical activity of the heart muscle about the potentials of the body surface [
5,
6,
7,
8]. The use of an
is nowadays widespread because of its non-invasiveness and easiness of performing as a test. Furthermore, the presence of slight cardiac anomalies produce distortions of the
signal with the consequent possibility of accurate diagnosis [
9,
10,
11]. During the years, the scientific community has been actively engaged in the development, implementation and validation of models and algorithms for the analysis and interpretation of
signals [
12]. In particular, many researchers have been involved in the study of wave morphology and wave complexes, forming a complete cardiac cycle [
13]. In addition, to explain the cardiac conductivity, a lot of models of the ECG wave have been proposed. Among them stands [
14] in which the
model was constructed starting from Gaussian formulations. Moreover, special indexes, such as the bicoherence-derived index, have been proposed for assessing nonlinear processes in cardiac regulation [
15]. At the same time, other researchers have focused their attention on the analysis and variations of the models’ outputs on a suitable number of heartbeats [
12,
13,
16]. The main reason that guides researchers to implement and validate physical-mathematical cardiac models concerns the impossibility of distorting cardiac activity directly on the patient to study its effects on the
signal [
17]. Furthermore, the creation of cardiac models has the undoubted advantage of characterizing the entire cardiac cycle, with the possibility of simulating highly dangerous cardiac disorders [
17]. With the aim of generating
s, many papers have been published, including [
18], in which, to generate
s, discretized reaction-diffusion systems to produce a set of three nonlinear oscillators simulating the main pacemakers in the heart were exploited. Moreover, the availability of synthetic
signals with or without distortions allows the exploitation of consolidated signal processing algorithms [
19,
20,
21]. Finally, the development of models and algorithms for the study of
also turns out to be a valuable aid in the evaluation of the fetal
[
22,
23]. Researchers are also interested in the synthesis of
in which distortions are present to simulate as many heart diseases as possible. This allows for laboratory analysis of signals without the need to sample signals from patients who are often difficult to transport due to severe heart disease. In this context, McSharry et al. in 2003, propose a dynamic model for the reconstruction of
signals combining Gaussian functions with different parameters [
24]. In particular, this model was derived from coupling a dynamic first-order model with a differential equation in which the respiratory artifact was taken into account. This model was solved numerically by Runge–Kutta procedures obtaining a typical ECG trajectory without diseases [
24]. It is worth noting that the Runge–Kutta methods, in addition to being a valid numerical tool for solving linear differential problems, are able to numerically solve problems characterized by strong nonlinearities when the nonlinear functions are smooth, such as the McSharry model [
25]. Although the McSharry model is interesting from a theoretical point of view, it does not supply
s with heart diseases. From which, it is imperative to make new models for
s where the heart diseases can be easily implemented exploiting suitable numerical procedures. Although many papers have addressed the problem of generalizing the McSharry model to generate
s with pathologies (see, for example, [
26]), the main objective of this study concerns the generalization of the model [
24] showing how it can be extended to simulate the effects on
affected by heart disease exploiting a linear transformation and a rotation of two dynamic parameters of the model in [
24]. In particular, atrial and ventricular fibrillation and premature ventricle contraction were chosen in this paper as they represent frequent pathologies in patients who are often highly at risk, having no obvious symptoms. From our analysis, six different formulations of the model [
24] have been obtained. These have been solved numerically by a procedure based on the Four Stage Lobatto IIIa formula [
25] obtaining six different typical trajectories related to normal
(without diseases). Although the implicit fourth-order Runge–Kutta methods are suitable for solving nonlinear differential systems, they can be considered equivalent to the four-stage Lobatto IIIa formula both in terms of convergence and stability. In fact, all the above numerical procedures admit the implicit fourth order version and are stable [
25]. However, the four-stage Lobatto IIIa formula, unlike the implicit fourth-order Runge–Kutta method, is a numerical collocation method which, when applied to the problem under study, gave slightly better qualitative results than those obtained in [
24] who exploited the implicit fourth-order Runge–Kutta procedure. This is evident from the qualitative analysis of the ECGs obtained in our work compared to the ECGs obtained in [
24], which requires a, albeit mild, filtering operation. Moreover, in this paper, we have verified whether the model [
24] admits existence and uniqueness of the solution (in order to avoid any numerical ghost solutions). Moreover, unlike the model [
24], in our work, the result provided by the autonomous system consisting of the first two equations in [
24] represents the input for the third equation in [
24] where the
signal is performed. So, before studying the complete system [
24], we need to study the stability of the system constituted by the first two equations in [
24]. This is because we have to verify that there were no instability points (which generate unbounded perturbations) on points admitted by the third equation in [
24]. In this paper, we have shown that the only position that generates instability, fortunately, is not in the domain of the third equation in [
24]. Thus, a matrix procedure here proposed, consisting of a linear transformation and a rotation depending on two characteristic parameters, distorted the
signal for implementing the heart diseases. Also, in this case, six different formulations of this new generalized model, named
modified heart dipole model (MHDM), have been obtained, reconstructing their typical trajectories, and verifying that, also in this case, the same unstable equilibrium position is obtained, which is discarded since it does not belong to the set of points allowed by the third equation. The results obtained can be considered encouraging because the
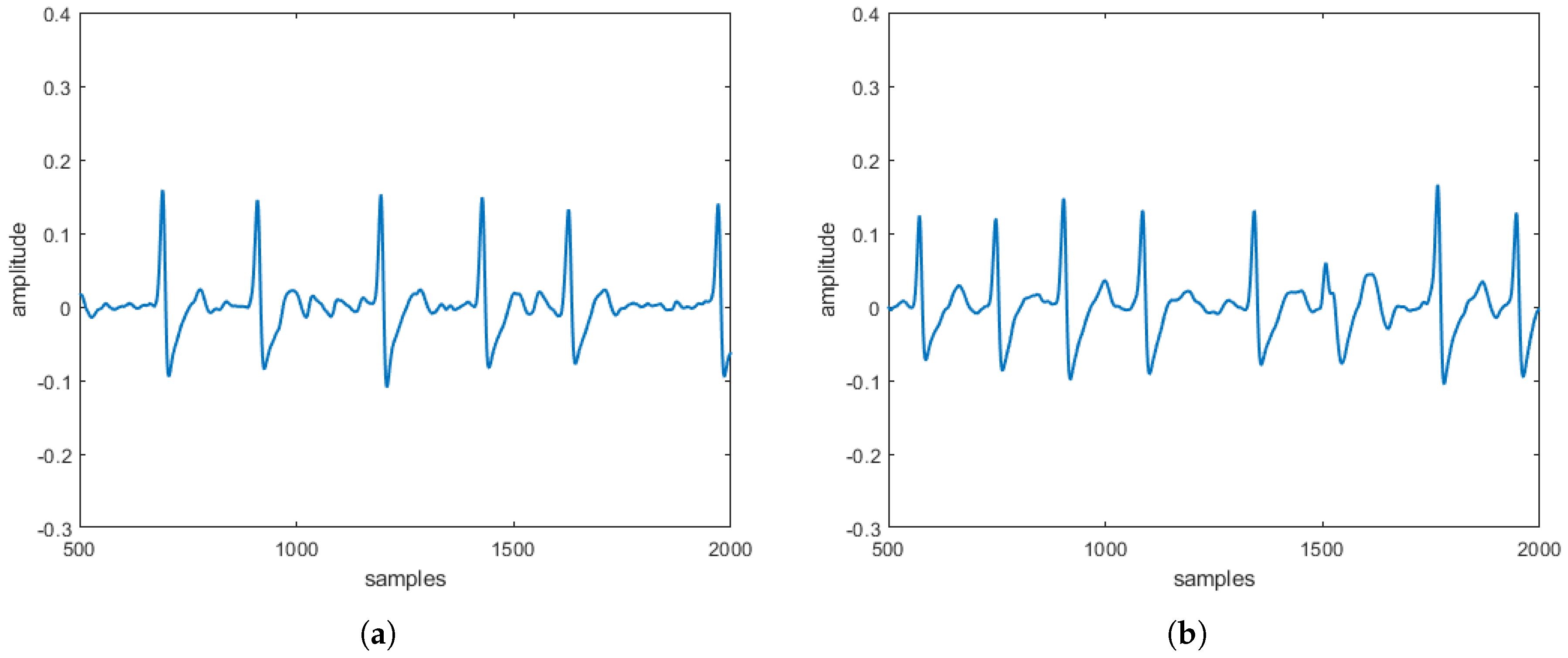
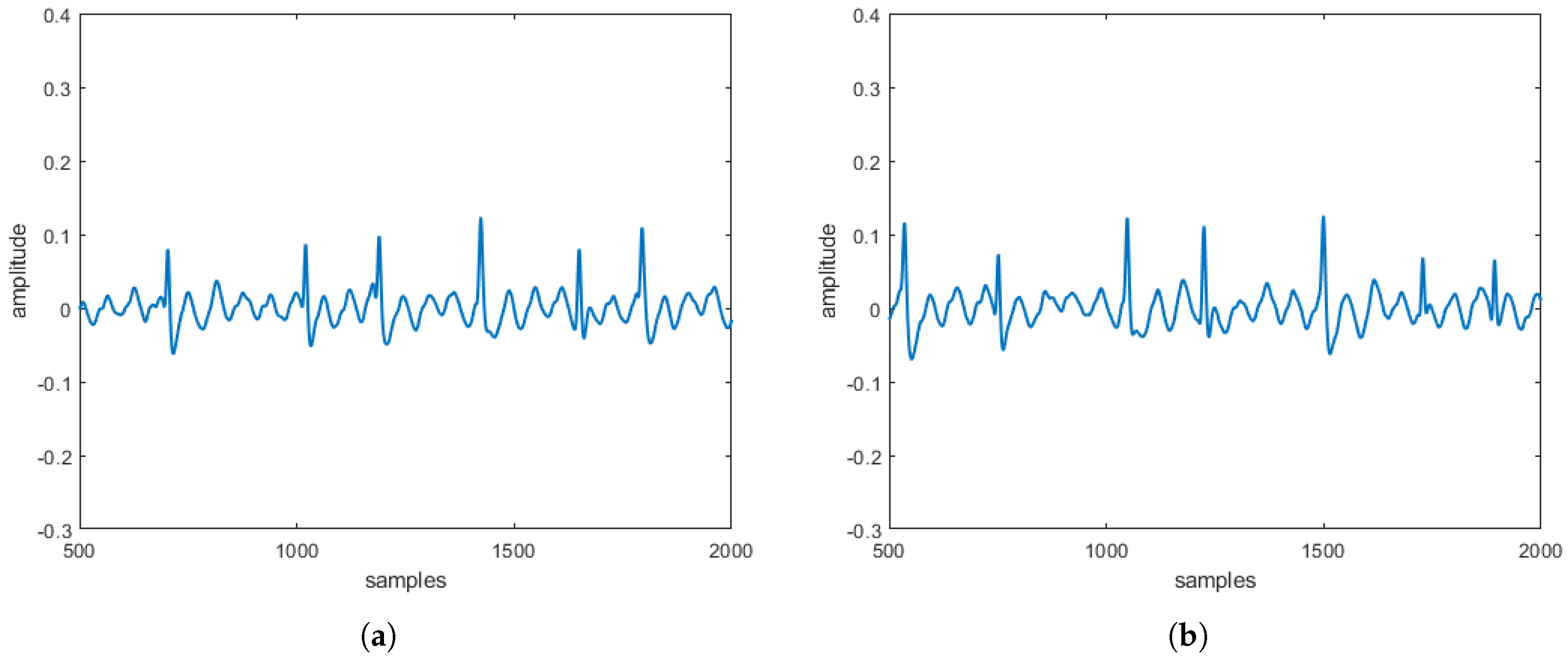
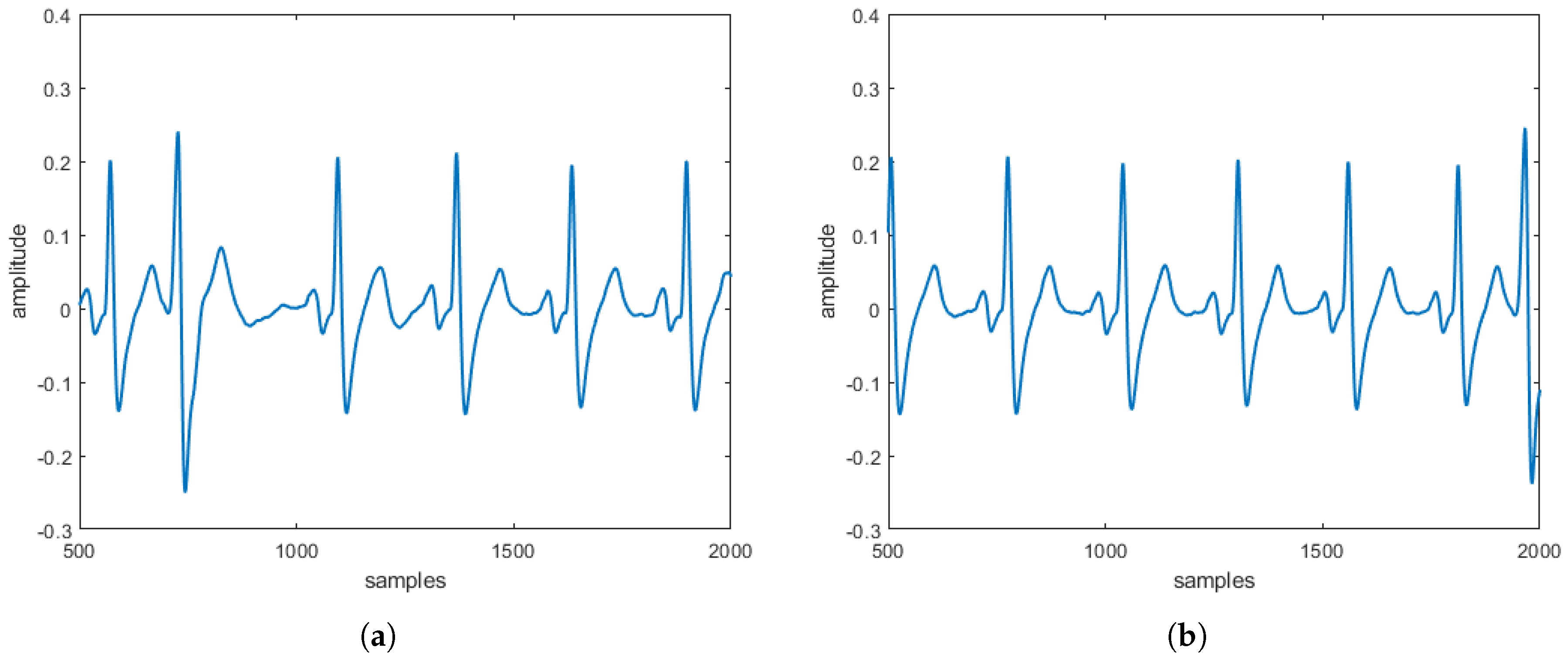
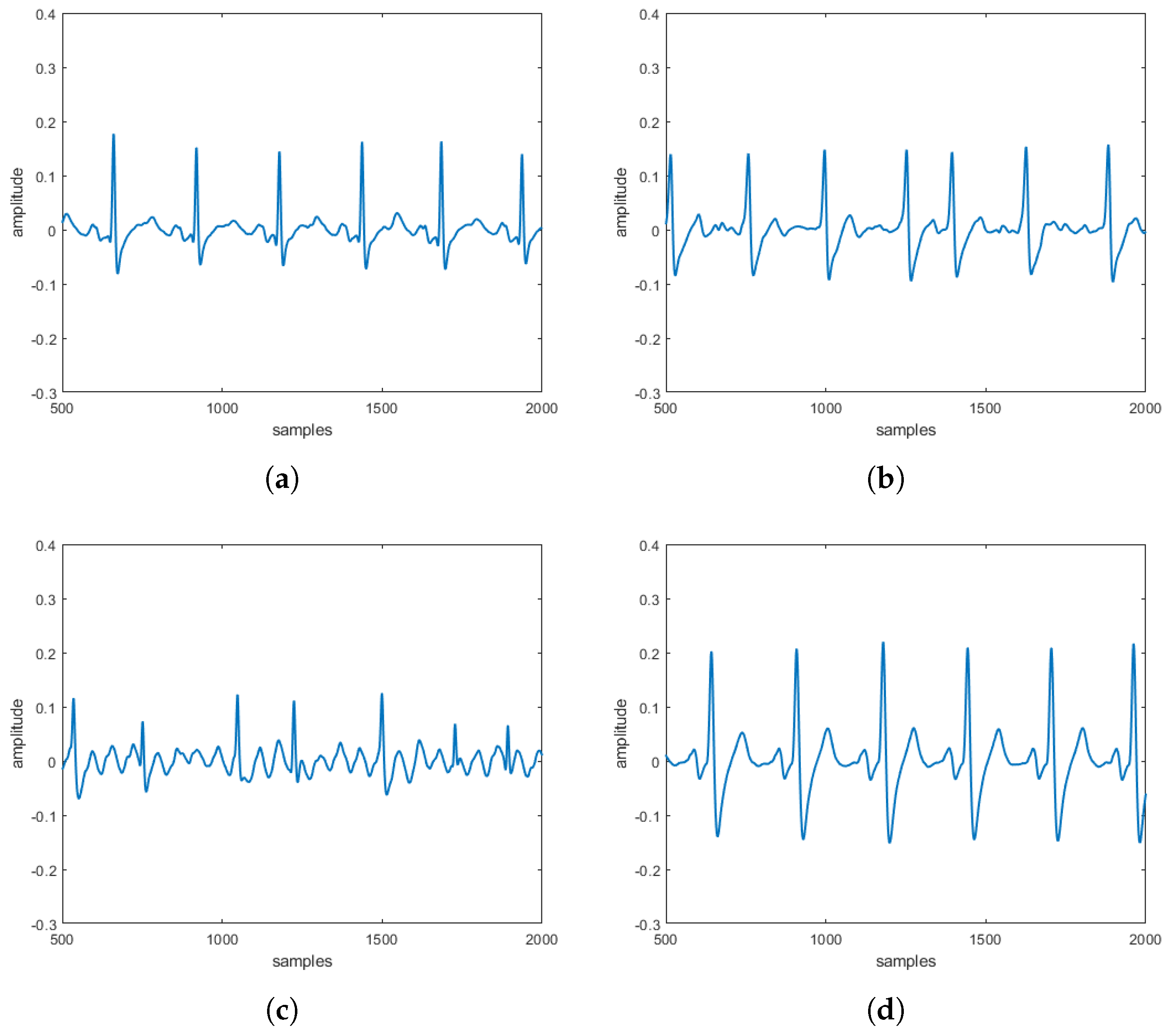
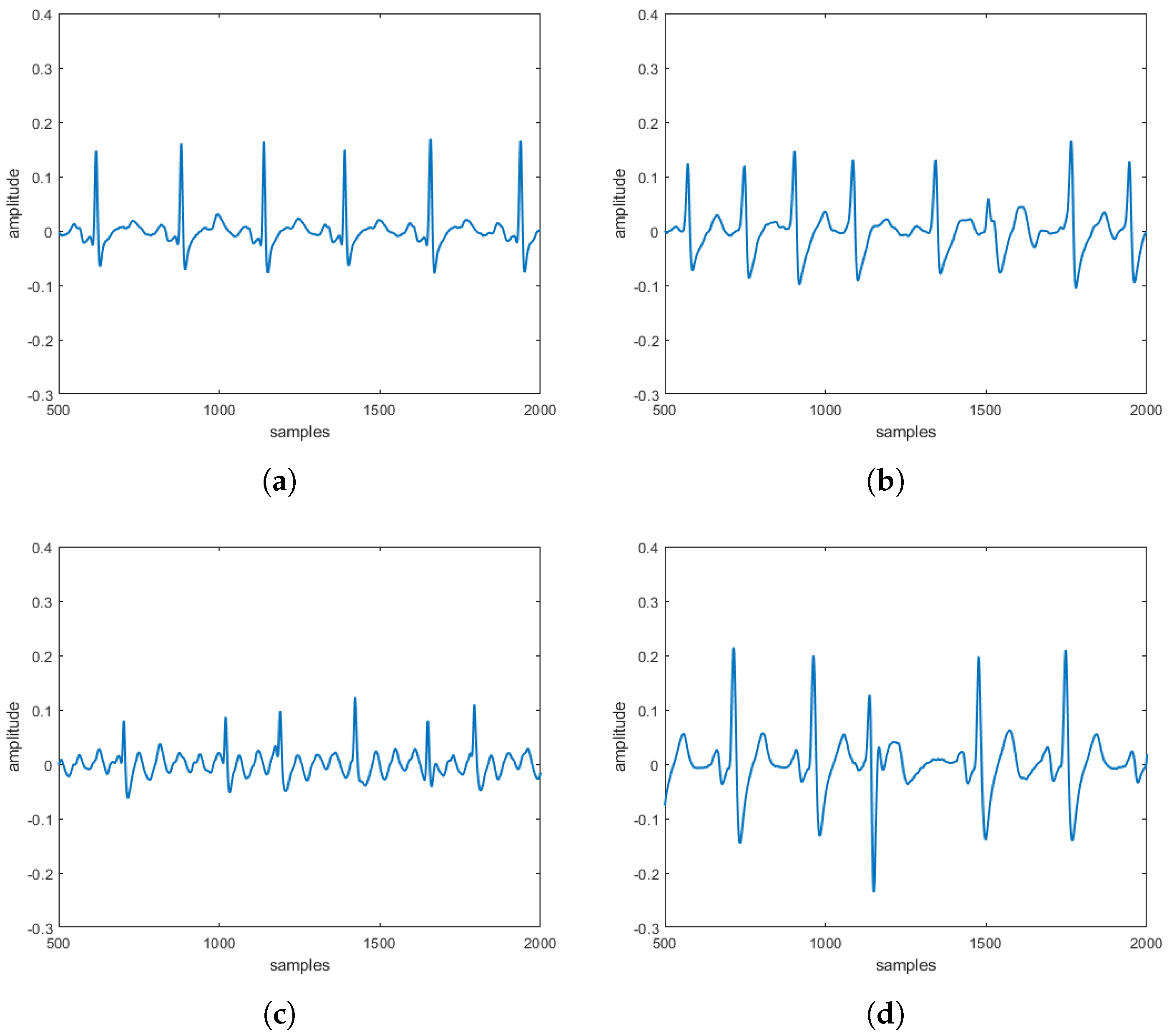
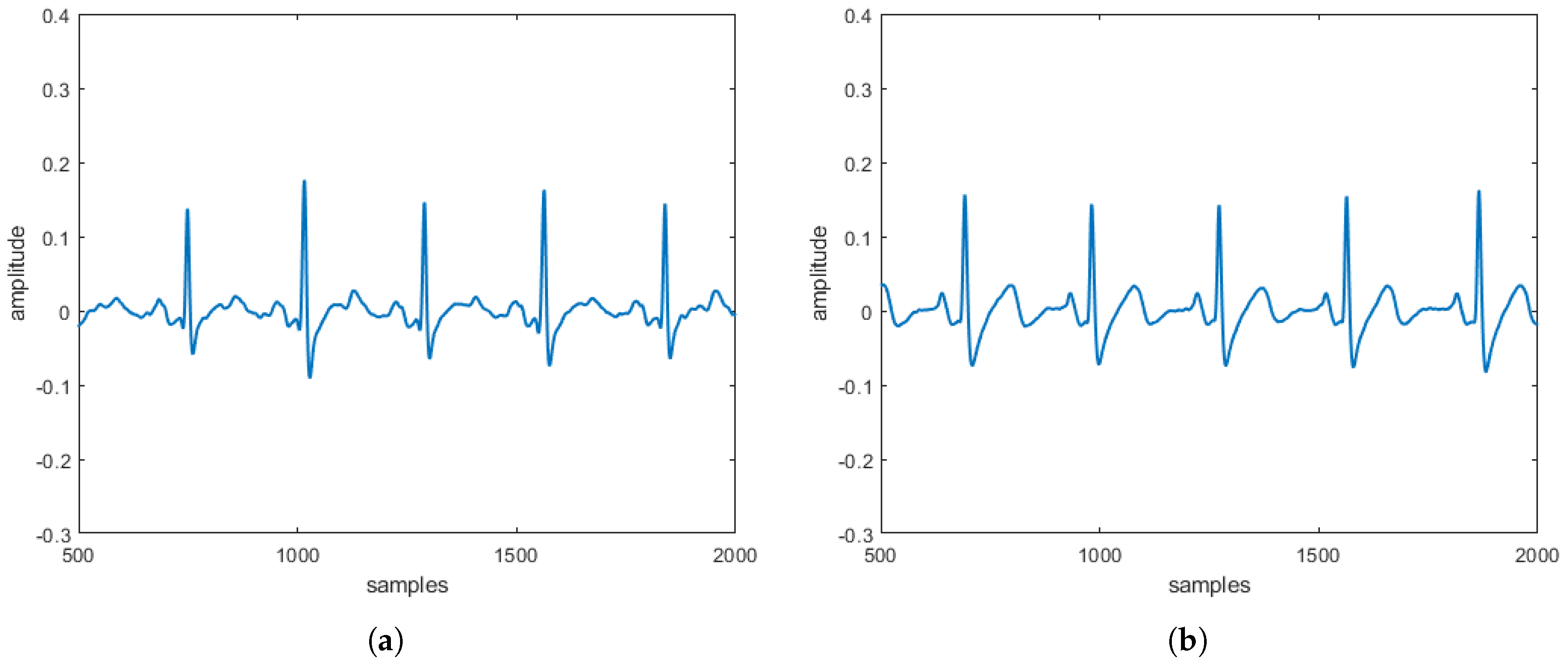
reconstructed in the absence and the presence of three specific pathologies are entirely comparable to the
obtained directly on patients. These comparisons, taking into account that
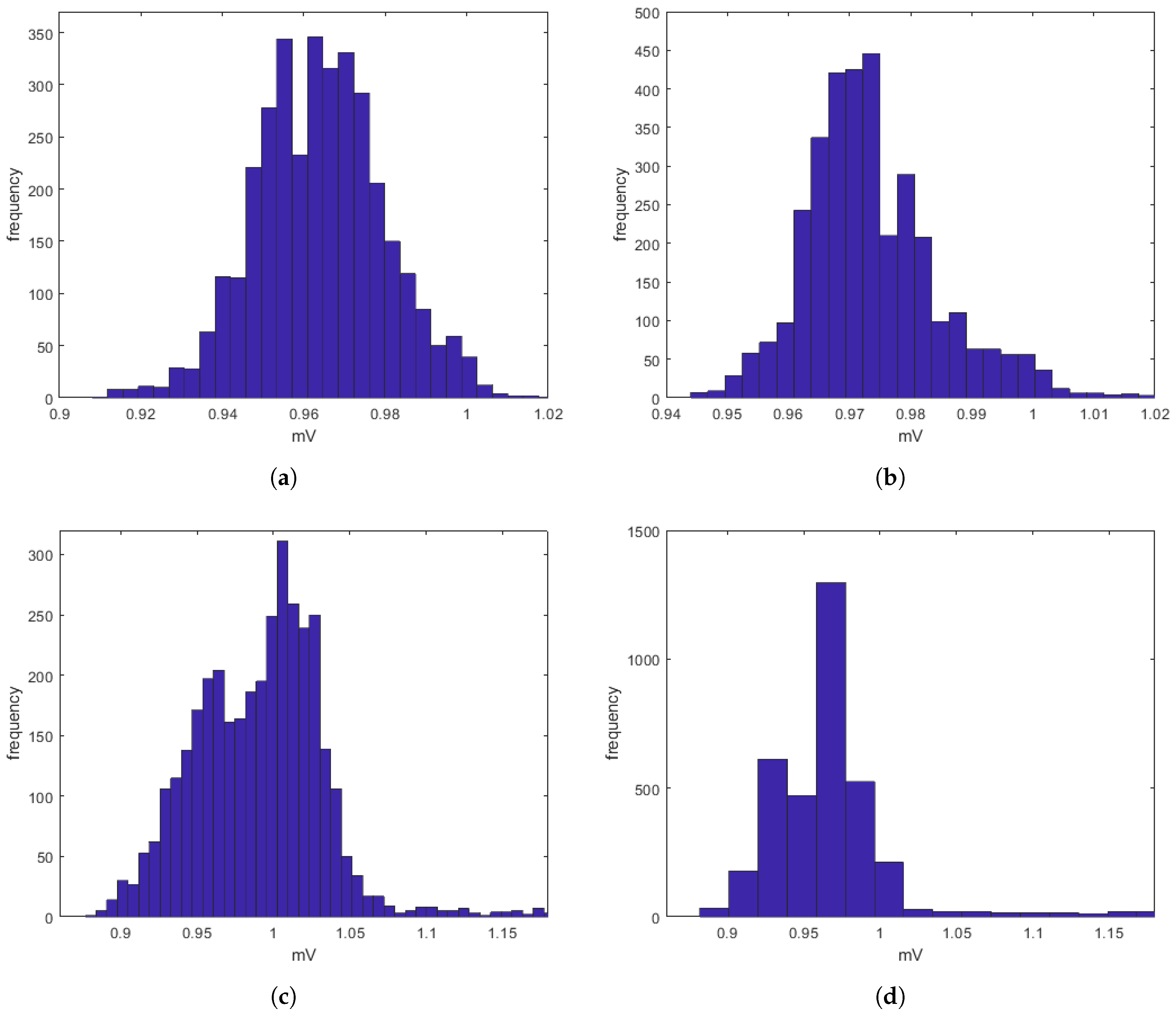
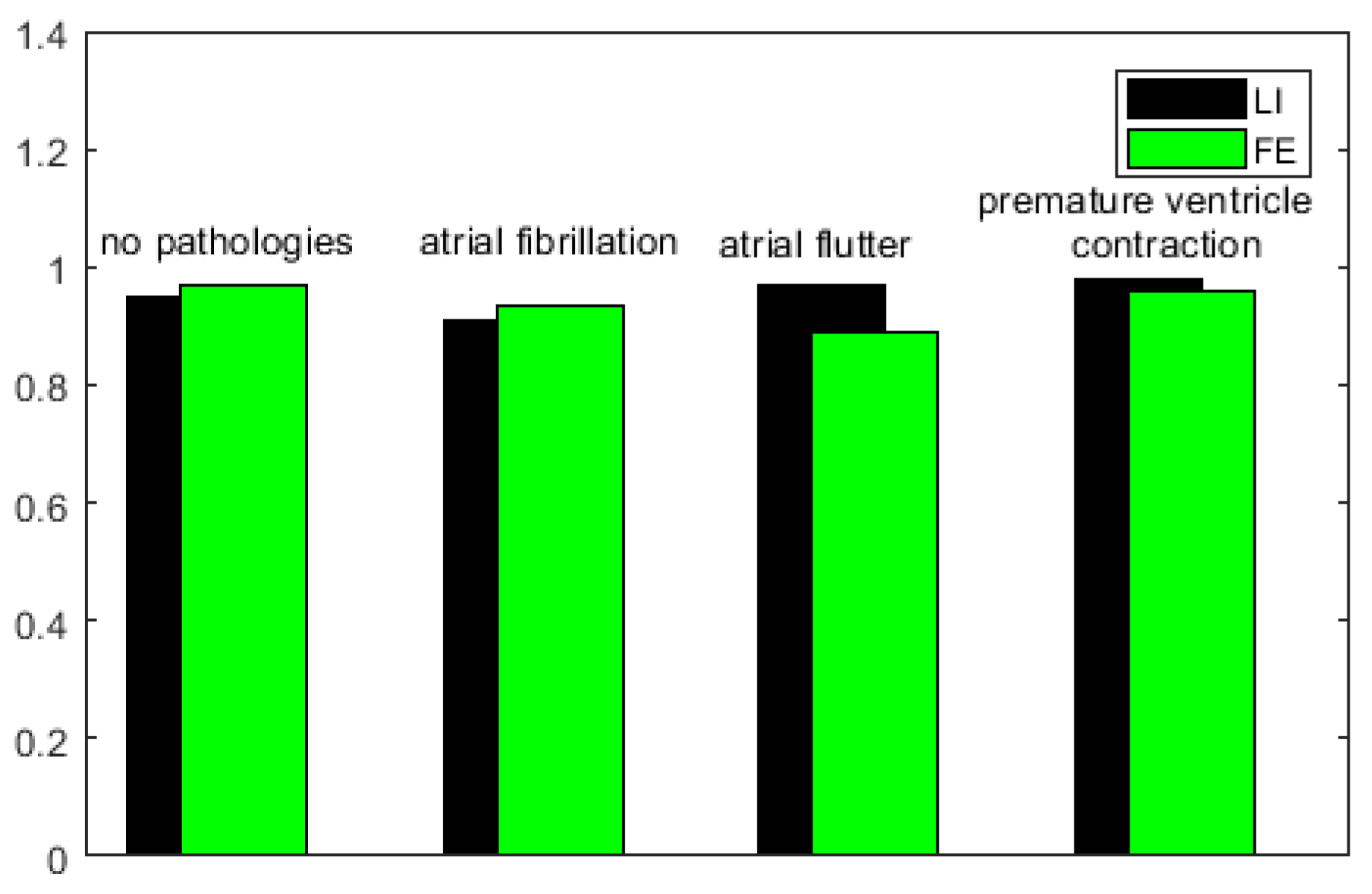
s can be often affected by uncertainty and/or imprecision (due, for example, to artifacts), have also been evaluated by a procedure based on fuzzy similarity measuring how similar is, in a fuzzy sense, an
(with or without heart diseases) obtained by the proposed model with an
(with or without heart diseases) sampled directly on a patient. Based on these results, our model should allow building a complete database of
without the need to carry out a measurement campaign directly on patients (some of which, due to the severity of the pathology they suffer from, are not transportable).