Modeling Interactions between Graphene and Heterogeneous Molecules
Abstract
1. Introduction
2. Methods
2.1. Atomic Density Function
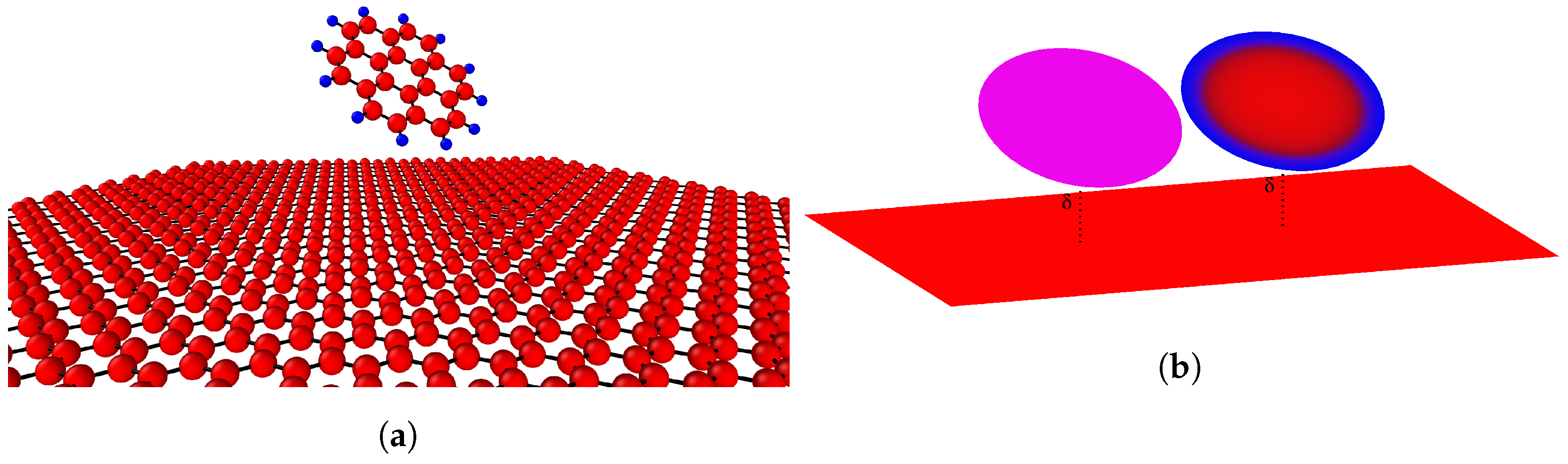
2.2. Disk and Plane Interaction
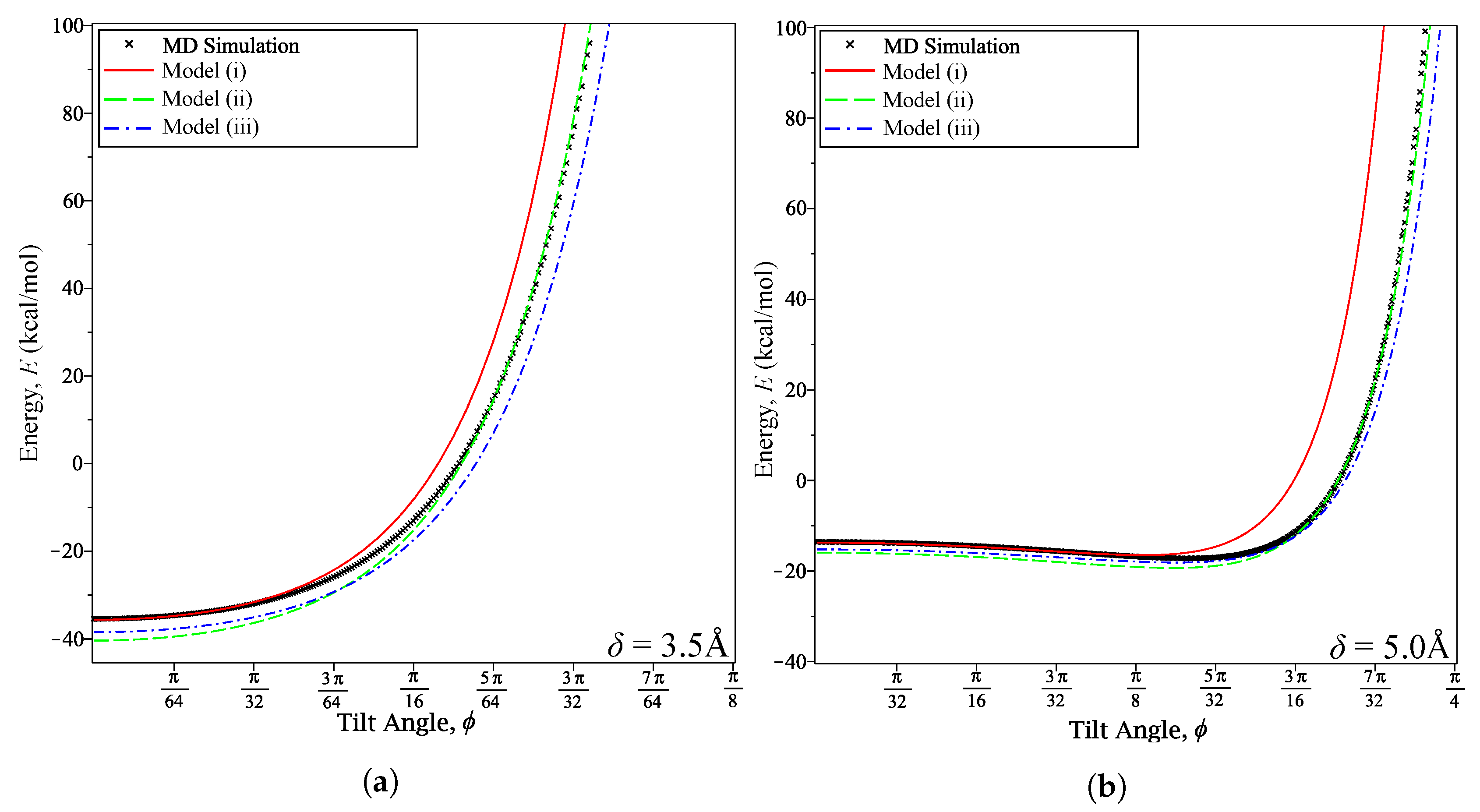
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LJ | Lennard–Jones |
| MD | Molecular dynamics |
Appendix A. Evaluation of the Integral I Defined by (10)
Including a Density Function
Appendix B. Explanation to Why MODEL (i) Is Accurate at ϕ = 0
Appendix B.1. Model (i)
Appendix B.2. Atomistic Model
References
- Rzepka, M.; Lamp, P.; De la Casa-Lillo, M. Physisorption of hydrogen on microporous carbon and carbon nanotubes. J. Phys. Chem. B 1998, 102, 10894–10898. [Google Scholar] [CrossRef]
- Bénard, P.; Chahine, R. Storage of hydrogen by physisorption on carbon and nanostructured materials. Scr. Mater. 2007, 56, 803–808. [Google Scholar] [CrossRef]
- Ren, H.; Ben, T.; Sun, F.; Guo, M.; Jing, X.; Ma, H.; Cai, K.; Qiu, S.; Zhu, G. Synthesis of a porous aromatic framework for adsorbing organic pollutants application. J. Mater. Chem. 2011, 21, 10348–10353. [Google Scholar] [CrossRef]
- Llanos, J.; Fertitta, A.; Flores, E.; Bottani, E. SO2 physisorption on exfoliated graphite. J. Phys. Chem. B 2003, 107, 8448–8453. [Google Scholar] [CrossRef]
- Steinmüller-Nethl, D.; Kloss, F.R.; Najam-Ul-Haq, M.; Rainer, M.; Larsson, K.; Linsmeier, C.; Köhler, G.; Fehrer, C.; Lepperdinger, G.; Liu, X.; et al. Strong binding of bioactive BMP-2 to nanocrystalline diamond by physisorption. Biomaterials 2006, 27, 4547–4556. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ceulemans, A. Physisorption of adenine DNA nucleosides on zigzag and armchair single-walled carbon nanotubes: A first-principles study. Phys. Rev. B 2009, 79, 195419. [Google Scholar] [CrossRef]
- Ulbricht, H.; Moos, G.; Hertel, T. Physisorption of molecular oxygen on single-wall carbon nanotube bundles and graphite. Phys. Rev. B 2002, 66, 075404. [Google Scholar] [CrossRef]
- Smith, D.G.; Patkowski, K. Toward an accurate description of methane physisorption on carbon nanotubes. J. Phys. Chem. C 2014, 118, 544–550. [Google Scholar] [CrossRef]
- Vela, S.; Huarte-Larra naga, F. A molecular dynamics simulation of methane adsorption in single walled carbon nanotube bundles. Carbon 2011, 49, 4544–4553. [Google Scholar] [CrossRef]
- Hentschke, R.; Schürmann, B.L.; Rabe, J.P. Molecular dynamics simulations of ordered alkane chains physisorbed on graphite. J. Chem. Phys. 1992, 96, 6213–6221. [Google Scholar] [CrossRef]
- Qin, Q.; Sun, T.; Wang, H.; Brault, P.; An, H.; Xie, L.; Peng, Q. Adsorption and diffusion of hydrogen in carbon honeycomb. Nanomaterials 2020, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Gao, Z.; Wang, H.; Wang, J.; Cheng, M. Computational insights into the sorption mechanism of polycyclic aromatic hydrocarbons by carbon nanotube through density functional theory calculation and molecular dynamics simulation. Comput. Mater. Sci. 2020, 179, 109677. [Google Scholar] [CrossRef]
- Darkrim, F.; Levesque, D. Monte Carlo simulations of hydrogen adsorption in single-walled carbon nanotubes. J. Chem. Phys. 1998, 109, 4981–4984. [Google Scholar] [CrossRef]
- Gatica, S.M.; Nekhai, A.; Scrivener, A. Adsorption and gas separation of molecules by carbon nanohorns. Molecules 2016, 21, 662. [Google Scholar] [CrossRef]
- Thornton, A.W. Mathematical Modelling of Gas Separation and Storage Using Advanced Materials. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2009. [Google Scholar]
- Adisa, O.O.; Cox, B.J.; Hill, J.M. Encapsulation of methane in nanotube bundles. Micro Nano Lett. 2010, 5, 291–295. [Google Scholar] [CrossRef]
- Adisa, O.O.; Cox, B.J.; Hill, J.M. Encapsulation of methane molecules into carbon nanotubes. Phys. B Condens. Matter 2011, 406, 88–93. [Google Scholar] [CrossRef]
- Tran-Duc, T.; Thamwattana, N.; Baowan, D. Modelling gas storage capacity for porous aromatic frameworks. J. Comput. Theor. Nanosci. 2014, 11, 234–241. [Google Scholar] [CrossRef]
- Stevens, K.; Tran-Duc, T.; Thamwattana, N.; Hill, J. Continuum Modelling for Interacting Coronene Molecules with a Carbon Nanotube. Nanomaterials 2020, 10, 152. [Google Scholar] [CrossRef]
- Garalleh, H.A.; Thamwattana, N.; Cox, B.J.; Hill, J.M. Encapsulation of L-Histidine amino acid inside single-walled carbon nanotubes. J. Biomater. Tissue Eng. 2016, 6, 362–369. [Google Scholar] [CrossRef]
- Thamwattana, N.; Sarapat, P.; Chan, Y. Mechanics and dynamics of lysozyme immobilisation inside nanotubes. J. Phys. Condens. Matter 2019, 31, 265901. [Google Scholar] [CrossRef]
- Thamwattana, N.; Baowan, D.; Cox, B.J. Modelling bovine serum albumin inside carbon nanotubes. RSC Adv. 2013, 3, 23482–23488. [Google Scholar] [CrossRef][Green Version]
- Tran-Duc, T.; Thamwattana, N.; Hill, J.M. Orientation of a benzene molecule inside a carbon nanotube. J. Math. Chem. 2011, 49, 1115–1127. [Google Scholar] [CrossRef]
- Hilder, T.A.; Hill, J.M. Modelling the encapsulation of the anticancer drug cisplatin into carbon nanotubes. Nanotechnology 2007, 18, 275704. [Google Scholar] [CrossRef]
- Chan, Y.; Hill, J. Modelling interaction of atoms and ions with graphene. Micro Nano Lett. 2010, 5, 247–250. [Google Scholar] [CrossRef]
- Sarapat, P.; Hill, J.M.; Baowan, D. Mechanics of atoms interacting with a carbon nanotorus: Optimal configuration and oscillation behaviour. Philos. Mag. 2019, 99, 1386–1399. [Google Scholar] [CrossRef]
- Baowan, D.; Cox, B.J.; Hilder, T.A.; Hill, J.M.; Thamwattana, N. Modelling and Mechanics of Carbon-Based Nanostructured Materials, 1st ed.; William Andrew: Norwich, NY, USA, 2017. [Google Scholar]
- Girifalco, L.A. Molecular properties of fullerene in the gas and solid phases. J. Phys. Chem. 1992, 96, 858–861. [Google Scholar] [CrossRef]
- Alshehri, M.H.; Cox, B.J.; Hill, J.M. DNA adsorption on graphene. Eur. Phys. J. D 2013, 67, 226. [Google Scholar] [CrossRef]
- Rahmat, F.; Thamwattana, N.; Cox, B.J. Modelling peptide nanotubes for artificial ion channels. Nanotechnology 2011, 22, 445707. [Google Scholar] [CrossRef]
- Stevens, K.; Thamwattana, N.; Tran-Duc, T. New functional Lennard-Jones parameters for heterogeneous molecules. J. Appl. Phys. 2020, 128, 204301. [Google Scholar] [CrossRef]
- Tran-Duc, T.; Thamwattana, N.; Cox, B.J.; Hill, J.M. Adsorption of polycyclic aromatic hydrocarbons on graphite surfaces. Comput. Mater. Sci. 2010, 49, S307–S312. [Google Scholar] [CrossRef]
- Gradshteyn, I.S.; Ryzhik, I.M. Table of Integrals, Series, and Products; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]



| Interaction | ||
|---|---|---|
| Carbon–Carbon | 112,175,566 | |
| Carbon–Hydrogen | 91,727.95 |
| Radius (Å) | Normalised Radius r | Density () |
|---|---|---|
| Model | (Å) | () | |
|---|---|---|---|
| (i) | 0 | ||
| (ii) | 0 | ||
| (iii) | 0 | ||
| MD Simulation | 0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stevens, K.; Tran-Duc, T.; Thamwattana, N.; Hill, J.M. Modeling Interactions between Graphene and Heterogeneous Molecules. Computation 2020, 8, 107. https://doi.org/10.3390/computation8040107
Stevens K, Tran-Duc T, Thamwattana N, Hill JM. Modeling Interactions between Graphene and Heterogeneous Molecules. Computation. 2020; 8(4):107. https://doi.org/10.3390/computation8040107
Chicago/Turabian StyleStevens, Kyle, Thien Tran-Duc, Ngamta Thamwattana, and James M. Hill. 2020. "Modeling Interactions between Graphene and Heterogeneous Molecules" Computation 8, no. 4: 107. https://doi.org/10.3390/computation8040107
APA StyleStevens, K., Tran-Duc, T., Thamwattana, N., & Hill, J. M. (2020). Modeling Interactions between Graphene and Heterogeneous Molecules. Computation, 8(4), 107. https://doi.org/10.3390/computation8040107






