Feature Selection of Non-Dermoscopic Skin Lesion Images for Nevus and Melanoma Classification
Abstract
1. Introduction
2. Materials and Methods
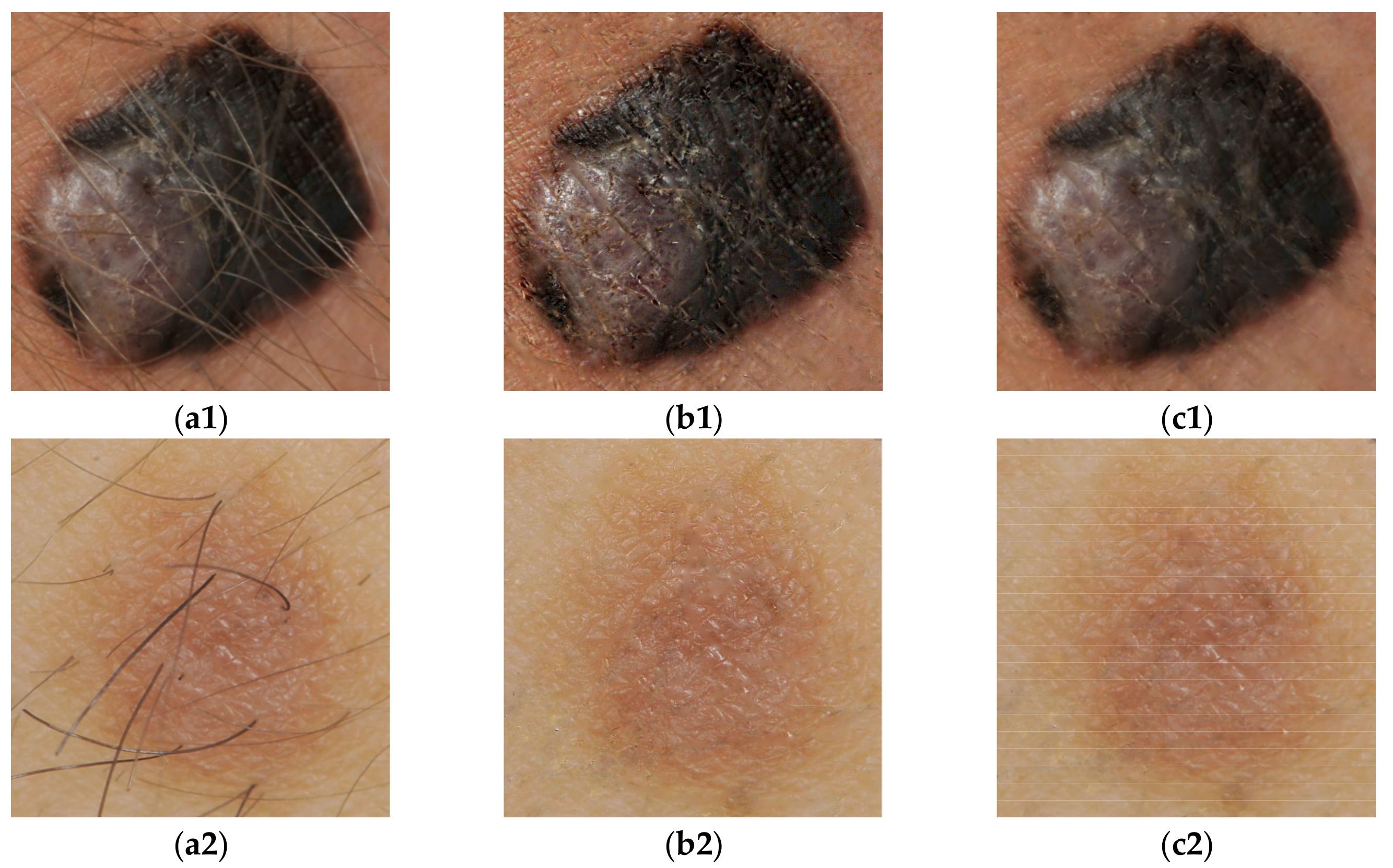
2.1. Preprocessing Operations
2.2. Segmentation Process
2.3. Description of Features
2.4. The Proposed Feature Selection Method
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saleh, J. Murine models of melanoma. Pathol. Res. Pract. 2018, 214, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Matthews, N.H.; Li, W.-Q.; Qureshi, A.A.; Weinstock, M.A.; Cho, E. Epidemiology of melanoma. In Cutaneous Melanoma: Etiology and Therapy, 2nd ed.; Ward, W.H., Farma, J.M., Eds.; Codon Publications: Brisbane, Australia, 2017; pp. 3–22. [Google Scholar]
- Abbasi, N.R.; Shaw, H.M.; Rigel, D.S.; Friedman, R.J.; McCarthy, W.H.; Osman, I.; Kopf, A.W.; Polsky, D. Early diagnosis of cutaneous melanoma: Revisiting the ABCD criteria. JAMA 2004, 292, 2771–2776. [Google Scholar] [CrossRef] [PubMed]
- Celebi, M.E.; Codella, N.; Halpern, A. Dermoscopy image analysis: Overview and future directions. IEEE J. Biomed. Health Inform. 2019, 23, 474–478. [Google Scholar] [CrossRef]
- Almaraz-Damian, J.A.; Ponomaryov, V.; Rendon-Gonzalez, E. Melanoma CADe based on ABCD rule and haralick texture features. In Proceedings of the 9th International Kharkiv Symposium on Physics and Engineering of Microwaves, Millimeter and Submillimeter Waves (MSMW), Kharkiv, Ukraine, 20–24 June 2016; pp. 1–4. [Google Scholar]
- Korotkov, K.; Garcia, R. Computerized analysis of pigmented skin lesions: A review. Artif. Intell. Med. 2012, 56, 69–90. [Google Scholar] [CrossRef]
- ESMO Guidelines Working Group; Dummer, R.; Hauschild, A.; Guggenheim, M.; Jost, L.; Pentheroudakis, G. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. J. Eur. Soc. Med. Oncol. 2010, 21, 194–197. [Google Scholar]
- Smaoui, N.; Bessassi, S. A developed system for melanoma diagnosis. IJCVSP 2013, 3, 10–17. [Google Scholar]
- Khan, A.; Gupta, K.; Stanley, R.J.; Stoecker, W.V.; Moss, R.H.; Argenziano, G.; Soyer, H.P.; Rabinovitz, H.S.; Cognetta, A.B. Fuzzy logic techniques for blotch feature evaluation in dermoscopy images. Comput. Med. Imaging Graph. 2009, 33, 50–57. [Google Scholar] [CrossRef][Green Version]
- Premaladha, J.; Ravichandran, K.S. Asymmetry analysis of malignant melanoma using image processing: A survey. J. Artif. Intell. 2014, 7, 45–53. [Google Scholar] [CrossRef]
- Hu, M.-K. Visual pattern recognition by moment invariants. IRE Trans. Inf. Theory 1962, 8, 179–187. [Google Scholar] [CrossRef]
- Wood, J. Invariant pattern recognition: A review. Pattern Recognit. 1996, 29, 1–17. [Google Scholar] [CrossRef]
- Vaishnavdev, A.; Suganraj, S.; Mareeswaran, L.; Subaraja, S.K.; Srinivas, K. Analysis of Brodatz image using GLCM and Hu’s moments. SSRG Int. J. Electron. C 2017, 54–58. [Google Scholar]
- Yuan, Z.; Li, F.; Zhang, P.; Chen, B. Description of shape characteristics through Fourier and wavelet analysis. Chin. J. Aeronaut. 2014, 27, 160–168. [Google Scholar] [CrossRef]
- Moldovanu, S.; Moraru, L.; Bibicu, D. Computerized decision support in liver steatosis investigation. Int. J. Biol. Biomed. Eng. 2012, 6, 69–76. [Google Scholar]
- Otsu, N. A Threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Moraru, L.; Moldovanu, S.; Biswas, A. Optimization of breast lesion segmentation in texture feature space approach. Med. Eng. Phys. 2014, 36, 129–135. [Google Scholar] [CrossRef]
- Moldovanu, S.; Moraru, L.; Bibicu, D. Characterization of myocardium muscle biostructure using first order features. Dig. J. Nanomater. Bios. 2011, 6, 1357–1365. [Google Scholar]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Li, H.; Chen, X.; Lu, H.; Ma, Y.; Peng, C.; Wang, Y.; Tang, L. Morphological background detection and illumination normalization of text image with poor lighting. PLoS ONE 2014, 9, e110991. [Google Scholar] [CrossRef]
- Soille, P. Morphological Image Analysis: Principles and Applications, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; pp. 1211–1227. [Google Scholar]
- Kaveri, E.; Chakravarthy, T. Skin images filtering using gaussian and median filtering techniques. IJIRCCE 2016, 4, 162–165. [Google Scholar]
- Garrido, A. Symmetry and asymmetry level measures. Symmetry 2010, 2, 707–721. [Google Scholar] [CrossRef]
- Sheeju, D.B.; Ramamurthy, B. Skin cancer detection and stage prediction using image processing techniques. Int. J. Eng. Technol. 2018, 7, 204–209. [Google Scholar]
- Xie, F.; Wu, Y.; Jiang, Z.; Meng, R. Dermoscopy image processing for Chinese. In Computer Vision Techniques for the Diagnosis of Skin Cancer. Series in BioEngineering; Scharcanski, J., Celebi, M.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 109–137. [Google Scholar]
- Giotis, I.; Molders, N.; Land, S.; Biehl, M.; Jonkman, M.; Petkov, N. MED-NODE: A computer-assisted melanoma diagnosis system using non-dermoscopic images. Expert Syst. Appl. 2015, 42, 6578–6585. [Google Scholar] [CrossRef]
- Almaraz-Damian, J.-A.; Ponomaryov, V.; Sadovnychiy, S.; Castillejos-Fernandez, H. Melanoma and nevus skin lesion classification using handcraft and deep learning feature fusion via mutual information measures. Entropy 2020, 22, 484. [Google Scholar] [CrossRef]
- Chaki, J.; Dey, N. Texture Feature Extraction Techniques for Image Recognition; Springer: Singapore, 2020. [Google Scholar]
- Chaki, J.; Dey, N. A Beginner’s Guide to Image Preprocessing Techniques, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Dey, N.; Rajinikanth, V.; Ashour, A.S.; Tavares, J.M.R. Social group optimization supported segmentation and evaluation of skin melanoma images. Symmetry 2018, 10, 51. [Google Scholar] [CrossRef]
- Rajinikanth, V.; Satapathy, S.C.; Dey, N.; Fernandes, S.L.; Manic, K.S. Skin melanoma assessment using Kapur’s entropy and level set—A study with bat algorithm. In Smart Intelligent Computing and Applications; Suresh, C.S., Vikrant, B., Swagatam, D., Eds.; Springer: Singapore, 2018; Volume 1, pp. 193–202. [Google Scholar]
- Wang, R.; Wang, G. Web text categorization based on statistical merging algorithm in big data environment. IJACI 2019, 10, 17–32. [Google Scholar] [CrossRef]



| AI Feature | E Feature | CIRC Feature | FFF Feature | Q Feature | ƛ Feature | F6 Feature | F7 Feature | |
|---|---|---|---|---|---|---|---|---|
| Sensitivity (D1) | 0.98 | 0.90 | 0.81 | 0.53 | 0.61 | 0.67 | 0.68 | 0.76 |
| Sensitivity (D2) | 0.84 | 0.94 | 0.81 | 0.80 | 0.63 | 0.65 | 0.72 | 0.62 |
| Specificity (D1) | 0.64 | 0.66 | 0.32 | 0.76 | 0.57 | 0.46 | 0.80 | 0.51 |
| Specificity (D2) | 0.87 | 0.63 | 0.35 | 0.51 | 0.58 | 0.53 | 0.82 | 0.71 |
| Accuracy (D1) | 0.82 | 0.78 | 0.57 | 0.65 | 0.59 | 0.57 | 0.74 | 0.64 |
| Accuracy (D2) | 0.85 | 0.79 | 0.58 | 0.66 | 0.61 | 0.59 | 0.77 | 0.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damian, F.A.; Moldovanu, S.; Dey, N.; Ashour, A.S.; Moraru, L. Feature Selection of Non-Dermoscopic Skin Lesion Images for Nevus and Melanoma Classification. Computation 2020, 8, 41. https://doi.org/10.3390/computation8020041
Damian FA, Moldovanu S, Dey N, Ashour AS, Moraru L. Feature Selection of Non-Dermoscopic Skin Lesion Images for Nevus and Melanoma Classification. Computation. 2020; 8(2):41. https://doi.org/10.3390/computation8020041
Chicago/Turabian StyleDamian, Felicia Anisoara, Simona Moldovanu, Nilanjan Dey, Amira S. Ashour, and Luminita Moraru. 2020. "Feature Selection of Non-Dermoscopic Skin Lesion Images for Nevus and Melanoma Classification" Computation 8, no. 2: 41. https://doi.org/10.3390/computation8020041
APA StyleDamian, F. A., Moldovanu, S., Dey, N., Ashour, A. S., & Moraru, L. (2020). Feature Selection of Non-Dermoscopic Skin Lesion Images for Nevus and Melanoma Classification. Computation, 8(2), 41. https://doi.org/10.3390/computation8020041








