Abstract
Gas adsorption in nanoscale pores is one of the key theoretical bases for shale gas development. However, the influence mechanisms of gas adsorption capacity and the second adsorption layer in nanoscale pores are very complex, and are difficult to directly observe by using traditional experimental methods. Therefore, multilayer graphene is used to model the nanopores in a shale reservoir, and the molecular dynamics method is carried out to study the adsorption dynamics of methane molecules. The results show that the adsorption density of methane molecules is inversely proportional to the temperature and pore size, and it positively correlates to the graphene layer number and pressure. The smaller adsorption region will reach the adsorption equilibrium state earlier, and the adsorption layer thickness is smaller. When the pore size is larger than 1.7 nm, the single-layer adsorption becomes double-layer adsorption of methane molecules. The peak of the second adsorption layer depends on the pressure and temperature, while the position of the second adsorption layer depends on the pore size. The present work is useful for understanding the dynamics mechanism of gas molecules in a nanoscale confined space, and may provide a theoretical basis for the development of unconventional natural gas.
1. Introduction
Shale gas has become one of the most important unconventional gas resources due to advances in horizontal drilling and hydraulic fracturing technology [1,2,3]. However, the storage and transport of shale gas are different from those of conventional gas reservoirs. Shale with abundant nanopores is both hydrocarbon source rock and gas reservoir rock, which is a self-generating and self-storage system [4,5]. Shale gas is mainly stored in organic matter and clay minerals on the surface of nanopores in the form of adsorbed gas [6,7,8]. Therefore, an accurate understanding of the adsorption behavior of shale gas in nanopores is significant for shale gas exploitation.
It has been proven by experimental studies that the shale gas content depends on the organic carbon content, and the gas adsorption capacity is related to the temperature, pressure, and pore structures [9,10]. However, due to the wide distribution of organic matter pores on the nanometer scale, the dynamic behavior of shale gas is extremely complex. Molecular dynamics (MD) simulation has been applied as a powerful tool to study the adsorption and transport behaviors of shale gas at the microscopic level intuitively and accurately [11,12,13,14]. Ambrose et al. studied the methane adsorption in carbon slit pores of varying size and temperature by using equilibrium MD, and they found that the change in methane adsorption behavior is dramatic at low slit width and high temperature [15]. Li et al. used grand canonical Monte Carlo (GCMC) and MD to study the methane/ethane mixture adsorption in kerogen nanopores at a temperature of 327 K and with pressure ranging from 1 to 50 MPa, and they proposed a multicomponent Langmuir model for binary mixture sorption in shale [16]. Zha et al. employed MD and density function theory (DFT) to study the methane adsorption in single-wall carbon nanolayers, slit graphene layers, and bituminous coal pore models, and they stated that methane molecules cannot be adsorbed in carbon nanotubes below 0.5 nm [17]. Zhang et al. examined sequentially the influence of the pore sizes on methane adsorption in kaolinite at a temperature of 293 K and with a pressure of 20 MPa [18]. They discovered that the absolute adsorption amount of CH4 decreases exponentially with the increase in pore size, which reaches a constant value when the pore size is in the range of 6–8 nm. Guo et al. carried out GCMC and MD on the methane adsorption and absorption in a shale kerogen slit at supercritical conditions and analyzed the influence of slit aperture, pressure, temperature, and water content on the adsorption and absorption capacities [19]. Their results indicate that the ratio of the capacity of adsorption to adsorption increases with the increase in pressure, the increase in slit aperture enhances the adsorbed gas content, and both increased temperature and increased water content impede methane adsorption. It can be found that the shale gas adsorption in nanopores depends on both pore structure and pressure as well as temperature.
The density peak of methane molecules corresponding to the two lowest potential energy points near the pore wall is generally named the first adsorption layer or the main adsorption layer. Recently, a second density peak that is much lower than the first density peak was observed about one molecular diameter from the first adsorption layer, and it is called the second adsorption layer or the secondary adsorption layer. Kowalczyk et al. used 0.7–7.5 nm graphene slits to simulate organic nanopores in shale reservoirs and studied the density distribution of methane molecules at temperature of 318.15 K and with a pressure of 0.1~10 MPa by using GCMC and MD [20]. They reported that there is an evident separation of the methane molecule density in the graphene slits. Huang et al. used the Monte Carlo method and MD to study the adsorption behavior of methane molecules in pores composed of graphite sheets [21]. The results show that pores with a size of 1–50 nm are the main adsorption pores, and the adsorption tends to split into multiple layers as the pressure increases. Liu et al. used MD to simulate the methane adsorption in graphite slits and reported that the methane molecules are stratified in graphite slits [22]. They stated that the intermolecular binding capacity of methane molecules in the adsorption layer is greater than that of the free layer, and the position of the density peak in the first adsorption layer is slightly different from that of the second adsorption layer. Wu et al. investigated the adsorption behavior in a slit of methane at a temperature of 298 K by using MD, and found that the methane adsorption changes from the single layer to a four-layer adsorption with the increase in the slit pore width [23]. Zhu and Zhao studied methane gas adsorption in carbon nanotubes with MD and proposed an adsorption-phase equation [24]. It was found that as the diameter of carbon nanotubes increases, the methane adsorption changes from single-layer adsorption to double-layer adsorption. With the increase in pressure, the density of the second layer and the first layer increases rapidly and slowly, respectively. Yang et al. used fractal topography to characterize the rough pore surfaces and studied the effect of rough surface on the adsorption density in slit pores by combining MD and GCMC [25]. They found that the adsorption mode changes from single-layer to multi-layer at a pressure of 10 MPa and a temperature of 303 K.
It can be found that the adsorption behavior of shale gas in the organic nanopores, especially under high temperature and pressure, are extremely complex. Also, the influencing factors and physical mechanisms of the second adsorption layer of methane molecules in nanopores have not been fully understood. Therefore, MD is performed on three-dimensional nanopores composed of multi-layer graphite lattice to explore the influence mechanisms of pore size, pressure, and temperature on the adsorption capacity and second adsorption layer. This paper is organized into four sections. In the Section 2, the geometrical pore structure and mathematical model of shale gas adsorption are introduced. In the Section 3, the effect of the graphene-layer number, temperature, pressure, and pore size on adsorption, and the influencing factors of the second adsorption layer of methane molecules, are discussed. The conclusions are provided in the Section 4.
2. Materials and Methods
Because the adsorption energy of methane molecules on graphene is close to that of shale [26,27], the graphene plate channels were designed by Lammps [28] (Figure 1). The slit pore skeleton in shale was approximated as a carbon–graphite structure, which consists of multiple layers of graphite lattice arranged in a six-lattice structure. The graphite slit wall and methane molecule are shown in Figure 2. In order to prevent interactions between adjacent layers of graphene, the thickness of the vacuum layer was taken as d = 3.35 Å. The Nose/Hoover method was used for NVT integration modeling, and OPLS force fields were used to calculate the interactions between atoms. The electrostatic long-range force action was calculated using the particle–particle particle–mesh (PPPM) method, and the interaction potential function between methane molecules and carbon skeleton atoms in the simulation was Lennard–Jones (LJ) 12–6 action potential [29].
where is the distance between atoms when the energy is zero, is the depth of the potential well, − is the lowest value of the potential energy, and is the distance between atom pairs. In the right of Equation (1), the first item represents the repulsive force, while the second item is the attractive force. The critical point for the transition between attraction and repulsion forces is . The truncation radius is set to 10 Å, and the Lorentz−Berthelot rule was used to calculate the interaction between methane atoms and carbon atoms. The values of the key–value pair parameters are summarized in Table 1.
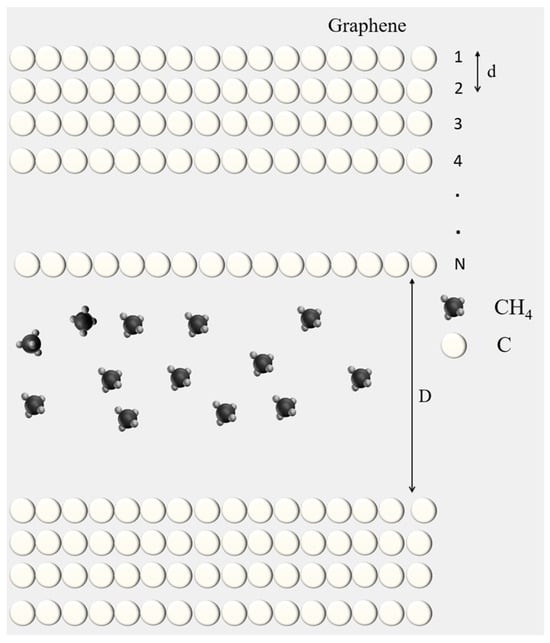
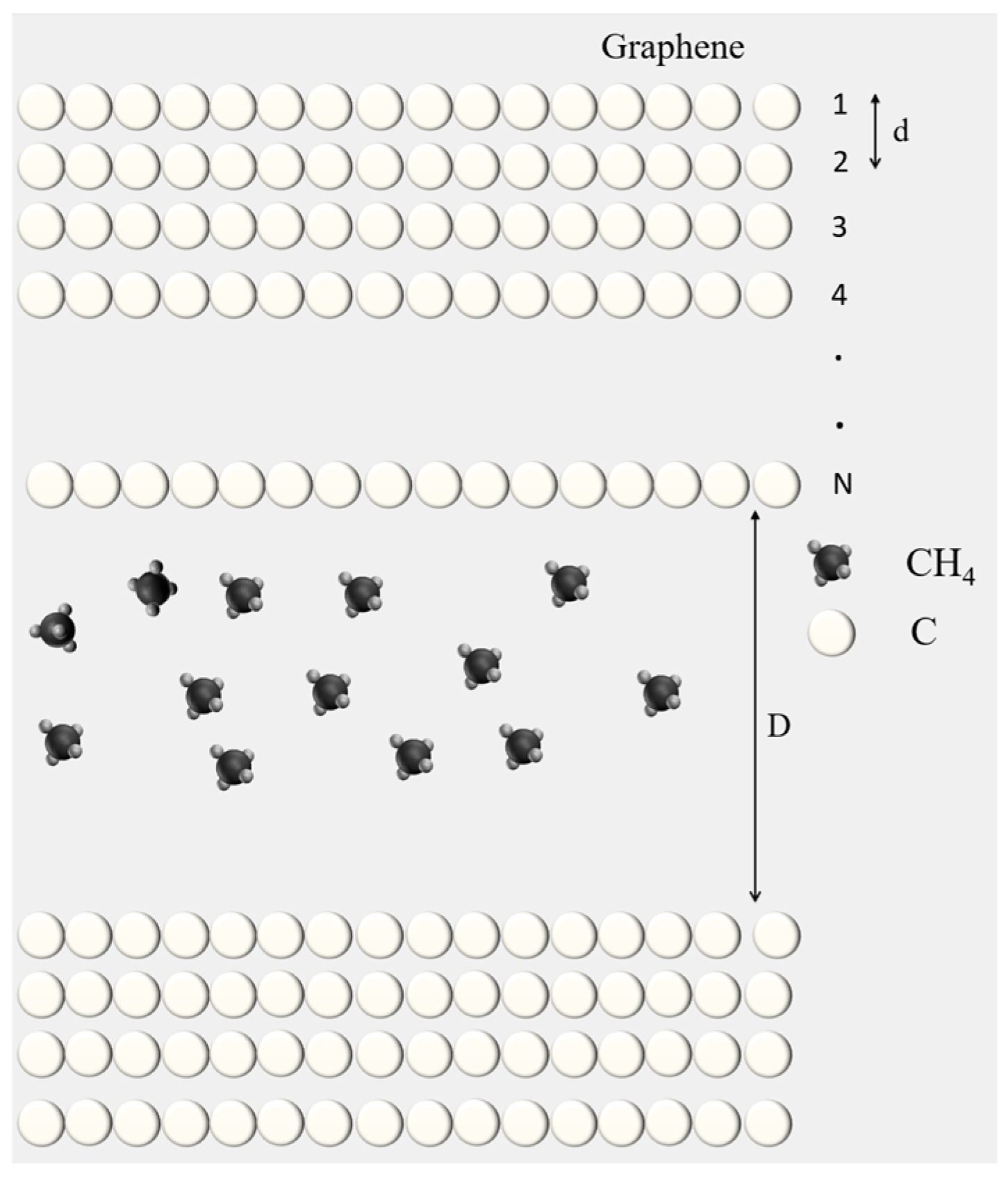
Figure 1.
The physical model with a multi-layer graphite wall and methane molecules (front view).
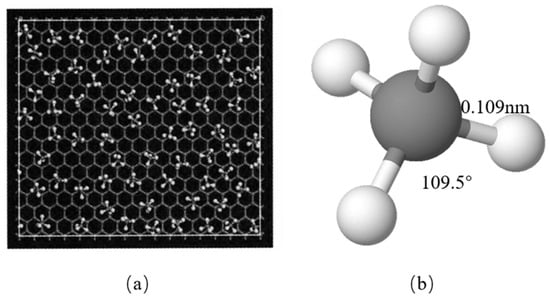
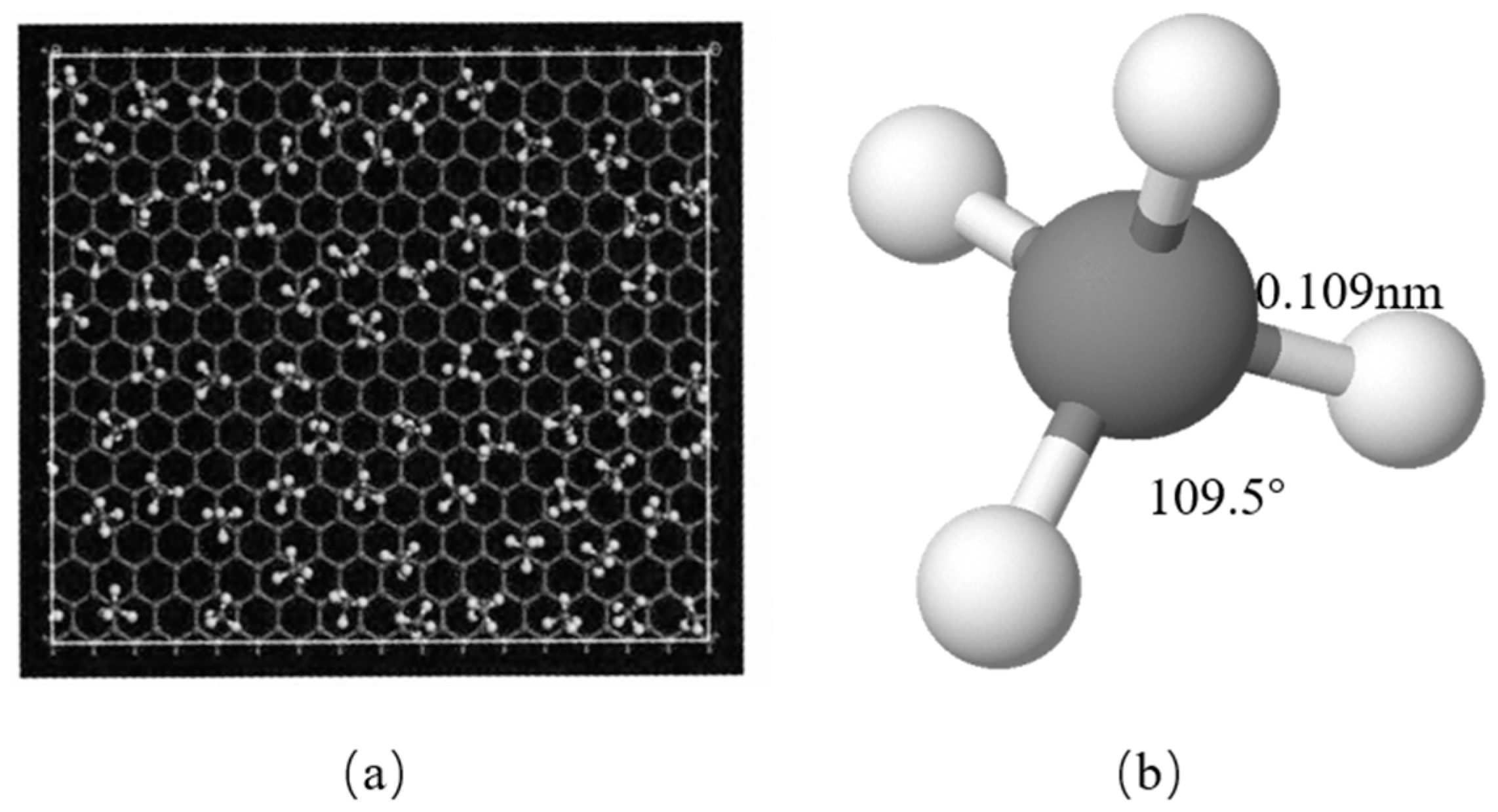
Figure 2.
(a) The methane molecules in the first adsorption layer onto the graphite slit walls. (b) A single methane molecule.

Table 1.
The value of key–value pair parameters.
In order to ensure accuracy, the model was relaxed to obtain a stable configuration with minimal energy, and then the formal simulation step was conducted. In order to explore the effects of pore size, temperature, and pressure on the adsorption and absorption behavior of CH4 in graphene, specific simulation parameters were used and are shown in Table 2. It should be noted that pressure was controlled by the gas density of methane. A time step of 0.1 fs was used. Each simulation group was first performed for 100 ps to balance, and the data acquisition was carried out in the following 200 ps. All the simulations were performed on a computer with CPU Xeon Silver 4210R.

Table 2.
The values of the condition parameters.
3. Results
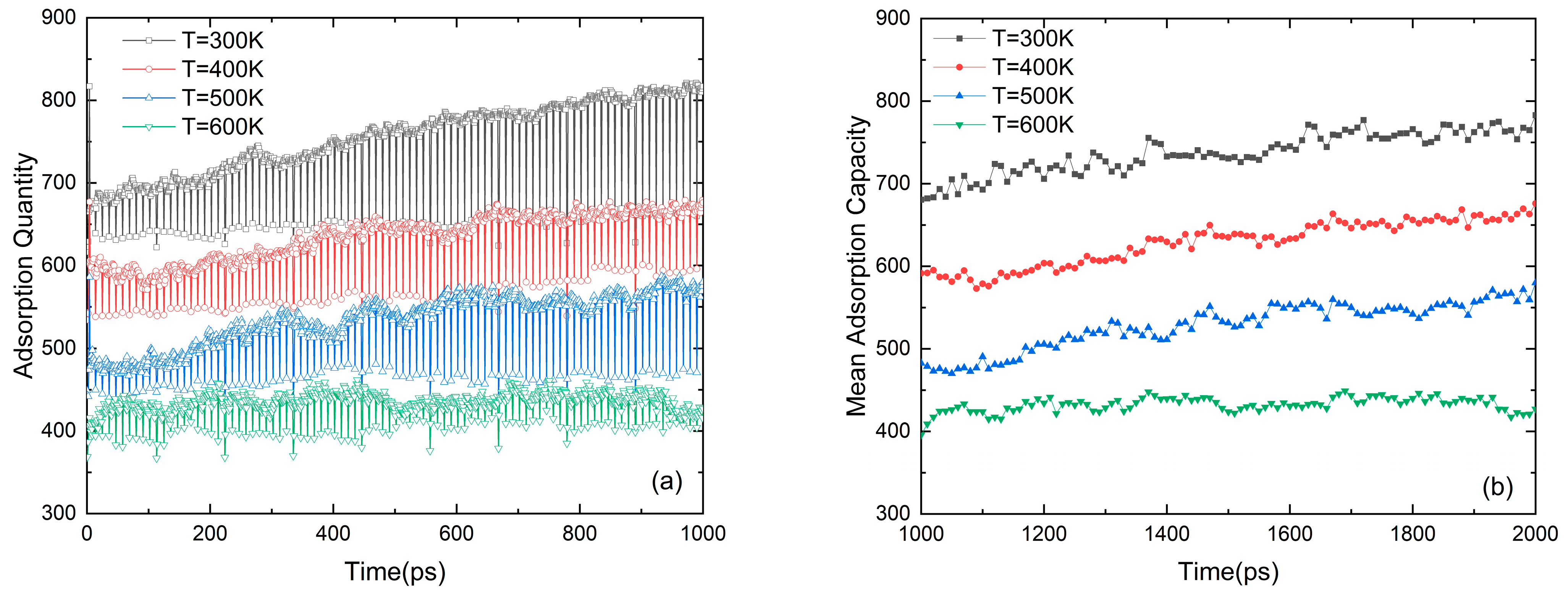
Figure 3 shows the dynamic adsorption process of methane in nanopores. Due to the adsorption potential between the methane molecules and the wall [30], the methane molecules are in the process of adsorption, desorption, and re-adsorption. It can be seen in Figure 3a that the density of the adsorbed gas decreases with the increase in temperature, and the variation amplitude of the gas adsorption quantity under high temperature gradually tends to stabilize with time. It can be found in Figure 3b that the adsorption capacity maintains a relatively stable level over time after a fixed relaxation time.
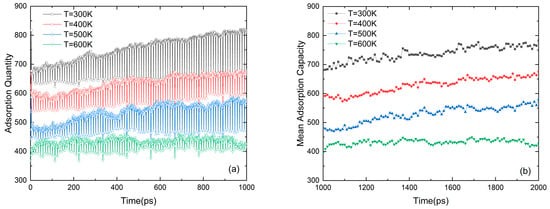
Figure 3.
(a) The adsorption quantity with time in an ensemble. (b) Mean adsorption capacity (N = 5, D = 7.2 nm and p = 5 MPa).
3.1. Effect of Graphene-Layer Number on Adsorption
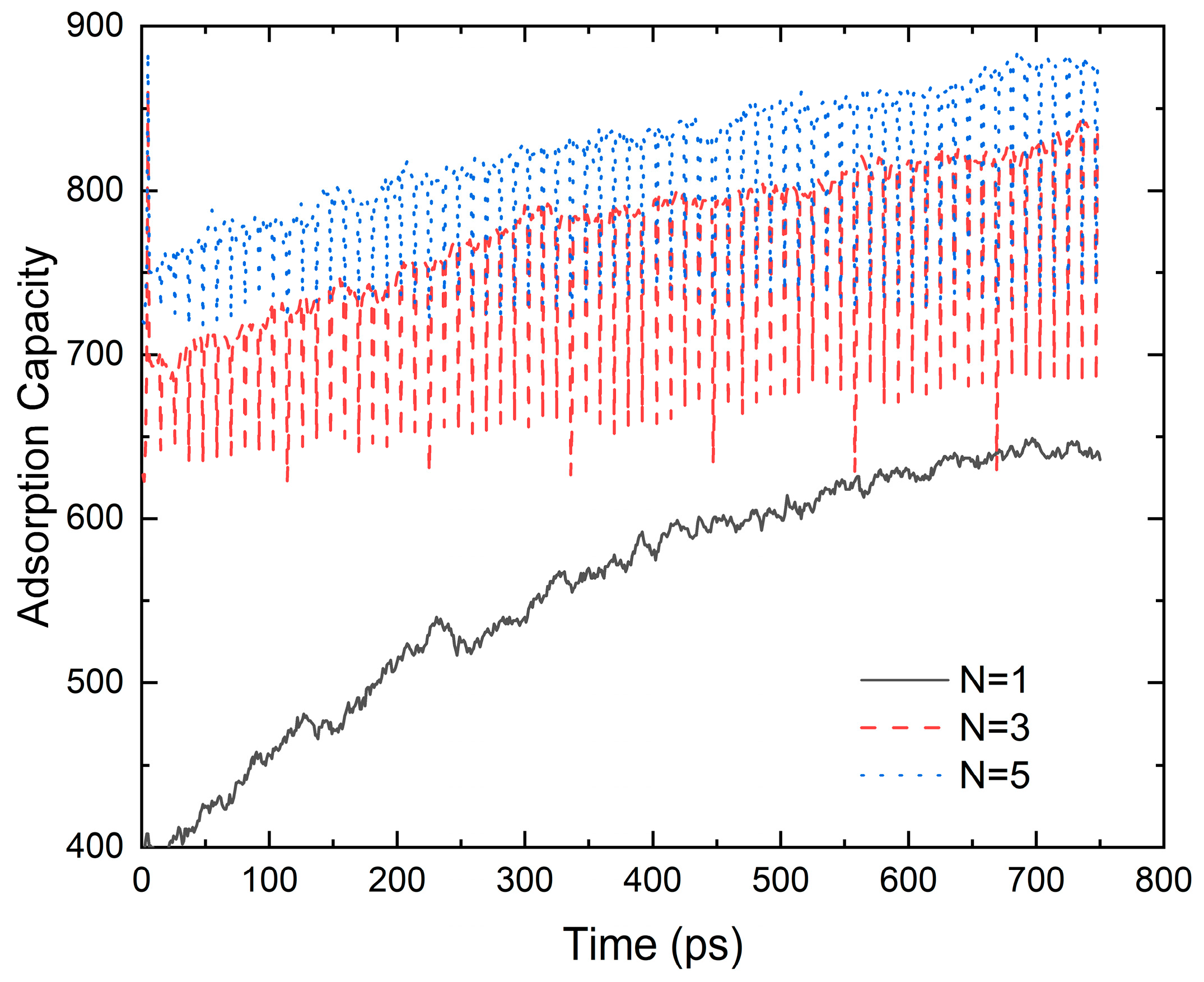
Figure 4 shows that the graphene-layer number evidently affects the adsorbed gas density. The adsorption force of a multi-layer wall on methane is much larger than that of a single-layer wall; thus, the maximum adsorption capacity of the pores with a multi-layer wall is higher than that of pores with a single-layer wall. When the adsorption layer number increases from N = 1 to N = 3 and N = 5, the adsorption capacity is enhanced by 40.72% and 49.37%, respectively. Also, under the same temperature and relaxation time, the methane adsorption by the single-layer wall takes longer to reach dynamic equilibrium. The methane in the adsorption layer on the multi-layer wall is more stable. Because the adsorption force between the carbon skeleton and methane is smaller than that between methane molecules, a second layer of CH4 adsorption is formed near the middle of the pore. The density of the second layer of CH4 is slightly higher than that of the free layer, but significantly lower than that of the first adsorption layer.
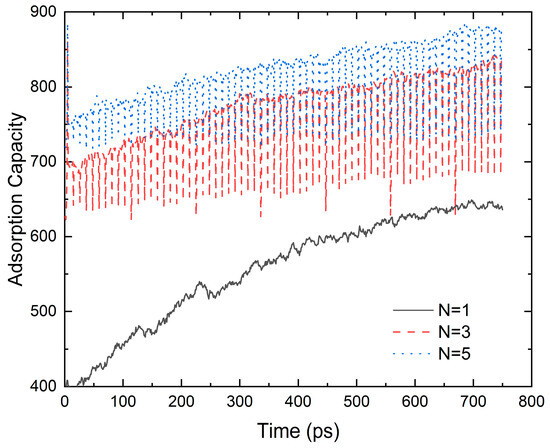
Figure 4.
The influence of the graphene layer on the adsorption gas density (D = 7.9 nm, p = 5 MPa and T = 300 K).
3.2. Effect of Temperature and Pressure on Adsorption
The Langmuir adsorption curve is generally used to describe the adsorption characteristics of gas:
where is the temperature, is the adsorption capacity, is the maximum adsorption capacity of Langmuir, and is the Langmuir pressure. The isothermal adsorption function by NIST was used here [31].
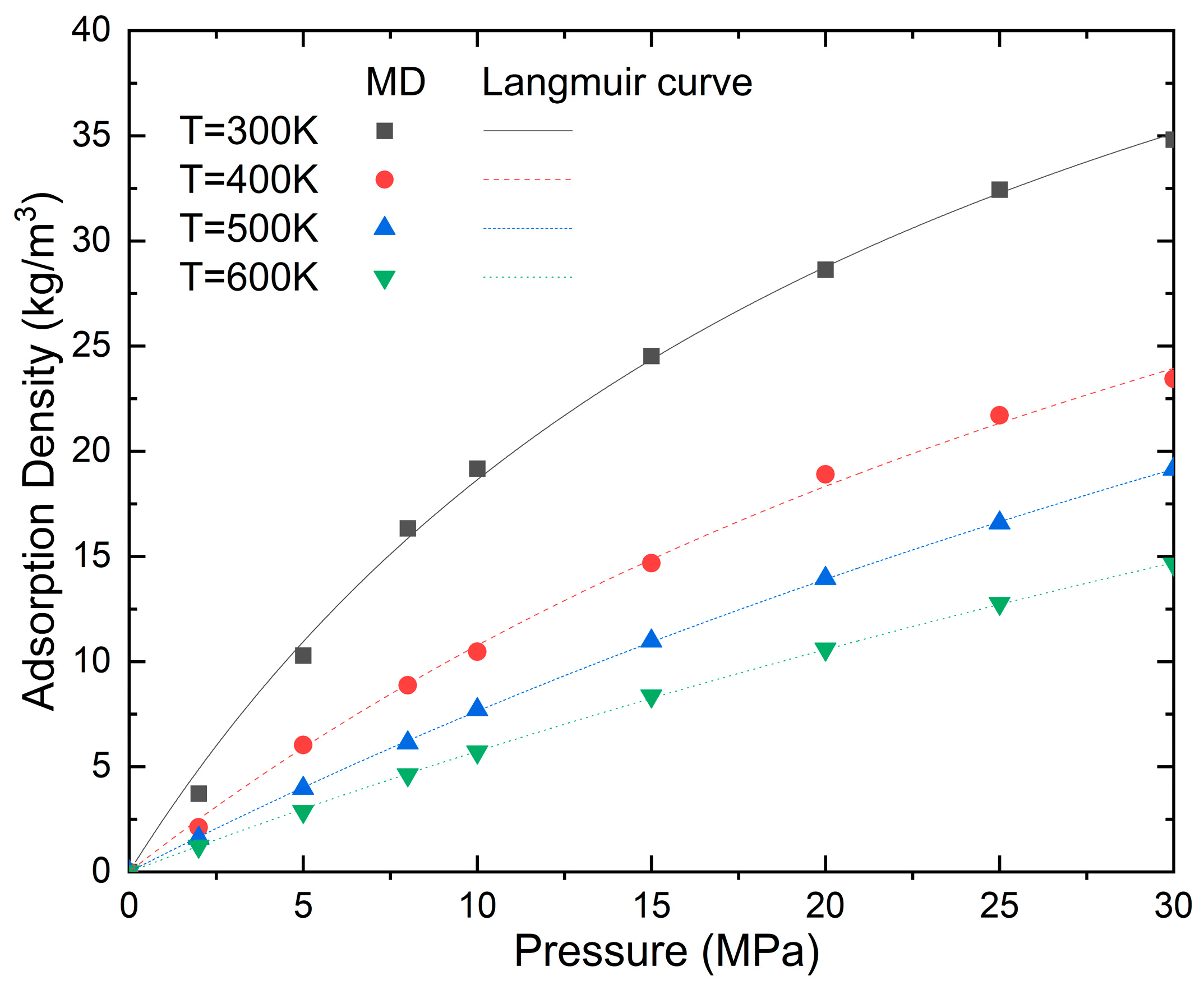
It can be clearly seen from Figure 5 that the adsorption of shale gas in nanopores is consistent with the Langmuir isothermal adsorption. The adsorption capacity decreases with the increase in temperature. When the temperature increases from 300 K to 400 K, 500 K, and 600 K at a pressure of 30 MPa, the adsorption density decreases by 48.71%, 22.19%, and 30.89%, respectively. This is mainly because the increase in temperature causes the kinetic energy of the methane molecules to increase, enabling more adsorbed methane molecules on the pore surfaces to gain sufficient energy to escape their adsorbed states and transition into a free (gaseous) state. Also, the van der Waals interaction gradually decreases as the temperature increases. As the pressure increases from zero, the methane molecules are rapidly adsorbed on the empty adsorption sites distributed on the wall of the carbon nanotubes. The adsorption amount increases with the increase in pressure; however, the increasing gradient of adsorption slows down as the pressure and temperature increase. For example, the adsorption density increases by 415% when the pressure increases from 2 to 10 MPa at a temperature of 300 K; however, it only increases by 49% and 21% when the pressure increases from 10 to 20 and 30 Mpa, respectively. At a temperature of 600 K, the adsorption density increases by 371%, 85%, and 38% when the pressure increases from 2 to 10, 20, and 30 MPa, respectively.
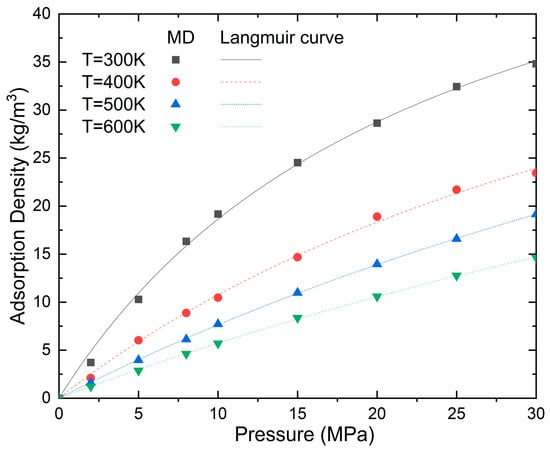
Figure 5.
Effect of temperature and pressure on the adsorption density (N = 5 and D = 5.6 nm).
3.3. Effect of Pore Size on Adsorption
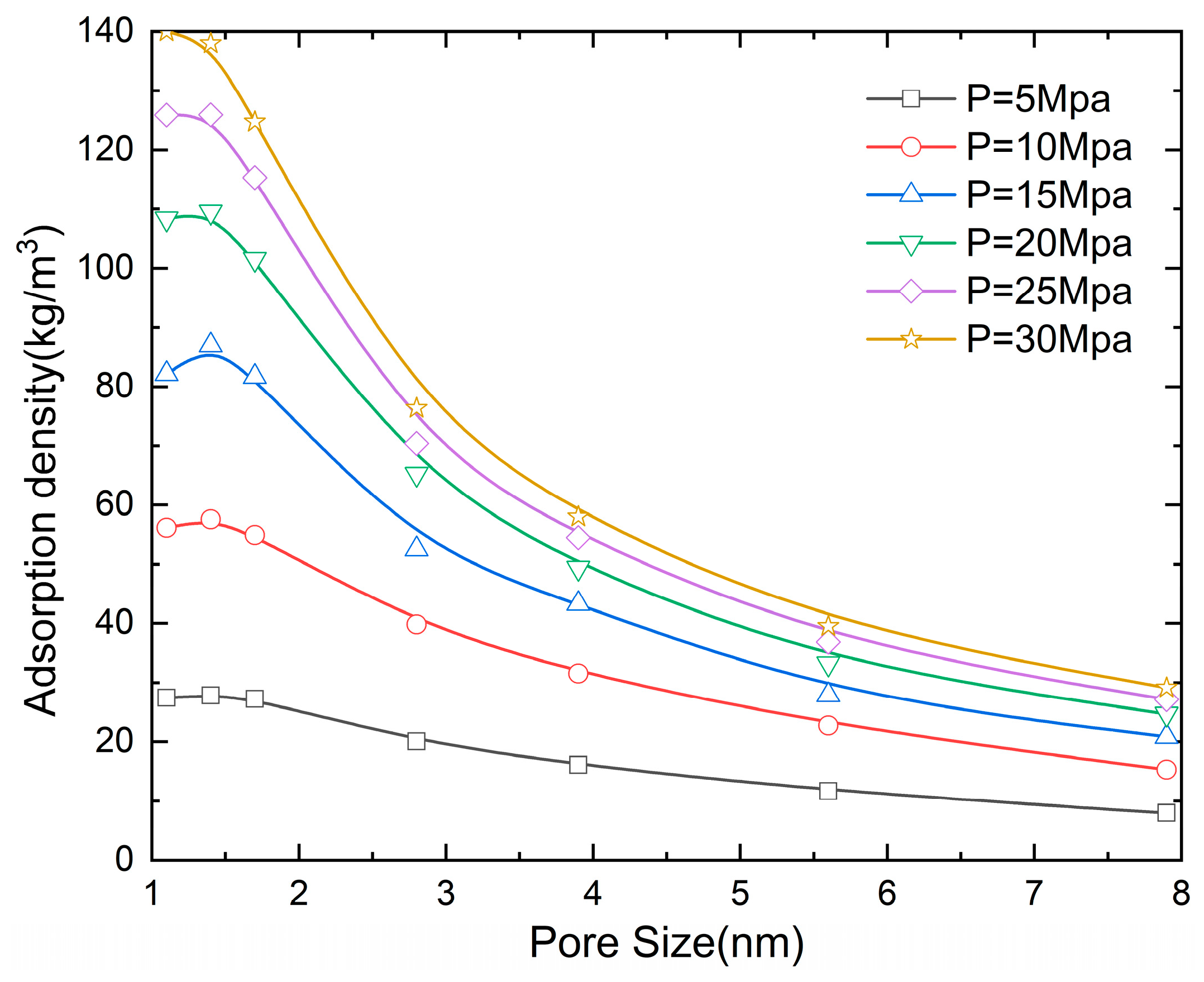
Figure 6 reveals the influence of pore size on the methane adsorption density. It can be found that the adsorption density decreases with the increase in the slit pore diameter. At a pressure of 5 MPa, when the pore size increases from 1.1 to 1.7, 2.8, 3.9, 5.6, and 7.9 nm, the adsorption density decreases by 1.8%, 27.6%, 41.9%, 58%, and 71.4%, respectively. The phenomenon primarily originates from the synergistic effect of the van der Waals potential field between confining walls and the van der Waals force between the graphite wall and methane molecules in spatially restricted systems. When the pore size is D = 2.8 nm, as the pressure increases from 5 to 10, 15, 20, 25, and 30 MPa, the adsorption density increases by 98.7%, 162%, 225.7%, 251.4%, and 281.1%, respectively.
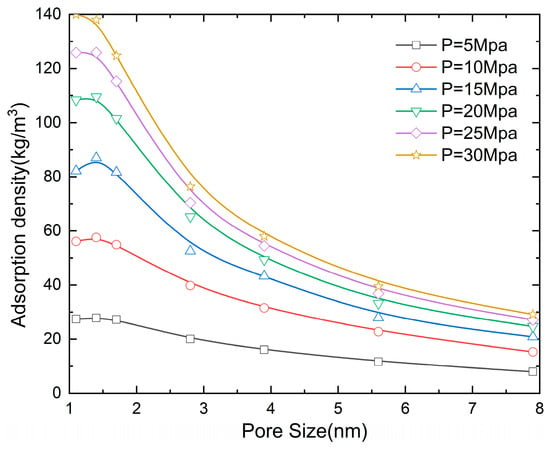
Figure 6.
The influence of pore size on the adsorption density (N = 5 and T = 300 K).
3.4. The Influencing Factors of the Second Adsorption Layer
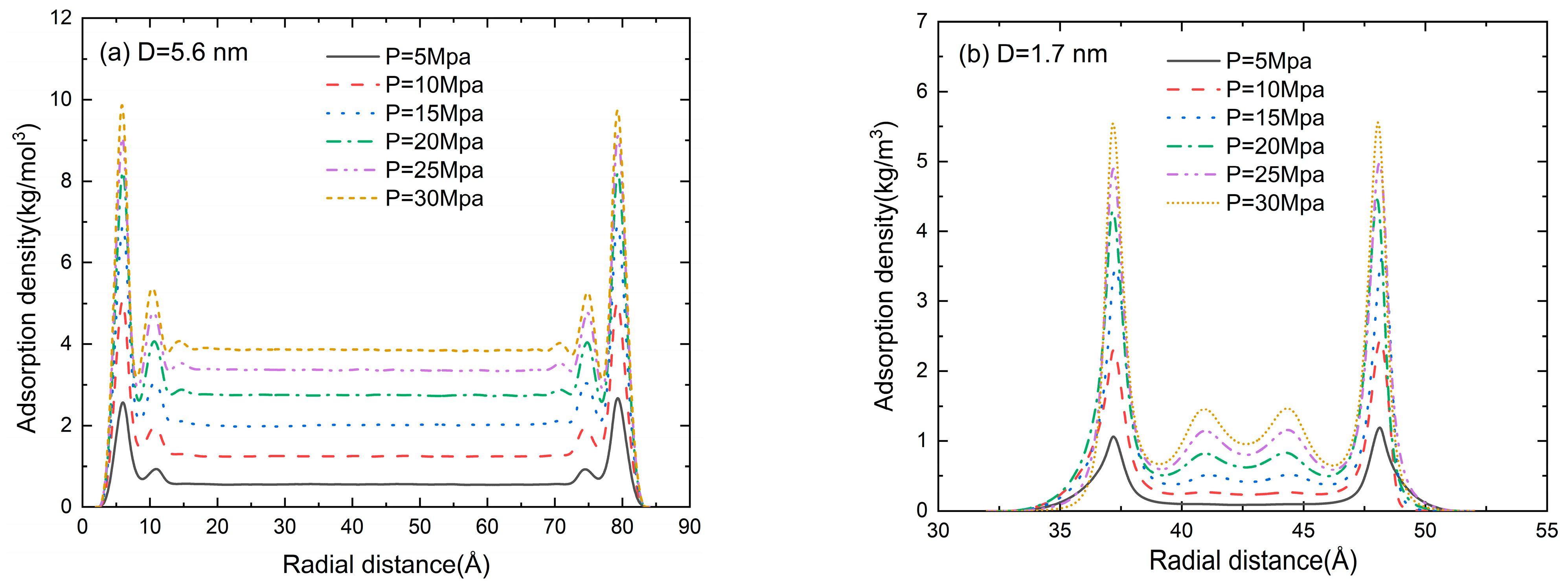
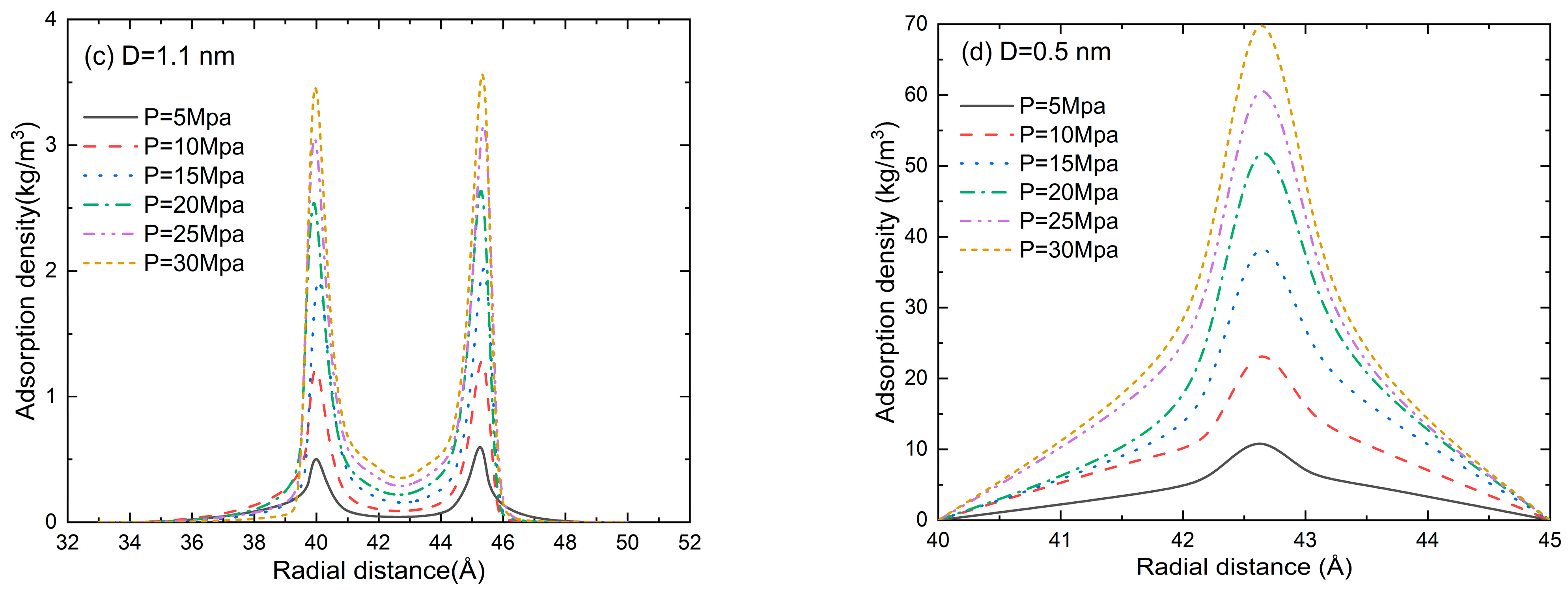
The radial distribution function (RDF) in the pore size of D = 5.6, 1.7, 1.1, and 0.5 nm at a pressure of 5–30 MPa is shown in Figure 7. An accumulation layer of methane molecules can be clearly seen near the inner wall of the slit. The methane molecules in the central region are randomly distributed, and the fluctuation in adsorption density is marginal. Two adsorption layers can be clearly seen in Figure 7a,b. When the pore size is comparable to the gas molecule size, the repulsion between the pore wall and the gas molecules dominates the adsorption process. Thus, the second adsorption layer disappears in Figure 7c and the gas adsorption density is much lower than that in Figure 7a,b. With the further reduction in pore size (D = 0.5 nm in Figure 7d), the adsorption potentials of the two walls are superimposed on each other and the peak value of the adsorption gas density is much higher than that in Figure 7a–c. It can be found from Figure 7 that the gas density increases as the pressure increases. The position of the second adsorption layer depends on the pore size, while the pressure only affects the peak value of gas density.
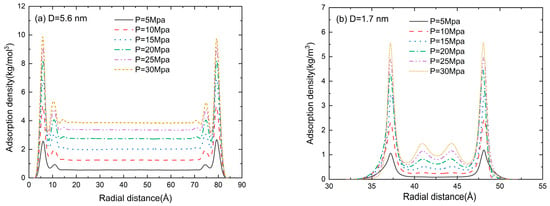
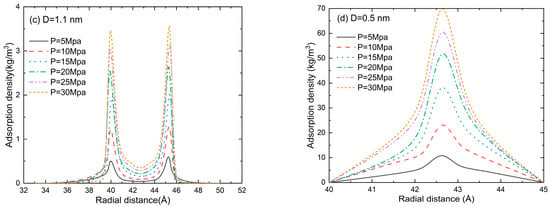
Figure 7.
The density distribution (RDF) of methane at a pore size of (a) D = 5.6 nm, (b) D = 1.7 nm, (c) D = 1.1 nm, and (d) D = 0.5 nm.
4. Conclusions
In this paper, the MD was performed on the gas adsorption in nanopores composed of multi-layer graphene. The effect of the graphene layer, pressure, temperature, and pore size on the adsorption density and second adsorption layer were explored. The main results are summarized as follows. (1) The gas adsorption in nanopores fits well with Langmuir isothermal adsorption, and the correlation coefficient is larger than 0.98. The density of adsorbed gas is inversely proportional to the temperature and pore size, and it is positively correlated to the wall-layer number and pressure. When the pore size is comparable to the gas molecule size, the adsorption density is high due to the superposition effect of the two pore walls. (2) When the pore size is larger than 1.7 nm, a second adsorption layer of methane molecules can be found. The peak of the second adsorption layer depends on the pressure and temperature, and the position of the second adsorption layer depends on the pore size. The present results may help with understanding the gas adsorption mechanisms of shale gas, and provide a theoretical basis for unconventional natural gas exploration.
Author Contributions
Conceptualization, Q.Y. and S.Q.; methodology, Q.Y.; software, J.Y.; validation, Q.Y.; formal analysis, S.Q.; investigation, Q.Y.; resources, P.X.; data curation, Q.Y.; writing—original draft preparation, Q.Y.; writing—review and editing, S.Q. and P.X.; visualization, P.X.; supervision, P.X.; project administration, S.Q.; funding acquisition, P.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China, grant number 52376079, and the Zhejiang Provincial Natural Science Foundation of China, grant number LZ24E060002.
Data Availability Statement
All data are contained within the paper, and a report of any other data is not included.
Acknowledgments
The authors thank the editor and referees for detailed reading and comments that were both helpful and insightful.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| MD | Molecular dynamics |
| GCMC | Grand canonical Monte Carlo |
| DFT | Density function theory |
| PPPM | Particle–particle particle–mesh |
| RDF | Radial distribution function |
References
- Nie, H.; Dang, W.; Zhang, Q.; Zhang, J.; Li, P.; Zhang, S.; Ding, J.; Chen, Q.; Feng, Y.; Zhang, X. Evaluation of gas content in organic-rich shale: A review of the history, current status, and future directions. Geosci. Front. 2024, 15, 101921. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhang, Z.; Yao, S.; Zhang, H.; Zheng, G.; Luo, F.; Feng, L.; Liu, K.; Jiang, L. Recent techniques on analyses and characterizations of shale gas and oil reservoir. Energy Rev. 2024, 3, 100067. [Google Scholar] [CrossRef]
- Bowker, K.A. Barnett Shale gas production, Fort Worth Basin: Issues and discussion. AAPG Bull. 2007, 91, 523–533. [Google Scholar] [CrossRef]
- Ren, W.; Guo, J.; Zeng, F.; Wang, T. Modeling of high-pressure methane adsorption on wet shales. Energy Fuels 2019, 33, 7043–7051. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Jarvie, D.M. Morphology, genesis, and distribution of nanometer-scale pores in siliceous mudstones of the Mississippian Barnett Shale. J. Sediment. Res. 2009, 79, 848–861. [Google Scholar] [CrossRef]
- He, X.; Zhang, K.; Jiang, S.; Jiang, Z.; Wang, X.; Jiang, W.; Li, J.; Wu, Y.; Gao, Z.; Tang, T.; et al. Influencing factors and quantitative prediction of gas content of deep marine shale in Luzhou block. Sci. Rep. 2025, 15, 1896. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Cheng, H. Gas adsorption characterization of pore structure of organic-rich shale: Insights into contribution of organic matter to shale pore network. Nat. Resour. Res. 2021, 30, 2377–2395. [Google Scholar] [CrossRef]
- Sharma, A.; Namsani, S.; Singh, J.K. Molecular simulation of shale gas adsorption and diffusion in inorganic nanopores. Mol. Simul. 2015, 41, 414–422. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Qu, Z.; Yin, Y.; Kang, Q.; Yu, B.; Tao, W.-Q. Modeling of multi-scale transport phenomena in shale gas production—A critical review. Appl. Energy 2020, 262, 114575. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, W.; Zhu, H.; Yin, Y.; Qu, Z. Review of shale gas transport prediction: Basic theory, numerical simulation, application of AI methods, and perspectives. Energy Fuels 2023, 37, 2520–2538. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, Y.; Yang, Q.; Chen, Q.; Zhang, Y.; Xu, Z.; Feng, X.; Tian, B.; Wang, L.; Liu, Y. Gas sorption in shale media by molecular simulation: Advances, challenges and perspectives. Chem. Eng. J. 2024, 25, 150742. [Google Scholar] [CrossRef]
- Shao, B.; Wang, S.; Li, T.; Chen, X.; Ma, Y. GCMC-MD prediction of adsorption and diffusion behavior of shale gas in nanopores. Fuel 2024, 377, 132696. [Google Scholar] [CrossRef]
- Wang, T.; Tian, S.; Li, G.; Zhang, L.; Sheng, M.; Ren, W. Molecular simulation of gas adsorption in shale nanopores: A critical review. Renew. Sustain. Energy Rev. 2021, 149, 111391. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, M.; Liu, L.; Wu, J.; Chen, M.; Zhang, L. Molecular dynamics simulations of shale gas transport in rough nanopores. J. Pet. Sci. Eng. 2022, 217, 110884. [Google Scholar] [CrossRef]
- Ambrose, R.J.; Hartman, R.C.; Diaz-Campos, M.; Akkutlu, I.Y.; Sondergeld, C.H. New pore-scale considerations for shale gas in place calculations. In Proceedings of the SPE Unconventional Resources Conference/Gas Technology Symposium, Calgary, AB, Canada, 19–21 October 2010. [Google Scholar]
- Li, W.; Cao, J.; Liang, Y.; Masuda, Y.; Tsuji, T.; Tamura, K.; Ishiwata, T.; Kuramoto, D.; Matsuoka, T. Molecular simulation of methane/ethane mixture adsorption behavior in shale nanopore systems with micropores and mesopores. Fuel 2024, 358, 130294. [Google Scholar] [CrossRef]
- Zha, W.; Lin, B.; Liu, T.; Liu, T.; Yang, W.; Zhang, X. Modeling methane adsorption distance using carbon nanotubes and bituminous coal pore models. Energy Fuels 2024, 38, 948–960. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, J.; Kang, T. Monte Carlo simulations of methane adsorption on kaolinite as a function of pore size. J. Nat. Gas Sci. Eng. 2018, 49, 410416. [Google Scholar] [CrossRef]
- Guo, F.; Wang, S.; Feng, Q.; Yao, X.; Xue, Q.; Li, X. Adsorption and absorption of supercritical methane within shale kerogen slit. J. Mol. Liq. 2020, 320, 114364. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Tanaka, H.; Kaneko, K.; Terzyk, A.P.; Do, D.D. Grand canonical Monte Carlo simulation study of methane adsorption at an open graphite surface and in slitlike carbon pores at 273 K. Langmuir 2005, 21, 5639–5646. [Google Scholar] [CrossRef]
- Huang, L.; Ning, Z.; Wang, Q.; Qi, R.; Zeng, Y.; Qin, H.; Ye, H.; Zhang, W. Molecular simulation of adsorption behaviors of methane, carbon dioxide and their mixtures on kerogen: Effect of kerogen maturity and moisture content. Fuel 2018, 211, 159–172. [Google Scholar] [CrossRef]
- Liu, B.; Shi, J.; Shen, Y.; Zhang, J. A molecular dynamics simulation of methane adsorption in graphite slit-pores. Chin. J. Comput. Phys. 2013, 30, 692–699. [Google Scholar]
- Wu, H.; Chen, J.; Liu, H. Molecular dynamics simulations about adsorption and displacement of methane in carbon nanochannels. J. Phys. Chem. C 2015, 119, 13652–13657. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Y.P. Atomic mechanisms and equation of state of methane adsorption in carbon nanopores. J. Phys. Chem. C 2014, 118, 17737–17744. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, Y.; Dong, J.; Song, H.; Chen, Z.; Zhao, B. Theoretical analysis and numerical simulation of methane adsorption behavior on rough surfaces featuring fractal property. Fuel 2024, 362, 130884. [Google Scholar] [CrossRef]
- Lin, K.; Yuan, Q.; Zhao, Y.P. Using graphene to simplify the adsorption of methane on shale in MD simulations. Comput. Mater. Sci. 2017, 133, 99–107. [Google Scholar] [CrossRef]
- Tan, Z.; Gubbins, K.E. Adsorption in carbon micropores at supercritical temperatures. J. Phys. Chem. 1990, 94, 6061–6069. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in ‘t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS-a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Sun, S. Review of deep learning algorithms in molecular simulations and perspective applications on petroleum engineering. Geosci. Front. 2024, 15, 101735. [Google Scholar] [CrossRef]
- Delavar, M.; Ghoreyshi, A.A.; Jahanshahi, M.; Khalili, S.; Nabian, N. Equilibria and kinetics of natural gas adsorption on multi-walled carbon nanotube material. RSC Adv. 2012, 2, 4490–4497. [Google Scholar] [CrossRef]
- Rhoderick, G.C.; Carney, J.; Guenther, F.R. NIST Gravimetrically prepared atmospheric level methane in dry air standards suite. Anal. Chem. 2012, 84, 3802–3810. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).