Assessing the Impact of Psyllid Pesticide Resistance on the Spread of Citrus Huanglongbing and Its Ecological Paradox
Abstract
:1. Introduction
2. Materials and Methods
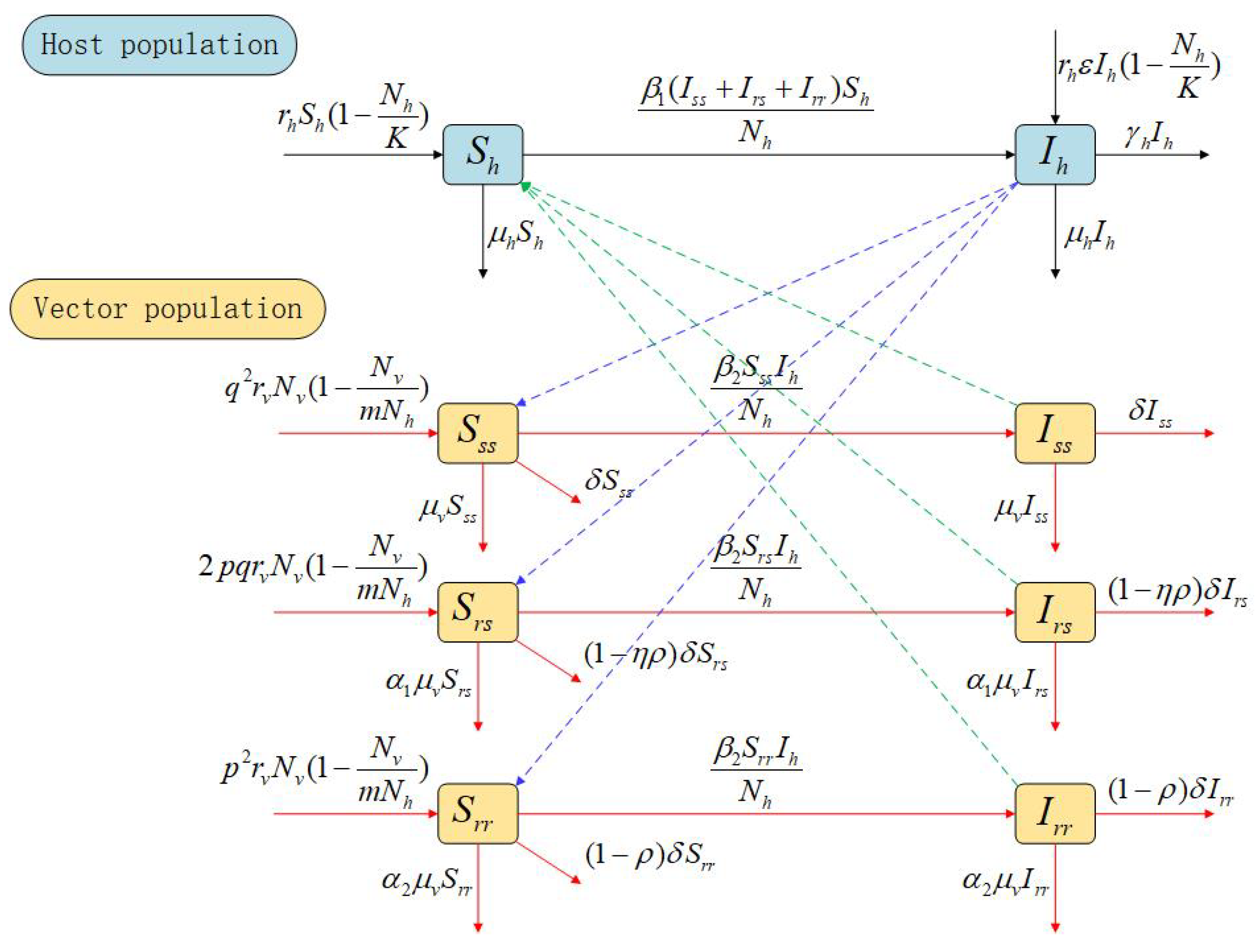
- We assume that the replanting rate is determined by the abundance or density of plants, using and to describe the logistic growth of healthy and infected plants, respectively. In these equations, r represents the intrinsic growth rate of healthy plants, () denotes the proportional reduction in the replacement rate for infected plants, and K is the maximum capacity for planting citrus trees in the orchard;
- The forces of infection for HLB transmission, namely the infection rates, are defined as follows: represents the vector-to-host transmission rate, while represents the host-to-vector transmission rate, where is the transmission probability from infectious ACP to susceptible citrus trees, and is the transmission probability from infectious citrus trees to susceptible ACP. Additionally, the natural mortality rate of citrus trees is given by , and the roguing rate of infected citrus trees is represented by ;
- It is assumed that mating among adult ACPs (between individuals of opposite sexes) occurs randomly [16]. In other words, all adult ACPs have an equal chance of reproducing and mate with any other adult ACP of the opposite sex with the same probability. The presence or absence of insecticide resistance is determined by a pair of alleles in the ACP population, which in turn affects the parameters for ACP mortality and growth rates. The insecticide-sensitive allele is denoted by S, and the insecticide-resistant allele by R. Thus, following the idea outlined in [16,19], we take the frequency of each allele using the following formulas:where and represent the frequencies of S and R alleles at time t, respectively. Therefore, the probability of forming an genotype is given . Similarly, the probability of forming an -genotype is (which accounts for for plus for ), while the probability of forming an -genotype is . Consequently, the proportions of the , , and genotypes in the next generation can be calculated as , , and , respectively, [16]. It is important to note that , and for all time (which reflects the Hardy–Weinberg principle in population genetics [16,19]). The following Verhulst–Pearl logistic birth functions for the , , and genotypes (denoted as , , and , respectively) are adopted:where is the oviposition rate of adult ACP, and m is the average carrying capacity of ACPs per citrus tree;
- Note that ACP insecticide resistance is inherited (vertically) [16,20]; that is, an insecticide-resistant adult ACP produces insecticide-resistant offspring. Susceptible ACPs of all genotypes (, , and ) acquire HLB infection at a rate of . ACPs of the -genotype ( and ) experience natural mortality at a rate of , as well as additional mortality due to insecticide use at a rate of ;
- It is further assumed that there is a mortality fitness cost associated with both homozygous resistant and heterozygous ACPs [21,22,23]. Specifically, ACPs of the genotype experience natural mortality at a rate of (with reflecting the increased mortality rate of genotype ACPs due to the fitness cost, compared to the natural mortality rate of genotype ACPs). ACPs of the genotype suffer natural mortality at a rate of (where represents the increased mortality rate of genotype ACPs due to the fitness cost, compared to genotype ACPs). Note that if the resistant allele (R) is dominant, and if the R allele is recessive;
- The population of ACPs with the genotype decreases due to insecticide use at a rate of . Similarly, the population of genotype decreases at a rate of , where is a modification parameter that accounts for the assumed decrease in mortality rate of genotype vectors due to insecticides, compared to genotype vectors (due to the mortality fitness cost). Additionally, genotype vectors experience mortality from insecticide use at a rate of , where is a modification parameter that reflects the degree of dominance of the resistant allele (i.e., models the case where the resistant allele is dominant, while represents the scenario when it is recessive). The parameter of the resistant allele measures the relative impact of the heterozygote compared to the two corresponding homozygote genotypes, and (see [18]).
3. Model Analysis
3.1. Basic Properties
3.2. Existence of Disease-Free Equilibria
- (i)
- and ;
- (ii)
- and ;
- (iii)
- and , provided that ;
- (iv)
- any value in with , provided that
- (1)
- The NTSDFE () exists if and only if ;
- (2)
- The NTRDFE () exists if and only if ;
- (3)
- The NTCDFE () exists if and only if and , or if and only if and
- (i)
- If , then , and ;
- (ii)
- If , then , and ;
- (iii)
- If , then , and ;
- (iv)
- If , then , and .
3.3. Local Asymptotic Stability of Equilibria
- (a)
- When or and , the NTCDFE is locally asymptotically stable if and unstable if .
- (b)
- When , the NTSDFE is locally asymptotically stable if and unstable if ;
- (c)
- When , the NTRDFE is locally asymptotically stable if and unstable if ;
- (d)
- The TDFE is always locally asymptotically stable.
3.4. Global Asymptotic Stability of Equilibria
- (a)
- If or , then the set is positively invariant provided that and .
- (b)
- If and , then the set is positively invariant.
- (c)
- If and , then the set is positively invariant.
- (a)
- When or , the NTCDFE () is globally asymptotically stable in if , and .
- (b)
- If , and , then the NTSDFE () is globally asymptotically stable in .
- (c)
- If , and , then the NTRDFE () is globally asymptotically stable in .
4. Global Sensitivity Analysis of the Reproduction Threshold
5. Numerical Simulation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Batool, A.; Iftikhar, Y.; Mughal, S.; Khan, M.; Jaskani, M.; Abbas, M.; Khan, I. Citrus greening disease-a major cause of citrus decline in the world—A review. Hort. Sci. 2007, 34, 159–166. [Google Scholar] [CrossRef]
- Inoue, H.; Ohnishi, J.; Ito, T.; Tomimura, K.; Miyata, S.; Iwanami, T.; Ashihara, W. Enhanced proliferation and efficient transmission of Candidatus Liberibacter asiaticus by adult Diaphorina citri after acquisition feeding in the nymphal stage. Ann. Appl. Biol. 2009, 155, 29–36. [Google Scholar] [CrossRef]
- Marutani-Hert, M.; Hunter, W.B.; Hall, D.G. Establishment of Asian citrus psyllid (Diaphorina citri) primary cultures. Vitr. Cell. Dev. Biol.-Anim. 2009, 45, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C. The status of citrus Huanglongbing in China. Trop. Plant Pathol. 2020, 45, 279–284. [Google Scholar] [CrossRef]
- Tian, F.; Mo, X.; Rizvi, S.A.H.; Li, C.; Zeng, X. Detection and biochemical characterization of insecticide resistance in field populations of Asian citrus psyllid in Guangdong of China. Sci. Rep. 2018, 8, 12587. [Google Scholar] [CrossRef]
- Tiwari, S.; Mann, R.S.; Rogers, M.E.; Stelinski, L.L. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manag. Sci. 2011, 67, 1258–1268. [Google Scholar] [CrossRef]
- Feyereisen, R. Molecular biology of insecticide resistance. Toxicol. Lett. 1995, 82, 83–90. [Google Scholar] [CrossRef]
- Tiwari, S.; Gondhalekar, A.; Mann, R.; Scharf, M.; Stelinski, L. Characterization of five CYP4 genes from Asian citrus psyllid and their expression levels in Candidatus Liberibacter asiaticus-infected and uninfected psyllids. Insect Mol. Biol. 2011, 20, 733–744. [Google Scholar] [CrossRef]
- Tiwari, S.; Pelz-Stelinski, K.; Mann, R.S.; Stelinski, L.L. Glutathione transferase and cytochrome P450 (general oxidase) activity levels in Candidatus Liberibacter asiaticus-infected and uninfected Asian citrus psyllid (Hemiptera: Psyllidae). Ann. Entomol. Soc. Am. 2011, 104, 297–305. [Google Scholar] [CrossRef]
- Yu, X.; Killiny, N. RNA interference of two glutathione S-transferase genes, Diaphorina citri DcGSTe2 and DcGSTd1, increases the susceptibility of Asian citrus psyllid (Hemiptera: Liviidae) to the pesticides fenpropathrin and thiamethoxam. Pest Manag. Sci. 2018, 74, 638–647. [Google Scholar] [CrossRef]
- Liang, J.; Tang, S.; Nieto, J.J.; Cheke, R.A. Analytical methods for detecting pesticide switches with evolution of pesticide resistance. Math. Biosci. 2013, 245, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Gao, S.; Zhang, F.; Liu, Y. Role of vector resistance and grafting infection in Huanglongbing control models. Infect. Dis. Model. 2023, 8, 491–513. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, F.; Liu, Y.; Gao, S. Analysis and optimal control of a Huanglongbing mathematical model with resistant vector. Infect. Dis. Model. 2021, 6, 782–804. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, A.J.; Wessler, S.R.; Lewontin, R.C.; Carroll, S.B. Introduction to Genetic Analysis (Loose-Leaf); Macmillan: New York, NY, USA, 2008. [Google Scholar]
- Freeman, S.; Herron, J.C. Evolutionary Analysis; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- Kuniyoshi, M.L.G.; Santos, F.L.P.d. Mathematical modelling of vector-borne diseases and insecticide resistance evolution. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 34. [Google Scholar] [CrossRef]
- Mohammed-Awel, J.; Gumel, A.B. Mathematics of an epidemiology-genetics model for assessing the role of insecticides resistance on malaria transmission dynamics. Math. Biosci. 2019, 312, 33–49. [Google Scholar] [CrossRef]
- Bourguet, D.; Genissel, A.; Raymond, M. Insecticide resistance and dominance levels. J. Econ. Entomol. 2000, 93, 1588–1595. [Google Scholar] [CrossRef]
- Hastings, A. Population Biology: Concepts and Models; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Luz, P.; Codeco, C.; Medlock, J.; Struchiner, C.; Valle, D.; Galvani, A. Impact of insecticide interventions on the abundance and resistance profile of Aedes aegypti. Epidemiol. Infect. 2009, 137, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Alout, H.; Dabiré, R.K.; Djogbénou, L.S.; Abate, L.; Corbel, V.; Chandre, F.; Cohuet, A. Interactive cost of Plasmodium infection and insecticide resistance in the malaria vector Anopheles gambiae. Sci. Rep. 2016, 6, 29755. [Google Scholar] [CrossRef]
- Anderson, R.A.; Knols, B.; Koella, J. Plasmodium falciparum sporozoites increase feeding-associated mortality of their mosquito hosts Anopheles gambiae sl. Parasitology 2000, 120, 329–333. [Google Scholar] [CrossRef]
- Barbosa, S.; Hastings, I.M. The importance of modelling the spread of insecticide resistance in a heterogeneous environment: The example of adding synergists to bed nets. Malar. J. 2012, 11, 258. [Google Scholar] [CrossRef]
- Van den Driessche, P.; Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002, 180, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Blower, S.M.; Dowlatabadi, H. Sensitivity and uncertainty analysis of complex models of disease transmission: An HIV model, as an example. Int. Stat. Rev./Rev. Int. Stat. 1994, 62, 229–243. [Google Scholar] [CrossRef]
- Agusto, F.; Khan, M. Optimal control strategies for dengue transmission in Pakistan. Math. Biosci. 2018, 305, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qiu, Z.; Zhong, B.; Feng, T.; Huang, A. Modeling Citrus Huanglongbing transmission within an orchard and its optimal control. Math. Biosci. Eng. 2020, 17, 2048–2069. [Google Scholar] [CrossRef]
- Taylor, R.A.; Mordecai, E.A.; Gilligan, C.A.; Rohr, J.R.; Johnson, L.R. Mathematical models are a powerful method to understand and control the spread of Huanglongbing. PeerJ 2016, 4, 2642–2660. [Google Scholar] [CrossRef]







| Variable | Description |
| Population of susceptible citrus trees | |
| Population of infected citrus trees | |
| Population of susceptible sensitive ACP | |
| Population of infected sensitive ACP | |
| Population of susceptible resistant ACP | |
| Population of infected sensitive ACP | |
| Population of susceptible sensitive ACP | |
| Population of infected resistant ACP | |
| Parameter | Description |
| K | Environmental carrying capacity of citrus trees |
| Recruitment rate of citrus trees | |
| Transmission probability from infected ACP to susceptible citrus trees | |
| Natural mortality rate in citrus trees | |
| Probability that a diseased citrus trees sapling is not removed | |
| The roguing rate of infected citrus trees | |
| Recruitment rate of ACP | |
| m | Average carrying capacity of ACP per citrus tree |
| Transmission probability from infected citrus trees to susceptible ACP | |
| Natural mortality rate of ACP | |
| Death rate due to the (encounter with) insecticides for genotype ACP | |
| Modification parameter in natural mortality rate of genotype ACP due to fitness cost in comparison to the natural mortality rate of genotype ACP | |
| Modification parameter in natural mortality rate of genotype ACP due to fitness cost in comparison to the natural mortality rate of genotype ACP | |
| Modification parameter in death rate of genotype ACP due to the insecticides in comparison to the death rate of genotype ACP | |
| Modification parameter in death rate of genotype ACP due to the insecticides in comparison to the death rate of genotype ACP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, R.; Luo, Y.; Gao, S. Assessing the Impact of Psyllid Pesticide Resistance on the Spread of Citrus Huanglongbing and Its Ecological Paradox. Computation 2024, 12, 242. https://doi.org/10.3390/computation12120242
Gan R, Luo Y, Gao S. Assessing the Impact of Psyllid Pesticide Resistance on the Spread of Citrus Huanglongbing and Its Ecological Paradox. Computation. 2024; 12(12):242. https://doi.org/10.3390/computation12120242
Chicago/Turabian StyleGan, Runyun, Youquan Luo, and Shujing Gao. 2024. "Assessing the Impact of Psyllid Pesticide Resistance on the Spread of Citrus Huanglongbing and Its Ecological Paradox" Computation 12, no. 12: 242. https://doi.org/10.3390/computation12120242
APA StyleGan, R., Luo, Y., & Gao, S. (2024). Assessing the Impact of Psyllid Pesticide Resistance on the Spread of Citrus Huanglongbing and Its Ecological Paradox. Computation, 12(12), 242. https://doi.org/10.3390/computation12120242





