Design of Trabecular Bone Mimicking Voronoi Lattice-Based Scaffolds and CFD Modelling of Non-Newtonian Power Law Blood Flow Behaviour
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of Voronoi Lattice-Based Scaffolds
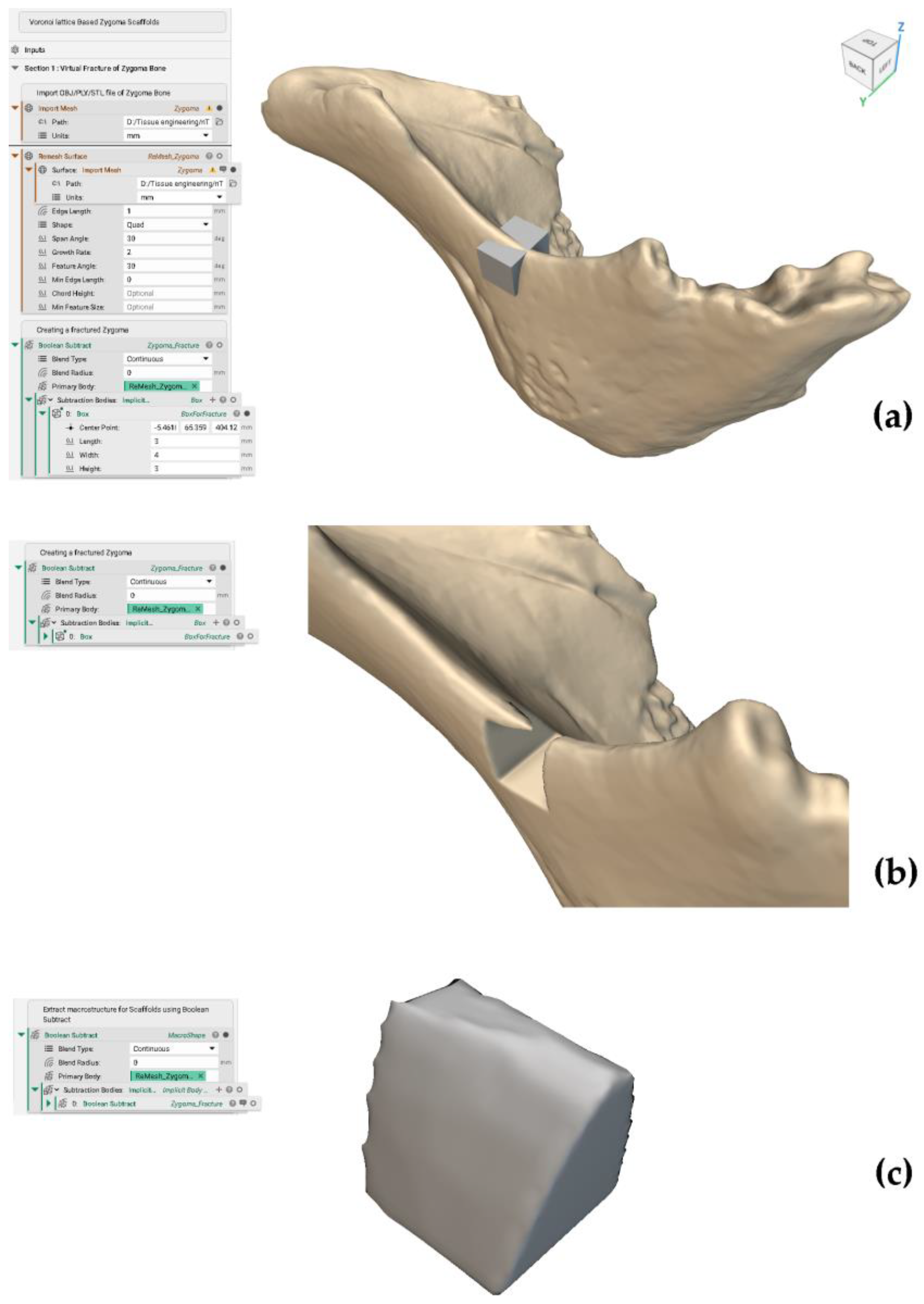
2.1.1. Extraction of Shape (Macrostructure) for Scaffolds
2.1.2. Design of Voronoi Lattice (Microstructure) for Scaffolds
- (i.)
- Creation of random seed points inside the implicit body of scaffold shape using point spacing.
- (ii.)
- Creation of a boundary volume from the shape of the scaffold
- (iii.)
- Creation of Voronoi lattice (microstructure) by adding strut thickness
2.1.3. Conversion of the Implicit Body (Voronoi Lattice) to CAD Body
2.2. CFD Modelling of Scaffolds
2.2.1. Geometry Preparation and Fluid Domain Generation for CFD Simulation
2.2.2. Setting Known Values for CFD Simulation
- (i.)
- Material Properties
- (ii.)
- Boundary Conditions
- (iii.)
- Volume Mesh Generation
2.2.3. Setting FEM-Based Solver for CFD Simulation
2.2.4. Visualisation of Results and Analysis
3. Results and Discussion
3.1. Design of Voronoi Lattice-Based Scaffolds
3.1.1. Gradient Pore Size Distribution
3.1.2. Consequences of Surface Area and Surface Area to Volume Ratio
3.2. CFD Simulation
3.2.1. Reliability of CFD Models
3.2.2. Velocity and Pressure Distributions Within Voronoi Scaffolds
3.2.3. Permeability Evaluation
3.3. Validation of Non-Newtonian Power Law Models with Newtonian Models
3.4. Limitations and Future Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A. Virtual Fracture of the Zygoma Bone
Appendix B. Creating a Voronoi Lattice (V70 Scaffold)


Appendix C. Calculation of Porosity, Volume Fraction and Surface Area to Volume Ratio

References
- Torres, A.M.; Trikanad, A.A.; Aubin, C.A.; Lambers, F.M.; Luna, M.; Rimnac, C.M.; Zavattieri, P.; Hernandez, C.J. Bone-inspired microarchitectures achieve enhanced fatigue life. Proc. Natl. Acad. Sci. USA 2019, 116, 24457–24462. [Google Scholar] [CrossRef] [PubMed]
- Chinnasami, H.; Dey, M.K.; Devireddy, R. Three-Dimensional Scaffolds for Bone Tissue Engineering. Bioengineering 2023, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Han, Q.; Chen, H.; Li, Y.; Guo, X.; Zhang, A.; Liu, Y.; Sun, Y.; Wang, J. Additive manufactured trabecular-like Ti-6Al-4V scaffolds for promoting bone regeneration. J. Mater. Sci. Technol. 2024, 188, 116–130. [Google Scholar] [CrossRef]
- Sun, L.; Niu, H.; Wu, Y.; Dong, S.; Li, X.; Kim, B.Y.; Liu, C.; Ma, Y.; Jiang, W.; Yuan, Y. Bio-integrated scaffold facilitates large bone regeneration dominated by endochondral ossification. Bioact. Mater. 2024, 35, 208–227. [Google Scholar] [CrossRef] [PubMed]
- Laubach, M.; Herath, B.; Bock, N.; Suresh, S.; Saifzadeh, S.; Dargaville, B.L.; McGovern, J.; Wille, M.-L.; Hutmacher, D.W.; Savi, F.M. In vivo characterization of 3D-printed polycaprolactone-hydroxyapatite scaffolds with Voronoi design to advance the concept of scaffold-guided bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1272348. [Google Scholar] [CrossRef]
- Laubach, M.; Suresh, S.; Herath, B.; Wille, M.-L.; Delbrück, H.; Alabdulrahman, H.; Hutmacher, D.W.; Hildebrand, F. Clinical translation of a patient-specific scaffold-guided bone regeneration concept in four cases with large long bone defects. J. Orthop. Transl. 2022, 34, 73–84. [Google Scholar] [CrossRef]
- Yousof, K.; Darwich, M.A.; Darwich, K.; Alassah, G.; Imran, A.; Nazha, H.M. A Case Report of Zygomatic Fracture Reconstruction: Evaluation with Orbital Measurements and Models Registration. Appl. Sci. 2023, 13, 6154. [Google Scholar] [CrossRef]
- Liu, G.; Wei, X.; Zhai, Y.; Zhang, J.; Li, J.; Zhao, Z.; Guan, T.; Zhao, D. 3D printed osteochondral scaffolds: Design strategies, present applications and future perspectives. Front. Bioeng. Biotechnol. 2024, 12, 1339916. [Google Scholar] [CrossRef]
- Hou, G.; Liu, B.; Tian, Y.; Liu, Z.; Zhou, F. Reconstruction of Ipsilateral Femoral and Tibial Bone Defect by 3D Printed Porous Scaffold Without Bone GraftL: A Case Report. J. Bone Jt. Surg. 2022, 12, e20.00592. [Google Scholar] [CrossRef]
- Parisien, A.; ElSayed, M.S.; Frei, H. Mature bone mechanoregulation modelling for the characterization of the osseointegration performance of periodic cellular solids. Materialia 2022, 25, 101552. [Google Scholar] [CrossRef]
- Alshammari, A.; Alabdah, F.; Wang, W.; Cooper, G. Virtual Design of 3D-Printed Bone Tissue Engineered Scaffold Shape Using Mechanobiological Modeling: Relationship of Scaffold Pore Architecture to Bone Tissue Formation. Polymers 2023, 15, 3918. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Ockermann, P.N.; Schwarz, T.; Mustafa, K.; Hansmann, J. Translation of biophysical environment in bone into dynamic cell culture under flow for bone tissue engineering. Comput. Struct. Biotechnol. J. 2023, 21, 4395–4407. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K. Bone Substitute Fabrication Based on Dissolution-Precipitation Reactions. Materials 2010, 3, 1138–1155. [Google Scholar] [CrossRef]
- Edelmers, E.; Kazoka, D.; Bolocko, K.; Pilmane, M. Different Techniques of Creating Bone Digital 3D Models from Natural Specimens. Appl. Syst. Innov. 2022, 5, 85. [Google Scholar] [CrossRef]
- Yoshino, M.; Kato, Y.; Kizu, Y.; Tonogi, M.; Abe, S.; Ide, Y.; Yamane, G.-Y. Study on internal structure of zygomatic bone using micro-finite element analysis model—Differences between dentulous and edentulous dentition in Japanese cadavers. Bull. Tokyo Dent. Coll. 2007, 48, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kizuo, Y.; Tonogi, M.; Ide, Y.; Yamane, G.-Y. Observation of the Internal Structure of the Zygomatic Bone by Micro-Computed Tomography. J. Oral Biosci. 2004, 46, 523–529. [Google Scholar] [CrossRef]
- Gupta, K.; Meena, K. Artificial bone scaffolds and bone joints by additive manufacturing: A review. Bioprinting 2023, 31, e00268. [Google Scholar] [CrossRef]
- Kouhi, M.; Araújo, I.J.d.S.; Asa’ad, F.; Zeenat, L.; Bojedla, S.S.R.; Pati, F.; Zolfagharian, A.; Watts, D.C.; Bottino, M.C.; Bodaghi, M. Recent advances in additive manufacturing of patient-specific devices for dental and maxillofacial rehabilitation. Dent. Mater. 2024, 40, 700–715. [Google Scholar] [CrossRef]
- Huang, X.; Lou, Y.; Duan, Y.; Liu, H.; Tian, J.; Shen, Y.; Wei, X. Biomaterial scaffolds in maxillofacial bone tissue engineering: A review of recent advances. Bioact. Mater. 2023, 33, 129–156. [Google Scholar] [CrossRef]
- Huo, Y.; Lu, Y.; Meng, L.; Wu, J.; Gong, T.; Zou, J.; Bosiakov, S.; Cheng, L. A Critical Review on the Design, Manufacturing and Assessment of the Bone Scaffold for Large Bone Defects. Front. Bioeng. Biotechnol. 2021, 9, 753715. [Google Scholar] [CrossRef]
- Boucetta, A.; Ramtani, S.; Garzón-Alvarado, D.A.; Spadavecchia, J. Computational study promoting engineering biomaterial pre-design to well adapt pores distribution on bone/scaffold assembly section. Comput. Methods Appl. Mech. Eng. 2024, 425, 116934. [Google Scholar] [CrossRef]
- Rojas-Rojas, L.; Tozzi, G.; Guillén-Girón, T. A Comprehensive Mechanical Characterization of Subject-Specific 3D Printed Scaffolds Mimicking Trabecular Bone Architecture Biomechanics. Life 2023, 13, 2141. [Google Scholar] [CrossRef] [PubMed]
- Garot, C.; Schoffit, S.; Monfoulet, C.; Machillot, P.; Deroy, C.; Roques, S.; Vial, J.; Vollaire, J.; Renard, M.; Ghanem, H.; et al. 3D-Printed Osteoinductive Polymeric Scaffolds with Optimized Architecture to Repair a Sheep Metatarsal Critical-Size Bone Defect. Adv. Health Mater. 2023, 12, e2301692. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.G.; Callahan, L.A.S.; Ma, P.X. Biodegradable Honeycomb-Mimic Scaffolds Consisting of Nanofibrous Walls. Macromol. Biosci. 2024, 24, e2300540. [Google Scholar] [CrossRef]
- Li, B.; Hesar, B.D.; Zhao, Y.; Ding, L. Design and additive manufacturing of porous titanium scaffolds for optimum cell viability in bone tissue engineering. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2020, 237, 2015–2024. [Google Scholar] [CrossRef]
- Liu, L.; Ma, S.; Zhang, Y.; Zhu, S.; Wu, S.; Liu, G.; Yang, G. Parametric Design of Porous Structure and Optimal Porosity Gradient Distribution Based on Root-Shaped Implants. Materials 2024, 17, 1137. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Chen, X.; Zhao, R. Design and evaluation of TPMS-inspired 3D-printed scaffolds for bone tissue engineering: Enabling tailored mechanical and mass transport properties. Compos. Struct. 2023, 327, 117638. [Google Scholar] [CrossRef]
- Yánez, A.; Cuadrado, A.; Martel, O.; Fiorucci, M.P.; Deviaene, S. Mechanical and permeability properties of skeletal and sheet triply periodic minimal surface scaffolds in bone defect reconstruction. Results Eng. 2024, 21, 101883. [Google Scholar] [CrossRef]
- Musthafa, H.-S.N.; Walker, J.; Rahman, T.; Bjørkum, A.; Mustafa, K.; Velauthapillai, D. In-Silico Prediction of Mechanical Behaviour of Uniform Gyroid Scaffolds Affected by Its Design Parameters for Bone Tissue Engineering Applications. Computation 2023, 11, 181. [Google Scholar] [CrossRef]
- Musthafa, H.-S.N.; Walker, J.; Domagala, M. Computational Modelling and Simulation of Scaffolds for Bone Tissue Engineering. Computation 2024, 12, 74. [Google Scholar] [CrossRef]
- Pokojski, W.; Pokojska, P. Voronoi diagrams—Inventor, method, applications. Pol. Cartogr. Rev. 2018, 50, 141–150. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, B.; Song, B.; Yao, Y.; Choi, S.-K.; Yang, C.; Shi, Y. 3D Printed Biomimetic Metamaterials with Graded Porosity and Tapering Topology for Improved Cell Seeding and Bone Regeneration. Bioact. Mater. 2022, 25, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, F.D.; García-Acosta, G.; Garzón-Alvarado, D.A.; Márquez-Flórez, K.; Quexada-Rodriguez, D.A.; Velasco, M.A. Design for the additive manufacturing of structural elements with cellular materials using Voronoi diagrams and Delaunay triangulations: Biological and structural applications. Mech. Adv. Mater. Struct. 2023, 1–21. [Google Scholar] [CrossRef]
- Gatto, M.L.; Cerqueni, G.; Furlani, M.; Riberti, N.; Tognoli, E.; Denti, L.; Leonardi, F.; Giuliani, A.; Mattioli-Belmonte, M.; Mengucci, P. Influence of Trabecular Geometry on Scaffold Mechanical Behavior and MG-63 Cell Viability. Materials 2023, 16, 2342. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chao, L.; Jiao, C.; Wang, H.; Xie, D.; Wu, G.; Wang, L.; Wang, C.; Zhao, J.; Shen, L.; et al. Sub-regional design of the bionic bone scaffolds using macrostructural topology. Int. J. Bioprinting 1970, 9, 222. [Google Scholar] [CrossRef]
- Nguyen, D.S. A Method for Generation of Random Lattice Structure for Additive Manufacturing. In Proceedings of the 2019 IEEE International Conference on Industrial Engineering and Engineering Management (IEEM), Macao, China, 15–18 December 2019; pp. 1275–1279. [Google Scholar]
- Rezapourian, M.; Hussainova, I. Optimal mechanical properties of Hydroxyapatite gradient Voronoi porous scaffolds for bone applications—A numerical study. J. Mech. Behav. Biomed. Mater. 2023, 148, 106232. [Google Scholar] [CrossRef]
- Liu, B.; Feng, J.; Chen, J.; He, Y.; Fu, J. A topology optimisation-based design method for 3D Voronoi porous structures and its application for medical pillows. Virtual Phys. Prototyp. 2023, 18, e2285392. [Google Scholar] [CrossRef]
- Xiao, F.; Yin, X. Geometry models of porous media based on Voronoi tessellations and their porosity–permeability relations. Comput. Math. Appl. 2016, 72, 328–348. [Google Scholar] [CrossRef]
- Voronoi Diagram Generator. Available online: https://cfbrasz.github.io/Voronoi.html (accessed on 29 October 2024).
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Peng, X.; Huo, Y.; Zhang, G.; Cheng, L.; Lu, Y.; Li, J.; Jin, Z. Controlled mechanical and mass-transport properties of porous scaffolds through hollow strut. Int. J. Mech. Sci. 2023, 248, 108202. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Sun, Z.; Peng, K.; Liu, K.; Xu, P.; Li, J.; Wei, X.; He, X. Integrated evaluation of biomechanical and biological properties of the biomimetic structural bone scaffold: Biomechanics, simulation analysis, and osteogenesis. Mater. Today Bio 2023, 24, 100934. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, S.; Ding, W.; Du, H.; Li, M.; Li, Z.; Chen, M. Additively-manufactured gradient porous bio-scaffolds: Permeability, cytocompatibility and mechanical properties. Compos. Struct. 2024, 336, 118021. [Google Scholar] [CrossRef]
- Jiao, J.; Hong, Q.; Zhang, D.; Wang, M.; Tang, H.; Yang, J.; Qu, X.; Yue, B. Influence of porosity on osteogenesis, bone growth and osteointegration in trabecular tantalum scaffolds fabricated by additive manufacturing. Front. Bioeng. Biotechnol. 2023, 11, 1117954. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.; Dunlop, J.W.C.; Fernandes, P.R.; Castro, A.P.G. Challenges in computational fluid dynamics applications for bone tissue engineering. Proc. R. Soc. A Math. Phys. Eng. Sci. 2022, 478, 20210607. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, L.; Wang, C.; Yin, H.; Raffaella, A.; Apicella, A.; Ji, P.; Zhang, H.; Fan, Y. Additively manufactured bioceramic scaffolds with 3D architecture for vertical bone augmentation: A proof-of-concept study. Mater. Des. 2024, 239, 112749. [Google Scholar] [CrossRef]
- Ji, G.; Zhang, M.; Lu, Y.; Dong, J. The Basic Theory of CFD Governing Equations and the Numerical Solution Methods for Reactive Flows. In Computational Fluid Dynamics; Chapter 1; Ji, G., Dong, J., Eds.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Lintermann, A. Computational Meshing for CFD Simulations. In Clinical and Biomedical Engineering in the Human Nose; Springer: Singapore, 2021; pp. 85–115. [Google Scholar] [CrossRef]
- Rice, J.; Schnipke, R. A monotone streamline upwind finite element method for convection-dominated flows. Comput. Methods Appl. Mech. Eng. 1985, 48, 313–327. [Google Scholar] [CrossRef]
- Khouya, B.; El-Amrani, M.; Seaid, M. A Conservative and Monotone Characteristic Finite Element Solver for Three-Dimensional Transport and Incompressible Navier-Stokes Equations on Unstructured Grids. Commun. Comput. Phys. 2022, 31, 224–256. [Google Scholar] [CrossRef]
- Cengizci, S.; Uğur, Ö.; Natesan, S. A stabilized finite element formulation with shock-capturing for solving advection-dominated convection–diffusion equations having time-fractional derivatives. J. Comput. Sci. 2024, 76, 102214. [Google Scholar] [CrossRef]
- Jia, D.; Esmaily, M. A time-consistent stabilized finite element method for fluids with applications to hemodynamics. Sci. Rep. 2023, 13, 19120. [Google Scholar] [CrossRef]
- Heydari, S.; Knobloch, P.; Wick, T. Flux-corrected transport stabilization of an evolutionary cross-diffusion cancer invasion model. J. Comput. Phys. 2024, 499, 112711. [Google Scholar] [CrossRef]
- Kuzmin, D. On the design of general-purpose flux limiters for finite element schemes. I. Scalar convection. J. Comput. Phys. 2006, 219, 513–531. [Google Scholar] [CrossRef]
- Rachowicz, W.; Zdunek, A.; Cecot, W. A discontinuous Petrov-Galerkin method for compressible Navier-Stokes equations in three dimensions. Comput. Math. Appl. 2021, 102, 113–136. [Google Scholar] [CrossRef]
- Help|Discretization Method|Autodesk. Available online: https://help.autodesk.com/view/SCDSE/2024/ENU/?guid=GUID-DEE0664D-771B-4446-9ED4-1498267D13FB (accessed on 9 July 2024).
- Gensler, M.; Malkmus, C.; Ockermann, P.; Möllmann, M.; Hahn, L.; Salehi, S.; Luxenhofer, R.; Boccaccini, A.R.; Hansmann, J. Perfusable Tissue Bioprinted into a 3D-Printed Tailored Bioreactor System. Bioengineering 2024, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Krasnyakov, I.; Bratsun, D. Cell-Based Modeling of Tissue Developing in the Scaffold Pores of Varying Cross-Sections. Biomimetics 2023, 8, 562. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Z.; Liu, L.; Huang, Z.; Li, Z.; Liu, J.; Hao, Y. Honeycomb structure is promising for the repair of human bone defects. Mater. Des. 2021, 207, 109832. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, L.; Li, P.; Lin, Z.; Huang, S.; Zhou, C. Fabrication of 3D printed trabecular bone-templated scaffolds modified with rare earth europium (III)-based complex for enhancing mitochondrial function in bone regeneration. Appl. Mater. Today 2024, 37, 102130. [Google Scholar] [CrossRef]
- Hussein, N.A.K.; Noordin, M.A.; Saad, A.P.M. Influence of conical graded porous architecture on the mechanical, failure behavior and fluid-flow properties for bone scaffold application. Eng. Fail. Anal. 2024, 157, 107893. [Google Scholar] [CrossRef]
- Omar, A.M.; Hassan, M.H.; Daskalakis, E.; Ates, G.; Bright, C.J.; Xu, Z.; Powell, E.J.; Mirihanage, W.; Bartolo, P.J.D.S. Geometry-Based Computational Fluid Dynamic Model for Predicting the Biological Behavior of Bone Tissue Engineering Scaffolds. J. Funct. Biomater. 2022, 13, 104. [Google Scholar] [CrossRef]
- Seehanam, S.; Khrueaduangkham, S.; Sinthuvanich, C.; Sae-Ueng, U.; Srimaneepong, V.; Promoppatum, P. Evaluating the effect of pore size for 3d-printed bone scaffolds. Heliyon 2024, 10, e26005. [Google Scholar] [CrossRef]
- Seehanam, S.; Chanchareon, W.; Promoppatum, P. Assessing the effect of manufacturing defects and non-Newtonian blood model on flow behaviors of additively manufactured Gyroid TPMS structures. Heliyon 2023, 9, e15711. [Google Scholar] [CrossRef]
- Wajihah, S.A.; Sankar, D.S. A review on non-Newtonian fluid models for multi-layered blood rheology in constricted arteries. Arch. Appl. Mech. 2023, 93, 1771–1796. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Kar, S.; Puniyani, R.R. Relationship between power law coefficients and major blood constituents affecting the whole blood viscosity. J. Biosci. 1999, 24, 329–337. [Google Scholar] [CrossRef]
- Marenzana, M.; Arnett, T.R. The Key Role of the Blood Supply to Bone. Bone Res. 2013, 1, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Sen, S. Permeability and fluid flow-induced wall shear stress of bone tissue scaffolds: Computational fluid dynamic analysis using Newtonian and non-Newtonian blood flow models. Comput. Biol. Med. 2018, 99, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Mahammod, B.P.; Barua, E.; Deoghare, A.B.; Pandey, K. Permeability quantification of porous polymer scaffold for bone tissue engineering. Mater. Today Proc. 2020, 22, 1687–1693. [Google Scholar] [CrossRef]
- Singh, S.; Yadav, S.K.; Meena, V.K.; Vashisth, P.; Kalyanasundaram, D. Orthopedic Scaffolds: Evaluation of Structural Strength and Permeability of Fluid Flow via an Open Cell Neovius Structure for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2023, 9, 5900–5911. [Google Scholar] [CrossRef]
- Li, J.; Guo, D.; Li, J.; Wei, X.; Sun, Z.; Yang, B.; Lu, T.; Ouyang, P.; Chang, S.; Liu, W.; et al. Irregular pore size of degradable bioceramic Voronoi scaffolds prepared by stereolithography: Osteogenesis and computational fluid dynamics analysis. Mater. Des. 2022, 224, 111414. [Google Scholar] [CrossRef]
- Lu, T.; Sun, Z.; Jia, C.; Ren, J.; Li, J.; Ma, Z.; Zhang, J.; Li, J.; Zhang, T.; Zang, Q.; et al. Roles of irregularity of pore morphology in osteogenesis of Voronoi scaffolds: From the perspectives of MSC adhesion and mechano-regulated osteoblast differentiation. J. Biomech. 2023, 151, 111542. [Google Scholar] [CrossRef]
- Katzenbach, A.; Handschuh, S.; Vettermann, S. JT Format (ISO 14306) and AP 242 (ISO 10303): The Step to the Next Gen-eration Collaborative Product Creation. IFIP Adv. Inf. Commun. Technol. 2013, 411, 41–52. [Google Scholar] [CrossRef]
- Bixel, M.G.; Kusumbe, A.P.; Ramasamy, S.K.; Sivaraj, K.K.; Butz, S.; Vestweber, D.; Adams, R.H. Flow Dynamics and HSPC Homing in Bone Marrow Microvessels. Cell Rep. 2017, 18, 1804–1816. [Google Scholar] [CrossRef]
- Ali, D.; Ozalp, M.; Blanquer, S.B.; Onel, S. Permeability and fluid flow-induced wall shear stress in bone scaffolds with TPMS and lattice architectures: A CFD analysis. Eur. J. Mech.-B/Fluids 2019, 79, 376–385. [Google Scholar] [CrossRef]
- Help|Advection Schemes|Autodesk. Available online: https://help.autodesk.com/view/SCDSE/2024/ENU/?guid=GUID-F691B334-CCE2-47E9-B6C4-21666712C163 (accessed on 9 July 2024).
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Watanabe, S.; Takabatake, K.; Tsujigiwa, H.; Watanabe, T.; Tokuyama, E.; Ito, S.; Nagatsuka, H.; Kimata, Y. Efficacy of Honeycomb TCP-induced Microenvironment on Bone Tissue Regeneration in Craniofacial Area. Int. J. Med. Sci. 2016, 13, 466–476. [Google Scholar] [CrossRef]
- Brennan, C.M.; Eichholz, K.F.; A Hoey, D. The effect of pore size within fibrous scaffolds fabricated using melt electrowriting on human bone marrow stem cell osteogenesis. Biomed. Mater. 2019, 14, 065016. [Google Scholar] [CrossRef]
- Ai, C.; Liu, L.; Goh, J.C.-H. Pore size modulates in vitro osteogenesis of bone marrow mesenchymal stem cells in fibronectin/gelatin coated silk fibroin scaffolds. Mater. Sci. Eng. C 2021, 124, 112088. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Chao, L.; Jiao, C.; Liang, H.; Xie, D.; Shen, L.; Liu, Z. Analysis of Mechanical Properties and Permeability of Trabecular-Like Porous Scaffold by Additive Manufacturing. Front. Bioeng. Biotechnol. 2021, 9, 779854. [Google Scholar] [CrossRef]
- Wang, C.; Xu, D.; Lin, L.; Li, S.; Hou, W.; He, Y.; Sheng, L.; Yi, C.; Zhang, X.; Li, H.; et al. Large-pore-size Ti6Al4V scaffolds with different pore structures for vascularized bone regeneration. Mater. Sci. Eng. C 2021, 131, 112499. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, S.; Liu, M.; Yang, H.; Wei, P.; Yi, F.; Ouyang, M.; Xi, H.; Long, Z.; Liu, Y.; et al. Study on the influence of scaffold morphology and structure on osteogenic performance. Front. Bioeng. Biotechnol. 2023, 11, 1127162. [Google Scholar] [CrossRef] [PubMed]
- Koushik, T.M.; Miller, C.M.; Antunes, E. Bone Tissue Engineering Scaffolds: Function of Multi-Material Hierarchically Structured Scaffolds. Adv. Heal. Mater. 2023, 12, e2202766. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Kadri, O.E.; Sikavitsas, V.I.; Voronov, R.S. Scaffolds with a High Surface Area-to-Volume Ratio and Cultured Under Fast Flow Perfusion Result in Optimal O2 Delivery to the Cells in Artificial Bone Tissues. Appl. Sci. 2019, 9, 2381. [Google Scholar] [CrossRef]
- Grimm, M.J.; Williams, J.L. Measurements of permeability in human calcaneal trabecular bone. J. Biomech. 1997, 30, 743–745. [Google Scholar] [CrossRef]
- Nauman, E.A.; Fong, K.E.; Keaveny, T.M. Dependence of Intertrabecular Permeability on Flow Direction and Anatomic Site. Ann. Biomed. Eng. 1999, 27, 517–524. [Google Scholar] [CrossRef]
- Ma, S.; Tang, Q.; Han, X.; Feng, Q.; Song, J.; Setchi, R.; Liu, Y.; Liu, Y.; Goulas, A.; Engstrøm, D.S.; et al. Manufacturability, Mechanical Properties, Mass-Transport Properties and Biocompatibility of Triply Periodic Minimal Surface (TPMS) Porous Scaffolds Fabricated by Selective Laser Melting. Mater. Des. 2020, 195, 109034. [Google Scholar] [CrossRef]
- Santos, J.; Pires, T.; Gouveia, B.P.; Castro, A.P.; Fernandes, P.R. On the permeability of TPMS scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 110, 103932. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous Scaffolds for Bone Regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Kanwar, S.; Al-Ketan, O.; Vijayavenkataraman, S. A novel method to design biomimetic, 3D printable stochastic scaffolds with controlled porosity for bone tissue engineering. Mater. Des. 2022, 220, 110857. [Google Scholar] [CrossRef]
- Amini, A.R.; Adams, D.J.; Laurencin, C.T.; Nukavarapu, S.P. Optimally Porous and Biomechanically Compatible Scaffolds for Large-Area Bone Regeneration. Tissue Eng. Part A 2012, 18, 1376–1388. [Google Scholar] [CrossRef]
- Lynch, S.; Nama, N.; Figueroa, C.A. Effects of non-Newtonian viscosity on arterial and venous flow and transport. Sci. Rep. 2022, 12, 20568. [Google Scholar] [CrossRef] [PubMed]
- Suffo, M.; López-Marín, C.J. A Comparative Study of Turbulence Methods Applied to the Design of a 3D-Printed Scaffold and the Selection of the Appropriate Numerical Scheme to Simulate the Scaffold for Tissue Engineering. Appl. Sci. 2021, 12, 191. [Google Scholar] [CrossRef]





















| Voronoi Scaffolds | Porosity (%) | Strut Thickness ST (mm) | Point Spacing PS (mm) | Voronoi Seed Points | Voronoi Cell Numbers | Surface Area * SA (mm2) | Surface Area/Volume SA: V (mm−1) |
|---|---|---|---|---|---|---|---|
| V90 | 90 | 0.11 | 0.851 | 65 | 11 | 70.5 | 2.29 |
| V85 | 85 | 0.11 | 0.668 | 124 | 35 | 102 | 3.32 |
| V80 | 80 | 0.11 | 0.570 | 206 | 70 | 131 | 4.26 |
| V75 | 75 | 0.11 | 0.502 | 314 | 121 | 160 | 5.19 |
| V70 | 70 | 0.11 | 0.449 | 433 | 180 | 185 | 6.01 |
| Voronoi Scaffolds | Number of Pores | Range of Pore Sizes (µm) |
|---|---|---|
| V90 | 216 | 22.2 to 923 |
| V85 | 455 | 20.2 to 669 |
| V80 | 668 | 20.7 to 618 |
| V75 | 983 | 20.1 to 532 |
| V70 | 1362 | 19.8 to 482 |
| Voronoi Scaffolds | Porosity (%) | Pressure Drop * (Pa) | Permeability (×10−9 m2) |
|---|---|---|---|
| V90 | 90 | 1.52 | 2.17 |
| V85 | 85 | 2.42 | 1.13 |
| V80 | 80 | 3.13 | 0.78 |
| V75 | 75 | 4.73 | 0.44 |
| V70 | 70 | 5.81 | 0.33 |
| Scaffolds/Bones | Permeability (×10−9 m2) | References |
|---|---|---|
| Voronoi scaffolds * | 0.33 to 2.17 | This study * |
| Human Trabecular bone | 0.40 to 11 | Grimm et al. [88] |
| Trabecular bone specimens | 0.0268 to 20 | Nauman et al. [89] |
| Gyroid scaffolds | 0.29 to 3.91 | Ma et al. [90] |
| Schwartz gyroid/diamond/primitive scaffolds | 0.431 to 8.44 | Santos et al. [91] |
| Sheet and skeletal gyroid scaffolds | 0.61 to 3.34 | Yanez et al. [28] |
| Diamond/Octet/Truncate-Octahedron/Double-Diamond/TPMS Scaffolds | 1.85 to 5.62 | Ali et al. [76] |
| Voronoi Scaffolds | Newtonian Model (µ = 0.0045 Pa.s) | Non-Newtonian Power Law Model (n = 1, K = 0.0045 Pa.s) | Non-Newtonian Power Law Model * (n = 0.708, K = 0.0017 Pa.s) |
|---|---|---|---|
| Pressure Drop (Pa) | Pressure Drop (Pa) | Pressure Drop (Pa) | |
| V90 | 0.726 | 0.726 | 1.52 |
| V85 | 1.26 | 1.26 | 2.42 |
| V80 | 1.75 | 1.75 | 3.13 |
| V75 | 2.88 | 2.88 | 4.73 |
| V70 | 3.76 | 3.76 | 5.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
N. Musthafa, H.-S.; Walker, J. Design of Trabecular Bone Mimicking Voronoi Lattice-Based Scaffolds and CFD Modelling of Non-Newtonian Power Law Blood Flow Behaviour. Computation 2024, 12, 241. https://doi.org/10.3390/computation12120241
N. Musthafa H-S, Walker J. Design of Trabecular Bone Mimicking Voronoi Lattice-Based Scaffolds and CFD Modelling of Non-Newtonian Power Law Blood Flow Behaviour. Computation. 2024; 12(12):241. https://doi.org/10.3390/computation12120241
Chicago/Turabian StyleN. Musthafa, Haja-Sherief, and Jason Walker. 2024. "Design of Trabecular Bone Mimicking Voronoi Lattice-Based Scaffolds and CFD Modelling of Non-Newtonian Power Law Blood Flow Behaviour" Computation 12, no. 12: 241. https://doi.org/10.3390/computation12120241
APA StyleN. Musthafa, H.-S., & Walker, J. (2024). Design of Trabecular Bone Mimicking Voronoi Lattice-Based Scaffolds and CFD Modelling of Non-Newtonian Power Law Blood Flow Behaviour. Computation, 12(12), 241. https://doi.org/10.3390/computation12120241






