Color Variability Constrains Detection of Geometrically Perfect Mirror Symmetry
Abstract
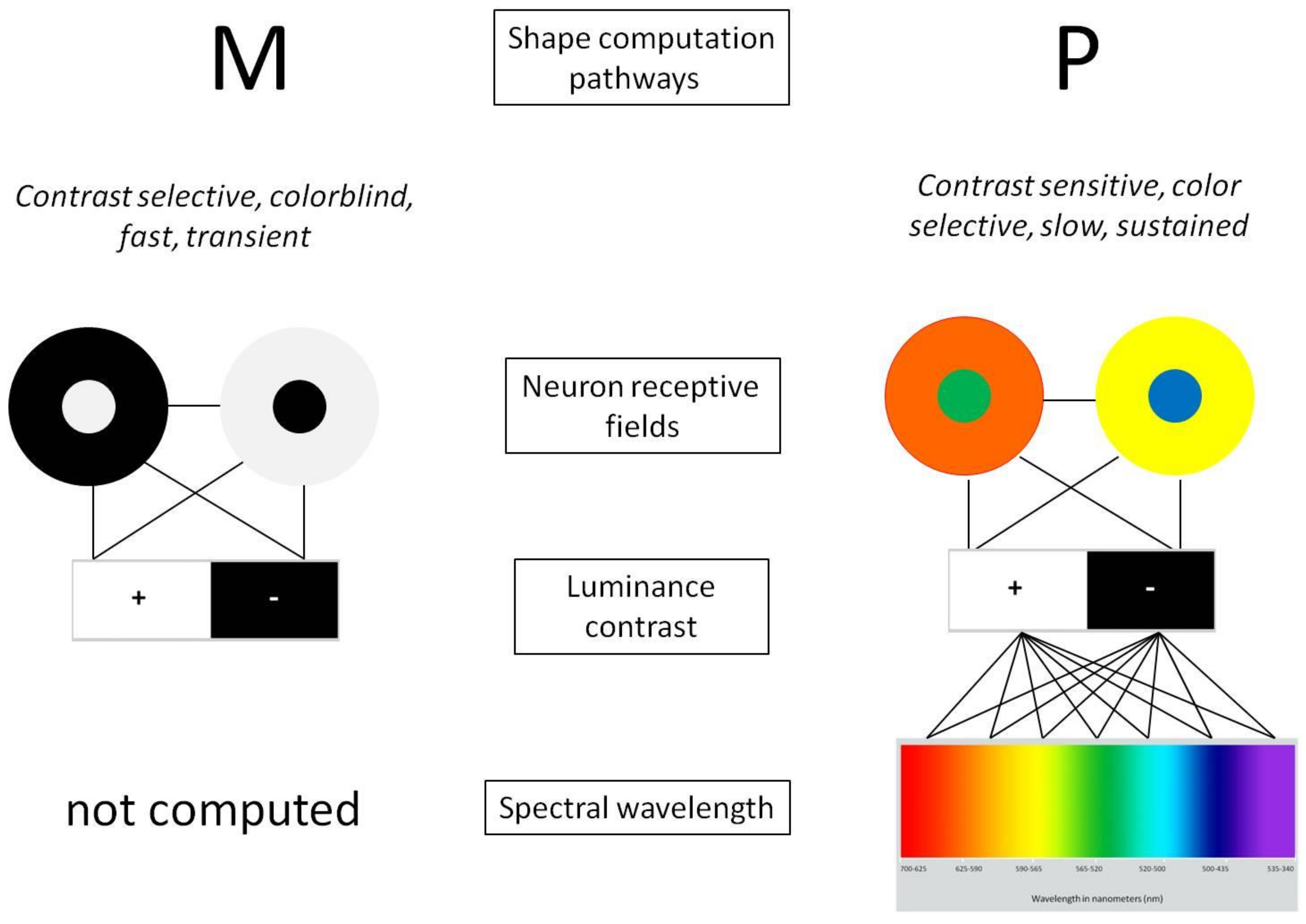
1. Introduction
2. Materials and Methods
2.1. Images and Display Calibration
2.2. Choice Response Time Experiment
2.2.1. Participants
2.2.2. Procedure
3. Results
3.1. Two-Way ANOVA
3.2. Linear Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schweisguth, F.; Corson, F. Self-Organization in Pattern Formation. Dev. Cell 2019, 49, 659–677. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.B. Chance and necessity: The evolution of morphological complexity and diversity. Nature 2001, 409, 1102–1109. [Google Scholar] [CrossRef]
- García-Bellido, A. Symmetries throughout organic evolution. Proc. Natl. Acad. Sci. USA 1996, 93, 14229–14232. [Google Scholar] [CrossRef] [PubMed]
- Groves, J.T. The physical chemistry of membrane curvature. Nat. Chem. Biol. 2009, 5, 783–784. [Google Scholar] [CrossRef] [PubMed]
- Dongen, S.V. Fluctuating asymmetry and developmental instability in evolutionary biology: Past, present and future. J. Evol. Biol. 2006, 19, 1727–1743. [Google Scholar] [CrossRef] [PubMed]
- Dresp-Langley, B. Affine Geometry, Visual Sensation, and Preference for Symmetry of Things in a Thing. Symmetry 2016, 8, 127. [Google Scholar] [CrossRef]
- Levy, K.; Lerner, A.; Shashar, N. Mate choice and body pattern variations in the Crown Butterfly fish Chaetodon paucifasciatus (Chaetodontidae). Biol. Open 2014, 3, 1245–1251. [Google Scholar] [CrossRef][Green Version]
- Gross, M.R.; Suk, H.Y.; Robertson, C.T. Courtship and genetic quality: Asymmetric males show their best side. Proc. R. Soc. B Boil. Sci. 2007, 274, 2115–2122. [Google Scholar] [CrossRef]
- Dresp, B.; Jouventin, P.; Langley, K. Ultraviolet reflecting photonic microstructures in the King Penguin beak horn. Biol. Lett. 2005, 1, 310–313. [Google Scholar] [CrossRef]
- Nolan, P.M.; Dobson, F.S.; Dresp, B.; Jouventin, P. Immunocompetence is signalled by ornamental color in King Penguins, Aptenodytes Patagonicus. Evol. Ecol. Res. 2006, 8, 1332–1335. [Google Scholar]
- Dresp, B.; Langley, K. Fine structural dependence of ultraviolet reflections in the King Penguin beak horn. Anat. Rec. A 2006, 288, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.A. Female preference for symmetrical males as a by-product of selection for mate recognition. Nature 1994, 372, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Deregowski, J.B. Symmetry, Gestalt and information theory. Q. J. Exp. Psychol. 1971, 23, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Eisenman, R. Complexity–simplicity: I. Preference for symmetry and rejection of complexity. Psychon. Sci. 1967, 8, 169–170. [Google Scholar] [CrossRef]
- Eisenman, R.; Rappaport, J. Complexity preference and semantic differential ratings of complexity-simplicity and symmetry-asymmetry. Psychon. Sci. 1967, 7, 147–148. [Google Scholar] [CrossRef]
- Deregowski, J.B. The Role of Symmetry in Pattern Reproduction by Zambian Children. J. Cross-Cult. Psychol. 1972, 3, 303–307. [Google Scholar] [CrossRef]
- Amir, O.; Biederman, I.; Hayworth, K.J. Sensitivity to non-accidental properties across various shape dimensions. Vis. Res. 2012, 62, 35–43. [Google Scholar] [CrossRef]
- Mach, E. On Symmetry. In Popular Scientific Lectures; Open Court Publishing: LaSalle, IL, USA, 1893. [Google Scholar]
- Arnheim, R. Visual Thinking; University of California Press: Berkeley, CA, USA, 1969. [Google Scholar]
- Bahnsen, P. Eine Untersuchung über Symmetrie und Asymmetrie bei visuellen Wahrnehmungen. Zeitschrift Psychol. 1928, 108, 129–154. [Google Scholar]
- Wagemans, J. Characteristics and models of human symmetry detection. Trends Cogn. Sci. 1997, 1, 346–352. [Google Scholar] [CrossRef]
- Cohen, E.H.; Zaidi, Q. Symmetry in context: Salience of mirror symmetry in natural patterns. J. Vis. 2013, 13, e22. [Google Scholar] [CrossRef]
- Barlow, H.B.; Reeves, B.C. The versatility and absolute efficiency of detecting mirror symmetry in random dot displays. Vis. Res. 1979, 19, 783–793. [Google Scholar] [CrossRef]
- Barrett, B.; Whitaker, D.; McGraw, P.; Herbert, A. Discriminating mirror symmetry in foveal and extra-foveal vision. Vis. Res. 1999, 39, 3737–3744. [Google Scholar] [CrossRef]
- Machilsen, B.; Pauwels, M.; Wagemans, J. The role of vertical mirror symmetry in visual shape perception. J. Vis. 2009, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Baylis, G.C.; Driver, J. Perception of symmetry and repetition within and across visual shapes: Part-descriptions and object-based attention. Vis. Cognit. 2001, 8, 163–196. [Google Scholar] [CrossRef]
- Dresp-Langley, B. Bilateral Symmetry Strengthens the Perceptual Salience of Figure against Ground. Symmetry 2019, 11, 225. [Google Scholar] [CrossRef]
- Sabatelli, H.; Lawandow, A.; Kopra, A.R. Asymmetry, symmetry and beauty. Symmetry 2010, 2, 1591–1624. [Google Scholar] [CrossRef]
- Giurfa, M.; Eichmann, B.; Menzel, R. Symmetry perception in an insect. Nature 1996, 382, 458–461. [Google Scholar] [CrossRef]
- Plowright, C.M.S.; Bridger, J.J.M.; Xu, V.; Herlehy, R.A.; Collin, C.A. Floral guidance of learning a preference for symmetry by bumblebees. Anim. Cogn. 2017, 20, 1115–1127. [Google Scholar] [CrossRef]
- Gheorghiu, E.; Kingdom, F.A.A.; Remkes, A.; Li, H.-C.O.; Rainville, S. The role of color and attention-to-color in mirror-symmetry perception. Sci. Rep. 2016, 6, 29287. [Google Scholar] [CrossRef]
- Martinovic, J.; Jennings, B.J.; Makin, A.D.J.; Bertamini, M.; Angelescu, I. Symmetry perception for patterns defined by color and luminance. J. Vis. 2018, 18, 4. [Google Scholar] [CrossRef]
- Turatto, M.; Galfano, G. Attentional capture by color without any relevant attentional set. Percept. Psychophys. 2001, 63, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, G. Focus-of-attention from local color symmetries. IEEE Trans. Pattern Anal. Mach. Intell. 2004, 26, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-S.; Bryan, R.; Miki, A.; Woo, J.; Liu, G.; Elliott, M. Magnocellular and Parvocellular Visual Pathways Have Different Blood Oxygen Level–Dependent Signal Time Courses in Human Primary Visual Cortex. AJNR Am. J. Neuroradiol. 2006, 27, 1628–1634. [Google Scholar] [PubMed]
- Paulus, W.; Korinth, S.; Wischer, S.; Tergau, F. Differential inhibition of chromatic and achromatic perception by transcranial magnetic stimulation of the human visual cortex. NeuroReport 1999, 10, 1245–1248. [Google Scholar] [CrossRef]
- Maunsell, J.H.; Ghose, G.M.; Assad, J.A.; McADAMS, C.J.; Boudreau, C.E.; Noerager, B.D. Visual response latencies of magnocellular and parvocellular LGN neurons in macaque monkeys. Vis. Neurosci. 1999, 16, 1–14. [Google Scholar] [CrossRef]
- Ueno, T. Sustained and transient properties of chromatic and luminance systems. Vis. Res. 1992, 32, 1055–1065. [Google Scholar] [CrossRef]
- Stigliani, A.; Jeska, B.; Grill-Spector, K. Differential sustained and transient temporal processing across visual streams. PLoS Comput. Biol. 2019, 15, e1007011. [Google Scholar] [CrossRef]
- Pramod, R.T.; Arun, S.P. Symmetric Objects Become Special in Perception Because of Generic Computations in Neurons. Psychol. Sci. 2017, 29, 95–109. [Google Scholar] [CrossRef]
- Pramod, R.T.; Arun, S. Improving Machine Vision Using Human Perceptual Representations: The Case of Planar Reflection Symmetry for Object Classification. IEEE Trans. Pattern Anal. Mach. Intell. 2020, 44, 228–241. [Google Scholar] [CrossRef]
- Dresp-Langley, B. Seven Properties of Self-Organization in the Human Brain. Big Data Cogn. Comput. 2020, 4, 10. [Google Scholar] [CrossRef]
- Kohonen, T. Self-Organizing Maps. 2001. Available online: http://link.springer.com/10.1007/978-3-642-56927-2 (accessed on 8 January 2021).
- Kohonen, T. MATLAB Implementations and Applications of the Self-Organizing Map; Unigrafia Oy: Helsinki, Finland, 2014. [Google Scholar]
- Dresp-Langley, B.; Wandeto, J. Human Symmetry Uncertainty Detected by a Self-Organizing Neural Network Map. Symmetry 2021, 13, 299. [Google Scholar] [CrossRef]
- Wandeto, J.M.; Dresp-Langley, B. The quantization error in a Self-Organizing Map as a contrast and colour specific indicator of single-pixel change in large random patterns. Neural Netw. 2019, 119, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Tyler, C.W. Symmetry: Modeling the effects of masking noise, axial cueing and salience. PLoS ONE 2010, 5, e9840. [Google Scholar] [CrossRef]
- Close, F. Lucifer’s Legacy: The Meaning of Asymmetry; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Bertamini, M.; Tyson-Carr, J.; Makin, A.D.J. Perspective Slant Makes Symmetry Harder to Detect and Less Aesthetically Appealing. Symmetry 2022, 14, 475. [Google Scholar] [CrossRef]
- van Zoest, W.; Giesbrecht, B.; Enns, J.T.; Kingstone, A. New reflections on visual search: Interitem symmetry matters! Psychol. Sci. 2006, 17, 535–542. [Google Scholar] [CrossRef]
- Wenderoth, P. The Effects of the Contrast Polarity of Dot-Pair Partners on the Detection of Bilateral Symmetry. Perception 1996, 25, 757–771. [Google Scholar] [CrossRef]
- Hogeweg, L.; Sánchez, C.I.; Maduskar, P.; Philipsen, R.H.; Van Ginneken, B. Fast and effective quantification of symmetry in medical images for pathology detection: Application to chest radiography. Med. Phys. 2017, 44, 2242–2256. [Google Scholar] [CrossRef]
- Bonnet, C.; Fauquet, A.J.; Estaún Ferrer, S. Reaction times as a measure of uncertainty. Psicothema 2008, 20, 43–48. [Google Scholar]
- Brown, S.D.; Marley, A.A.J.; Donkin, C.; Heathcote, A. An integrated model of choices and response times in absolute identification. Psychol. Rev. 2008, 115, 396–425. [Google Scholar] [CrossRef]
- Proctor, R.W.; Schneider, D.W. Hick’s law for choice reaction time: A review. Q. J. Exp. Psychol. 2018, 71, 1281–1299. [Google Scholar] [CrossRef]
- Kvålseth, T.O. Hick’s law equivalent for reaction time to individual stimuli. Br. J. Math. Stat. Psychol. 2021, 74 (Suppl. S1), 275–293. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, H.; Rovamo, J.; Tiippana, K.; Näsänen, R. Michelson contrast, RMS contrast and energy of various spatial stimuli at threshold. Vis. Res. 1993, 33, 1431–1436. [Google Scholar] [CrossRef]
- Ishihara, S. Tests for Color-Blindness; Handaya: Tokyo, Japan, 1917. [Google Scholar]
- Dresp-Langley, B.; Monfouga, M. Combining Visual Contrast Information with Sound Can Produce Faster Decisions. Information 2019, 10, 346. [Google Scholar] [CrossRef]
- Treder, M.S. Behind the Looking-Glass: A Review on Human Symmetry Perception. Symmetry 2010, 2, 1510–1543. [Google Scholar] [CrossRef]
- Dresp-Langley, B.; Reeves, A. Simultaneous Brightness and Apparent Depth from True Colors on Grey: Chevreul Revisited. Seeing Perceiving 2012, 25, 597–618. [Google Scholar] [CrossRef] [PubMed]
- Dresp-Langley, B.; Reeves, A. Color and figure-ground: From signals to qualia. In Perception beyond Gestalt: Progress in Vision Research; Magnussen, S., Greenlee, M., Werner, J., Geremek, A., Eds.; Psychology Press: Abingdon, UK, 2014; pp. 159–171. [Google Scholar]
- Dresp-Langley, B.; Reeves, A. Effects of saturation and contrast polarity on the figure-ground organization of color on gray. Front. Psychol. 2014, 5, 1136. [Google Scholar] [CrossRef] [PubMed]
- Dresp-Langley, B.; Reeves, A. Color for the perceptual organization of the pictorial plane: Victor Vasarely’s legacy to Gestalt psychology. Heliyon 2020, 6, e04375. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Sun, H. On a gesture-computing technique using electromagnetic waves. Inverse Probl. Imaging 2018, 12, 677–696. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Yang, C. Mathematical Design of a Novel Gesture-Based Instruction/Input Device Using Wave Detection. SIAM J. Imaging Sci. 2016, 9, 822–841. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Liu, H.; Tsui, W.-Y.; Wang, X. An inverse scattering approach for geometric body generation: A machine learning perspective. Math. Eng. 2019, 1, 800–823. [Google Scholar] [CrossRef]
- George, J.K.; Soci, C.; Miscuglio, M.; Sorger, V.J. Symmetry perception with spiking neural networks. Sci. Rep. 2021, 11, 5776. [Google Scholar] [CrossRef] [PubMed]
- Sharman, R.J.; Gheorghiu, E. The role of motion and number of element locations in mirror symmetry perception. Sci. Rep. 2017, 7, 45679. [Google Scholar] [CrossRef] [PubMed]
- Dresp-Langley, B.; Wandeto, J.M. Unsupervised classification of cell imaging data using the quantization error in a Self Organizing Map. In Transactions on Computational Science and Computational Intelligence; Arabnia, H.R., Ferens, K., de la Fuente, D., Kozerenko, E.B., Olivas Varela, J.A., Tinetti, F.G., Eds.; Springer-Nature: Berlin/Heidelberg, Germany, 2021; pp. 201–210. [Google Scholar]
- Kim, A.S.; Cheng, W.-C.; Beams, R.; Badano, A. Color Rendering in Medical Extended-Reality Applications. J. Digit. Imaging 2020, 34, 16–26. [Google Scholar] [CrossRef] [PubMed]






| Appearance | Hue (deg) | Saturation (%) | L (cd/m2) | R-G-B | |
|---|---|---|---|---|---|
| Saturated Colors | Blue | 240 | 100 | 14 | 0-0-255 |
| Red | 0 | 100 | 36 | 255-0-0 | |
| Green | 120 | 100 | 79 | 0-255-0 | |
| Magenta | 300 | 100 | 50 | 255-0-255 | |
| Desaturated Colors | Yellow | 60 | 100 | 115 | 255-255-0 |
| Cyan | 180 | 100 | 75 | 0-255-255 | |
| Pale Blue | 240 | 25 | 62 | 190-190-255 | |
| Pale Red | 0 | 25 | 87 | 255-190-190 | |
| Pale Green | 120 | 25 | 90 | 190-255-190 | |
| Pale Magenta | 300 | 25 | 92 | 255-190-255 | |
| Pale Yellow | 60 | 25 | 100 | 255-255-190 | |
| Pale Cyan | 180 | 25 | 97 | 190-255-255 | |
| Achromatic Tones | Black | 0 | 0 | 0 | 0-0-0 |
| Dark Grey1 | 0 | 0 | 15 | 80-80-80 | |
| Dark Grey2 | 0 | 0 | 20 | 90-90-90 | |
| Medium Grey1 | 0 | 0 | 50 | 150-150-150 | |
| Medium Grey2 | 0 | 0 | 60 | 170-170-170 | |
| Light Grey1 | 0 | 0 | 70 | 190-190-190 | |
| Light Grey2 | 0 | 0 | 99 | 215-215-215 | |
| White | 0 | 0 | 129 | 255-255-255 |
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Color | 0.7/0.7 | 0.7/0.63 | 0.7/0.63 | 0.8/0.8 | 0.8/0.43 |
| d = 0 | d = 0.13 | d = 0.13 | d = 0 | d = 0.37 | |
| Achromatic | 0.53/0.53 | 0.53/1 | 0.53/1 | 1/1 | 1/0.74 |
| d = 0 | d = 0.47 | d = 0.47 | d = 0 | d = 0.26 |
| Hue | Mean | Standard Error |
|---|---|---|
| Chromatic | 1121.5 | 10.2 |
| Achromatic | 1033.1 | 8.9 |
| Variability | ||
| 1 | 589.6 | 9.6 |
| 2 | 954.1 | 10.4 |
| 3 | 1055.6 | 13.5 |
| 4 | 1298.1 | 13.6 |
| 5 | 1489.1 | 14.4 |
| Interactions | ||
| Chromatic × 1 | 512.6 | 11.9 |
| Chromatic × 2 | 885.6 | 12.4 |
| Chromatic × 3 | 1112.6 | 12.9 |
| Chromatic × 4 | 1408.9 | 13.5 |
| Chromatic × 5 | 1687.6 | 16.2 |
| Achromatic × 1 | 666.6 | 9.3 |
| Achromatic × 2 | 1022.6 | 11.0 |
| Achromatic × 3 | 998.6 | 11.9 |
| Achromatic × 4 | 1187.2 | 12.3 |
| Achromatic × 5 | 1290.6 | 12.9 |
| DF | SS | MS | F | p | |
|---|---|---|---|---|---|
| Hue | 1 | 292,640 | 292,604 | 64.27 | <0.001 |
| Variability | 4 | 14,754,969 | 3,538,742 | 777.3 | <0.001 |
| Interaction | 4 | 1,674,091 | 418,523 | 91.92 | <0.001 |
| Residual | 140 | 637,422 | 4553 | ||
| Total | 149 | 16,759,087 | 112,477 |
| Coefficients ‘Color’ | Model Function | Coefficients ‘Achromatic’ | Model Function | ||
|---|---|---|---|---|---|
| x | f(x) | x | f(x) | ||
| Intercept (a) = 546.8 Slope (b) = 288 R 2 = 0.9951972709 | 0 | 546.8 | Intercept (a) = 750.6 Slope (b) = 140.6 R 2 = 0.883791256 | 0 | 750.6 |
| 0.08 | 569.84 | 0.08 | 761.84 | ||
| 0.16 | 592.88 | 0.16 | 773.09 | ||
| 0.24 | 615.92 | 0.24 | 784.34 | ||
| 0.32 | 638.96 | 0.32 | 795.59 | ||
| 0.40 | 662.00 | 0.40 | 806.84 | ||
| 0.48 | 685.04 | 0.48 | 818.08 | ||
| 0.56 | 708.08 | 0.56 | 829.33 | ||
| 0.64 | 731.12 | 0.64 | 840.58 | ||
| 0.72 | 754.16 | 0.72 | 851.83 | ||
| 0.80 | 777.20 | 0.80 | 863.08 | ||
| 0.88 | 800.24 | 0.88 | 874.32 | ||
| 0.96 | 823.28 | 0.96 | 885.57 | ||
| 1.04 | 846.32 | 1.04 | 896.82 | ||
| 1.12 | 869.36 | 1.12 | 908.07 | ||
| 1.20 | 892.40 | 1.20 | 919.32 | ||
| 1.28 | 915.44 | 1.28 | 930.56 | ||
| 1.36 | 938.48 | 1.36 | 941.81 | ||
| 1.44 | 961.52 | 1.44 | 953.06 | ||
| 1.52 | 984.56 | 1.52 | 964.31 | ||
| 1.60 | 1007.60 | 1.60 | 975.56 | ||
| 1.68 | 1030.64 | 1.68 | 986.80 | ||
| 1.76 | 1053.68 | 1.76 | 998.05 | ||
| 1.84 | 1076.72 | 1.84 | 1009.30 | ||
| 1.92 | 1099.76 | 1.92 | 1020.55 | ||
| 2.00 | 1122.80 | 2.00 | 1031.80 | ||
| 2.08 | 1145.84 | 2.08 | 1043.04 | ||
| 2.16 | 1168.88 | 2.16 | 1054.29 | ||
| 2.24 | 1191.92 | 2.24 | 1065.54 | ||
| 2.32 | 1214.96 | 2.32 | 1076.79 | ||
| 2.04 | 1238.00 | 2.40 | 1088.04 | ||
| 2.48 | 1261.04 | 2.48 | 1099.28 | ||
| 2.56 | 1284.08 | 2.56 | 1110.53 | ||
| 2.64 | 1307.12 | 2.64 | 1121.78 | ||
| 2.72 | 1330.16 | 2.72 | 1133.03 | ||
| 2.80 | 1353.20 | 2.80 | 1144.28 | ||
| 2.88 | 1376.24 | 2.88 | 1155.52 | ||
| 2.96 | 1399.28 | 2.96 | 1166.77 | ||
| 3.04 | 1422.32 | 3.04 | 1178.02 | ||
| 3.12 | 1445.36 | 3.12 | 1189.27 | ||
| 3.20 | 1468.40 | 3.20 | 1200.52 | ||
| 3.28 | 1491.44 | 3.28 | 1211.76 | ||
| 3.36 | 1514.48 | 3.36 | 1223.01 | ||
| 3.44 | 1537.52 | 3.44 | 1234.26 | ||
| 3.52 | 1560.56 | 3.51 | 1245.51 | ||
| 3.60 | 1583.60 | 3.60 | 1256.76 | ||
| 3.68 | 1606.64 | 3.68 | 1268.00 | ||
| 3.76 | 1629.68 | 3.76 | 1279.25 | ||
| 3.84 | 1652.72 | 3.84 | 1290.50 | ||
| 3.92 | 1675.76 | 3.92 | 1301.75 | ||
| 4.00 | 1698.80 | 4.00 | 1313.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dresp-Langley, B. Color Variability Constrains Detection of Geometrically Perfect Mirror Symmetry. Computation 2022, 10, 99. https://doi.org/10.3390/computation10060099
Dresp-Langley B. Color Variability Constrains Detection of Geometrically Perfect Mirror Symmetry. Computation. 2022; 10(6):99. https://doi.org/10.3390/computation10060099
Chicago/Turabian StyleDresp-Langley, Birgitta. 2022. "Color Variability Constrains Detection of Geometrically Perfect Mirror Symmetry" Computation 10, no. 6: 99. https://doi.org/10.3390/computation10060099
APA StyleDresp-Langley, B. (2022). Color Variability Constrains Detection of Geometrically Perfect Mirror Symmetry. Computation, 10(6), 99. https://doi.org/10.3390/computation10060099





