2. The Limits of Chemistry
In traditional information-based—or “genetics-first”—theories for the origin of life, much of biological evolution is reduced to the chemistry of nucleic acids (such as RNA) [
18]. To accomplish this reduction, two important assumptions are made: (1) genes are the fundamental units of biological information, and (2) evolution is primarily concerned with the accumulation of (genetic) information through the processes of replication and selection. These two features can be captured by appealing to chemical models for the catalyzed replication of genetic polymers, such as RNA. This forms the foundation of the RNA world hypothesis [
19], which suggests that the early Earth was populated by “riboorganisms” who utilized RNA as both genetic material and the only genetically encoded biochemical catalyst.
Collapsing genetic information and catalytic function to the same molecule introduces a paradox. If genotype (genetic information) and phenotype (catalytic function) are encoded in the same molecule, there is an inherent chemical trade-off between genotypic (selection for replication) and phenotypic (selection for function) selection [
20]. For example, well-folded RNA sequences are likely to be poor templates for copying, conversely poorly folded RNA sequences are unlikely to be good ribozymes (catalytic RNAs) [
21]. This trade-off imposes a
physical limit on the information content of primitive genomes. This physical constraint imposed on information content should be viewed in contrast with the more widely discussed
informational limits of early replicators, defined by Eigen’s “error threshold” as the maximum amount of genomic information that can be reliably propagated from generation to generation for a fixed mutation rate [
22]. Both the informational limit(s) on physical systems and the physical limit(s) on informational systems are important factors shaping biological evolution.
Modern life has overcome the chemical limitations imposed on template-based replication through the decoupling of selection for replication from selection for function. In effect, this amounts to physically separating information propagation (replication) from the processing of that information (function) [
3]. Life achieves this decoupling by utilizing two species of biopolymer that play different biochemical roles: DNA stores genetic information and proteins execute the majority of catalytic and structural functions (with RNA playing a prominent dual role as an informational and functional intermediate). In modern life, replication of genetic information is not constrained by the physics or chemistry of particular DNA sequences. This is not to say that chemistry does not play a role, as it clearly must: thus, for example, some polymerases are more efficient and less error-prone for certain reading frames than others. However, in general the efficiency of copying sequences is not as widely varied as it is for non-enzymatic replicators. The utility of genes is that they carry
instructional information that can be read-out by the translation machinery of the cell to execute specific functions: selection is ultimately operating on the informational output, or function, of a gene and not strictly on the gene itself.
The above considerations have led many researches to distinguish replicators from reproducers, going as far back as the early work of von Neumann [
23]. An interesting side note, highlighting the power of logic-based views of living systems, is that von Neumann conceptually formalized a distinction between trivial and non-trivial replicators based solely on logical considerations, before the inner workings of cellular self-reproduction, such as the structure of DNA, were elucidated. von Neumann identified two classes of self-replicators: trivial and non-trivial.
Trivial replicators are constrained by strict physical limits imposed on their capacity to generate copies, such as the chemical trade-off between templating ability and catalytic function imposed on an RNA genome encoding RNA catalysts. In contrast,
non-trivial replicators—also commonly referred to as reproducers—have in some sense transcended many of these constraints due to the explicit use of programs, symbolic logic and codes to mediate self-reproduction. Non-trivial replicators require constructors, defined as entities that can cause some change in a physical system while retaining the ability to cause it again. Examples of constructors include heat engines and chemical catalysts. von Neumann was particularly interested in the idea of a
universal constructor, a machine capable of taking materials from its host environment to build any possible physical structure (consistent with the available resources and the laws of physics), including itself. Universal constructors are therefore, by definition, capable of self-reproduction. A key feature of a universal constructor is that it must be programmed with an explicit set of instructions to construct itself (these systems are therefore self-referencing, another distinctive feature of the living state [
24,
25,
26]).
Living systems are constructors in the sense proposed by von Neumann. A caveat is that known life may not be truly universal—it is an open question whether living systems possess the capacity to construct
any possible object consistent with available resources; however, technological civilizations may approach this ideal. While constructors certainly exist outside of living systems (e.g., the examples of the heat engine or catalyst cited above), life represents a unique class of physical systems in that the relevant constructors are
virtual. That is to say, in living systems the constructors are programs. For a living cell, it is the program that must be preserved through the process of construction and not necessarily the matter it is instantiated in (see, e.g., discussion on functional equivalence in
Section 5).
The emergence of life is therefore describable in physical terms as emergence of causally efficacious abstractions, or virtual constructors. As I discuss below, the emergence of virtual constructors is intimately tied to the notion of causal emergence as it requires the appearance of new “higher-level” causes that are necessarily abstract (e.g., programs). Identifying when such systems emerge therefore requires rigorous quantification of the notion of causal emergence, including “top-down” causation, which we discuss in detail in
Section 3.
Identifying the role of (virtual) constructors in the early evolution of life highlights the need for an information-based understanding of how life first emerged by identifying the limits of chemistry (hardware)—only origin of life scenarios. This is perhaps most strikingly evident when one considers the relevant complexity classes for describing replicative systems, which we turn to next.
Szathmáry and Maynard Smith, for example, have offered a classification scheme that distinguishes the complexity of replicators based on hereditary potential [
27]. They identify
limited heredity replicators as those where the number of types is smaller than, or roughly equal to, the number of individuals in a realistic physical system (
i.e., short oligonucleotides). Alternatively,
unlimited heredity replicators are identified as those where the number of types far exceeds the number of individuals that could possibly exist in any realistic system (
i.e., genes and genomes of extant life). By definition, only unlimited heredity replicators allow the potential for unlimited growth in complexity: unlimited heredity replicators occupy a vast state-space (much larger than anything physically achievable) that is mostly unoccupied by physical systems where “complex” states could, at least in principle, be populated through Darwinian evolution. The distinction between limited and unlimited heredity is therefore in the size of the relevant state space. Life is an example of the more “complex” unlimited heredity replicator, because the state space of hypothetically possible genomes is much larger than could ever be realized by summing over all genomes on Earth.
Szathmáry and Maynard Smith’s classification cannot distinguish trivial and non-trivial replicators. It is based solely on identifying the hypothetically possible state space for the chemical hardware and does not take into account the existence of counterfactuals. However, in determining the complexity of physical systems (including life) a distinction must be made between
possible states and
physically accessible states. Distinguishing between possible and impossible transformations is the basis of constructor theory, currently being developed by Deutsch and Marletto [
1,
28]. Their proposal is to recast all fundamental laws of nature entirely in terms of statements of which tasks (
i.e., classes of physical transformations or constructions) are possible and which are impossible, and why. Constructor theory therefore proposes to place counterfactual statements as first-class descriptors of physical systems, a position of prominence normally only awarded to laws of motion and initial conditions [
1]. Taking their proposal seriously, the subset of physically accessible states among all possible states is defined by the constructors present in a given system. This yields a new perspective on emergent phenomena—emergence can occur only when new constructors arise that open possible, but previously inaccessible, physical states (this is somewhat akin to the notion of the “adjacent possible” as popularized by Kauffman [
29]). In the context of the current discussion, the complexity of trivial and non-trivial replicators is distinguished by the size of the constructible state space. The size of the constructible state space, although difficult to quantify in practice, may be a natural complexity measure for the biosphere: humans are arguably more complex than other organisms because of the space of all possible constructions we can build (contrast with the length of our genome, which does not accurately capture our “complexity” relative to other organisms).
Table 1.
Intersection of trivial/non-trivial (software) and limited/unlimited heredity (hardware) replicator classification schemes. The limited/unlimited heredity classification scheme distinguishes replicators based on the total number of all possible states of the chemical substrate, whereas the trivial/non-trivial classification scheme distinguishes replicators based on the number of physically accessible states due to the presence of constructors. Living systems are members of the class of non-trivial replicators with virtual constructors, and are capable of unlimited heredity.
| | Trivial | Non-trivial |
|---|
| Limited heredity | short oligonucleotides such as non-enzymatic template replicators, crystals | physical constructors such as catalysts and autocatalytic sets |
| Unlimited heredity | monomolecular genes and genomes such as would exist in RNA-world riboorganisms | virtual constructors such as the cellular operating system of extant life and von Neumann automata |
The intersection of these two schema is shown in
Table 1. A trivial replicator such as an RNA replicase could, for example, be a limited or unlimited heredity replicator depending on its length. A trivial replicator with unlimited heredity may therefore appear to have open-ended evolutionary potential due to the large size of its hypothetically possible state space (e.g., consider the size of state space of a 50 nucleotide RNA—it would take more carbon than is available in the Earth’s crust to synthesize one picomole of all possible RNA sequences in this space [
30]). Genetics-first RNA world scenarios are based on the premise that limited heredity replicators will evolve into unlimited heredity replicators through the process of Darwinian evolution. However, as noted above, much of this state space is physically inaccessible due to chemical trade-offs. Conversely, metabolism-first scenarios that often involve autocatalytic sets (sets of mutually catalytic species that collectively reproduce) provided examples of non-trivial replicators in that they explicitly rely on the presence of constructors (catalysts). However, they are limited in their potential for open-ended growth in complexity (limited heredity) by the physical and chemical limitations imposed on the physical constructors: in short, they are not programmable. Life arises through evolutionary processes converging on the lower left-hand box of
Table 1. To understand the transition to non-trivial replicators with unlimited heredity—and thus the emergence of life—therefore requires explicit acknowledgement of the causal role of information in physical systems.
3. Top-Down Causation in Biological Systems
The forgoing discussion suggests that life first arose through the emergence of virtual constructors in chemical systems. The emergence of virtual constructors is intimately tied to the notion of causal emergence as it requires the appearance of new “higher-level” causes that are necessarily abstract (e.g., implemented through the use of symbolic logic and programs). Identifying when such systems emerge therefore requires rigorous quantification of the physics underlying causal emergence, and in particular “top-down” causation. We first discuss top-down causation as a conceptual framework for understanding living matter, and then turn to how quantifying causal emergence could provide new insights into how life first emerged.
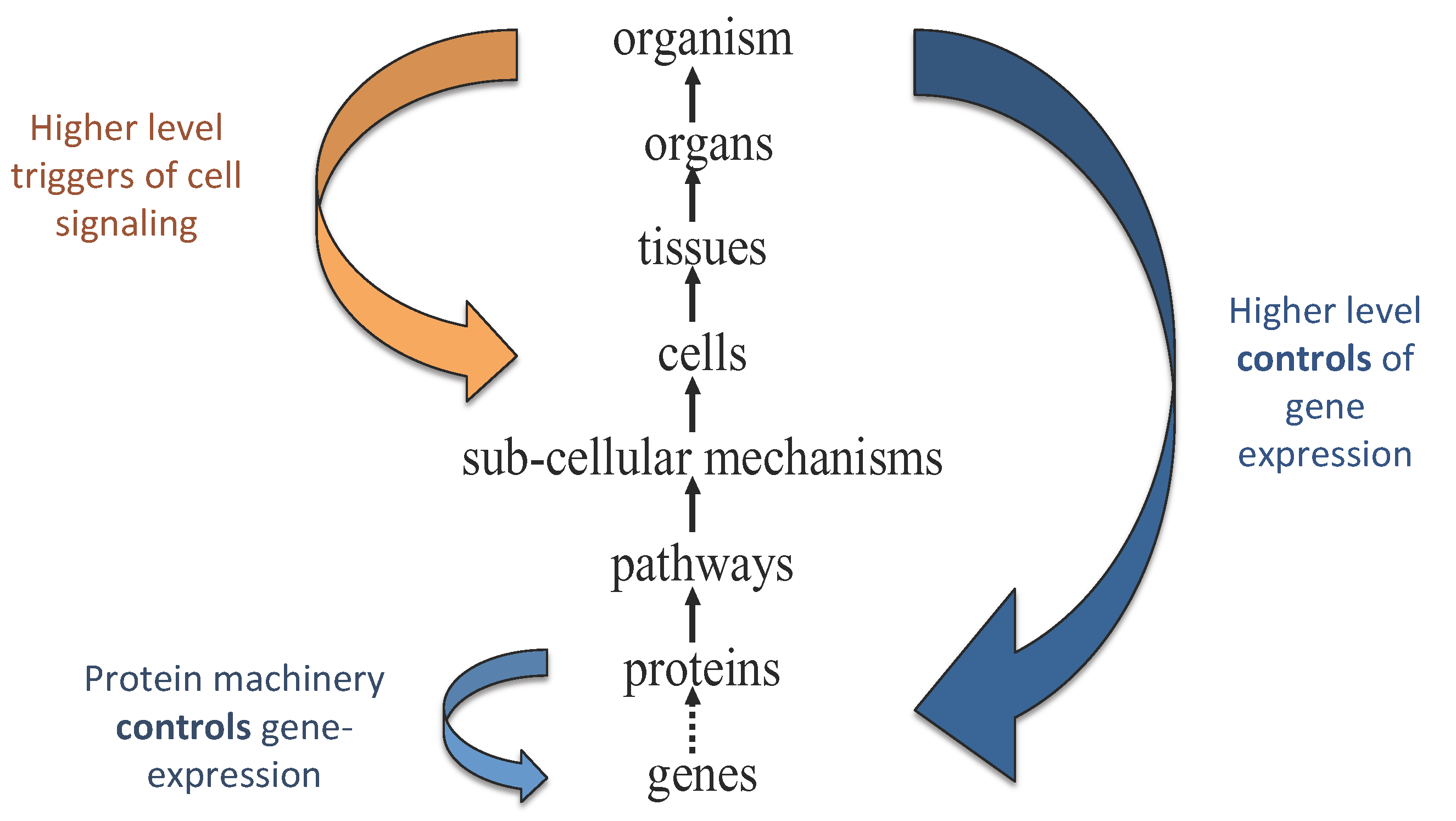
In living systems, causal influences run both up and down organizational hierarchies [
10] (see e.g.,
Figure 1). Characterizing living processes therefore requires both bottom-up causation—such as when a gene is read-out to make a protein that affects cellular behavior—and top-down causation—as occurs when changes in the environment initiate an organismal response that permeates all the way down to the level of individual genes [
5]. Bottom-up causation is the status quo in modern physics, whereas top-down causation is less familiar and much more challenging to quantify. Top-down (or downward) causation occurs when a higher hierarchical level exerts causal influence over a lower level by setting a context (for example, by changing some physical constraints) within which the lower level actions take place [
31,
32]. A relatively simple example of top-down causation is the three-dimensional folding of an RNA molecule. The global constraint imposed by the network of structural interactions between residues causes the molecule to acquire a specific three-dimensional conformation [
13]. RNA (or protein) folding therefore presents an example of whole-part causation, albeit localized to a single contiguous molecule.
Figure 1.
Information flows both up and down the hierarchy of structure and causation in biological systems. Figure adapted from Noble [
33].
Ellis has identified at least five different classes of top-down causation [
32]: algorithmic top-down causation, top-down causation by non-adaptive information control, top-down causation via adaptive selection, top-down causation via adaptive information control and intelligent top-down causation. Of these, top-down causation by information control and top-down causation via adaptive selection are essential to life [
13]. In top-down causation by information control, a higher hierarchal level influences lower level entities to achieve a specific functional outcome (goal) through the use of feedback control loops. Feedback control depends on the flow of information through the system, where information on the system’s current state is evaluated relative to a particular functional outcome. Top-down causation by information control thereby presents a framework for understanding how information—an abstraction—can play a causally efficacious role in biological systems. It is important to note that while information is abstract, in the sense that it involves one entity symbolically representing another, it is nonetheless physical and only exists when physically instantiated (it therefore holds similar ontological status to energy). There is thus no conflict with information playing a causal role in the dynamics of living systems.
Top-down causation is an important mechanism for adaptive evolution through natural selection, where the higher-level “goal” is survival [
32,
34]. The role of top-down causation in adaptive selection is particularly evident for cases of convergent evolution. A striking example is provided by the evolution of echolocation in dolphins and bats, where over 200 genes have independently changed in the same ways to confer both species with the ability to use sonar [
35]. A common high-level selection pressure (e.g., for the ability to navigate) lead independently to the same specific mutations in the DNA of both species. Convergent evolution thus provides a clear example of top-down causation via adaptive selection, where causal influences run from macroscopic environmental context to microscopic biochemical structure.
Top-down causation operates through functional equivalence classes. Functional equivalence occurs when a given “higher-level” state leads to the same high-level outcome, independent of which “lower-level” state instantiates it [
32]. Equivalence classes are defined in terms of their function, not their particular physical instantiation: operations are considered (functionally) equivalent (
i.e., in the same equivalence class) if they produce the same outcome for different lower-level mechanisms. Functional equivalence classes therefore represent the physical manifestations of virtual constructors. Functional equivalence is evident, for example, in the case of convergent evolution presented above, where convergence occurs because natural selection optimizes a functionally equivalent outcome (in this case, echolocation). Jaeger and Calkins present a more compelling example of functional equivalence [
14], citing an experiment where the RNA based RNase P in
Escherichia coli bacteria is replaced by a purely proteinaceous RNase P derived from plant organelles, which is completely functional in the host
E.coli cell [
36]. In this example, the RNA and protein versions of RNase P are structurally unrelated, but they clearly fulfill the same functional outcome and are thus members of the same functional equivalence class. This kind of functional equivalency between RNA and protein likely played a prominent role in the evolution of the RNA-peptide world. Further studies of this kind will be important to understanding transitions in chemical hardware that maintain high-level function that would have been particularly important throughout the early evolution of life (discussed in
Section 5).
Life may be defined as a self-reproducing system that functions via top-down causation by adaptive selection
and information control [
13,
14,
16]. Here information control is the critical factor distinguishing living systems. Using the notion of functional equivalence classes, Jaeger and Calkins define bacterial cell as the congruence of two master functional classes: replication (of the genome) and reproduction—or construction (of the cell machinery). Thus, their descriptive framework shares much in common with the logical structure of a von Neumann automata: the relevant functional equivalence classes are abstract (virtual) constructors of the state of the system. Here the “higher-level” is the information, or program, encoded in the two master functions. Top-down causation by information control operates via the flow of information among and between these two master functions, which acts to conserve their functions (though not necessarily the precise chemical substrate they are instantiated in). Thus,
a bacterial cell may be defined by its architecture of information flow. This insight provides a new framework for quantifying the emergence of life that captures the significance of abstract constructors in defining the living state.
4. The Emergence of Life as a Transition in Informational and Causal Architecture
Living and non-living systems may be distinguished by how information and causation are distributed. For living systems, information—in the form of programs, symbolic logic, and codes—plays an active, and therefore causally efficacious, role. Based on this observation, we previously identified several hallmarks of living systems that could lead to new productive modes of inquiry into understanding how life first emerged [
15,
16]. These hallmarks are reproduced in
Table 2. Many of these represent different ways of approaching the same key attribute(s) of life. For example, as discussed here, the logical structure of a universal constructor necessitates the presence of information as a causal agent, and in particular the presence of top-down causation, in that it requires “high-level” programs to direct the dynamics of its physical instantiation. Similarly, these concepts are intimately related to the notion of the co-evolution of laws and states (e.g., self-referential dynamics [
24,
25,
26]), where the current state of a physical system encodes information that determines its future state(s) (thus highlighting the fact that counterfactuals can, and do, matter in setting the dynamics of certain classes of physical systems). As such, we see
Table 2 as a framework for identifying what it is that distinguishes life as a unique state of matter, by approaching the problem from multiple directions that each hold promise to eventually converge on identifying the most fundamental attribute(s) of life.
Table 2.
The hallmarks of life. Table from Walker and Davies [16].
| Hallmarks of Life |
|---|
| Global organization |
| Information as a causal agency |
| Top-down causation |
| Analog and digital information processing |
| Laws and states co-evolve |
| Logical structure of a universal constructor |
| Dual hardware and software roles of genetic material |
| Non-trivial replication |
| Physical separation of instructions (algorithms) |
| from the mechanism that implements them |
In particular, the unique role of top-down causation in biological organization potentially enables a methodology for identifying a non-trivial distinction between life and non-life, identifiable as a fundamental difference in causal and informational architecture [
16]. Rigorously quantifying top-down causal information flow and causal emergence may therefore provide plausible candidate parameter(s) for tracking the transition from non-living to living matter. Furthermore, fundamental differences in the causal architecture of information flow may provide insights not only into differentiating non-life from life, but also in identifying intermediate states of the transitory regime that could include a gradation of states of “almost life”. This framework thus holds promise for identifying transitory phases in the evolution of chemical systems on the pathway to modern cells that have previously proved elusive to characterize.
We recently proposed, via a toy model, one such framework for identifying transitions in information flow, using Schreiber’s “transfer entropy” (TE) [
37] to explicitly study the transfer of information from local (individual nodes) to global (the mean-field) and from global to local degrees of freedom in a lattice of coupled logistic maps [
15]. TE was chosen for this study as it provides insight into the directionality of information flow that more traditional measures commonly used in the biological sciences such as mutual information (
i.e., static, symmetric measures) cannot capture. Non-trivial collective behavior was observed to emerge each time the dominant direction of TE transitioned from local-to-global (bottom-up) to global-to-local (top-down), indicating that the emergence of collective organization is correlated with a dominance of information transfer from global to local scales. Thus, collective behavior may be characterized by “top-down” information transfer for this toy model system. Similar dynamics have been observed applying TE to other coupled non-linear systems, including cases where individual nodes do not have direct access to global state information [
38,
39].
Implementing measures of information transfer such as TE, combined with the appropriate aggregate variables describing informational degrees of freedom, may provide a quantitative framework that accurately captures the causal and informational architecture of biological networks. However, TE is a measure of predictability and thus may capture the notion of emergent computation but not true causal emergence. Identifying causal influences requires an interventionist approach [
40], which is not capture by TE [
41]. These types of analyses therefore need to be extended to investigate the
causal flow of information. A measure of “information flow” (IF) that accurately captures casual effects has been proposed by Ay and Polani [
42], where causality is detected by using a measure similar to TE that replaces observational conditioning on the probability distributions (as used in TE) with interventional conditioning [
40]. However, thus far, IF has been applied to identifying local (micro)-level causation only and not to understanding global causal architecture.
A different measure of causal information that does capture global architecture is provided under the framework of integrated information theory (IIT). “Integrated information” (
) was originally devised by Tononi as a possible measure of consciousness [
43]. In addition to quantifying conscious experience, it may also be a contender for defining life.
quantifies how much information is generated by a physical system when it enters a particular state through the causal interactions among its elements, above and beyond the information generated independently by its parts [
44,
45]. More specifically,
is the effective information (
) of the minimum partition determined by the systems “wholeness” [
44,
46].
characterizes the information generated by the causal structure of a network, when it enters a particular state. The effective information for each physically realized state is the relative entropy of the
a posteriori repertoire with respect to the
a priori repertoire of the network. The
a priori repertoire is calculated by intervening on the system and perturbing it into all states with equal likelihood; in other words, it is the maximum entropy distribution. The
a posteriori repertoire is defined as the repertoire of possible states that could have led to the realized state through the causal mechanisms of the system. As such,
measures how much the causal mechanisms of the system reduce the uncertainty about the possible causes for a given state. IIT thus potentially provides a framework for understanding how constructors, which define the causal mechanisms of physical systems, can generate physically manifest information by eliminating counterfactuals.
can be calculated at any organizational level (e.g., by coarse-graining) and has been implemented by Hoel
et al. as a measure of causal emergence by comparing the
at microscopic and macroscopic levels of organization [
47]. They showed that in simple networks with fixed microscopic causal mechanisms
peaks at a macroscopic level in space and/or time rather than at a microscopic level for certain causal architectures. These measures hold promise for identifying the appropriate formalism for characterizing the causal structure of living systems. However, much work remains to be done.
5. Top-down Causation in Chemical Evolution
The prominent role of top-down causation in extant life suggests that many of the transitions in the complexity of chemical reaction networks leading to life’s emergence may be characterizable as transitions in informational and causal architecture. The hope is that these may eventually be identifiable using the results of approaches as outlined in
Section 4. In addition to the potential to measure states of life, non-life and almost-life, there are many other insights to be gained by considering explicitly the causal role of information in studies of the emergence of life. Here we briefly discuss a few of the insights that derive from this perspective, which may have the potential to shed new light into the processes driving prebiotic evolution and the origin of life.
Top-down causation through functional equivalency permits alternative chemistries for life to be viable functional alternatives to natural biochemical systems. These alternative biochemistries represent different physical instantiations that have not been explored by extant biology but can be studied through synthetic biology. This includes alternative nucleic acids (other than DNA or RNA), such as expanded genetic alphabets (
i.e., more than two base pairs) or nucleic acids with modified backbone sugars. A particularly striking example is the recent engineering of a bacterium to host an additional unnatural DNA base pair synthetically incorporated in its genome [
48]. This is the first successful demonstration of an organism stably incorporating an expanded genetic alphabet in its genome over successive generations. This represents an example of an alternative instantiation of the replication master function [
14,
17]: both the genomes of natural life and of this synthetically engineered life are members of the same functional equivalence class for replication within the cellular operating system.
The capacity for synthetic biologists to engineer functionally equivalent chemistries for life opens the possibility to explore the alternative chemical hardware that early life may have utilized. Typically the functional equivalence class most frequently studied by prebiotic chemists is replication of genetic material (though this effort is not typically labeled as such). The transition from RNA-based to DNA-based life forms represents a “genetic takeover” that required maintaining higher level functions—such as replication of genetic information—through functional equivalency. The notion of genetic takeover was first popularized by Graham Cairns-Smith to describe how life could have started with genetic information encoded in clays, which eventually transitioned to being stored in more familiar organic genetic polymers through information transfer between the inorganic and organic substrates [
49]. More generally, genetic takeover can apply to the transfer of information between any two genetic systems, including between different nucleic acid species [
30]. Examples of possible alternative nucleic acids include TNA (
α-L-threofuranosyl nucleic acid) and GNA (glycol nucleic acid), each of which can exchange genetic information with RNA [
50]. This suggests that either TNA or GNA could have preceded RNA in the evolution of the genetic system of terrestrial life. TNA, GNA and RNA—although structurally different molecules—are therefore members of the same functional equivalence class. Additionally, six synthetic genetic polymers (including TNA) have been shown to be capable of information storage, retrieval (through transcription onto DNA) and functional folding [
51], thus demonstrating the existence of a functional equivalence class of nucleic acids suitable for the replication master function in modern cells. Here, the members of the equivalence class do not need to have the capacity to exchange information with each other directly: information, in this case, is preserved and exchanged only through higher-level function.
The earliest stages of evolution may have exploited functional equivalency in a different manner than modern organisms do. Prebiotic synthesis is notorious for generating nonspecific products. An example is provided by the prebiotic synthesis of RNA, which typically yields 2’-5’ backbone linkages, whereas in modern life RNA polymers have exclusively 3’-5’ linkages. Early RNA would have therefore represented a mixed population of polymers with non-heritable information in the sequence of backbone linkages. RNA molecules with random compositions of mixed backbones of 2’-5’ and 3’-5’ linkages have been shown to fold and retain the capacity for molecular recognition [
52]. This suggests that in a prebiotic environment, low yield of nonspecific synthesis products may have provided sufficient fuel for evolution, with selection operating on classes of functional equivalent molecules, rather than on specific target structures. In this “top-down” view of prebiotic evolution, functional equivalence classes would have emerged first, as a result of selection on function, followed by refinement of the particular molecular instantiation as specificity evolved and the relevant functional equivalence class(es) became fixed in the population. This model for early evolution is advantageous in that it allows nascent life to take advantage of the mess of prebiotic chemistry, rather than viewing the nonspecific products of prebiotic synthesis solely as a hindrance to evolutionary progress [
53]. It is also amenable to laboratory study, for example by performing
in vitro selection experiments on nucleic acids of mixed composition and determining what compositions might enable robust selection for function over a large class of molecular compositions.
Selection for functional equivalence would have also operated at the level of entire reaction networks in addition to classes of molecular compounds within a network. The notion of functional equivalence is closely related to that of biological modularity, where a module is a separable functional unit within a biological network. Modules may be replaced by other members of their functional equivalence class without affecting the global network structure. Modularity has been demonstrated to emerge spontaneously in the formation of autocatalytic sets (collections of entities where production of each member is catalyzed by another member of the set) [
54]. This may have been an important step in the evolution of the decoupling of information storage and processing: modules would permit evolutionary refinement of particular instantiations of functional equivalence classes in the top-down manner described above, thus also permitting storage of information about particular functional equivalence classes, rather than their precise chemical instantiation.
While evolution has been shaped by functional equivalency, it does not necessarily mean that one could construct early life by replacing the parts of modern cells with their ancient counterparts. For example, one might wonder if riboorganisms could be resurrected in the laboratory by starting with replacing all functions mediated by protein or DNA in modern cells (see e.g., [
14]). In practice this would lead to several complications, the most significant of which is the potential to mix stored information with its execution. Riboorganisms populated a world prior to the origin of translation. Riboorganisms therefore represent a different functional equivalence class than modern life (constructor and replicator functions cannot be easily identified for riboorganisms, as they can for modern cells) and could therefore potentially represent a distinct instantiation of life from that of any known extant life.