Abstract
Pharmaceutical production typically focuses on individual drug types for each production line, which limits flexibility. However, the emergence of Industry 4.0 technologies presents new opportunities for more adaptable and customized manufacturing processes. Despite this promise, the development of innovative design techniques for pharmaceutical production equipment remains incomplete. Manufacturers encounter challenges due to rapid innovation cycles while adhering to stringent Good Manufacturing Practice (GMP) standards. Our research addresses this issue by introducing an information model that organizes the design, development, and testing of pharmaceutical manufacturing equipment. This model is based on an exploratory review of 176 articles concerning design principles in regulated industries and integrates concepts from Axiomatic Design, Quality by Design, Model-Based Systems Engineering, and the V-Model framework. Further refinement was achieved through insights from 10 industry experts. The resultant workflow-based information model can be implemented as software to enhance engineering and project management. This research offers a structured framework that enables pharmaceutical equipment manufacturers and users to collaboratively develop solutions in an iterative manner, effectively closing the gap between industry needs and systematic design methodologies.
1. Introduction
Industry 4.0 has been a common topic among scholars and professionals. There are ongoing discussions about different ways to approach the inherited idea of a smart factory that allows mass customization of consumer goods with little effort, and thus being affordable to a broad customer base [1,2].
Similar discussions occur within the pharmaceutical industry. Widely used technologies and production methods are challenged. Practices and business models for pharmaceutical manufacturing have been approached using new ways of thinking [3,4].
Compared to less regulated branches, pharma companies are often more cautious when it comes to the use of new technologies [3]. This behavior is a result of the rules governing the whole drug-producing industry, called current good manufacturing principles (cGMPs) [5]. This set of rules evolved over time as a reaction to different situations in which drugs caused harm to humans. The combination of strict rules and authorities that oversee the implementation and use of the measures given by the regulations is often considered counterproductive for new ways of thinking. Food and Drug Authority (FDA) representatives state the opposite by saying that these rules do not focus on the used technology but rather on the quality of the output, which has to be as expected by the patients [6]. Regulatory agencies also contribute actively to new ways of working, for example, by new guidelines for continuous manufacturing of drug substances and products [7].
Besides the official rules from organizations such as the World Health Organization (WHO), the FDA, or the European Medicines Agency (EMA), there are non-profit organizations that try to bring together regulators, equipment manufacturers, and productive companies. These associations help the industry with guidelines like the Good Automated Manufacturing Practice (GAMP5), published by the International Society for Pharmaceutical Engineering (ISPE) [8]. Another source of information is standardization organizations like the former American Society for Testing and Materials (ASTM), which released their Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment.
From where we stand, these rules and guidelines focus more on the specification of the best possible outcome of a design and verification process than on the development process for new hardware equipment and software itself. The need to implement new methods and ways of thinking to drive the design of smart factory equipment for the pharmaceutical shop floor motivates the creation of an information model to guide the engineering process [9].
This is precisely the focus of this research, which aims to propose and test an innovative and modular information model that brings together needs from technology development, manufacturing, and regulatory perspectives, to establish a basis of trust while implementing new ways of thinking in design and development. The existing techniques, like the V-Model proposed in the GAMP5 [8] guidelines, form a basis for our research, which concentrates on the thoughts and engineering work that have to be performed around the specifications. We focus on the connection of information to establish a transparent equipment development process for the regulated pharmaceutical industry.
The current literature and industry landscape heavily favor a waterfall-based approach to equipment specification and construction. In accordance with GAMP5 [8], the processes guided by the V-Model rely on static documents such as the User Requirement Specification (URS), which serves as a foundational document for manufacturers during design and development. Testing and validation processes depend on documents detailing functional specifications (FS) and installation (IQ), operation (OQ), or performance (PQ) qualifications. These documents lack interconnections and dependencies that would help navigate their correlations, posing challenges when changes in requirements affect the entire documentation chain. For equipment manufacturers, the demand for quicker innovation cycles and reduced time to market calls for new collaboration strategies between users and vendors in engineer-to-order (ETO) projects. Currently, collaboration in innovation projects is insufficiently represented in the design methods used within the regulated pharmaceutical sector.
This research creates an information model based on model-driven design principles to aid the agile-like engineering of pharmaceutical equipment compliant with pharma and Industry 4.0 standards. By shifting from conventional waterfall methods to an iterative, collaborative approach, the model promotes ongoing integration of regulatory requirements and technological progress. It supports structured data exchange and encourages innovation by offering a unified framework accessible to all stakeholders, thereby enhancing collaboration between pharmaceutical manufacturers and equipment suppliers.
To achieve the research objective, the literature review conducted to develop the first theoretical model is described. Then, the model is explained in detail. Afterward, the empirical methodology used to test the model with the experts is described, as well as the corresponding adjustments made to the model based on the experts’ feedback. Finally, the discussion and conclusion are presented.
2. Literature Review Process
To develop the initial model with a focus on the design process for innovative pharma equipment for drug production, scientific documents from ISI Web of Science—Current Contents Connect—were obtained. All searches were performed using the title, abstract, author, and keywords.
- “Design Principles AND Pharma* AND Equipment”
- “Quality by Design AND Pharma* AND Equipment”
- “Equipment Design AND GMP”
- “Modular Equipment Design AND Design Theory”
- “Design Theory AND Pharma”
- “Design Theory AND GMP”
- “Design AND Pharma 4.0”
These are topics that bring together design theory for software and hardware, relevant for machine equipment with a focus on modular solutions and Industry 4.0. Articles that discussed design principles for Internet of Things (IoT) development and testing approaches were also considered, together with articles studying tasks and achievements for drug production.
Articles that studied drug development or respective laboratory equipment were excluded since the research concentrates on pharma equipment for drug production, not research and development of a product. Also, if the main focus was laboratory automation or the construction of facilities, these articles were excluded.
After screening, the relevant articles were transferred to NVivo for coding and used as input for the initial model. The final visual representation of the initial model was inspired by a swimlane diagram containing the main logic structure and information. All the steps of the literature review process are presented in Figure 1.
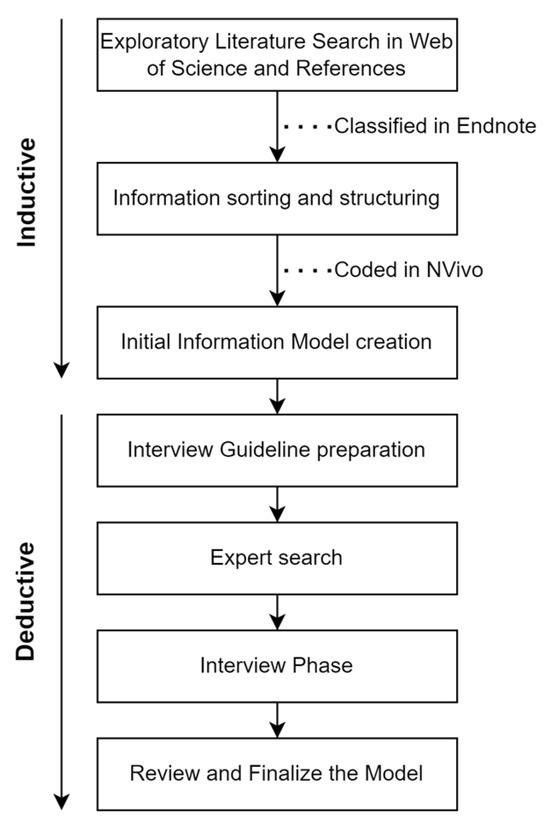
Figure 1.
The research approach used to develop the model.
3. Initial Model
The initial model was developed based on the literature and has as its main structure a set of sequential actions with information dependencies from initial specification to implementation and testing. It is presented in Figure 2.
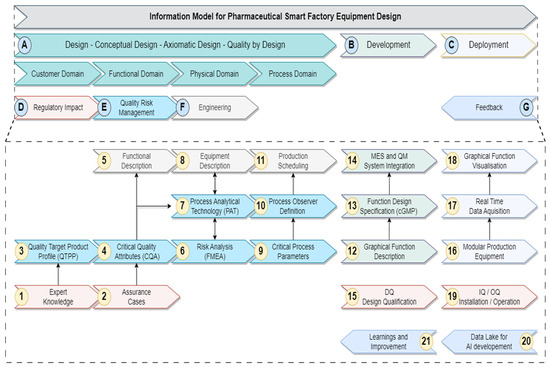
Figure 2.
Initial model.
The logical structure of this information model for smart pharmaceutical equipment design can be split into three main phases. First of all, the Design Phase is where user and product demands are transformed into equipment and process requirements. Quality risk management techniques are in place to guide the data gathering and evaluation process, which forms a valid basis for the following engineering tasks and later on validation tests. The second step is the Development Phase, in which the designed equipment and process concepts are implemented and verified. This includes qualification and validation actions. The third phase is the Deployment Phase, in which processes and machinery are operational and produce pharmaceutical goods in a modular, organized, and thus reconfigurable production.
Table A1, which can be found in Appendix A and Appendix B, explains the building blocks and information dependencies of the initial model. First, the core model is explained, indicated by capital letters. Second, the more detailed concept, which is derived from the core concept, is presented with numerical indicators.
Design and development of equipment for use in pharmaceutical production come with the need to document dependencies between the different engineering tasks, such as mechanical, electronic, and software design. Later changes to hardware—or software—need to be analyzed for risks to the overall process and the resulting product quality [8]. To have these interdependencies transparent to interdisciplinary teams, a model-based design and digital validation workflow can be a possible solution. The benefits of this combination can be auto-generated and tested source codes for automation equipment that comes with validation documentation of the underlying logic and its dependencies with the mechanical subsystems [10].
Systematic prospective analysis of product and process characteristics during their design phase is proposed under the term Quality by Design (QbD) [11]. The main contributions of QbD are specifications and control parameters that enable processes to achieve predefined quality characteristics [12].
The ICH guideline Q8 (R2) on pharmaceutical development (2017) [13] gives instructions on how to use a quality-risk-based approach for the development of drug substances and manufacturing processes. According to this guideline, the basis for development is a set of measurable product properties that are most relevant for the product quality, named Quality Target Product Profile (QTPP). Based on this set of data, Critical Quality Attributes (CQA) can be derived. These are physical, chemical, biological, or microbiological characteristics that are decisive for the product’s ability to meet the QTPP and need to be in a dedicated range during the production process or in the product itself.
Lean production, designed as a modular and reconfigurable factory, aims to shift the traditional make-to-stock production (push-production) to a make-to-order production (pull-production) [14]. In the pharma context, the same pattern has been discovered, as there is a change in production design from batch (push-production) to personalized drug production (pull-production). Kulak et al. [14] define the flexibility of a manufacturing system by its “speed and ability to respond to rapidly changing customer needs”. To achieve such a design in the highly regulated pharma environment, a design framework that supports decision-making along the solution-finding process is welcome. Axiomatic Design (AD) offers such guidelines and helps to reduce waste associated with the trial and error method, and is dedicated to flexible system design [15].
To accomplish the goal of pharma-ready production, AD offers a systematic search process through different design steps. With AD, a solution approach can be proposed that has the ability to fit very small and well-described problems as well as design studies for complete production facilities. Suh [16] emphasizes that his AD theory fits among others in manufacturing, software, or system design tasks. This set of implemented possibilities seems to be a promising approach for smart manufacturing design in the pharma context.
The principle of AD is based on two axioms with the definition that an axiom is a “principle or self-evident truth that cannot be derived or proven to be true except that there are no counter-examples or exceptions” [17]. The first axiom is the Independence Axiom, which states that the Functional Requirements (FR) defined within the Functional Domain need to be selected in a way that none of them influences any other. The second axiom is the Information Axiom, which declares that the best solution of the ones that satisfy the FR is the one with the least amount of information necessary to describe them [16]. AD knows four different domains that follow each other in consecutive order. From the Customer Domain, Functional Domain, and Physical Domain to the Process Domain. These four steps can be applied to many different facets of solution finding within the smart pharmaceutical equipment design process.
4. Methodology
In order to propose a new model, which took as its starting point a framework created based on the literature and explained above, a set of interviews was conducted with experts in the field of pharmaceutical production, quality management, and pharmaceutical production engineering.
Expert interviews are widely used when it comes to topics like digitalization or the development of new ways of working in the technology sector. Haselbock et al. [18] used ten domain experts to identify relevant design areas for a microservice design approach and assessed its importance. Liu et al. [19] used a systematic literature review and expert interviews to develop a design framework for smart connected products. Lutz et al. [20] used scoping reviews and expert interviews to elaborate on how social robots impact privacy. Mergel et al. [21] described in their article that a conceptual framework covering digital transformation in the public sector can be derived from expert interviews. Withanagamage et al. [22] used content analysis in existing literature and expert interviews to develop a conceptual framework to assess the necessity of being agile in an industrial environment.
The selection of interview partners resulted from talks at conferences or direct requests through project-based contacts. The experts were chosen based on their knowledge of the subject and professional experience in the industry or regulatory organizations. Thus, ten experts were selected to discuss and evaluate the initial model. After six interviews, the model was reviewed and updated. Four more interviews were conducted to finalize the model. All interview data are presented as anonymized.
The interview questions were based on the initial model and the context of its application, as shown in Table 1. All experts had the same interview guide.

Table 1.
Interview guideline.
The interviews were conducted in person and through online meetings. All participants were asked for their consent to the anonymized processing of their responses for scientific work. The interview and discussion generated were structured along the logical order given by the framework and started with general questions about the theoretical background and previous experiences of the interview partners with Industry 4.0 and equipment design for smart factories. After a brief graphic presentation of the theoretical framework by the researcher, an open discussion followed.
The interviews were anonymized, audiotaped, transcribed, and analyzed using content analysis. To structure the analysis, a table was set up carrying all the main statements of the initial model. The experts’ opinions on open questions or their reactions to partial aspects of the model were mapped to these predefined categories. If none of the prepared matched the experts’ statement, a new subset of information was created below the given main categories. The logical structure of this table was used to easily identify the reaction to individual model elements. The additional feedback on categories that did not exist in the initial table pointed directly to missing elements within the model and was used to improve it.
5. Results
5.1. Sample Profile
Table 2 summarizes the profile of the partners interviewed; 90% of respondents have professional experience in the pharmaceutical business and are ranked at a senior level or higher. Candidate 2 (C2) has only two years of experience, but his role in the company is market search and trend scouting for new technology, which was worth keeping the input from the author’s perspective. The peer group was selected based on their professional background to gain relevant input for the design framework. The chosen cluster to select from was Process or Production Engineering (Eng.) in combination with Industry 4.0 (Industry 4.0) knowledge, Project Management for the acquisition of automated pharmaceutical equipment (PM), or Quality Management for automated equipment (QM).

Table 2.
Interview participants.
5.2. Content Analysis
5.2.1. Digital Transformation in Pharma
The interviewees were asked to talk about their point of view on digitalization in pharma and the relevance of information integration during complex projects that aim for new machine concepts for smart factories. Overall, digitalization is seen as a way to capture, transfer, connect, evaluate, and present information, or more generally, to have information right at the time and location where it is needed:
- [Digitalization is] “a general way to share and connect knowledge” (C3);
- “Aggregation and processing of data to evaluate, optimise and describe processes” (C5);
- “Information at any time at any place by mobile devices” (C6).
5.2.2. Data Quality and Information Transparency
The quality of data is most important in that context, as wrong decisions based on incorrect datasets can negatively affect patients. Three of the candidates claim that there is a need for more standardized ways to share data among machines or projects through modularization and standardization of datasets:
- “Data is extremely relevant for product and issue tracking in Pharma” (C2);
- “Connection of machines via OPC UA is a must have in today’s digital shopfloor” (C7).
Usability and data transparency are still underrated as top priorities of different tools coming with digitalization approaches. Specification and documentation of decisions along projects is also a process that needs to be upgraded in terms of standardized documents with templates and ways to share knowledge and highlight decisions:
- “Technical documentation should be shared between both [supplier and user] in electronic way […] with searchable structures” (C7).
5.2.3. Forms of Sharing Information
Comparing direct and visual communication against messaging services or ticket systems was a task for all candidates. The feedback was to have human-to-human interaction when it comes to decisions because they trust more when there is visual feedback than plain text in an email. All agree that documentation of decisions is important. A written agreement among all involved is obligatory. As there is constant change in everything again, data quality is also important in this regard. Knowing what points were discussed and in what context is important for understanding the problem. A summary of these points shall be part of the project documentation. This kind of transparency is important for all candidates to ask. Some feedback related to this pointed to the different phases within an equipment lifecycle. The better documented a project is, the easier it is to find information when it comes to changes to the machine:
- “Ideal for qualification purposes in pharma, it really helps when it comes to changes” (C7).
Today’s way of documentation often does not consider that most of the people who have to work with that data are not part of the project team at that moment, and therefore, it is important to have a clear structure in how datasets are archived. To avoid databases becoming silos because of a complex structure or limited access possibilities, all candidates agree that this is an important but still underrated task:
- “A lifecycle documentation for equipment or devices is important when it comes to revalidation and the team changed meanwhile” (C6).
5.2.4. Information Model Principles
The principles of AD and QbD were understood very well during a short introduction. The concept of following a one-on-one relationship between functional description and physical implementation, as AD proposes, sounds very promising to be applicable; the more complex a task, the more relevant such a structured approach is:
- “By that [the use of a model-based engineering] easier to find the root cause of a problem” (C3).
The way the current framework defines its starting point in terms of information from experts is not enough because the definition of Regulatory Impact is not correct and shall be redefined, as there are general rules and regulations that force the production of drugs to be strict in their way of working. The feedback includes that overall, the executing organization, as one of the three pillars in pharma production (Production, Engineering, Quality Management), is underrated in this framework. In this context, Assurance Cases are described as unusual wording.
Overall positive feedback was obtained from the use of QbD, the interdependencies to FMEA and Process Parameters, including an Observer instance, and the clear structure. The different domains are commented on as suitable and promising for a well-structured design process.
The use of graphical means to explain and monitor process functionality and parameters is very well acknowledged as an innovative approach to bringing process knowledge to all stakeholders along the equipment lifecycle. The clear structure of the model can make a difference when it comes to the storage of data and using it as a recommendation system for upcoming tasks:
- “[This] Approach fits very well to a graphical description of the production timeline with trend graphs and images in one document” (C7).
During the open questions of the interviews, it became clear how important a clear project and information structure are to all the experts. The proposed model offers the possibility to link information to sequential steps, which covers the need for repeatability and data quality because the context is defined by the model.
- “When concepts are designed together, this approach is useful”, “When it comes to decisions for new products or equipment updates, [it] could be very good to have the model context” (C9).
6. Proposed Model
The use of AD was commented on as an innovative approach, and therefore, it remains a core principle for the conceptual design phase.
One important learning is the clear definition that a pharmaceutical production operation consists of three pillars, namely Quality Management, Engineering, and Production. To take this into account, the reviewed model (Figure 3) has an additional track covering the production information subset. The relevant actions in this track are the definition of the Product Requirements, Process Requirements, and specification of the requirements according to Modular Production Equipment.
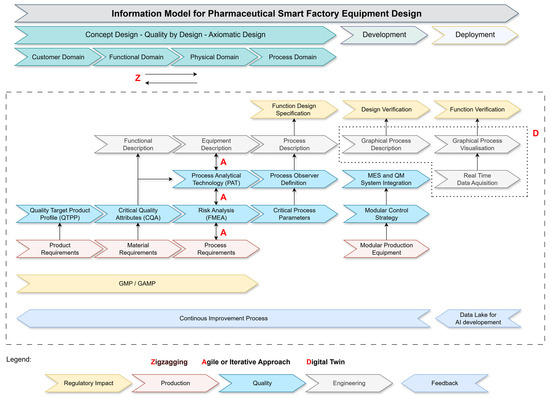
Figure 3.
Proposed model.
Regulations come from the GMP (Good Manufacturing Practice) and are enhanced by the GAMP (Good Automated Manufacturing Practice) framework and need to be followed by all persons and equipment in charge of the execution of the production. To follow the idea of a frequently discussed standard, the GAMP V-Model, the Regulatory Impact Track receives an updated visual representation of the model. As regulations are the backbone of every decision and the starting point of the design phase, testing of the equipment against the governing rules coming from regulations is mandatory. To achieve a visible representation of the underlying concept, the model now has GMP/GAMP as the basis for Design and Function Design Specification (FDS), Design Verification, and Function Verification as the last actions of the individual phase.
As there was feedback regarding the unclear definition of the term Assurance Cases as input at the very beginning of the model, the more general term product requirement is used in the improved framework.
The design steps within the Physical Domain were discussed as process steps that need iterative or agile methods to produce the best possible outcome. The reviewed model carries this information and, therefore, takes care of the methodical approach known as zigzagging in the Axiomatic Design principle.
The repeatedly expressed demand for modular production equipment in the pharmaceutical sector creates a key information dependency known as the Modular Control Strategy. This strategy is vital for ensuring smooth integration between Manufacturing Execution Systems (MESs) and Quality Management (QM) systems. While modularization can lead to challenges such as additional and more complicated interfaces, we refer to established industry standards like ISA-88 [23] for Batch Control and ISA-95 [24] for system architecture. The modular control method allows for the creation of standardized and easy-to-maintain interfaces interconnecting the various subfunctions integrated into the modules.
Within the context of Industry 4.0, the proposed model tackles this issue by incorporating Cyber-Physical Systems (CPSs) (also called Digital Twins (DTs)) as core components of the contemporary production equipment development architecture. These technologies enable the development of a digital twin for each physical asset, allowing for ongoing synchronization and contextual data exchange across the system’s lifecycle.
The revised model combines graphical process representation, real-time data collection, and smart process visualization into a cohesive information layer that fosters semantic interoperability and digital continuity. This methodology ensures a two-way information flow between physical equipment and its digital counterpart, which facilitates adaptive control strategies, improves traceability, and supports predictive decision-making. By aligning with Industry 4.0 principles, such as decentralized intelligence, plug-and-produce functionality, and data-driven lifecycle management, the model advances the development of intelligent, modular, and compliant pharmaceutical manufacturing environments.
To use this model to train improvement and recommendation systems, as discussed with the expert group, it is necessary to spread the feedback path across the complete design and development phases. With that change, the more common term in pharma, Continuous Improvement Process (CIP), is used.
7. Discussion
The initial model results from exploratory literature research covering many different facets of the tasks that come with the design of pharmaceutical equipment. The need to have a framework to support such development projects comes with the rising complexity and demand for short time-to-market solutions.
The main challenge in this research was the impact of Industry 4.0 on design and implementation strategies in the pharmaceutical shopfloor, which can be found in topics like modular production concepts or Digital Twin [9]. This model requires collaboration across various sectors and topics to determine the best approach, focusing on the pharmaceutical industry while also facilitating the production of specialized equipment. Both were addressed in our proposed model. Modular equipment is the target of the design process when it comes to the needs of the production as a stakeholder. To have a digital twin as a counterpart of the physical equipment is implemented as a beneficial part of supporting the design and function verification process within the project. The information model shall guide the equipment design to produce a graphical process description and visualization that can be coupled with real-time data to perform simulated tests to prove the equipment’s behavior without the need to run real experiments.
Developing new pharmaceutical equipment is a challenging endeavor that requires expertise from multiple disciplines, which is also true for creating this model. As the regulatory impact is a quality assurance method, there are helpful guidelines and tools to achieve conformity. One well-accepted technique to support high-quality development of customized applications is the V-Model described in the GAMP 5 guidelines [8]. Its main focus can be described as a model-based approach in which each component has the goal of detailing a more specific subpart of the overall application. According to the model, every specification has to come with a testing strategy to make sure that the implementation fits the requirements. The approach in GAMP 5 adds a risk-based approach to this well-known conceptual design strategy (Figure A1).
This model has limitations due to its general nature, which prevents it from supporting all applications in detail. However, similar approaches have proven effective in software development projects [25]. Model-based design can result in under- or oversimplification, especially if the model fails to accurately represent the real scenario. Consequently, this can leave unaddressed tasks that are essential for building and operating the equipment without further guidance. Additionally, collaborative strategies in complex projects can result in unmonitored decisions without a structured workflow to reduce this risk. This unintended use, often stemming from insufficient training or a lack of understanding of the model’s logic, can result in inaccurate system modeling, which subsequently causes quality deviations that the model cannot effectively handle. In particular, inadequate requirement analysis can lead to incorrect information processing throughout the model.
To believe that the pure V-Model precisely reflects a development process could lead to false classification of the project complexity; thus, adoption of the original model can be a measure to lower this risk [26]. Our proposed model instead takes the basic idea of the V-Model (user requirements as input, functional decompensation by AD, release testing as output) and fits the necessary information processing along the project lifecycle and its dependencies in between. It not only supports the idea of working with specifications and test requirements, but it is its built-in structure that enforces all involved parties to keep the origin and relevance of information in every step. Our proposed model is a guideline to perform actions that can be achieved with the V-Model, but with more structure and density of information. Our approach gives an in-depth view of the ideal design process for pharmaceutical equipment:
- “It describes the perfect process very well” (C6);
- “That would be the ideal development process” (C2).
Building smart factory-ready pharmaceutical equipment not only requires modular mechatronic design but also documentation and testing according to cGMPs [9]. The V-Model does not incorporate current trends like QbD or PAT in detail because it is very general. Our structure implements state-of-the-art techniques and their interrelation to achieve the best possible and transparent knowledge transfer along a development project. For example, the proposed model makes sure that the functional requirements coming from the product requirements are transferred to the design parameter, which becomes implemented in the development phase.
As a lack of process understanding is often the cause of over-dimensioned and thus time-intensive validation cycles, the FDA is calling for measures that bring this knowledge to the production and operation levels [27]. Our proposed model contributes to this initiative as it brings an understanding of the origin of design specifications and the interdependencies of information to the production and qualification personnel through its fully transparent information model. This also contributes to more trustworthy software as it combines test-driven and model-driven approaches within one framework [28].
Model-based design or engineering faces some disadvantages, like its cost-intensive implementation or low possibility in terms of early prototyping [26]. Our proposed model ensures that the engineering of pharma equipment is efficient. Through the information model, relevant information is available whenever needed for the various design steps. By that, the cost of implementation can be returned.
Further research should have a close look at the possible cost advantages of a model-based design over plan-driven engineering.
From an ecological viewpoint, reducing waste during development yields beneficial outcomes. Waste can encompass prototyping cycles, resolving errors due to product design flaws, or late project modifications affecting already built equipment components. Sustainability matrices can be incorporated into the developed framework to ensure the best possible results regarding emissions of various types. In the pharmaceutical sector, product quality will remain the highest priority among all measures. However, with a modeling approach, the positive impacts on environmental effects can be evaluated throughout the entire system lifecycle, starting even in the early design phase, as the model maintains various operational information that can be used to optimize emissions while preserving superior product quality.
Following a model-driven approach does not guarantee success; there are things like usage policies, training, and tool support that need to be taken care of. Especially for engineering teams trained in Waterfall methods, an agile or model-based approach can result in cultural resistance and misalignment if not implemented thoughtfully (e.g., applied change management). Also, a late change in the project requirements means that the complete model needs to be reviewed to be in line [28]. In the pharma context, this is not really a disadvantage of our model, as there is a need to specify and verify all product-relevant actions. It is more of a quality assurance mechanism of such an information model, which takes care of the origin and use of information.
By the use of qualitative research, there is still a risk that our model has not covered all relevant aspects. Furthermore, a larger number of experts could have led to deeper insights and statistical measures to improve the model.
Nevertheless, the common feedback from the expert group shows that the model represents the ideal work and information flow of a design process for pharmaceutical equipment. Further testing with real-world design tasks will evaluate applicability in different scenarios.
8. Conclusions
Today’s iterative development of pharma-relevant software, equipment, and processes seems not to be feasible for innovative production concepts that need to have in-concept quality assurance approaches with a short time-to-market. The goal of the conducted research was to formulate and test a novel information model to support a design framework that visualizes and structures the design process for pharma equipment.
The proposed concept shows that the design process can be structured as a workflow with an in-built information model. Evaluation through the lens of subject matter experts provides relevance and applicability for the desired circumstances in pharmaceutical production. The overall feedback showed that the model-based design approach is a future-proof concept in this regulated environment.
Further research is necessary to detail, improve, and test the information dependencies between the individual actions.
The newly developed design procedure establishes a strong foundation for testing the suggested information structure in actual development projects within the pharmaceutical manufacturing sector. The model’s behavior can be assessed through a case study focusing on innovative production concepts.
Future work involving this model could focus on integrating it into a user-friendly collaboration tool. We envision developing a design assistant based on an ontology (or language) model that utilizes our current research as thematic guidance. This forthcoming software could incorporate substantial information and quality mechanisms derived from our current research to offer a customized assistant for agile project and information management in complex pharma projects.
Our research contributes to filling the gap in the literature that does not yet give applicable instructions to transform the needs of pharmaceutical manufacturing into a structured design process for the supplying industry.
Author Contributions
Conceptualization, R.W.; methodology, R.W.; validation, L.T. and I.S.-A.; formal analysis, R.W.; investigation, R.W.; writing—original draft preparation, R.W.; writing—review and editing, L.T. and I.S.-A.; visualization, R.W.; supervision, L.T. and I.S.-A.; project administration, R.W. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was developed in the scope of IDU at pester pac automation GmbH, NECE-UBI, Research Centre for Business Sciences (UIDB/04630/2020), and the Institute of Electronics and Informatics Engineering of Aveiro (IEETA) (UIDB/00127/2020), both funded by national funds through FCT—Fundação para a Ciência e a Tecnologia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Since this study did not use sensitive data, but only collected the perceptions of professionals (based on interviews) to design a model of a system, no code of ethics was defined.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Roland Wölfle is employed by pester pac automation GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| cGMP | current Good Manufacturing Practice |
| FDA | Food and Drug Authority |
| WHO | World Health Organization |
| EMA | European Medicines Agency |
| ISPE | International Society for Pharmaceutical Engineering |
| ASTM | American Society for Testing and Materials |
| IOT | Internet of Things |
| QbD | Quality by Design |
| QTPP | Quality Target Product Profile |
| CQA | Critical Quality Attributes |
| AD | Axiomatic Design |
| FR | Functional Requirements |
| FMEA | Failure Modes and Effects Analysis |
| FDS | Function Design Specification |
| DT | Digital Twin |
| CPS | Cyber-Physical System |
| CIP | Continuous Improvement Process |
| PAT | Process Analytical Technology |
Appendix A
Table A1 explains the building blocks and information dependencies of the initial model. The core model is explained by capital letters. Second, the more detailed concept, which is derived from the core concept, is presented with numerical indicators.

Table A1.
Information dependencies table of the initial model.
Table A1.
Information dependencies table of the initial model.
| ID | Element | Definition | Informed by Element | Domain | Academic Source | Legal Source |
|---|---|---|---|---|---|---|
| A | Design | Conceptual design of the equipment that takes care of all GMP-relevant aspects to ensure the best possible information basis for the development process | - | Conceptual Design, Axiomatic Design, Quality by Design | Kulak, Durmusoglu and Tufekci [14], Vinodh [15], Suh [17], Cavique, et al. [29], Pecoraro, et al. [30], Puik and Ceglarek [31], Suh [16], Suh, et al. [32], Aulakh and Gill [33] | - |
| B | Development | Defined goals that lead engineering and manufacturing of the equipment by Computer Aided Engineering (CAE) and Software Development | Design | Development of hard- and software components | See A | - |
| C | Deployment | Commissioning and testing the equipment | Development | Implementation and testing of the developed components | See A | - |
| D | Regulation | Legal basis that forms the unique set of rules within the pharmaceutical branch | Legal Framework | Arden, Fisher, Tyner, Yu, Lee and Kopcha [3], Lee, et al. [34] | ISPE [8] | |
| E | Pharmaceutical Quality System (PQS), Quality Risk Management (QRM) | Set of measurements and processes that take care of pharmaceutical quality standards | Regulation | Implementation of the legal framework | Yu [12], Grangeia, et al. [35], Rantanen and Khinast [36], Schmidt, et al. [37], Su, et al. [38], Yu, et al. [39] | ICH [7,40,41] |
| F | Engineering | Technology-related subset of the design process that takes care of the applicability of requests given by the QRM | PQS, QRM | Puik and Ceglarek [31], Rantanen and Khinast [36], Bano, et al. [42], Both, et al. [43], Pokojski, et al. [44], Setti, et al. [45], Testa, et al. [46], Uysal and Mergen [47] | ||
| G | Feedback | Continuous feedback to all contributors to ensure the improvement of product and process quality | Design, Development, Deployment | Continual Improvement Strategy | Pokojski, Szustakiewicz, Woznicki, Oleksinski and Pruszynski [44], Duran [48] | ICH [41] |
| 1 | Expert Knowledge | All requirements that address the main goals that need to be achieved; these are not yet classified in the product or process domain | User Requirement Specification (URS) | Regulations | Weng and Jenq [49], Amorosi [50] | |
| 2 | Assurance Cases | Learnings from past events that led to problematic situations that must be avoided in future | Expert Knowledge | Regulations | Yu [12], Schmidt, Frey, Hillen, Horbelt, Schandar, Schneider and Sorokos [37], Yu, Amidon, Khan, Hoag, Polli, Raju and Woodcock [39], Zhang, et al. [51] | |
| 3 | Quality Target Product Profile (QTPP) | Description of the target product profile as best-case scenario that needs to be accomplished | Expert Knowledge | QbD | Yu [12], Lee, O’Connor, Yang, Cruz, Chatterjee, Madurawe, Moore, Yu and Woodcock [34], Grangeia, Silva, Simoes and Reis [35], Yu, Amidon, Khan, Hoag, Polli, Raju and Woodcock [39], Tian, et al. [52] | ICH [13] |
| 4 | Critical Quality Attributes (CQA) | Set of attributes that are directly impacting the QTPP | QTTP, Assurance Cases | QbD | ||
| 5 | Functional Description | Result of the Axiomatic Design process step, in which all functional requirements are listed | CQA | Axiomatic Design | Puik and Ceglarek [31], Suh [16], Guebitz, et al. [53], McCarthy, et al. [54] | |
| 6 | Risk Analysis | Systematic approach to find and classify risk that comes with the chosen function | CQA, PAT | QRM, QbD | Rantanen and Khinast [36], Gervais and D’Arcy [55], Topolski [56] | ICH [13,40] |
| 7 | Process Analytical Technology (PAT) | Selection of the technology that is able to sense critical parameters identified in the risk analysis | CQA, Risk Analysis, Equipment Description | QRM, QbD | Lee, O’Connor, Yang, Cruz, Chatterjee, Madurawe, Moore, Yu and Woodcock [34,40], Gerzon, et al. [57] | FDA [27], ICH [40] |
| 8 | Equipment Description | Result of the Axiomatic Design process in which all Functional Requirements (FR) are mapped to a design parameter (DP) | PAT, Functional Description | Axiomatic Design | Kulak, Durmusoglu and Tufekci [14], Vinodh [15], Suh [16], Guebitz, Schnedl and Khinast [53], McCarthy, Hinchy, O’Dowd, McCarthy and McMorrow [54], Puik, et al. [58] | |
| 9 | Critical Process Parameters (CPP) | Parameter that result from the risk analysis that affect the product quality | Risk Analysis | QRM, QbD | Yu, Amidon, Khan, Hoag, Polli, Raju and Woodcock [39], Xie, et al. [59], Mohammed, et al. [60] | ICH [13] |
| 10 | Process Observer Definition | Description of the necessary capabilities to feed process relevant data to an observing and decision-making instance. Can be seen as one building block towards a Cyber-Physical System (CPS) | PAT, CPP | QRM, QbD | Cavique, Cavique, Mendes and Cavique [29], O’Connor, et al. [61], Chindrus, et al. [62], Chen, et al. [63] | ICH [13] |
| 11 | Production Scheduling | Description of the combination of equipment to fulfil the user requirements | Process Observer Definition, Equipment Description | Axiomatic Design | Suh [16], Leuenberger [64], Leuenberger and Leuenberger [65], Awad, et al. [66] | |
| 12 | Graphical Function Description | Graphical way to specify the logical dependencies between the designed function | Conceptual Design | Development | Cavique, Cavique, Mendes and Cavique [29], Tian, Koolivand, Arden, Lee and O’Connor [52], Escotet-Espinoza, et al. [67] | |
| 13 | Function Design Specification (FDS) | Documentation that combines function and equipment description to prove the concept against the URS | Engineering, Graphical Function Description | Engineering, Regulation | ISPE [8], Guebitz, Schnedl and Khinast [53], McCarthy, Hinchy, O’Dowd, McCarthy and McMorrow [54] | |
| 14 | MES and QM System Integration | Definition of the Information that needs to be tracked in the QM System | Process Observer Definition, FDS | QRM | Duran [48], Xie, Chen, Fang and Chen [59] | ICH [40,41] |
| 15 | Design Qualification (DQ) | Document that has all the information to evaluate the design according to the URS | Design | Regulation | Amorosi [50] | ICH [5] |
| 16 | Modular Production Equipment | Modular equipment that supports the need for flexible or reconfigurable production systems | Development | Deployment | Reitze, Jurgensmeyer, Lier, Kohnke, Riese and Grunewald [1], Puik, Telgen, van Moergestel and Ceglarek [58], Mothes [68] | |
| 17 | Real Time Data Acquisition | Definition of the interfaces needed to transfer relevant data from the sensor level to the decision-making level, such as Real-Time Release Testing (RTRT) | Modular Production Equipment, QRM | Deployment | Xie, Chen, Fang and Chen [59], Ruppert and Abonyi [69], Leal, et al. [70] | ICH [7] |
| 18 | Graphical Function Visualisation | Graphical representation of the functions carried out by the modular production equipment | Real-Time Data Acquisition | Deployment | Cavique, Cavique, Mendes and Cavique [29], Pokojski, Szustakiewicz, Woznicki, Oleksinski and Pruszynski [44], Barenji, et al. [71] | |
| 19 | Installation and operation qualification (IQ, OQ) | Document that has all the information to test the designed equipment after the deployment phase | Development | Regulation | Amorosi [50], Gengenbach [72] | ICH [5] |
| 20 | Data Lake | Summary of all data that have been generated throughout the complete process | Design, Development, Deployment | Continual Improvement | Su, Ganesh, Moreno, Bommireddy, Gonzalez, Reklaitis and Nagy [38], Nagy, et al. [73], Sundarkumar, et al. [74] | |
| 21 | Learnings and Improvement | Learnings and potential for improvement collected throughout the complete process to be used as recommendation system for following projects | Design, Development, Deployment | Continual Improvement | Yu, Amidon, Khan, Hoag, Polli, Raju and Woodcock [39], Pramod, et al. [75] |
Appendix B
Figure A1 showing the risk-based GAMP 5 V-Model approach for applications that include custom implementations. The illustration was taken from the ISPE recommendations in the GAMP 5 [8].
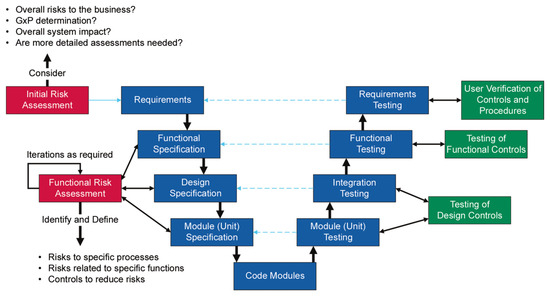
Figure A1.
GAMP 5 V-Model for custom application.
References
- Reitze, A.; Jurgensmeyer, N.; Lier, S.; Kohnke, M.; Riese, J.; Grunewald, M. Roadmap for a Smart Factory: A Modular, Intelligent Concept for the Production of Specialty Chemicals. Angew. Chem. Int. Ed. 2018, 57, 4242–4247. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.F.; Tang, S.L.; Li, D.; Imran, M.; Zhang, C.H.; Liu, C.L.; Pang, Z.B. Reconfigurable Smart Factory for Drug Packing in Healthcare Industry 4.0. IEEE Trans. Ind. Inform. 2019, 15, 507–516. [Google Scholar] [CrossRef]
- Arden, S.; Fisher, A.C.; Tyner, K.; Yu, L.C.X.; Lee, S.L.; Kopcha, M. Industry 4.0 for pharmaceutical manufacturing: Preparing for the smart factories of the future. Int. J. Pharm. 2021, 602, 20554. [Google Scholar] [CrossRef]
- Reinhardt, I.C.; Oliveira, J.C.; Ring, D.T. Current Perspectives on the Development of Industry 4.0 in the Pharmaceutical Sector. J. Ind. Inf. Integr. 2020, 18, 131. [Google Scholar] [CrossRef]
- ICH. ICH Guideline Q7 on Good Manufacturing Practice for Active Pharmaceutical Ingredients. Available online: https://database.ich.org/sites/default/files/Q7%20Guideline.pdf (accessed on 14 March 2025).
- Rosa, C. Regulatory Panel A: Panel Discussion with Regulators on Sustainability, Annex 1 Implementation, Good Engineering Practice. In Proceedings of the 2022 ISPE Europe Annual Conference, Madrid, Spain, 26 April 2022. [Google Scholar]
- ICH. ICH Guideline Q13 on Continous Manufacturing of Drug Substances and Drug Products. Available online: https://database.ich.org/sites/default/files/ICH_Q13_Step4_Guideline_2022_1116.pdf (accessed on 1 April 2025).
- ISPE. GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized Systems; International Society for Pharmaceutical Engineering: North Bethesda, MD, USA, 2008. [Google Scholar]
- Wölfle, R.; Saur Amaral, I.; Teixeira, L. Pharma in the Context of Industry 4.0—Challenges and Opportunities Based on Literature Review. In Proceedings of the 5th European International Conference on Industrial Engineering and Operations Management, Rome, Italy, 26–28 July 2022. [Google Scholar]
- McCarthy, D.; McMorrow, D.; O’Dowd, N.P.; McCarthy, C.T.; Hinchy, E.P. A Model-Based Approach to Automated Validation and Generation of PLC Code for Manufacturing Equipment in Regulated Environments. Appl. Sci. 2022, 12, 7506. [Google Scholar] [CrossRef]
- Pallagi, E.; Ambrus, R.; Szabo-Revesz, P.; Csoka, I. Adaptation of the quality by design concept in early pharmaceutical development of an intranasal nanosized formulation. Int. J. Pharm. 2015, 491, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 2463. [Google Scholar] [CrossRef]
- ICH. ICH Guideline Q8 (R2) on Pharmaceutical Development. Available online: https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf (accessed on 31 March 2023).
- Kulak, O.; Durmusoglu, M.B.; Tufekci, S. A complete cellular manufacturing system design methodology based on axiomatic design principles. Comput. Ind. Eng. 2005, 48, 765–787. [Google Scholar] [CrossRef]
- Vinodh, S. Axiomatic modelling of agile production system design. Int. J. Prod. Res. 2011, 49, 3251–3269. [Google Scholar] [CrossRef]
- Suh, N.P. Axiomatic design of mechanical systems. J. Mech. Des. 1995, 117, 2–10. [Google Scholar] [CrossRef]
- Suh, N.P. Designing-in of quality through axiomatic design. IEEE Trans. Reliab. 1995, 44, 256–264. [Google Scholar] [CrossRef]
- Haselbock, S.; Weinreich, R.; Buchgeher, G.; IEEE. An Expert Interview Study on Areas of Micro service Design. In Proceedings of the 11th IEEE International Conference on Service-Oriented Computing and Applications (SOCA), Paris, France, 19–22 November 2018; pp. 137–144.
- Liu, S.X.; Zhang, M.T.; de Bont, C. The holistic frame of designing smart, connected products: A systematic literature review and expert interview. Des. J. 2022, 25, 334–352. [Google Scholar] [CrossRef]
- Lutz, C.; Schottler, M.; Hoffmann, C.P. The privacy implications of social robots: Scoping review and expert interviews. Mobile Media Commun. 2019, 7, 412–434. [Google Scholar] [CrossRef]
- Mergel, I.; Edelmann, N.; Haug, N. Defining digital transformation: Results from expert interviews. Gov. Inf. Q. 2019, 36, 101385. [Google Scholar] [CrossRef]
- Withanagamage, L.V.K.; Ratnayake, R.; Wattegama, E.J.; IEEE. A Conceptual Framework to assess the Applicability of Agile Manufacturing Techniques. In Proceedings of the International Conference on Production and Operations Management Society (POMS), Kandy, Sri Lanka, 14–16 December 2018. [Google Scholar]
- Automation, I.S.o. ISA-88 Series of Standards—Batch Process Control. Available online: https://www.isa.org/standards-and-publications/isa-standards/isa-88-standards (accessed on 26 February 2025).
- Automation, I.S.o. ISA-95 Series of Standards: Enterprise-Control System Integration. Available online: https://www.isa.org/standards-and-publications/isa-standards/isa-95-standard (accessed on 26 February 2025).
- Sarhadi, P.; Naeem, W.; Fraser, K.; Wilson, D. On the Application of Agile Project Management Techniques, V-Model and Recent Software Tools in Postgraduate Theses Supervision. In Proceedings of the 13th IFAC Symposium on Advances in Control Education (ACE), Hamburg, Germany, 24–27 July 2022; pp. 109–114. [Google Scholar]
- Durmus, M.S.; Ustoglu, I.; Tsarev, R.Y.; Borcsok, J. Enhanced V-Model. Inform.-J. Comput. Inform. 2018, 42, 577–585. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry PAT—A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. Available online: https://www.fda.gov/media/71012/download (accessed on 14 March 2025).
- Paulus, S.; Mohammadi, N.G.; Weyer, T. Trustworthy Software Development. In Proceedings of the 14th IFIP-TC 6 and TC-11 International Conference on Communications and Multimedia Security (CMS), Magdeburg, Germany, 25–26 September 2013; pp. 233–247. [Google Scholar]
- Cavique, L.; Cavique, M.; Mendes, A.; Cavique, M. Improving information system design: Using UML and axiomatic design. Comput. Ind. 2022, 135, 103569. [Google Scholar] [CrossRef]
- Pecoraro, F.; Pourabbas, E.; Rolli, F.; Parretti, C. Digitally Sustainable Information Systems in Axiomatic Design. Sustainability 2022, 14, 2598. [Google Scholar] [CrossRef]
- Puik, E.; Ceglarek, D. Application of Axiomatic Design for Agile Product Development. In Proceedings of the 12th International Conference on Axiomatic Design (ICAD), Reykjavik Univ, Reykjavik, Iceland, 9–12 October 2018. [Google Scholar]
- Suh, N.P.; Cochran, D.S.; Lima, P.C. Manufacturing system design. In Proceedings of the 48th General Assembly of CIRP, Athens, Greece, 23–29 August 1998; pp. 627–639. [Google Scholar]
- Aulakh, S.S.; Gill, J.S. Lean Manufacturing using Axiomatic Design. In Proceedings of the World Congress on Engineering, Imperial Coll London, London, UK, 1–3 July 2009; pp. 673–678. [Google Scholar]
- Lee, S.L.; O’Connor, T.F.; Yang, X.C.; Cruz, C.N.; Chatterjee, S.; Madurawe, R.D.; Moore, C.M.V.; Yu, L.X.; Woodcock, J. Modernizing Pharmaceutical Manufacturing: From Batch to Continuous Production. J. Pharm. Innov. 2015, 10, 191–199. [Google Scholar] [CrossRef]
- Grangeia, H.B.; Silva, C.; Simoes, S.P.; Reis, M.S. Quality by design in pharmaceutical manufacturing: A systematic review of current status, challenges and future perspectives. Eur. J. Pharm. Biopharm. 2020, 147, 19–37. [Google Scholar] [CrossRef]
- Rantanen, J.; Khinast, J. The Future of Pharmaceutical Manufacturing Sciences. J. Pharm. Sci. 2015, 104, 3612–3638. [Google Scholar] [CrossRef]
- Schmidt, A.; Frey, J.; Hillen, D.; Horbelt, J.; Schandar, M.; Schneider, D.; Sorokos, I. A Framework for Automated Quality Assurance and Documentation for Pharma 4.0. In Proceedings of the 40th International Conference on Computer Safety, Reliability and Security (SAFECOMP), York, UK, 8–10 September 2021; pp. 226–239. [Google Scholar]
- Su, Q.; Ganesh, S.; Moreno, M.; Bommireddy, Y.; Gonzalez, M.; Reklaitis, G.V.; Nagy, Z.K. A perspective on Quality-by-Control (QbC) in pharmaceutical continuous manufacturing. Comput. Chem. Eng. 2019, 125, 216–231. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding Pharmaceutical Quality by Design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [PubMed]
- ICH. ICH Guideline Q9 on Quality Risk Management. Available online: https://database.ich.org/sites/default/files/Q9%20Guideline.pdf (accessed on 14 March 2025).
- ICH. ICH Guideline Q10 on Pharmaceutical Quality System. Available online: https://database.ich.org/sites/default/files/Q10%20Guideline.pdf (accessed on 14 March 2025).
- Bano, G.; Wang, Z.L.; Facco, P.; Bezzo, F.; Barolo, M.; Ierapetritou, M. A novel and systematic approach to identify the design space of pharmaceutical processes. Comput. Chem. Eng. 2018, 115, 309–322. [Google Scholar] [CrossRef]
- Both, S.; Koudous, I.; Jenelten, U.; Strube, J. Model-based equipment-design for plant-based extraction processes—Considering botanic and thermodynamic aspects. Comptes Rendus Chim. 2014, 17, 187–196. [Google Scholar] [CrossRef]
- Pokojski, J.; Szustakiewicz, K.; Woznicki, L.; Oleksinski, K.; Pruszynski, J. Industrial application of knowledge-based engineering in commercial CAD/CAE systems. J. Ind. Inf. Integr. 2022, 25, 255. [Google Scholar] [CrossRef]
- Setti, P.H.P.; Canciglieri, O.; Estorilio, C.C.A. Integrated product development method based on Value Engineering and design for assembly concepts. J. Ind. Inf. Integr. 2021, 21, 199. [Google Scholar] [CrossRef]
- Testa, C.J.; Hu, C.; Shvedova, K.; Wu, W.; Sayin, R.; Casati, F.; Halkude, B.S.; Hermant, P.; Shen, D.E.; Ramnath, A.; et al. Design and Commercialization of an End-to-End Continuous Pharmaceutical Production Process: A Pilot Plant Case Study. Org. Process Res. Dev. 2020, 24, 2874–2889. [Google Scholar] [CrossRef]
- Uysal, M.P.; Mergen, A.E. Smart manufacturing in intelligent digital mesh: Integration of enterprise architecture and software product line engineering. J. Ind. Inf. Integr. 2021, 22, 202. [Google Scholar] [CrossRef]
- Duran, R. Improving pharma and biotech quality systems with fewer audits The link between a well-established quality system and fewer audits. Chim. Oggi-Chem. Today 2014, 32, 64–65. [Google Scholar]
- Weng, F.T.; Jenq, S.M. Application integrating axiomatic design and agile manufacturing unit in product evaluation. Int. J. Adv. Manuf. Technol. 2012, 63, 181–189. [Google Scholar] [CrossRef]
- Amorosi, F. Process Mapping, a key milestone in Engineering for Health. In Proceedings of the 4th European Conference of the International Federation for Medical and Biological Engineering (ECIFMBE), Antwerp, Belgium, 23–27 November 2008; pp. 1726–1729. [Google Scholar]
- Zhang, Y.; Larson, B.; Hatcliff, J. Assurance Case Considerations for Interoperable Medical Systems. In Proceedings of the 37th International Conference on Computer Safety, Reliability, and Security (SAFECOMP), Vasteras, Sweden, 18–21 September 2018; pp. 42–48. [Google Scholar]
- Tian, G.; Koolivand, A.; Arden, N.S.; Lee, S.; O’Connor, T.F. Quality risk assessment and mitigation of pharmaceutical continuous manufacturing using flowsheet modeling approach. Comput. Chem. Eng. 2019, 129, 10. [Google Scholar] [CrossRef]
- Guebitz, B.; Schnedl, H.; Khinast, J.G. A risk management ontology for Quality-by-Design based on a new development approach according GAMP 5.0. Expert Syst. Appl. 2012, 39, 7291–7301. [Google Scholar] [CrossRef]
- McCarthy, D.J.; Hinchy, E.P.; O’Dowd, N.P.; McCarthy, C.T.; McMorrow, D. Using Model Based Design as an Enabler for Digital Validation of Discrete State Machines in Regulated Manufacturing Environments. In Proceedings of the 30th International Conference on Flexible Automation and Intelligent Manufacturing (FAIM), School of Mechanical Engineering, National Technical University of Athens, Athens, Greece, 15–18 June 2021; pp. 365–370. [Google Scholar]
- Gervais, B.; D’Arcy, D.M. Quality risk analysis in a cGMP environment: Multiple models for comprehensive failure mode identification during the computer system lifecycle. Drug Dev. Ind. Pharm. 2014, 40, 46–60. [Google Scholar] [CrossRef]
- Topolski, M. Application of Feature Extraction Methods for Chemical Risk Classification in the Pharmaceutical Industry. Sensors 2021, 21, 5753. [Google Scholar] [CrossRef] [PubMed]
- Gerzon, G.; Sheng, Y.; Kirkitadze, M. Process Analytical Technologies—Advances in bioprocess integration and future perspectives. J. Pharm. Biomed. Anal. 2022, 207, 14379. [Google Scholar] [CrossRef]
- Puik, E.; Telgen, D.; van Moergestel, L.; Ceglarek, D. Assessment of reconfiguration schemes for Reconfigurable Manufacturing Systems based on resources and lead time. Robot. Comput.-Integr. Manuf. 2017, 43, 30–38. [Google Scholar] [CrossRef]
- Xie, X.W.; Chen, L.; Fang, H.Z.; Chen, Y.X. Research on Process Supervision and Quality Control System of Modular Pharmaceutical Production Line. In Proceedings of the 2nd IEEE International Conference on Computer Science and Information Technology, Beijing, China, 8–11 August 2009; p. 69. [Google Scholar]
- Mohammed, A.Q.; Sunkari, P.K.; Srinivas, P.; Roy, A.K. Quality by Design in Action 1: Controlling Critical Quality Attributes of an Active Pharmaceutical Ingredient. Org. Process Res. Dev. 2015, 19, 1634–1644. [Google Scholar] [CrossRef]
- O’Connor, T.; Yang, X.; Tian, G.; Chatterjee, S.; Lee, S. 2—Quality risk management for pharmaceutical manufacturing: The role of process modeling and simulations. In Predictive Modeling of Pharmaceutical Unit Operations; Pandey, P., Bharadwaj, R., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 15–37. [Google Scholar]
- Chindrus, A.; Copott, D.; Caruntu, C.E. Continuous Manufacturing using Linear Quadratic Regulator in the Context of Cyber-Physical Systems. In Proceedings of the 26th International Conference on System Theory, Control and Computing (ICSTCC), Sinaia, Romania, 19–21 October 2022; pp. 231–236. [Google Scholar]
- Chen, Y.J.; Yang, O.; Sampat, C.; Bhalode, P.; Ramachandran, R.; Ierapetritou, M. Digital Twins in Pharmaceutical and Biopharmaceutical Manufacturing: A Literature Review. Processes 2020, 8, 1088. [Google Scholar] [CrossRef]
- Leuenberger, H. New trends in the production of pharmaceutical granules: Batch versus continuous processing. Eur. J. Pharm. Biopharm. 2001, 52, 289–296. [Google Scholar] [CrossRef]
- Leuenberger, H.; Leuenberger, M.N. Impact of the digital revolution on the future of pharmaceutical formulation science. Eur. J. Pharm. Sci. 2016, 87, 100–111. [Google Scholar] [CrossRef]
- Awad, M.; Mulrennan, K.; Donovan, J.; Macpherson, R.; Tormey, D. A constraint programming model for makespan minimisation in batch manufacturing pharmaceutical facilities. Comput. Chem. Eng. 2022, 156, 7565. [Google Scholar] [CrossRef]
- Escotet-Espinoza, M.S.; Vadodaria, S.; Singh, R.; Muzzio, F.J.; Ierapetritou, M.G. Modeling the effects of material properties on tablet compaction: A building block for controlling both batch and continuous pharmaceutical manufacturing processes. Int. J. Pharm. 2018, 543, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Mothes, H. No-Regret Solutions—Modular Production Concepts in Times of Complexity and Uncertainty. Chem. Ing. Tech. 2015, 87, 1159–1172. [Google Scholar] [CrossRef]
- Ruppert, T.; Abonyi, J. Integration of real-time locating systems into digital twins. J. Ind. Inf. Integr. 2020, 20, 174. [Google Scholar] [CrossRef]
- Leal, F.; Chis, A.E.; Caton, S.; Gonzalez-Velez, H.; Garcia-Gomez, J.M.; Dura, M.; Sanchez-Garcia, A.; Saez, C.; Karageorgos, A.; Gerogiannis, V.C.; et al. Smart Pharmaceutical Manufacturing: Ensuring End-to-End Traceability and Data Integrity in Medicine Production. Big Data Res. 2021, 24, 172. [Google Scholar] [CrossRef]
- Barenji, R.V.; Akdag, Y.; Yet, B.; Oner, L. Cyber-physical-based PAT (CPbPAT) framework for Pharma 4.0. Int. J. Pharm. 2019, 567, 118445. [Google Scholar] [CrossRef] [PubMed]
- Gengenbach, R. FDA-compliant qualification of manufacturing plants for active pharmaceutical ingredients. Pharm. Ind. 2003, 65, 704–711. [Google Scholar]
- Nagy, B.; Szabados-Nacsa, A.; Fulop, G.; Nagyne, A.T.; Galata, D.L.; Farkas, A.; Meszaros, L.A.; Nagy, Z.K.; Marosi, G. Interpretable artificial neural networks for retrospective QbD of pharmaceutical tablet manufacturing based on a pilot-scale developmental dataset. Int. J. Pharm. 2023, 633, 122620. [Google Scholar] [CrossRef]
- Sundarkumar, V.; Nagy, Z.K.; Reklaitis, G.V. Machine learning enabled integrated formulation and process design framework for a pharmaceutical 3D printing platform. AIChE J. 2022, 69, e17990. [Google Scholar] [CrossRef]
- Pramod, K.; Abu Tahir, M.; Charoo, N.A.; Ansari, S.H.; Ali, J. Pharmaceutical product development: A quality by design approach. Int. J. Pharm. Investig. 2016, 6, 129–138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).