Test–Retest Reliability of Deep Learning Analysis of Brain Volumes in Adolescent Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participant Recruitment
2.3. Imaging Protocol
2.4. Data Analysis
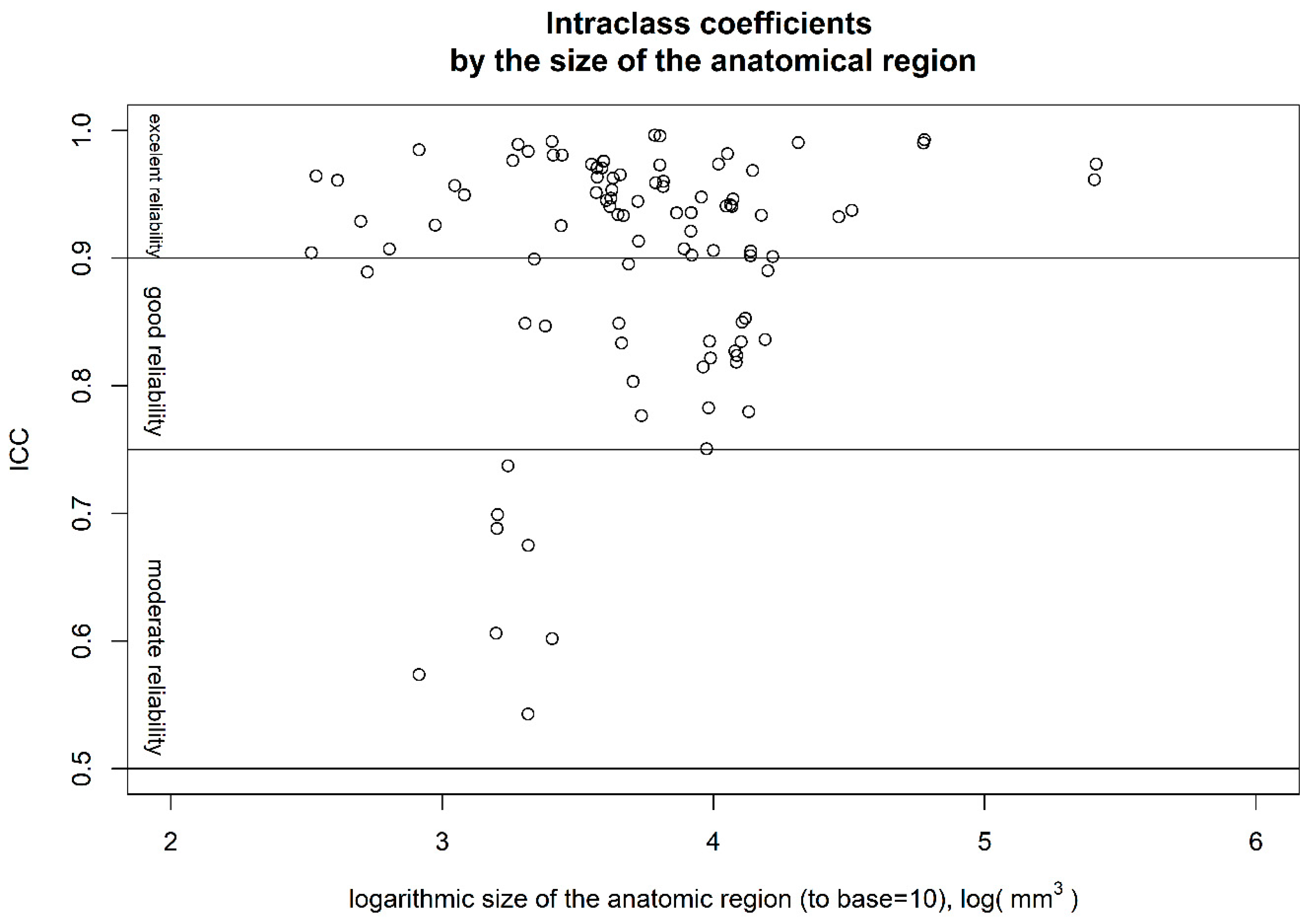
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thapar, A.; Riglin, L. The Importance of a Developmental Perspective in Psychiatry: What Do Recent Genetic-Epidemiological Findings Show? Mol. Psychiatry 2020, 25, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Radua, J.; Olivola, M.; Croce, E.; Soardo, L.; Salazar de Pablo, G.; Il Shin, J.; Kirkbride, J.B.; Jones, P.; Kim, J.H.; et al. Age at Onset of Mental Disorders Worldwide: Large-Scale Meta-Analysis of 192 Epidemiological Studies. Mol. Psychiatry 2022, 27, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Bethlehem, R.A.I.; Seidlitz, J.; White, S.R.; Vogel, J.W.; Anderson, K.M.; Adamson, C.; Adler, S.; Alexopoulos, G.S.; Anagnostou, E.; Areces-Gonzalez, A.; et al. Brain Charts for the Human Lifespan. Nature 2022, 604, 525–533. [Google Scholar] [CrossRef]
- Vijayakumar, N.; Allen, N.B.; Youssef, G.; Dennison, M.; Yücel, M.; Simmons, J.G.; Whittle, S. Brain Development during Adolescence: A Mixed-Longitudinal Investigation of Cortical Thickness, Surface Area, and Volume. Hum. Brain Mapp. 2016, 37, 2027–2038. [Google Scholar] [CrossRef]
- Gałecki, P.; Talarowska, M. Neurodevelopmental Theory of Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Paus, T.; Keshavan, M.; Giedd, J.N. Why Do Many Psychiatric Disorders Emerge during Adolescence? Nat. Rev. Neurosci. 2008, 9, 947–957. [Google Scholar] [CrossRef]
- Lai, C.-H. Promising Neuroimaging Biomarkers in Depression. Psychiatry Investig. 2019, 16, 662–670. [Google Scholar] [CrossRef]
- Fonseka, T.M.; MacQueen, G.M.; Kennedy, S.H. Neuroimaging Biomarkers as Predictors of Treatment Outcome in Major Depressive Disorder. J. Affect. Disord. 2018, 233, 21–35. [Google Scholar] [CrossRef]
- Lener, M.S.; Iosifescu, D.V. In Pursuit of Neuroimaging Biomarkers to Guide Treatment Selection in Major Depressive Disorder: A Review of the Literature. Ann. N. Y. Acad. Sci. 2015, 1344, 50–65. [Google Scholar] [CrossRef]
- Hu, J.; Huang, Y.; Zhang, X.; Liao, B.; Hou, G.; Xu, Z.; Dong, S.; Li, P. Identifying Suicide Attempts, Ideation, and Non-Ideation in Major Depressive Disorder from Structural MRI Data Using Deep Learning. Asian J. Psychiatr. 2023, 82, 103511. [Google Scholar] [CrossRef]
- Akkus, Z.; Galimzianova, A.; Hoogi, A.; Rubin, D.L.; Erickson, B.J. Deep Learning for Brain MRI Segmentation: State of the Art and Future Directions. J. Digit. Imaging 2017, 30, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, P.; Singh, A.R. Deep Learning Models and Traditional Automated Techniques for Brain Tumor Segmentation in MRI: A Review. Artif. Intell. Rev. 2023, 56, 2923–2969. [Google Scholar] [CrossRef]
- Estrada, S.; Kügler, D.; Bahrami, E.; Xu, P.; Mousa, D.; Breteler, M.M.B.; Aziz, N.A.; Reuter, M. FastSurfer-HypVINN: Automated Sub-Segmentation of the Hypothalamus and Adjacent Structures on High-Resolutional Brain MRI. Imaging Neurosci. 2023, 1, 1–32. [Google Scholar] [CrossRef]
- Faber, J.; Kügler, D.; Bahrami, E.; Heinz, L.-S.; Timmann, D.; Ernst, T.M.; Deike-Hofmann, K.; Klockgether, T.; van de Warrenburg, B.; van Gaalen, J.; et al. CerebNet: A Fast and Reliable Deep-Learning Pipeline for Detailed Cerebellum Sub-Segmentation. Neuroimage 2022, 264, 119703. [Google Scholar] [CrossRef]
- Henschel, L.; Kügler, D.; Reuter, M. FastSurferVINN: Building Resolution-Independence into Deep Learning Segmentation Methods—A Solution for HighRes Brain MRI. Neuroimage 2022, 251, 118933. [Google Scholar] [CrossRef]
- Henschel, L.; Conjeti, S.; Estrada, S.; Diers, K.; Fischl, B.; Reuter, M. FastSurfer—A Fast and Accurate Deep Learning Based Neuroimaging Pipeline. Neuroimage 2020, 219, 117012. [Google Scholar] [CrossRef]
- Guha Roy, A.; Conjeti, S.; Navab, N.; Wachinger, C. QuickNAT: A Fully Convolutional Network for Quick and Accurate Segmentation of Neuroanatomy. Neuroimage 2019, 186, 713–727. [Google Scholar] [CrossRef]
- Ducharme, S.; Albaugh, M.D.; Nguyen, T.-V.; Hudziak, J.J.; Mateos-Pérez, J.M.; Labbe, A.; Evans, A.C.; Karama, S. Trajectories of Cortical Thickness Maturation in Normal Brain Development—The Importance of Quality Control Procedures. Neuroimage 2016, 125, 267–279. [Google Scholar] [CrossRef]
- Paus, T.; Collins, D.L.; Evans, A.C.; Leonard, G.; Pike, B.; Zijdenbos, A. Maturation of White Matter in the Human Brain: A Review of Magnetic Resonance Studies. Brain Res. Bull. 2001, 54, 255–266. [Google Scholar] [CrossRef]
- Satterthwaite, T.D.; Wolf, D.H.; Loughead, J.; Ruparel, K.; Elliott, M.A.; Hakonarson, H.; Gur, R.C.; Gur, R.E. Impact of In-Scanner Head Motion on Multiple Measures of Functional Connectivity: Relevance for Studies of Neurodevelopment in Youth. Neuroimage 2012, 60, 623–632. [Google Scholar] [CrossRef]
- Schoemaker, D.; Buss, C.; Head, K.; Sandman, C.A.; Davis, E.P.; Chakravarty, M.M.; Gauthier, S.; Pruessner, J.C. Hippocampus and Amygdala Volumes from Magnetic Resonance Images in Children: Assessing Accuracy of FreeSurfer and FSL against Manual Segmentation. Neuroimage 2016, 129, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Drobinin, V.; Van Gestel, H.; Helmick, C.A.; Schmidt, M.H.; Bowen, C.V.; Uher, R. Reliability of Multimodal MRI Brain Measures in Youth at Risk for Mental Illness. Brain Behav. 2020, 10, e01609. [Google Scholar] [CrossRef]
- Lundervold, A.S.; Lundervold, A. An Overview of Deep Learning in Medical Imaging Focusing on MRI. Z. Med. Phys. 2019, 29, 102–127. [Google Scholar] [CrossRef]
- Reuter, M.; Tisdall, M.D.; Qureshi, A.; Buckner, R.L.; van der Kouwe, A.J.W.; Fischl, B. Head Motion during MRI Acquisition Reduces Gray Matter Volume and Thickness Estimates. Neuroimage 2015, 107, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Wunram, H.L.; Hamacher, S.; Hellmich, M.; Volk, M.; Jänicke, F.; Reinhard, F.; Bloch, W.; Zimmer, P.; Graf, C.; Schönau, E.; et al. Whole Body Vibration Added to Treatment as Usual Is Effective in Adolescents with Depression: A Partly Randomized, Three-Armed Clinical Trial in Inpatients. Eur. Child. Adolesc. Psychiatry 2018, 27, 645–662. [Google Scholar] [CrossRef]
- Steer, R.A.; Clark, D.A.; Beck, A.T.; Ranieri, W.F. Common and Specific Dimensions of Self-Reported Anxiety and Depression: The BDI-II versus the BDI-IA. Behav. Res. Ther. 1999, 37, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Stiensmeier-Pelster, J.; Schürmann, M.; Duda, K. Depressions-Inventar Für Kinder Und Jugendliche: (DIKJ); Verlag für Psychologie Dr. CJ Hogrefe: Göttingen, Germany, 1989. [Google Scholar]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An Automated Labeling System for Subdividing the Human Cerebral Cortex on MRI Scans into Gyral Based Regions of Interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Buimer, E.E.L.; Pas, P.; Brouwer, R.M.; Froeling, M.; Hoogduin, H.; Leemans, A.; Luijten, P.; van Nierop, B.J.; Raemaekers, M.; Schnack, H.G.; et al. The YOUth Cohort Study: MRI Protocol and Test-Retest Reliability in Adults. Dev. Cogn. Neurosci. 2020, 45, 100816. [Google Scholar] [CrossRef]
- Iscan, Z.; Jin, T.B.; Kendrick, A.; Szeglin, B.; Lu, H.; Trivedi, M.; Fava, M.; McGrath, P.J.; Weissman, M.; Kurian, B.T.; et al. Test–Retest Reliability of Freesurfer Measurements within and between Sites: Effects of Visual Approval Process. Hum. Brain Mapp. 2015, 36, 3472–3485. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, S.; Jiang, C.; He, Y.; Zuo, X.N. Charting the Human Amygdala Development across Childhood and Adolescence: Manual and Automatic Segmentation. Dev. Cogn. Neurosci. 2021, 52, 101028. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.C.; Dvorak, D.; Sartin-Tarm, A.; Botsford, C.; Cogswell, I.; Hoffstetter, A.; Putnam, O.; Schomaker, C.; Smith, P.; Stalsberg, A.; et al. Gray Matter Volume Correlates of Adolescent Posttraumatic Stress Disorder: A Comparison of Manual Intervention and Automated Segmentation in FreeSurfer. Psychiatry Res. Neuroimaging 2021, 313, 111297. [Google Scholar] [CrossRef] [PubMed]
- Hedges, E.P.; Dimitrov, M.; Zahid, U.; Brito Vega, B.; Si, S.; Dickson, H.; McGuire, P.; Williams, S.; Barker, G.J.; Kempton, M.J. Reliability of Structural MRI Measurements: The Effects of Scan Session, Head Tilt, Inter-Scan Interval, Acquisition Sequence, FreeSurfer Version and Processing Stream. Neuroimage 2022, 246, 118751. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Liu, H.-M.; Chen, S.-K.; Chen, Y.-F.; Lee, C.-W.; Yeh, L.-R. Reproducibility of Brain Morphometry from Short-Term Repeat Clinical MRI Examinations: A Retrospective Study. PLoS ONE 2016, 11, e0146913. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Tran, T.; Chu, K.; Borzage, M.T.; Braskie, M.N.; Harrington, M.G.; King, K.S. White Matter Hypointensities and Hyperintensities Have Equivalent Correlations with Age and CSF Β-amyloid in the Nondemented Elderly. Brain Behav. 2019, 9, e01457. [Google Scholar] [CrossRef]
- Marsh, R.; Gerber, A.J.; Peterson, B.S. Neuroimaging Studies of Normal Brain Development and Their Relevance for Understanding Childhood Neuropsychiatric Disorders. J. Am. Acad. Child. Adolesc. Psychiatry 2008, 47, 1233–1251. [Google Scholar] [CrossRef]
- Wei, Y.; Jagtap, J.M.; Singh, Y.; Khosravi, B.; Cai, J.; Gunter, J.L.; Erickson, B.J. Comprehensive Segmentation of Gray Matter Structures on T1-Weighted Brain MRI: A Comparative Study of CNN, CNN Hybrid-Transformer or -Mamba Architectures. Am. J. Neuroradiol. 2024, ajnr.A8544. [Google Scholar] [CrossRef]
| N = 42 | Mean ± Standard Deviation |
|---|---|
| Sex | 11 Male |
| Age | 15.02 ± 1.13 |
| BMI | 25.13 ± 6.94 |
| IQ | 102.22 ± 11.06 |
| Depression Score (BDI-II 1) | 28.88 ± 12.23 |
| Paired Regions | ||||
|---|---|---|---|---|
| Anatomical Region * | Left | Right | ||
| ICC | Median Absolute Error, % | ICC | Median Absolute Error, % | |
| Cerebral White Matter | 0.973 (0.951; 0.985) | 2.14 (1.43; 2.91) | 0.961 (0.929; 0.979) | 1.76 (1.36; 2.73) |
| Lateral Ventricle | 0.995 (0.992; 0.997) | 3.32 (2.21; 4.58) | 0.996 (0.993; 0.997) | 3.12 (1.69; 3.97) |
| Inf Lat Ventricle | 0.904 (0.828; 0.947) | 7.4 (4.38; 9.92) | 0.964 (0.934; 0.98) | 5.56 (4.52; 9.11) |
| Cerebellum White Matter | 0.933 (0.879; 0.963) | 1.92 (1; 2.97) | 0.968 (0.942; 0.982) | 1.5 (0.86; 2.24) |
| Cerebellum Cortex | 0.99 (0.981; 0.994) | 0.71 (0.52; 0.98) | 0.992 (0.986; 0.996) | 0.79 (0.5; 1.17) |
| Thalamus | 0.947 (0.905; 0.971) | 1.53 (0.69; 1.82) | 0.902 (0.825; 0.946) | 1.67 (0.96; 2.57) |
| Caudate | 0.97 (0.945; 0.984) | 1.04 (0.65; 1.75) | 0.975 (0.955; 0.986) | 1.03 (0.66; 1.55) |
| Putamen | 0.944 (0.899; 0.969) | 1.54 (1.1; 2.14) | 0.776 (0.62; 0.873) | 1.34 (0.86; 1.73) |
| Pallidum | 0.674 (0.469; 0.811) | 2.72 (1.81; 5.1) | 0.542 (0.289; 0.725) | 2.24 (1.41; 3.14) |
| Hippocampus | 0.933 (0.88; 0.963) | 1.28 (0.81; 2.12) | 0.965 (0.936; 0.981) | 1.19 (0.68; 1.63) |
| Amygdala | 0.688 (0.488; 0.819) | 3.42 (1.57; 5.41) | 0.737 (0.56; 0.849) | 3.16 (2.11; 6.23) |
| Accumbens area | 0.889 (0.803; 0.938) | 3.11 (1.93; 5.17) | 0.907 (0.833; 0.948) | 3.8 (2.58; 4.85) |
| Ventral diencephalon | 0.962 (0.931; 0.979) | 1.63 (0.95; 2.4) | 0.94 (0.891; 0.967) | 2.15 (1.56; 2.52) |
| choroid plexus | 0.96 (0.928; 0.978) | 7.34 (6.51; 11.98) | 0.928 (0.871; 0.961) | 8.5 (5.23; 10.41) |
| ctx caudalanteriorcing. | 0.973 (0.951; 0.985) | 2.03 (1.54; 3.76) | 0.98 (0.964; 0.989) | 2.39 (1.96; 3.61) |
| ctx caudalmiddlefrontal | 0.907 (0.834; 0.949) | 3.04 (2.04; 5.81) | 0.935 (0.883; 0.964) | 3.29 (2.33; 4.23) |
| ctx cuneus | 0.976 (0.956; 0.987) | 2.39 (1.67; 3.54) | 0.951 (0.911; 0.973) | 2.38 (1.33; 3.35) |
| ctx entorhinal | 0.698 (0.504; 0.826) | 6.51 (4.28; 9.27) | 0.606 (0.373; 0.767) | 8.39 (4.56; 10.93) |
| ctx fusiform | 0.921 (0.857; 0.956) | 2.59 (1.18; 3.63) | 0.935 (0.883; 0.964) | 1.9 (1.3; 2.54) |
| ctx inferiorparietal | 0.849 (0.737; 0.916) | 2.94 (2.09; 5.19) | 0.89 (0.805; 0.939) | 3.72 (2.54; 5.4) |
| ctx inferiortemporal | 0.823 (0.695; 0.901) | 5.05 (2.67; 6.57) | 0.834 (0.712; 0.907) | 3.57 (2.45; 4.38) |
| ctx isthmuscingulate | 0.98 (0.964; 0.989) | 1.35 (0.96; 2.07) | 0.991 (0.984; 0.995) | 1.9 (1.42; 2.66) |
| ctx lateraloccipital | 0.94 (0.892; 0.967) | 3.17 (2.28; 3.87) | 0.946 (0.902; 0.97) | 2.44 (1.28; 3.42) |
| ctx lateralorbitofrontal | 0.75 (0.58; 0.857) | 2.53 (1.53; 3.89) | 0.782 (0.63; 0.877) | 2.58 (1.53; 5.87) |
| ctx lingual | 0.958 (0.925; 0.977) | 2.17 (1.35; 3.06) | 0.972 (0.95; 0.985) | 1.46 (1.13; 2.39) |
| ctx medialorbitofrontal | 0.913 (0.844; 0.952) | 1.99 (1.21; 3.69) | 0.803 (0.662; 0.889) | 1.69 (1.14; 3.13) |
| ctx middletemporal | 0.852 (0.742; 0.918) | 5.57 (3.42; 8.18) | 0.779 (0.625; 0.875) | 5.19 (2.57; 6.74) |
| ctx parahippocampal | 0.899 (0.82; 0.944) | 3.83 (2.53; 5.45) | 0.848 (0.735; 0.915) | 2.58 (1.91; 4.45) |
| ctx paracentral | 0.953 (0.915; 0.974) | 2.65 (1.75; 3.86) | 0.946 (0.903; 0.971) | 2.31 (1.94; 3.44) |
| ctx parsopercularis | 0.933 (0.879; 0.963) | 3.68 (2.3; 6.03) | 0.833 (0.71; 0.906) | 4.2 (3.03; 7.11) |
| ctx parsorbitalis | 0.846 (0.732; 0.914) | 3.78 (2.08; 4.77) | 0.601 (0.367; 0.764) | 3.62 (2.57; 5.54) |
| ctx parstriangularis | 0.895 (0.813; 0.942) | 3.72 (2.13; 6.35) | 0.848 (0.735; 0.915) | 4.81 (2.89; 6.73) |
| ctx pericalcarine | 0.976 (0.956; 0.987) | 3.4 (2.34; 4.62) | 0.983 (0.969; 0.991) | 2.28 (1.38; 3.2) |
| ctx postcentral | 0.905 (0.831; 0.948) | 4.06 (2.51; 6.33) | 0.834 (0.713; 0.907) | 3.42 (2.33; 4.98) |
| ctx posteriorcingulate | 0.963 (0.933; 0.98) | 1.98 (1.35; 2.63) | 0.97 (0.946; 0.984) | 1.5 (0.86; 1.96) |
| ctx precentral | 0.818 (0.686; 0.898) | 3.53 (2.92; 6.5) | 0.827 (0.7; 0.903) | 4.77 (2.79; 6.6) |
| ctx precuneus | 0.973 (0.951; 0.985) | 1.56 (1.09; 2.72) | 0.981 (0.966; 0.99) | 1.32 (0.85; 2.05) |
| ctx rostralanteriorcing. | 0.945 (0.900; 0.970) | 2.76 (2.1; 3.54) | 0.925 (0.865; 0.959) | 3.03 (1.51; 4.4) |
| ctx rostralmiddlefrontal | 0.901 (0.824; 0.945) | 3.23 (2.45; 5.17) | 0.905 (0.83; 0.948) | 2.95 (1.99; 4.14) |
| ctx superiorfrontal | 0.932 (0.877; 0.963) | 1.59 (0.94; 2.39) | 0.937 (0.886; 0.965) | 1.96 (1.23; 3.35) |
| ctx superiorparietal | 0.94 (0.891; 0.967) | 2.88 (1.45; 3.98) | 0.941 (0.894; 0.968) | 2.53 (1.25; 3.35) |
| ctx superiortemporal | 0.901 (0.823; 0.945) | 3.57 (2.94; 4.87) | 0.836 (0.715; 0.908) | 3.99 (2.41; 5.43) |
| ctx supramarginal | 0.821 (0.691; 0.9) | 6.53 (5.17; 8.28) | 0.814 (0.68; 0.895) | 5.7 (3.54; 6.39) |
| ctx transversetemporal | 0.949 (0.908; 0.972) | 4.53 (3; 6.61) | 0.925 (0.866; 0.959) | 3.19 (2.27; 4.67) |
| ctx insula | 0.956 (0.919; 0.976) | 1.42 (0.79; 2.37) | 0.96 (0.927; 0.978) | 1.22 (0.8; 1.78) |
| Unpaired Regions | ||||
| ICC | Median Absolut Error, % | |||
| 3rd Ventricle | 0.984 (0.971; 0.991) | 2.29 (1.23; 3.6) | ||
| 4th Ventricle | 0.989 (0.979; 0.994) | 2.18 (1.91; 2.74) | ||
| Brain Stem | 0.99 (0.982; 0.994) | 0.86 (0.65; 1.2) | ||
| CSF | 0.956 (0.921; 0.976) | 2.94 (2.21; 3.99) | ||
| white matter hypointensities | 0.573 (0.329; 0.745) | 12.8 (6.81; 18.49) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasparbauer, A.-M.; Wunram, H.L.; Abuhsin, F.; Körber, F.; Schönau, E.; Bender, S.; Duran, I. Test–Retest Reliability of Deep Learning Analysis of Brain Volumes in Adolescent Brain. Information 2024, 15, 748. https://doi.org/10.3390/info15120748
Kasparbauer A-M, Wunram HL, Abuhsin F, Körber F, Schönau E, Bender S, Duran I. Test–Retest Reliability of Deep Learning Analysis of Brain Volumes in Adolescent Brain. Information. 2024; 15(12):748. https://doi.org/10.3390/info15120748
Chicago/Turabian StyleKasparbauer, Anna-Maria, Heidrun Lioba Wunram, Fabian Abuhsin, Friederike Körber, Eckhard Schönau, Stephan Bender, and Ibrahim Duran. 2024. "Test–Retest Reliability of Deep Learning Analysis of Brain Volumes in Adolescent Brain" Information 15, no. 12: 748. https://doi.org/10.3390/info15120748
APA StyleKasparbauer, A.-M., Wunram, H. L., Abuhsin, F., Körber, F., Schönau, E., Bender, S., & Duran, I. (2024). Test–Retest Reliability of Deep Learning Analysis of Brain Volumes in Adolescent Brain. Information, 15(12), 748. https://doi.org/10.3390/info15120748








