A Review of Imaging Methods and Recent Nanoparticles for Breast Cancer Diagnosis
Abstract
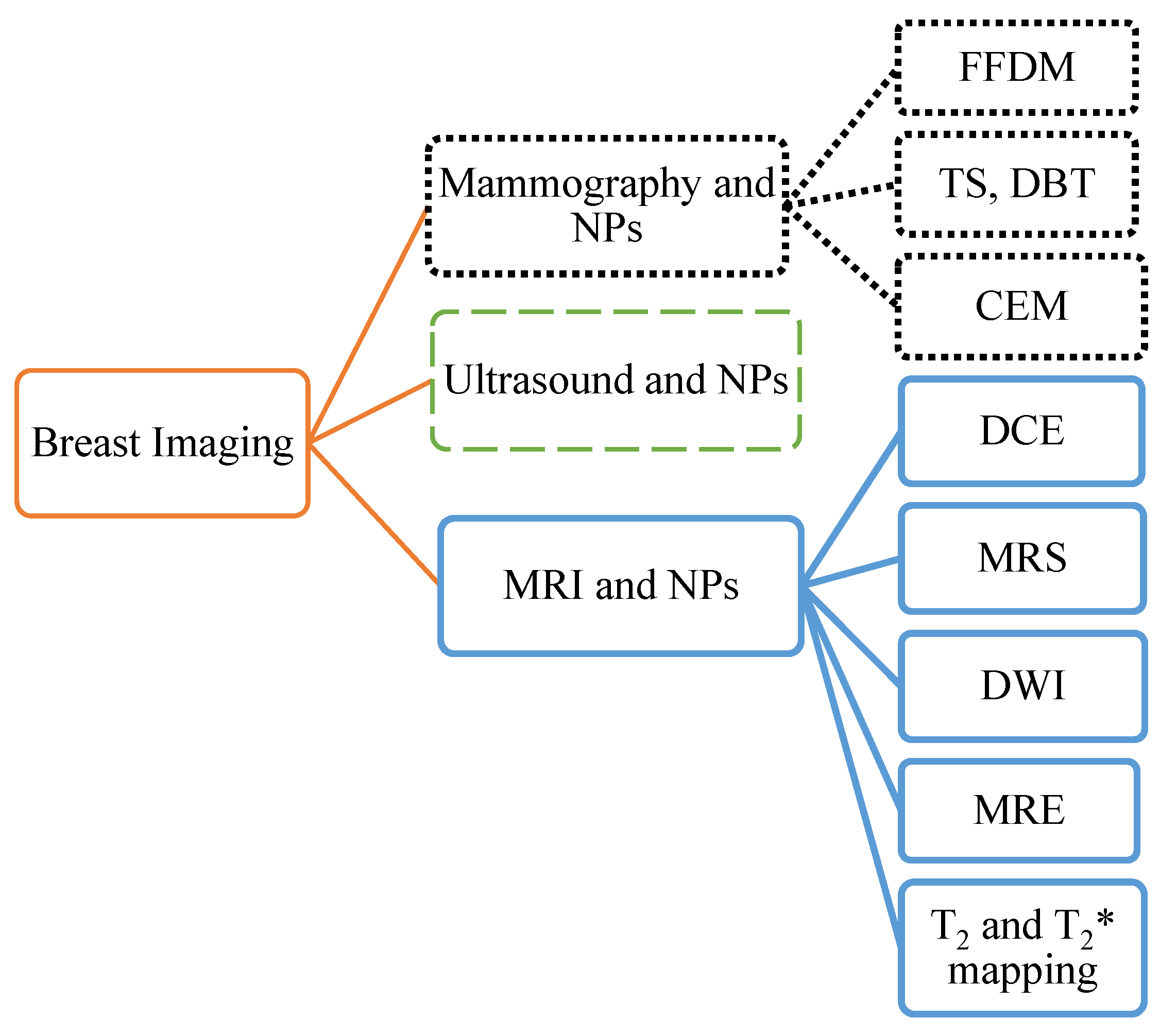
1. Introduction
2. Mammography (MG)
2.1. Full-Field Digital Mammography (FFDM)
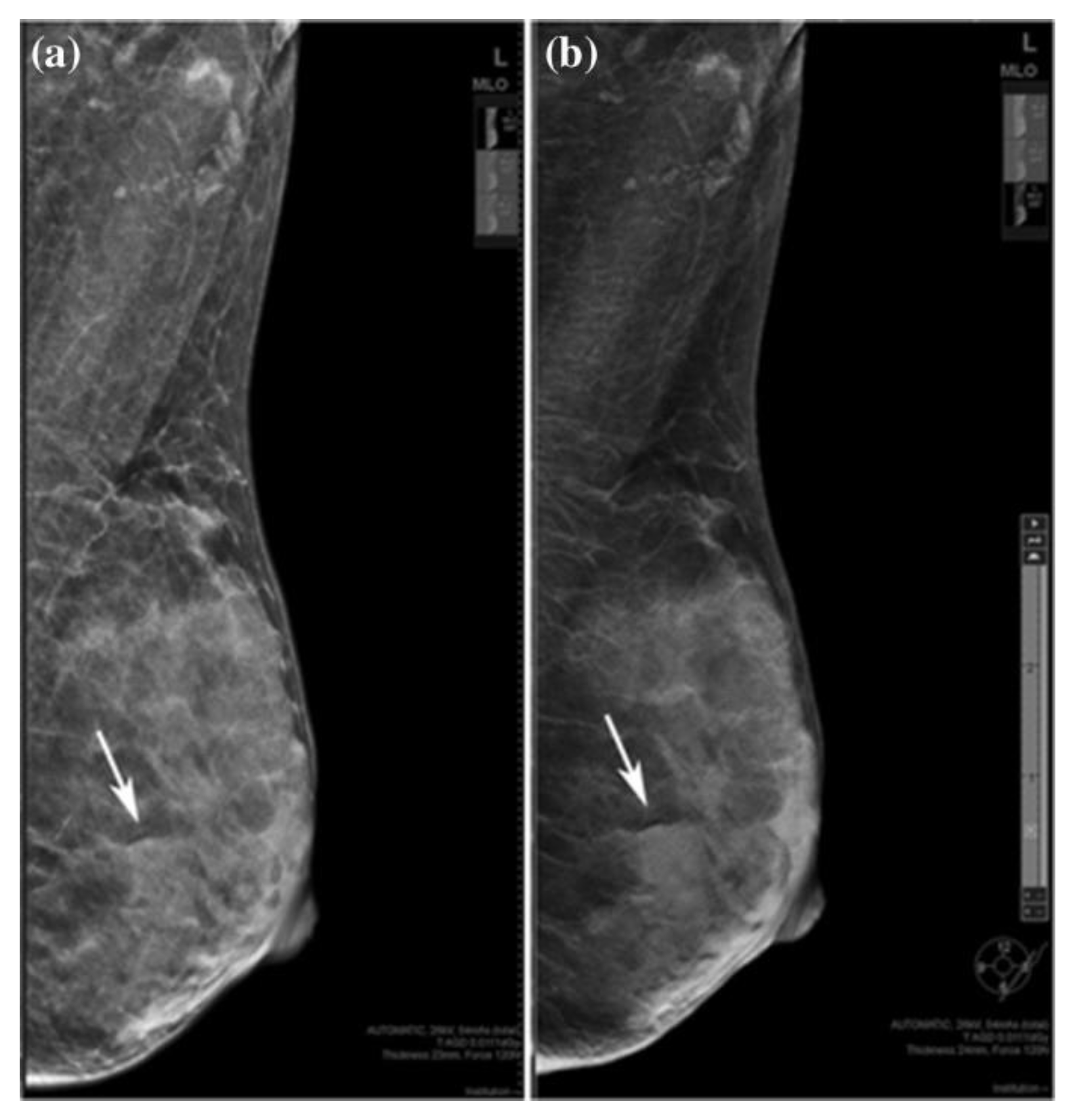
2.2. Digital Breast Tomosynthesis (DBT)
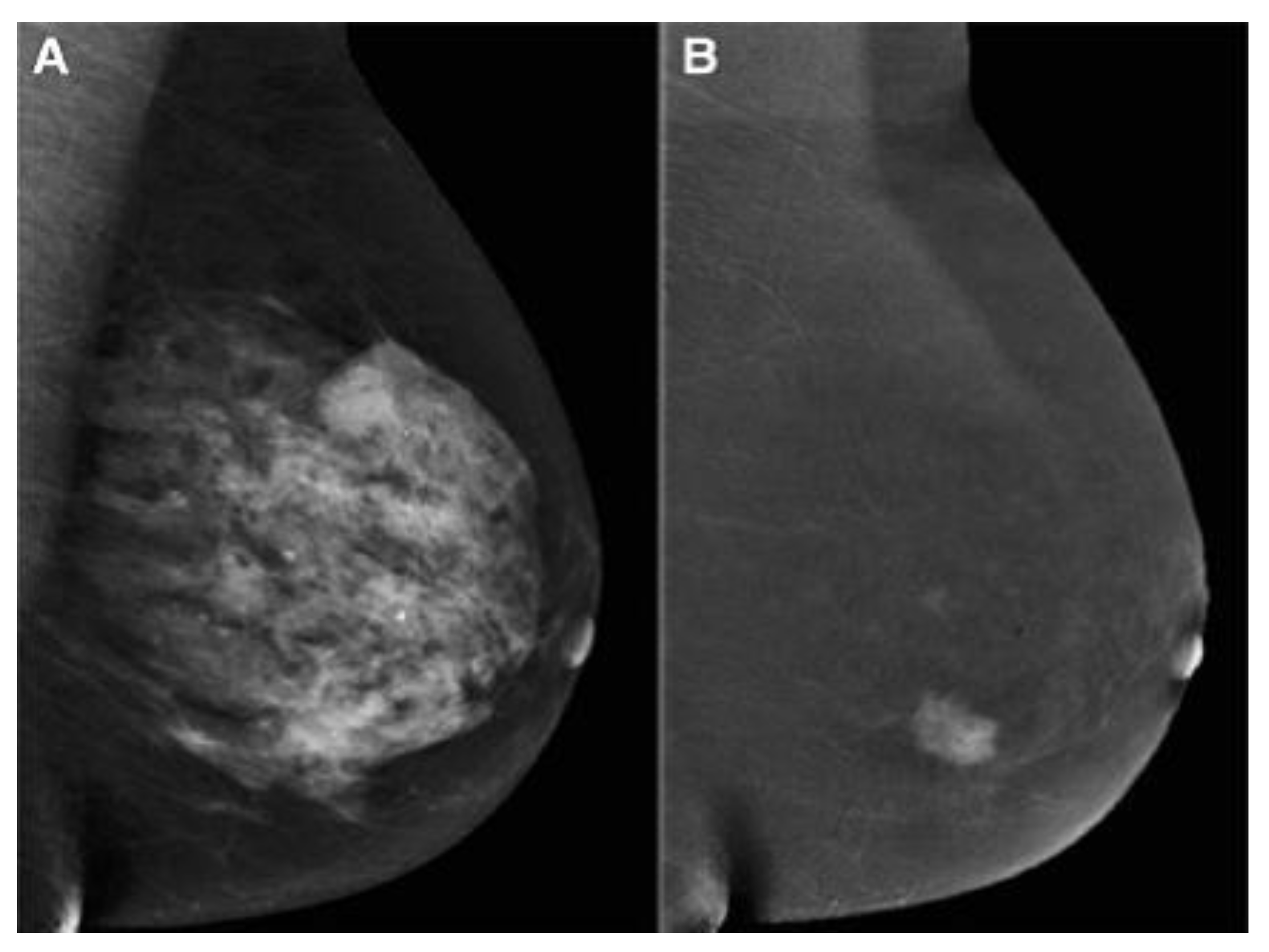
2.3. Contrast-Enhanced Mammography (CEM)
2.4. Nanoparticles in Mammography
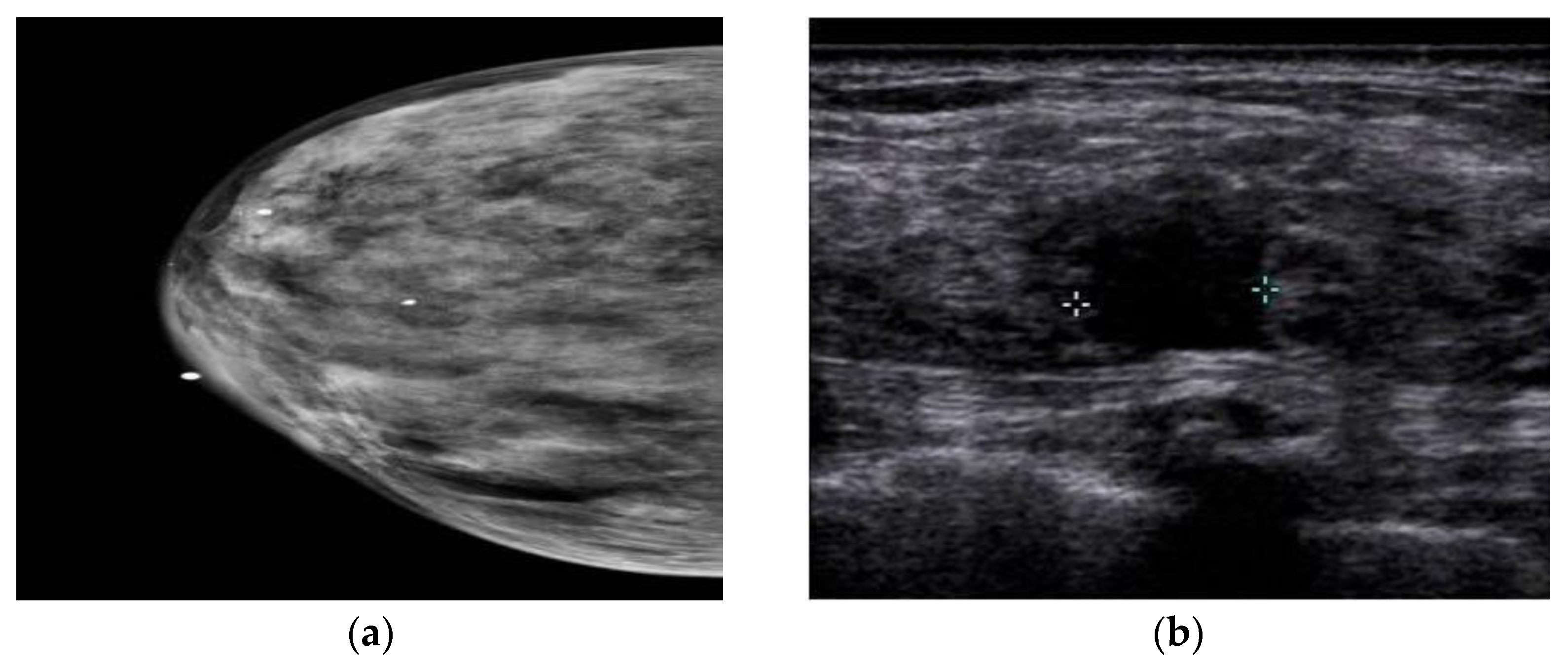
3. Ultrasound Imaging (US)
Nanoparticles in US
4. Magnetic Resonance Imaging (MRI)
4.1. Dynamic Contrast-Enhanced MRI
4.2. Diffusion-Weighted Imaging (DWI)
4.3. Magnetic Resonance Spectroscopy
4.4. Magnetic Resonance Elastography
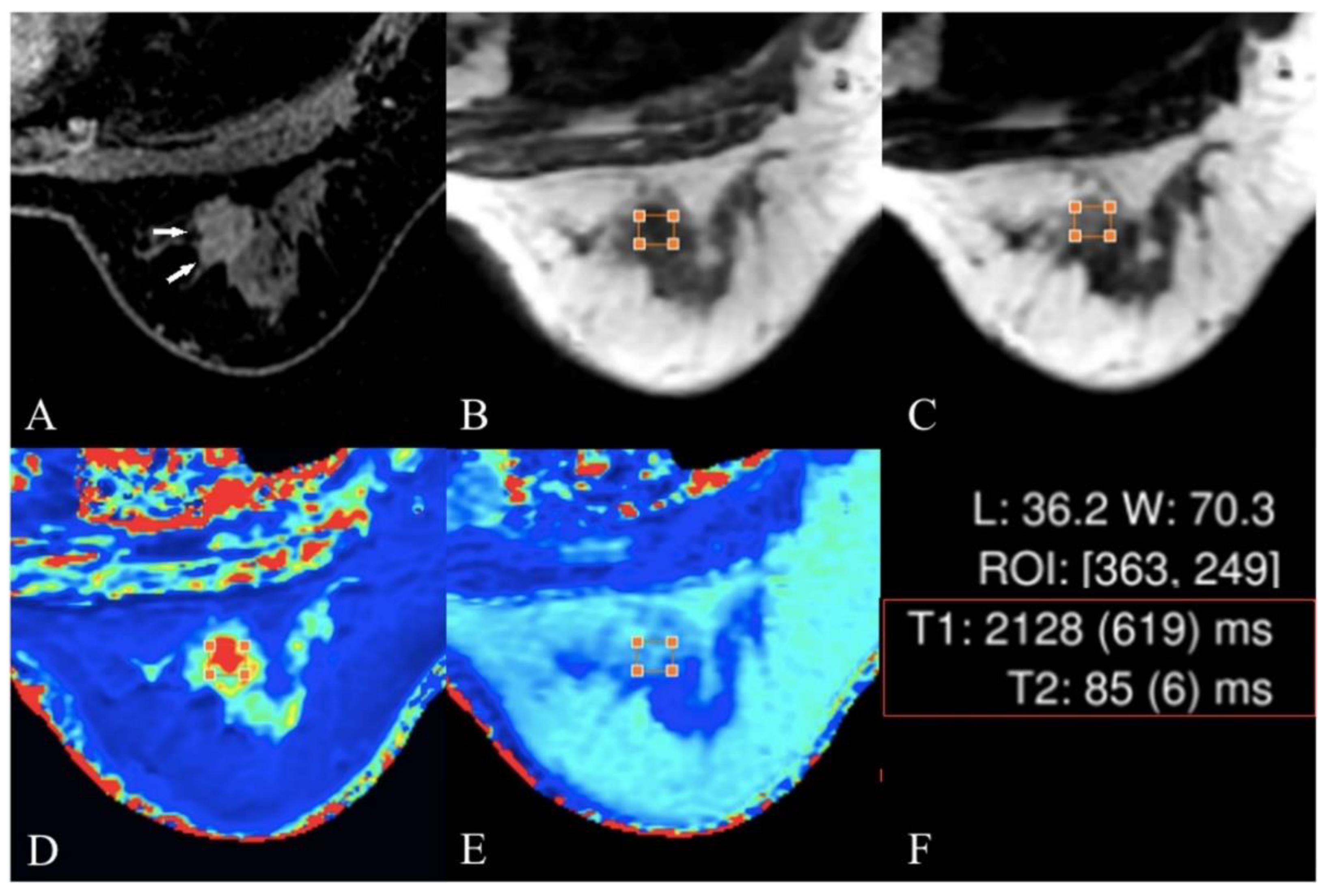
4.5. T2 and T2* Mapping
4.6. Nanoparticles in MRI
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Singletary, S.E. Rating the risk factors for breast cancer. Ann. Surg. 2003, 237, 474–482. [Google Scholar] [CrossRef]
- Le Boulc’h, M.; Bekhouche, A.; Kermarrec, E.; Milon, A.; Abdel Wahab, C.; Zilberman, S.; Chabbert-Buffet, N.; Thomassin-Naggara, I. Comparison of breast density assessment between human eye and automated software on digital and synthetic mammography: Impact on breast cancer risk. Diagn. Interv. Imaging 2020, 101, 811–819. [Google Scholar] [CrossRef]
- Huang, J.; Chan, P.S.; Lok, V.; Chen, X.; Ding, H.; Jin, Y.; Yuan, J.; Lao, X.Q.; Zheng, Z.J.; Wong, M.C. Global incidence and mortality of breast cancer: A trend analysis. Aging 2021, 13, 5748–5803. [Google Scholar] [CrossRef]
- Duggento, A.; Conti, A.; Mauriello, A.; Guerrisi, M.; Toschi, N. Deep computational pathology in breast cancer. Semin. Cancer Biol. 2021, 72, 226–237. [Google Scholar] [CrossRef]
- Ding, K.; Zhou, M.; Wang, H.; Gevaert, O.; Metaxas, D.; Zhang, S. A large-scale synthetic pathological dataset for deep learning-enabled segmentation of breast cancer. Sci. Data 2023, 10, 231. [Google Scholar] [CrossRef]
- Qu, H.; Zhou, M.; Yan, Z.; Wang, H.; Rustgi, V.K.; Zhang, S.; Gevaert, O.; Metaxas, D.N. Genetic mutation and biological pathway prediction based on whole slide images in breast carcinoma using deep learning. NPJ Precis. Oncol. 2021, 5, 87. [Google Scholar] [CrossRef]
- Ibrahim, A.; Gamble, P.; Jaroensri, R.; Abdelsamea, M.M.; Mermel, C.H.; Chen, P.-H.C.; Rakha, E.A. Artificial intelligence in digital breast pathology: Techniques and applications. Breast 2020, 49, 267–273. [Google Scholar] [CrossRef]
- Bhushan, A.; Gonsalves, A.; Menon, J.U. Current state of breast cancer diagnosis, treatment, and theranostics. Pharmaceutics 2021, 13, 723. [Google Scholar] [CrossRef]
- Moy, L.; Heller, S.L.; Bailey, L.; D’Orsi, C.; DiFlorio, R.M.; Green, E.D.; Holbrook, A.I.; Lee, S.J.; Lourenco, A.P.; Mainiero, M.B.; et al. ACR Appropriateness Criteria(®) Palpable Breast Masses. J. Am. Coll. Radiol. 2017, 14, S203–S224. [Google Scholar] [CrossRef]
- Nikolova, N.K. Microwave imaging for breast cancer. IEEE Microw. Mag. 2011, 12, 78–94. [Google Scholar] [CrossRef]
- Løberg, M.; Lousdal, M.L.; Bretthauer, M.; Kalager, M. Benefits and harms of mammography screening. Breast Cancer Res. 2015, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Dibden, A.; Offman, J.; Duffy, S.W.; Gabe, R. Worldwide review and meta-analysis of cohort studies measuring the effect of mammography screening programmes on incidence-based breast cancer mortality. Cancers 2020, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, R.E. Radiation Doses and Risks in Breast Screening. J. Breast Imaging 2020, 2, 188–200. [Google Scholar] [CrossRef]
- Zeeshan, M.; Salam, B.; Khalid, Q.S.B.; Alam, S.; Sayani, R. Diagnostic accuracy of digital mammography in the detection of breast cancer. Cureus 2018, 10, e2448. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, Z.; Tan, M.; Elingarami, S.; Liu, Y.; Li, T.; Deng, Y.; He, N.; Li, S.; Fu, J. A review on methods for diagnosis of breast cancer cells and tissues. Cell Prolif. 2020, 53, e12822. [Google Scholar] [CrossRef] [PubMed]
- Mandelson, M.T.; Oestreicher, N.; Porter, P.L.; White, D.; Finder, C.A.; Taplin, S.H.; White, E. Breast density as a predictor of mammographic detection: Comparison of interval-and screen-detected cancers. J. Natl. Cancer Inst. 2000, 92, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Seeram, E. Full-Field Digital Mammography. In Digital Radiography: Physical Principles and Quality Control; Seeram, E., Ed.; Springer: Singapore, 2019; pp. 111–123. [Google Scholar] [CrossRef]
- Song, S.Y.; Park, B.; Hong, S.; Kim, M.J.; Lee, E.H.; Jun, J.K. Comparison of Digital and Screen-Film Mammography for Breast-Cancer Screening: A Systematic Review and Meta-Analysis. J. Breast Cancer 2019, 22, 311–325. [Google Scholar] [CrossRef]
- Farber, R.; Houssami, N.; Wortley, S.; Jacklyn, G.; Marinovich, M.L.; McGeechan, K.; Barratt, A.; Bell, K. Impact of Full-Field Digital Mammography Versus Film-Screen Mammography in Population Screening: A Meta-Analysis. JNCI J. Natl. Cancer Inst. 2020, 113, 16–26. [Google Scholar] [CrossRef]
- Pisano, E.D.; Gatsonis, C.; Hendrick, E.; Yaffe, M.; Baum, J.K.; Acharyya, S.; Conant, E.F.; Fajardo, L.L.; Bassett, L.; D’Orsi, C.; et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N. Engl. J. Med. 2005, 353, 1773–1783. [Google Scholar] [CrossRef]
- Posso, M.; Louro, J.; Sánchez, M.; Román, M.; Vidal, C.; Sala, M.; Baré, M.; Castells, X.; Group, B.S. Mammographic breast density: How it affects performance indicators in screening programmes? Eur. J. Radiol. 2019, 110, 81–87. [Google Scholar] [CrossRef]
- Kerlikowske, K.; Hubbard, R.A.; Miglioretti, D.L.; Geller, B.M.; Yankaskas, B.C.; Lehman, C.D.; Taplin, S.H.; Sickles, E.A.; Consortium, B.C.S. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: A cohort study. Ann. Intern. Med. 2011, 155, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, K.E.; Weinstein, S.P.; McDonald, E.S.; Conant, E.F. Strategies to increase cancer detection: Review of true-positive and false-negative results at digital breast tomosynthesis screening. Radiographics 2016, 36, 1954. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.A.; Lo, J.Y. Breast tomosynthesis: State-of-the-art and review of the literature. Acad. Radiol. 2011, 18, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, G.; Bernardi, D.; Houssami, N. Radiation dose with digital breast tomosynthesis compared to digital mammography: Per-view analysis. Eur. Radiol. 2018, 28, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Georgian-Smith, D.; Obuchowski, N.A.; Lo, J.Y.; Brem, R.F.; Baker, J.A.; Fisher, P.R.; Rim, A.; Zhao, W.; Fajardo, L.L.; Mertelmeier, T. Can Digital Breast Tomosynthesis Replace Full-Field Digital Mammography? A Multireader, Multicase Study of Wide-Angle Tomosynthesis. Am. J. Roentgenol. 2019, 212, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.A.; Adel, L. Study of role of digital breast tomosynthesis over digital mammography in the assessment of BIRADS 3 breast lesions. Egypt. J. Radiol. Nucl. Med. 2019, 50, 48. [Google Scholar] [CrossRef]
- Østerås, B.H.; Martinsen, A.C.T.; Gullien, R.; Skaane, P. Digital Mammography versus Breast Tomosynthesis: Impact of Breast Density on Diagnostic Performance in Population-based Screening. Radiology 2019, 293, 60–68. [Google Scholar] [CrossRef]
- Dang, P.A.; Wang, A.; Senapati, G.M.; Ip, I.K.; Lacson, R.; Khorasani, R.; Giess, C.S. Comparing Tumor Characteristics and Rates of Breast Cancers Detected by Screening Digital Breast Tomosynthesis and Full-Field Digital Mammography. Am. J. Roentgenol. 2019, 214, 701–706. [Google Scholar] [CrossRef]
- Lee, S.H.; Jang, M.J.; Kim, S.M.; Yun, B.L.; Rim, J.; Chang, J.M.; Kim, B.; Choi, H.Y. Factors affecting breast cancer detectability on digital breast tomosynthesis and two-dimensional digital mammography in patients with dense breasts. Korean J. Radiol. 2019, 20, 58–68. [Google Scholar] [CrossRef]
- Romanucci, G.; Mercogliano, S.; Carucci, E.; Cina, A.; Zantedeschi, E.; Caneva, A.; Benassuti, C.; Fornasa, F. Diagnostic accuracy of resection margin in specimen radiography: Digital breast tomosynthesis versus full-field digital mammography. La Radiol. Medica 2021, 126, 768–773. [Google Scholar] [CrossRef]
- Heindel, W.; Weigel, S.; Gerß, J.; Hense, H.-W.; Sommer, A.; Krischke, M.; Kerschke, L. Digital breast tomosynthesis plus synthesised mammography versus digital screening mammography for the detection of invasive breast cancer (TOSYMA): A multicentre, open-label, randomised, controlled, superiority trial. Lancet Oncol. 2022, 23, 601–611. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Zhang, Y.; Gu, Y.; Xiao, Q.; Liu, G.; Shen, X.; Yang, W.; Peng, W. Comparison of the diagnostic performance of synthesized two-dimensional mammography and full-field digital mammography alone or in combination with digital breast tomosynthesis. Breast Cancer 2020, 27, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Han, B.-K.; Ko, E.Y.; Kim, G.R.; Ko, E.S.; Park, K.W. Comparison of synthetic and digital mammography with digital breast tomosynthesis or alone for the detection and classification of microcalcifications. Eur. Radiol. 2019, 29, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Woo, O.-h.; Shin, H.-s.; Cho, K.R.; Seo, B.K.; Choi, G.-Y. Quantitative analysis of radiation dosage and image quality between digital breast tomosynthesis (DBT) with two-dimensional synthetic mammography and full-field digital mammography (FFDM). Clin. Imaging 2019, 55, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Falomo, E.; Myers, K.; Reichel, K.F.; Carson, K.A.; Mullen, L.; Di Carlo, P.; Harvey, S. Impact of insurance coverage and socioeconomic factors on screening mammography patients’ selection of digital breast tomosynthesis versus full-field digital mammography. Breast J. 2018, 24, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Barca, P.; Lamastra, R.; Aringhieri, G.; Tucciariello, R.M.; Traino, A.; Fantacci, M.E. Comprehensive assessment of image quality in synthetic and digital mammography: A quantitative comparison. Australas. Phys. Eng. Sci. Med. 2019, 42, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.; Uchiyama, N.; Tani, H.; Yoshida, T.; Kumita, S. Comparative analysis between synthetic mammography reconstructed from digital breast tomosynthesis and full-field digital mammography for breast cancer detection and visibility. Eur. J. Radiol. Open 2020, 7, 100207. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.; Chaturvedi, A.K.; Aggarwal, A.; Rao, S.A.; Hazarika, D.; Mahawar, V. Comparing the diagnostic efficacy of full field digital mammography with digital breast tomosynthesis using BIRADS score in a tertiary cancer care hospital. Indian J. Radiol. Imaging 2018, 28, 115–122. [Google Scholar] [CrossRef]
- Skaane, P.; Bandos, A.I.; Niklason, L.T.; Sebuødegård, S.; Østerås, B.H.; Gullien, R.; Gur, D.; Hofvind, S. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: The Oslo Tomosynthesis Screening Trial. Radiology 2019, 291, 23–30. [Google Scholar] [CrossRef]
- Yi, A.; Chang, J.M.; Shin, S.U.; Chu, A.J.; Cho, N.; Noh, D.-Y.; Moon, W.K. Detection of noncalcified breast cancer in patients with extremely dense breasts using digital breast tomosynthesis compared with full-field digital mammography. Br. J. Radiol. 2019, 92, 20180101. [Google Scholar] [CrossRef]
- Alabousi, M.; Wadera, A.; Kashif Al-Ghita, M.; Kashef Al-Ghetaa, R.; Salameh, J.P.; Pozdnyakov, A.; Zha, N.; Samoilov, L.; Dehmoobad Sharifabadi, A.; Sadeghirad, B.; et al. Performance of Digital Breast Tomosynthesis, Synthetic Mammography, and Digital Mammography in Breast Cancer Screening: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2021, 113, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Khanani, S.; Hruska, C.; Lazar, A.; Hoernig, M.; Hebecker, A.; Obuchowski, N. Performance of Wide-Angle Tomosynthesis with Synthetic Mammography in Comparison to Full Field Digital Mammography. Acad. Radiol. 2022, 30, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, S.P.; Conant, E.F.; Keller, B.M.; Maidment, A.D.; Barufaldi, B.; Weinstein, S.P.; Synnestvedt, M.; McDonald, E.S. Implementation of synthesized two-dimensional mammography in a population-based digital breast tomosynthesis screening program. Radiology 2016, 281, 730. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, D.; Macaskill, P.; Pellegrini, M.; Valentini, M.; Fantò, C.; Ostillio, L.; Tuttobene, P.; Luparia, A.; Houssami, N. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): A population-based prospective study. Lancet Oncol. 2016, 17, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Tamam, N.; Salah, H.; Rabbaa, M.; Abuljoud, M.; Sulieman, A.; Alkhorayef, M.; Bradley, D.A. Evaluation of patients radiation dose during mammography imaging procedure. Radiat. Phys. Chem. 2021, 188, 109680. [Google Scholar] [CrossRef]
- Ghaderi, K.F.; Phillips, J.; Perry, H.; Lotfi, P.; Mehta, T.S. Contrast-enhanced mammography: Current applications and future directions. Radiographics 2019, 39, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Diekmann, F.; Lawaczeck, R. Contrast Media in CEDM. In Contrast-Enhanced Digital Mammography (CEDM); Nori, J., Kaur, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 25–33. [Google Scholar] [CrossRef]
- Jochelson, M.S.; Dershaw, D.D.; Sung, J.S.; Heerdt, A.S.; Thornton, C.; Moskowitz, C.S.; Ferrara, J.; Morris, E.A. Bilateral contrast-enhanced dual-energy digital mammography: Feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013, 266, 743–751. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y. Performance evaluation of total variation (TV) denoising technique for dual-energy contrast-enhanced digital mammography (CEDM) with photon counting detector (PCD): Monte Carlo simulation study. Radiat. Phys. Chem. 2019, 156, 94–100. [Google Scholar] [CrossRef]
- Mori, M.; Akashi-Tanaka, S.; Suzuki, S.; Daniels, M.I.; Watanabe, C.; Hirose, M.; Nakamura, S. Diagnostic accuracy of contrast-enhanced spectral mammography in comparison to conventional full-field digital mammography in a population of women with dense breasts. Breast Cancer 2017, 24, 104–110. [Google Scholar] [CrossRef]
- Kim, G.; Phillips, J.; Cole, E.; Brook, A.; Mehta, T.; Slanetz, P.; Fishman, M.D.C.; Karimova, E.; Mehta, R.; Lotfi, P.; et al. Comparison of Contrast-Enhanced Mammography With Conventional Digital Mammography in Breast Cancer Screening: A Pilot Study. J. Am. Coll. Radiol. 2019, 16, 1456–1463. [Google Scholar] [CrossRef]
- Sorin, V.; Yagil, Y.; Yosepovich, A.; Shalmon, A.; Gotlieb, M.; Neiman, O.H.; Sklair-Levy, M. Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts. AJR Am. J. Roentgenol. 2018, 211, W267–W274. [Google Scholar] [CrossRef] [PubMed]
- Sudhir, R.; Sannapareddy, K.; Potlapalli, A.; Krishnamurthy, P.B.; Buddha, S.; Koppula, V. Diagnostic accuracy of contrast-enhanced digital mammography in breast cancer detection in comparison to tomosynthesis, synthetic 2D mammography and tomosynthesis combined with ultrasound in women with dense breast. Br. J. Radiol. 2021, 94, 20201046. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Roth, R.; Germaine, P.; Ren, S.; Lee, M.; Hunter, K.; Tinney, E.; Liao, L. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): A retrospective comparison in 66 breast lesions. Diagn. Interv. Imaging 2017, 98, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; Miller, M.M.; Mehta, T.S.; Fein-Zachary, V.; Nathanson, A.; Hori, W.; Monahan-Earley, R.; Slanetz, P.J. Contrast-enhanced spectral mammography (CESM) versus MRI in the high-risk screening setting: Patient preferences and attitudes. Clin. Imaging 2017, 42, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Bozzini, A.; Nicosia, L.; Pruneri, G.; Maisonneuve, P.; Meneghetti, L.; Renne, G.; Vingiani, A.; Cassano, E.; Mastropasqua, M.G. Clinical performance of contrast-enhanced spectral mammography in pre-surgical evaluation of breast malignant lesions in dense breasts: A single center study. Breast Cancer Res. Treat. 2020, 184, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-P.; Lewin, J.M.; Chiang, C.-L.; Hung, B.-H.; Yang, T.-L.; Huang, J.-S.; Liao, J.-B.; Pan, H.-B. Clinical evaluation of contrast-enhanced digital mammography and contrast enhanced tomosynthesis—Comparison to contrast-enhanced breast MRI. Eur. J. Radiol. 2015, 84, 2501–2508. [Google Scholar] [CrossRef]
- Huang, J.-S.; Pan, H.-B.; Yang, T.-L.; Hung, B.-H.; Chiang, C.-L.; Tsai, M.-Y.; Chou, C.-P. Kinetic patterns of benign and malignant breast lesions on contrast enhanced digital mammogram. PLoS ONE 2020, 15, e0239271. [Google Scholar] [CrossRef]
- De Silva, F.; Alcorn, J. A tale of two cancers: A current concise overview of breast and prostate cancer. Cancers 2022, 14, 2954. [Google Scholar] [CrossRef]
- Hogan, M.P.; Horvat, J.V.; Ross, D.S.; Sevilimedu, V.; Jochelson, M.S.; Kirstein, L.J.; Goldfarb, S.B.; Comstock, C.E.; Sung, J.S. Contrast-enhanced mammography in the assessment of residual disease after neoadjuvant treatment. Breast Cancer Res. Treat. 2023, 198, 349–359. [Google Scholar] [CrossRef]
- Bicchierai, G.; Tonelli, P.; Piacenti, A.; De Benedetto, D.; Boeri, C.; Vanzi, E.; Bianchi, S.; Cirone, D.; Kaur Gill, M.; Miele, V. Evaluation of contrast-enhanced digital mammography (CEDM) in the preoperative staging of breast cancer: Large-scale single-center experience. Breast J. 2020, 26, 1276–1283. [Google Scholar] [CrossRef]
- Bicchierai, G.; Amato, F.; Vanzi, B.; De Benedetto, D.; Boeri, C.; Vanzi, E.; Di Naro, F.; Bianchi, S.; Cirone, D.; Cozzi, D. Which clinical, radiological, histological, and molecular parameters are associated with the absence of enhancement of known breast cancers with Contrast Enhanced Digital Mammography (CEDM)? Breast 2020, 54, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.K.; Naylor, M.E.; Kosiorek, H.E.; Lopez-Alvarez, Y.M.; Miller, A.M.; Pizzitola, V.J.; Pockaj, B.A. Clinical utility of contrast-enhanced spectral mammography as an adjunct for tomosynthesis-detected architectural distortion. Clin. Imaging 2017, 46, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.C.; Lau, K.C.; Hsu, J.C.; Hajfathalian, M.; Mian, S.; Chhour, P.; Uppuluri, L.; McDonald, E.S.; Maidment, A.D.; Cormode, D.P. Gold silver alloy nanoparticles (GSAN): An imaging probe for breast cancer screening with dual-energy mammography or computed tomography. Nanoscale 2016, 8, 13740–13754. [Google Scholar] [CrossRef] [PubMed]
- Nieves, L.M.; Hsu, J.C.; Lau, K.C.; Maidment, A.D.; Cormode, D.P. Silver telluride nanoparticles as biocompatible and enhanced contrast agents for X-ray imaging: An in vivo breast cancer screening study. Nanoscale 2021, 13, 163–174. [Google Scholar] [CrossRef]
- Karunamuni, R.; Naha, P.C.; Lau, K.C.; Al-Zaki, A.; Popov, A.V.; Delikatny, E.J.; Tsourkas, A.; Cormode, D.P.; Maidment, A.D. Development of silica-encapsulated silver nanoparticles as contrast agents intended for dual-energy mammography. Eur. Radiol. 2016, 26, 3301–3309. [Google Scholar] [CrossRef]
- Cole, L.E.; Vargo-Gogola, T.; Roeder, R.K. Contrast-enhanced X-ray detection of breast microcalcifications in a murine model using targeted gold nanoparticles. ACS Nano 2014, 8, 7486–7496. [Google Scholar] [CrossRef]
- Cole, L.E.; Vargo-Gogola, T.; Roeder, R.K. Contrast-enhanced x-ray detection of microcalcifications in radiographically dense mammary tissue using targeted gold nanoparticles. ACS Nano 2015, 9, 8923–8932. [Google Scholar] [CrossRef]
- Choudhery, S.; Axmacher, J.; Conners, A.L.; Geske, J.; Brandt, K. Masses in the era of screening tomosynthesis: Is diagnostic ultrasound sufficient? Br. J. Radiol. 2019, 92, 20180801. [Google Scholar] [CrossRef]
- Vourtsis, A.; Kachulis, A. The performance of 3D ABUS versus HHUS in the visualisation and BI-RADS characterisation of breast lesions in a large cohort of 1886 women. Eur. Radiol. 2018, 28, 592–601. [Google Scholar] [CrossRef]
- Lin, X.; Wang, J.; Han, F.; Fu, J.; Li, A. Analysis of eighty-one cases with breast lesions using automated breast volume scanner and comparison with handheld ultrasound. Eur. J. Radiol. 2012, 81, 873–878. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, H.H.; Cha, J.H. Current status of automated breast ultrasonography. Ultrasonography 2015, 34, 165. [Google Scholar] [CrossRef] [PubMed]
- Melnikow, J.; Fenton, J.J.; Whitlock, E.P.; Miglioretti, D.L.; Weyrich, M.S.; Thompson, J.H.; Shah, K. Supplemental Screening for Breast Cancer in Women With Dense Breasts: A Systematic Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Partridge, S.C.; Liao, G.J.; Hippe, D.S.; Kim, A.E.; Lee, C.I.; Rahbar, H.; Scheel, J.R.; Lehman, C.D. Double reading of automated breast ultrasound with digital mammography or digital breast tomosynthesis for breast cancer screening. Clin. Imaging 2019, 55, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Sood, R.; Rositch, A.F.; Shakoor, D.; Ambinder, E.; Pool, K.-L.; Pollack, E.; Mollura, D.J.; Mullen, L.A.; Harvey, S.C. Ultrasound for breast cancer detection globally: A systematic review and meta-analysis. J. Glob. Oncol. 2019, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Badu-Peprah, A.; Adu-Sarkodie, Y. Accuracy of clinical diagnosis, mammography and ultrasonography in preoperative assessment of breast cancer. Ghana Med. J. 2018, 52, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Harada-Shoji, N.; Suzuki, A.; Ishida, T.; Zheng, Y.-F.; Narikawa-Shiono, Y.; Sato-Tadano, A.; Ohta, R.; Ohuchi, N. Evaluation of adjunctive ultrasonography for breast cancer detection among women aged 40–49 years with varying breast density undergoing screening mammography: A secondary analysis of a randomized clinical trial. JAMA Netw. Open 2021, 4, e2121505. [Google Scholar] [CrossRef] [PubMed]
- Yi, A.; Jang, M.-j.; Yim, D.; Kwon, B.R.; Shin, S.U.; Chang, J.M. Addition of screening breast US to digital mammography and digital breast Tomosynthesis for breast cancer screening in women at average risk. Radiology 2021, 298, 568–575. [Google Scholar] [CrossRef]
- Dibble, E.H.; Singer, T.M.; Jimoh, N.; Baird, G.L.; Lourenco, A.P. Dense Breast Ultrasound Screening After Digital Mammography Versus After Digital Breast Tomosynthesis. Am. J. Roentgenol. 2019, 213, 1397–1402. [Google Scholar] [CrossRef]
- Choi, H.Y.; Park, M.; Seo, M.; Song, E.; Shin, S.Y.; Sohn, Y.-M. Preoperative axillary lymph node evaluation in breast cancer: Current issues and literature review. Ultrasound Q. 2017, 33, 6–14. [Google Scholar] [CrossRef]
- Lu, Z.; Hao, C.; Pan, Y.; Mao, N.; Wang, X.; Yin, X. Contrast-enhanced spectral mammography versus ultrasonography: Diagnostic performance in symptomatic patients with dense breasts. Korean J. Radiol. 2020, 21, 442–449. [Google Scholar] [CrossRef]
- Boyd, N.F.; Guo, H.; Martin, L.J.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.A.; Hislop, G.; Chiarelli, A.; Minkin, S. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 2007, 356, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Thigpen, D.; Kappler, A.; Brem, R. The Role of Ultrasound in Screening Dense Breasts—A Review of the Literature and Practical Solutions for Implementation. Diagnostics 2018, 8, 20. [Google Scholar] [CrossRef]
- Berg, W.A.; Gutierrez, L.; NessAiver, M.S.; Carter, W.B.; Bhargavan, M.; Lewis, R.S.; Ioffe, O.B. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology 2004, 233, 830–849. [Google Scholar] [CrossRef] [PubMed]
- Teh, W.; Wilson, A. The role of ultrasound in breast cancer screening. A consensus statement by the European Group for Breast Cancer Screening. Eur. J. Cancer 1998, 34, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Iranmakani, S.; Mortezazadeh, T.; Sajadian, F.; Ghaziani, M.F.; Ghafari, A.; Khezerloo, D.; Musa, A.E. A review of various modalities in breast imaging: Technical aspects and clinical outcomes. Egypt. J. Radiol. Nucl. Med. 2020, 51, 57. [Google Scholar] [CrossRef]
- Shin, S.U.; Chang, J.M.; Park, J.; Lee, H.B.; Han, W.; Moon, W.K. The Usefulness of Ultrasound Surveillance for Axillary Recurrence in Women With Personal History of Breast Cancer. J. Breast Cancer 2022, 25, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Cho, N.; Kim, S.Y.; Choi, Y.; Kim, E.S.; Ha, S.M.; Lee, S.H.; Chang, J.M.; Moon, W.K. Supplemental Breast US Screening in Women with a Personal History of Breast Cancer: A Matched Cohort Study. Radiology 2020, 295, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Tan-Chiu, E.; Wang, J.; Costantino, J.P.; Paik, S.; Butch, C.; Wickerham, D.L.; Fisher, B.; Wolmark, N. Effects of tamoxifen on benign breast disease in women at high risk for breast cancer. J. Natl. Cancer Inst. 2003, 95, 302–307. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Milgroom, A.; Intrator, M.; Madhavan, K.; Mazzaro, L.; Shandas, R.; Liu, B.; Park, D. Mesoporous silica nanoparticles as a breast-cancer targeting ultrasound contrast agent. Colloids Surf. B Biointerfaces 2014, 116, 652–657. [Google Scholar] [CrossRef]
- Subhan, M.A. Advances with metal oxide-based nanoparticles as MDR metastatic breast cancer therapeutics and diagnostics. RSC Adv. 2022, 12, 32956–32978. [Google Scholar] [CrossRef]
- Nguyen Cao, T.G.; Kang, J.H.; You, J.Y.; Kang, H.C.; Rhee, W.J.; Ko, Y.T.; Shim, M.S. Safe and Targeted Sonodynamic Cancer Therapy Using Biocompatible Exosome-Based Nanosonosensitizers. ACS Appl. Mater. Interfaces 2021, 13, 25575–25588. [Google Scholar] [CrossRef] [PubMed]
- Morrow, M.; Waters, J.; Morris, E. MRI for breast cancer screening, diagnosis, and treatment. Lancet 2011, 378, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Pilarski, R.; Berry, M.; Buys, S.S.; Farmer, M.; Friedman, S.; Garber, J.E.; Kauff, N.D.; Khan, S.; Klein, C. NCCN guidelines insights: Genetic/familial high-risk assessment: Breast and ovarian, version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Anderson, B.O.; Balassanian, R.; Blair, S.L.; Burstein, H.J.; Cyr, A.; Elias, A.D.; Farrar, W.B.; Forero, A.; Giordano, S.H.; et al. NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J. Natl. Compr. Cancer Netw. JNCCN 2017, 15, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Riedl, C.C.; Luft, N.; Bernhart, C.; Weber, M.; Bernathova, M.; Tea, M.K.; Rudas, M.; Singer, C.F.; Helbich, T.H. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Benndorf, M.; Baltzer, P.A.; Vag, T.; Gajda, M.; Runnebaum, I.B.; Kaiser, W.A. Breast MRI as an adjunct to mammography: Does it really suffer from low specificity? A retrospective analysis stratified by mammographic BI-RADS classes. Acta Radiol. 2010, 51, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, C.G.; Dietzel, M.; Vag, T.; Froelich, M.F. Cost-effectiveness of MR-mammography vs. conventional mammography in screening patients at intermediate risk of breast cancer—A model-based economic evaluation. Eur. J. Radiol. 2021, 136, 109355. [Google Scholar] [CrossRef]
- Sippo, D.A.; Burk, K.S.; Mercaldo, S.F.; Rutledge, G.M.; Edmonds, C.; Guan, Z.; Hughes, K.S.; Lehman, C.D. Performance of screening breast MRI across women with different elevated breast cancer risk indications. Radiology 2019, 292, 51–59. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Cho, N.; Hong, H.; Lee, Y.; Yoen, H.; Kim, Y.S.; Park, A.R.; Ha, S.M.; Lee, S.H.; Chang, J.M.; et al. Abbreviated Screening MRI for Women with a History of Breast Cancer: Comparison with Full-Protocol Breast MRI. Radiology 2022, 305, 36–45. [Google Scholar] [CrossRef]
- Plana, M.N.; Carreira, C.; Muriel, A.; Chiva, M.; Abraira, V.; Emparanza, J.I.; Bonfill, X.; Zamora, J. Magnetic resonance imaging in the preoperative assessment of patients with primary breast cancer: Systematic review of diagnostic accuracy and meta-analysis. Eur. Radiol. 2012, 22, 26–38. [Google Scholar] [CrossRef]
- Comstock, C.E.; Gatsonis, C.; Newstead, G.M.; Snyder, B.S.; Gareen, I.F.; Bergin, J.T.; Rahbar, H.; Sung, J.S.; Jacobs, C.; Harvey, J.A.; et al. Comparison of Abbreviated Breast MRI vs Digital Breast Tomosynthesis for Breast Cancer Detection among Women With Dense Breasts Undergoing Screening. JAMA 2020, 323, 746–756. [Google Scholar] [CrossRef]
- Vreemann, S.; van Zelst, J.C.M.; Schlooz-Vries, M.; Bult, P.; Hoogerbrugge, N.; Karssemeijer, N.; Gubern-Mérida, A.; Mann, R.M. The added value of mammography in different age-groups of women with and without BRCA mutation screened with breast MRI. Breast Cancer Res. 2018, 20, 84. [Google Scholar] [CrossRef]
- Gu, W.Q.; Cai, S.M.; Liu, W.D.; Zhang, Q.; Shi, Y.; Du, L.J. Combined molybdenum target X-ray and magnetic resonance imaging examinations improve breast cancer diagnostic efficacy. World J. Clin. Cases 2022, 10, 485–491. [Google Scholar] [CrossRef]
- Wernli, K.J.; Ichikawa, L.; Kerlikowske, K.; Buist, D.S.M.; Brandzel, S.D.; Bush, M.; Johnson, D.; Henderson, L.M.; Nekhlyudov, L.; Onega, T.; et al. Surveillance Breast MRI and Mammography: Comparison in Women with a Personal History of Breast Cancer. Radiology 2019, 292, 311–318. [Google Scholar] [CrossRef]
- Xiang, W.; Rao, H.; Zhou, L. A meta-analysis of contrast-enhanced spectral mammography versus MRI in the diagnosis of breast cancer. Thorac. Cancer 2020, 11, 1423–1432. [Google Scholar] [CrossRef]
- Covington, M.F.; Pizzitola, V.J.; Lorans, R.; Pockaj, B.A.; Northfelt, D.W.; Appleton, C.M.; Patel, B.K. The future of contrast-enhanced mammography. Am. J. Roentgenol. 2018, 210, 292–300. [Google Scholar] [CrossRef]
- Shahbazi-Gahrouei, D.; Aminolroayaei, F.; Nematollahi, H.; Ghaderian, M.; Shahbazi Gahrouei, S. Advanced magnetic resonance imaging modalities for breast cancer diagnosis: An overview of recent findings and perspectives. Diagnostics 2022, 12, 2741. [Google Scholar] [CrossRef]
- Kaiser, C.G.; Dietzel, M.; Vag, T.; Rübenthaler, J.; Froelich, M.F.; Tollens, F. Impact of specificity on cost-effectiveness of screening women at high risk of breast cancer with magnetic resonance imaging, mammography and ultrasound. Eur. J. Radiol. 2021, 137, 109576. [Google Scholar] [CrossRef]
- Graeser, M.; Schrading, S.; Gluz, O.; Strobel, K.; Herzog, C.; Umutlu, L.; Frydrychowicz, A.; Rjosk-Dendorfer, D.; Würstlein, R.; Culemann, R. Magnetic resonance imaging and ultrasound for prediction of residual tumor size in early breast cancer within the ADAPT subtrials. Breast Cancer Res. 2021, 23, 36. [Google Scholar] [CrossRef]
- Romeo, V.; Helbich, T.H.; Pinker, K. Breast PET/MRI Hybrid imaging and targeted tracers. J. Magn. Reson. Imaging 2023, 57, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Morawitz, J.; Bruckmann, N.-M.; Dietzel, F.; Ullrich, T.; Bittner, A.-K.; Hoffmann, O.; Ruckhäberle, E.; Mohrmann, S.; Häberle, L.; Ingenwerth, M. Comparison of nodal staging between CT, MRI, and [18 F]-FDG PET/MRI in patients with newly diagnosed breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 992–1001. [Google Scholar] [CrossRef]
- Choi, E.J.; Choi, H.; Choi, S.A.; Youk, J.H. Dynamic contrast-enhanced breast magnetic resonance imaging for the prediction of early and late recurrences in breast cancer. Medicine 2016, 95, e5330. [Google Scholar] [CrossRef]
- Amornsiripanitch, N.; Bickelhaupt, S.; Shin, H.J.; Dang, M.; Rahbar, H.; Pinker, K.; Partridge, S.C. Diffusion-weighted MRI for unenhanced breast cancer screening. Radiology 2019, 293, 504. [Google Scholar] [CrossRef]
- Millet, I.; Pages, E.; Hoa, D.; Merigeaud, S.; Curros Doyon, F.; Prat, X.; Taourel, P. Pearls and pitfalls in breast MRI. Br. J. Radiol. 2012, 85, 197–207. [Google Scholar] [CrossRef]
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B. Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 2017, 16, 564–570. [Google Scholar] [CrossRef]
- Layne, K.A.; Dargan, P.I.; Archer, J.R.; Wood, D.M. Gadolinium deposition and the potential for toxicological sequelae–A literature review of issues surrounding gadolinium-based contrast agents. Br. J. Clin. Pharmacol. 2018, 84, 2522–2534. [Google Scholar] [CrossRef]
- Sharma, U.; Agarwal, K.; Hari, S.; Mathur, S.R.; Seenu, V.; Parshad, R.; Jagannathan, N.R. Role of diffusion weighted imaging and magnetic resonance spectroscopy in breast cancer patients with indeterminate dynamic contrast enhanced magnetic resonance imaging findings. Magn. Reson. Imaging 2019, 61, 66–72. [Google Scholar] [CrossRef]
- Chotai, N.; Kulkarni, S. Breast Imaging Essentials; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Suh, J.; Kim, J.-H.; Kim, S.-Y.; Cho, N.; Kim, D.-H.; Kim, R.; Kim, E.S.; Jang, M.-j.; Ha, S.M.; Lee, S.H.; et al. Noncontrast-Enhanced MR-Based Conductivity Imaging for Breast Cancer Detection and Lesion Differentiation. J. Magn. Reson. Imaging 2021, 54, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.A.; Leithner, D.; Sung, J.; Avendano, D.; Morris, E.A.; Pinker, K.; Jochelson, M.S. Radiomics for tumor characterization in breast cancer patients: A feasibility study comparing contrast-enhanced mammography and magnetic resonance imaging. Diagnostics 2020, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Mansour, S.; Farouk, A.; Hanafy, M.; Elhatw, A.; Goma, M.M. Contrast-enhanced mammography in comparison with dynamic contrast-enhanced MRI: Which modality is appropriate for whom? Egypt. J. Radiol. Nucl. Med. 2021, 52, 216. [Google Scholar] [CrossRef]
- Pötsch, N.; Vatteroni, G.; Clauser, P.; Helbich, T.H.; Baltzer, P.A. Contrast-enhanced mammography versus contrast-enhanced breast MRI: A systematic review and meta-analysis. Radiology 2022, 305, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.M.; Kuhl, C.K.; Moy, L. Contrast-enhanced MRI for breast cancer screening. J. Magn. Reson. Imaging 2019, 50, 377–390. [Google Scholar] [CrossRef]
- Barkhausen, J.; Bischof, A.; Haverstock, D.; Klemens, M.; Brueggenwerth, G.; Weber, O.; Endrikat, J. Diagnostic efficacy of contrast-enhanced breast MRI versus X-ray mammography in women with different degrees of breast density. Acta Radiol. 2021, 62, 586–593. [Google Scholar] [CrossRef]
- Woitek, R.; McLean, M.A.; Gill, A.B.; Grist, J.T.; Provenzano, E.; Patterson, A.J.; Ursprung, S.; Torheim, T.; Zaccagna, F.; Locke, M.; et al. Hyperpolarized 13C MRI of Tumor Metabolism Demonstrates Early Metabolic Response to Neoadjuvant Chemotherapy in Breast Cancer. Radiol. Imaging Cancer 2020, 2, e200017. [Google Scholar] [CrossRef]
- Moy, L.; Noz, M.E.; Maguire Jr, G.Q.; Melsaether, A.; Deans, A.E.; Murphy-Walcott, A.D.; Ponzo, F. Role of fusion of prone FDG-PET and magnetic resonance imaging of the breasts in the evaluation of breast cancer. Breast J. 2010, 16, 369–376. [Google Scholar] [CrossRef]
- Rabasco, P.; Caivano, R.; Simeon, V.; Dinardo, G.; Lotumolo, A.; Gioioso, M.; Villonio, A.; Iannelli, G.; D’Antuono, F.; Zandolino, A. Can diffusion-weighted imaging and related apparent diffusion coefficient be a prognostic value in women with breast cancer? Cancer Investig. 2017, 35, 92–99. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Wienke, A. Can apparent diffusion coefficient (ADC) distinguish breast cancer from benign breast findings? A meta-analysis based on 13 847 lesions. BMC Cancer 2019, 19, 955. [Google Scholar] [CrossRef]
- Sharma, U.; Jagannathan, N.R. Characterization of breast tissues by diffusion weighted MR imaging. Biomed. Spectrosc. Imaging 2014, 3, 1–13. [Google Scholar] [CrossRef]
- Pinker, K.; Moy, L.; Sutton, E.J.; Mann, R.M.; Weber, M.; Thakur, S.B.; Jochelson, M.S.; Bago-Horvath, Z.; Morris, E.A.; Baltzer, P.A. Diffusion-weighted imaging with apparent diffusion coefficient mapping for breast cancer detection as a stand-alone-parameter: Comparison with dynamic contrast-enhanced and multiparametric magnetic resonance imaging. Investig. Radiol. 2018, 53, 587. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Lee, J.H.; Baek, H.J.; Ha, J.Y.; Ryu, K.H.; Park, S.E.; Moon, J.I.; Gho, S.-M.; Wakayama, T. Clinical Feasibility of Reduced Field-of-View Diffusion-Weighted Magnetic Resonance Imaging with Computed Diffusion-Weighted Imaging Technique in Breast Cancer Patients. Diagnostics 2020, 10, 538. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.C.; Nissan, N.; Rahbar, H.; Kitsch, A.E.; Sigmund, E.E. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J. Magn. Reson. Imaging JMRI 2017, 45, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Galati, F.; Trimboli, R.M.; Pediconi, F. Special Issue “Advances in Breast MRI&rdquo. Diagnostics 2021, 11, 2297. [Google Scholar] [PubMed]
- Montemezzi, S.; Cavedon, C.; Camera, L.; Meliadò, G.; Caumo, F.; Baglio, I.; Sardanelli, F. 1H-MR spectroscopy of suspicious breast mass lesions at 3T: A clinical experience. La Radiol. Medica 2017, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Prvulovic Bunovic, N.; Sveljo, O.; Kozic, D.; Boban, J. Is Elevated Choline on Magnetic Resonance Spectroscopy a Reliable Marker of Breast Lesion Malignancy? Front. Oncol. 2021, 11, 610354. [Google Scholar] [CrossRef] [PubMed]
- Sodano, C.; Clauser, P.; Dietzel, M.; Kapetas, P.; Pinker, K.; Helbich, T.H.; Gussew, A.; Baltzer, P.A. Clinical relevance of total choline (tCho) quantification in suspicious lesions on multiparametric breast MRI. Eur. Radiol. 2020, 30, 3371–3382. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.R.; Kalra, P.; Mo, X.; Raterman, B.; Yee, L.D.; Kolipaka, A. Quantification of breast stiffness using MR elastography at 3 Tesla with a soft sternal driver: A reproducibility study. J. Magn. Reson. Imaging JMRI 2017, 45, 1379–1384. [Google Scholar] [CrossRef]
- Patel, B.K.; Samreen, N.; Zhou, Y.; Chen, J.; Brandt, K.; Ehman, R.; Pepin, K. MR Elastography of the Breast: Evolution of Technique, Case Examples, and Future Directions. Clin. Breast Cancer 2021, 21, e102–e111. [Google Scholar] [CrossRef]
- Pepin, K.M.; Ehman, R.L.; McGee, K.P. Magnetic resonance elastography (MRE) in cancer: Technique, analysis, and applications. Prog. Nucl. Magn. Reson. Spectrosc. 2015, 90, 32–48. [Google Scholar] [CrossRef]
- Lorenzen, J.; Sinkus, R.; Lorenzen, M.; Dargatz, M.; Leussler, C.; Röschmann, P.; Adam, G. MR elastography of the breast:preliminary clinical results. Rofo 2002, 174, 830–834. [Google Scholar] [CrossRef]
- Liu, L.; Yin, B.; Geng, D.Y.; Lu, Y.P.; Peng, W.J. Changes of T2 relaxation time from neoadjuvant chemotherapy in breast cancer lesions. Iran. J. Radiol. 2016, 13, e24014. [Google Scholar] [CrossRef]
- Seo, M.; Ryu, J.K.; Jahng, G.-H.; Sohn, Y.-M.; Rhee, S.J.; Oh, J.-H.; Won, K.-Y. Estimation of T2* relaxation time of breast cancer: Correlation with clinical, imaging and pathological features. Korean J. Radiol. 2017, 18, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yin, B.; Shek, K.; Geng, D.; Lu, Y.; Wen, J.; Kuai, X.; Peng, W. Role of quantitative analysis of T2 relaxation time in differentiating benign from malignant breast lesions. J. Int. Med. Res. 2018, 46, 1928–1935. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; He, N.; He, H.; Liu, K.; Ke, L.; Liu, H.; Zhong, L.; Huang, C.; Yang, A.; Zhou, C. The diagnostic performance of quantitative mapping in breast cancer patients: A preliminary study using synthetic MRI. Cancer Imaging 2020, 20, 88. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Sarkar, S.; Saber, R.; Delavari, H.; Alizadeh, A.M.; Mulder, H.T. Magnetic hyperthermia of breast cancer cells and MRI relaxometry with dendrimer-coated iron-oxide nanoparticles. Cancer Nanotechnol 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Núñez, C.; Estévez, S.V.; del Pilar Chantada, M. Inorganic nanoparticles in diagnosis and treatment of breast cancer. JBIC J. Biol. Inorg. Chem. 2018, 23, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron Oxide Nanoparticles as T(1) Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2021, 33, e1906539. [Google Scholar] [CrossRef]
- Xiao, S.; Yu, X.; Zhang, L.; Zhang, Y.; Fan, W.; Sun, T.; Zhou, C.; Liu, Y.; Liu, Y.; Gong, M.; et al. Synthesis Of PEG-Coated, Ultrasmall, Manganese-Doped Iron Oxide Nanoparticles With High Relaxivity For T(1)/T(2) Dual-Contrast Magnetic Resonance Imaging. Int. J. Nanomed. 2019, 14, 8499–8507. [Google Scholar] [CrossRef]
- Huang, H.; Yue, T.; Xu, K.; Golzarian, J.; Yu, J.; Huang, J. Fabrication and evaluation of tumor-targeted positive MRI contrast agent based on ultrasmall MnO nanoparticles. Colloids Surf. B Biointerfaces 2015, 131, 148–154. [Google Scholar] [CrossRef]
- Ye, Y.J.; Huang, X.J.; Luo, B.C.; Wang, X.Y.; Cai, X.R. Application of multiparametric magnetic resonance imaging to monitor the early antitumor effect of CuS@ GOD nanoparticles in a 4 T1 breast cancer xenograft model. J. Magn. Reson. Imaging 2022, 55, 301–310. [Google Scholar] [CrossRef]
- Tao, Y.; Li, Y.; Wei, D.; Liang, M.; Ren, P.; Dai, J.; Zhang, T.; Lei, J.; Liu, P. Fe3O4 Nanoparticles Embedded in Pectin–Doxorubicin Composites as pH-Responsive Nanoplatforms for Tumor Diagnosis and Therapy by T 1-Weighted Magnetic Imaging. ACS Appl. Nano Mater. 2022, 6, 633–645. [Google Scholar] [CrossRef]
- Cheng, K.; Peng, S.; Xu, C.; Sun, S. Porous hollow Fe3O4 nanoparticles for targeted delivery and controlled release of cisplatin. J. Am. Chem. Soc. 2009, 131, 10637–10644. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, N.; Kim, H.; Kim, J.; Choi, S.H.; Kim, J.H.; Kim, T.; Song, I.C.; Park, S.P.; Moon, W.K. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. J. Am. Chem. Soc. 2010, 132, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.; Semelka, R.; Ramalho, M.; Nunes, R.; AlObaidy, M.; Castillo, M. Gadolinium-based contrast agent accumulation and toxicity: An update. Am. J. Neuroradiol. 2016, 37, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Liu, F.; Nasr, K.; Misra, P.; Bawendi, M.G.; Frangioni, J.V. Design considerations for tumour-targeted nanoparticles. Nat. Nanotechnol. 2010, 5, 42–47. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef]
- Poplack, S.P.; Park, E.-Y.; Ferrara, K.W. Optical breast imaging: A review of physical principles, technologies, and clinical applications. J. Breast Imaging 2023, 5, 520–537. [Google Scholar] [CrossRef]
| Modality | Advantages | Disadvantages | Diagnostic Performance | Ref. | |||
|---|---|---|---|---|---|---|---|
| AUC | Sensitivity | Specificity | Diagnostic Performance for: | ||||
| MG |
|
| N/A | 97% | 64.5% | Breast cancer detection | [14,15,16] |
| DBT |
|
| N/A | 95.5% | 78.8% | Malignancy detection | [27,30,31,35] |
| CEM |
|
| (0.768–0.924) | (86.2–98%) | (57.9–94.1%) | Cancer detection and breast cancer classification into malignant and benign | [49,51,53,54,57,62,63,64] |
| US |
|
| N/A | (49–90.6%) | (34–88.4%) | Screening of dense breast and breast cancer classification into the malignant and benign | [76,81,82,84,85,86] |
| MRI |
|
| 0.93 | (51–100%) | (94.9–96.1%) | Breast cancer detection | [15,94,98,102,103,108,109,122] |
| DCE-MRI |
|
| N/A | (81–100%) | ~97% | Breast cancer detection | [49,115,116,117,118,119,120,123,124,125] |
| DWI |
|
| 0.85 | (63–100%) | (46–97%) | Breast cancer classification into malignant and benign | [115,119,128,129,131,132] |
| Authors and Ref. | Nanoparticles | Imaging Modality | Application | Conclusion | |
|---|---|---|---|---|---|
| Imaging | Therapy | ||||
| Naha et al. [65] | Gold–silver alloy nanoparticles (GSAN) | DEM and CT | ✓ | GSAN produces strong DEM and CT contrast in images and has potential for breast cancer screening. | |
| Nieves et al. [66] | Silver telluride NPs (Ag2Te NPs) | DEM and CT | Strong X-ray contrast for breast cancer screening. | ||
| Karunamuni et al. [67] | Silica-encapsulated silver NPs | DEM | ✓ | Silver nanoparticles produce strong contrast in vivo using DEM imaging systems for breast cancer detection. | |
| Cole et al. [68] | Bisphosphonate-functionalized gold NPs (BP-Au NPs) | CT and X-ray imaging | ✓ | Targeted BP-Au NPs enabled improved sensitivity and specificity for the detection of microcalcifications in breast cancer with CT imaging. | |
| Cole et al. [69] | Bisphosphonate-functionalized gold NPs (BP-Au NPs) | CT and X-ray imaging | ✓ | Improved sensitivity and specificity for microcalcification detection in radiographically dense mammary tissues. | |
| Milgroom et al. [91] | Mesoporous silica nanoparticles (MSNs), functionalized with the monoclonal antibody Herceptin® | US | ✓ | ✓ | The results demonstrated that MSNs are a stable, biocompatible, and effective diagnostic and therapeutic agent for US breast cancer imaging, diagnosis, and treatment. |
| Cao et al. [93] | Exosome-based NPs | US | ✓ | Exosome-based NPs could serve as effective nanosonosensitizers for safe and targeted cancer treatment. | |
| Salimi et al. [148] | Fourth-generation dendrimer-coated iron-oxide nanoparticles (G4@IONPs) | MRI | ✓ | ✓ | The results showed that G4@IONPs improved transverse relaxivity (r2) significantly. |
| Xiao et al. [150] | Poly (ethylene glycol) (PEG)-coated, manganese-doped iron oxide nanocomposites (Mn-IONPs@PEG) | MRI | ✓ | The Mn-IONPs@PEG exhibited good properties for MRI imaging as a T1/T2 dual-contrast MRI contrast agent for cancer detection. | |
| Huang et al. [151] | PEGylated ultrasmall MnO NPs | MRI | ✓ | MnO NPs showed a great potential for the T1-weighted MRI diagnosis of tumors. | |
| Tao et al. [153] | Small Fe3O4 NPs | MRI | ✓ | ✓ | Fe3O4@PD-based system has the potential to be a multifunctional nanodrug delivery system and a smart theragnostic platform for cancer detection and treatment. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aminolroayaei, F.; Shahbazi-Gahrouei, S.; Khorasani, A.; Shahbazi-Gahrouei, D. A Review of Imaging Methods and Recent Nanoparticles for Breast Cancer Diagnosis. Information 2024, 15, 10. https://doi.org/10.3390/info15010010
Aminolroayaei F, Shahbazi-Gahrouei S, Khorasani A, Shahbazi-Gahrouei D. A Review of Imaging Methods and Recent Nanoparticles for Breast Cancer Diagnosis. Information. 2024; 15(1):10. https://doi.org/10.3390/info15010010
Chicago/Turabian StyleAminolroayaei, Fahimeh, Saghar Shahbazi-Gahrouei, Amir Khorasani, and Daryoush Shahbazi-Gahrouei. 2024. "A Review of Imaging Methods and Recent Nanoparticles for Breast Cancer Diagnosis" Information 15, no. 1: 10. https://doi.org/10.3390/info15010010
APA StyleAminolroayaei, F., Shahbazi-Gahrouei, S., Khorasani, A., & Shahbazi-Gahrouei, D. (2024). A Review of Imaging Methods and Recent Nanoparticles for Breast Cancer Diagnosis. Information, 15(1), 10. https://doi.org/10.3390/info15010010







