Ontology-Driven Knowledge Sharing in Alzheimer’s Disease Research
Abstract
1. Introduction
2. Materials and Methods
2.1. Purpose and Scope
- What are the stages within the Alzheimer’s disease spectrum and their differences?
- How is Alzheimer’s disease diagnosed?
- Why is the preclinical stage important and how can we do preclinical research?
- What are the assessments that clinicians usually use whenever Alzheimer’s disease is suspected and which are the most informative ones?
- What are the symptoms and pathological hallmarks of AD?
2.2. Building AD-DPC
2.3. Ontology Evaluation
2.4. Procedure and Participants
2.5. Questionnaires and Scales
3. Results
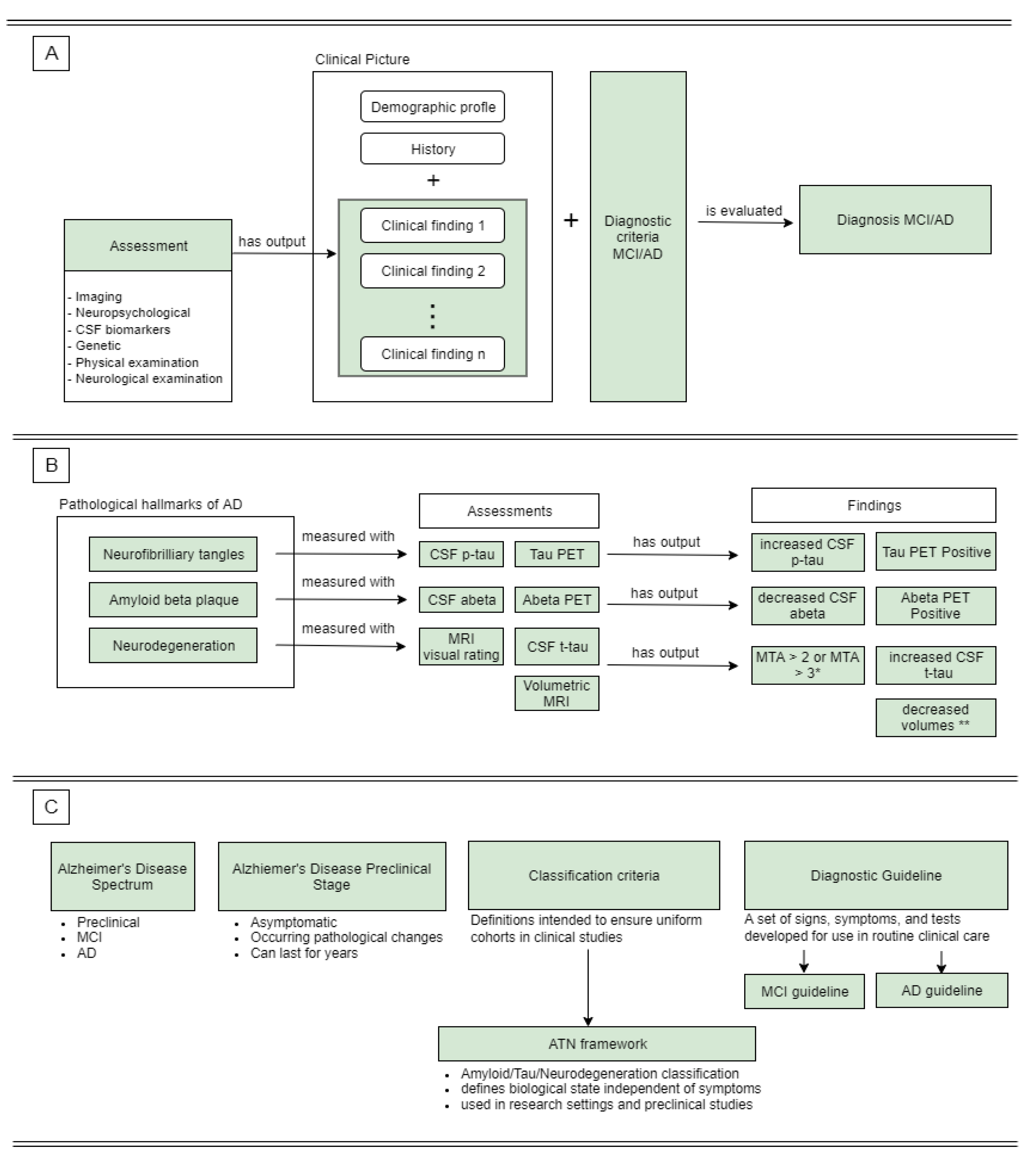
3.1. AD-DPC Definition
3.1.1. Alzheimer’s Disease Pathology
3.1.2. Alzheimer’s Disease Spectrum
3.1.3. Diagnostic Process
3.1.4. Symptoms
3.1.5. Assessments
3.1.6. Relevant Clinical Findings
AD-DPC Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Heal. 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef]
- Silva, M.V.F.; Loures, C.D.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; Carvalho, M.D.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Z.K.; Zhao, R.C.; Chakrabarti, S.; Stambler, I.; Jin, K.; Lim, L.W. Interdisciplinary Research in Alzheimer’s Disease and the Roles International Societies Can Play. Aging Dis. 2021, 12, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Littmann, M.; Selig, K.; Cohen-Lavi, L.; Frank, Y.; Hönigschmid, P.; Kataka, E.; Mösch, A.; Qian, K.; Ron, A.; Schmid, S.; et al. Validity of machine learning in biology and medicine increased through collaborations across fields of expertise. Nat. Mach. Intell. 2020, 2, 18–24. [Google Scholar] [CrossRef]
- Grassi, M.; Loewenstein, D.A.; Caldirola, D.; Schruers, K.; Duara, R.; Perna, G. A clinically-translatable machine learning algorithm for the prediction of Alzheimer’s disease conversion: Further evidence of its accuracy via a transfer learning approach. Int. Psychogeriatrics 2018, 31, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Antor, M.B.; Jamil, A.H.M.S.; Mamtaz, M.; Khan, M.M.; Aljahdali, S.; Kaur, M.; Singh, P.; Masud, M. A Comparative Analysis of Machine Learning Algorithms to Predict Alzheimer’s Disease. J. Heal. Eng. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Leong, L.K.; Abdullah, A.A. Prediction of Alzheimer’s disease (AD) Using Machine Learning Techniques with Boruta Algorithm as Feature Selection Method. J. Phys. Conf. Ser. 2019, 1372, 012065. [Google Scholar] [CrossRef]
- Liu, S.; Liu, S.; Cai, W.; Pujol, S.; Kikinis, R.; Feng, D. Early diagnosis of Alzheimer’s disease with deep learning. In Proceedings of the IEEE 11th International Symposium on Biomedical Imaging (ISBI), Beijing, China, 29 April–2 May 2014; pp. 1015–1018. [Google Scholar] [CrossRef]
- James, C.; Ranson, J.M.; Everson, R.; Llewellyn, D.J. Performance of Machine Learning Algorithms for Predicting Progression to Dementia in Memory Clinic Patients. JAMA Netw. Open 2021, 4, e2136553. [Google Scholar] [CrossRef]
- Savile, D.B.O. Communication problems in interdisciplinary research. Proc. Plant Sci. 1984, 93, 223–230. [Google Scholar] [CrossRef]
- Kumazawa, T.; Hara, K.; Endo, A.; Taniguchi, M. Supporting collaboration in interdisciplinary research of water–energy–food nexus by means of ontology engineering. J. Hydrol. Reg. Stud. 2017, 11, 31–43. [Google Scholar] [CrossRef]
- Menzel, C. Reference Ontologies-Application Ontologies: Either/Or or Both/And? In Proceedings of the KI2003 Workshop on Reference Ontologies and Application Ontologies, Hamburg, Germany, 26 September 2003; pp. 1–10. [Google Scholar]
- Whetzel, P.L.; Noy, N.F.; Shah, N.H.; Alexander, P.R.; Nyulas, C.; Tudorache, T.; Musen, M. BioPortal: Enhanced functionality via new Web services from the National Center for Biomedical Ontology to access and use ontologies in software applications. Nucleic Acids Res. 2011, 39, W541–W545. [Google Scholar] [CrossRef]
- Barton, A.; Rosier, A.; Burgun, A.; Ethier, J.-F. The Cardiovascular Disease Ontology. FOIS 2014, 267, 409–414. [Google Scholar] [CrossRef]
- Cole, N.I.; Liyanage, H.; Suckling, R.J.; Swift, P.A.; Gallagher, H.; Byford, R.; Williams, J.; Kumar, S.; De Lusignan, S. An ontological approach to identifying cases of chronic kidney disease from routine primary care data: A cross-sectional study. BMC Nephrol. 2018, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Babcock, S.; Beverley, J.; Cowell, L.G.; Smith, B. The Infectious Disease Ontology in the age of COVID-19. J. Biomed. Semant. 2021, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kostovska, A.; Initiative, T.A.D.N.; Tolovski, I.; Maikore, F.; Soldatova, L.; Panov, P. Neurodegenerative Disease Data Ontology. In Discovery Science; DS 2019; Lecture Notes in Computer Science; Kralj Novak, P., Šmuc, T., Džeroski, S., Eds.; Springer: Cham, Switzerland, 2019; pp. 235–245. [Google Scholar] [CrossRef]
- Younesi, E.; Malhotra, A.; Gündel, M.; Scordis, P.; Kodamullil, A.T.; Page, M.; Müller, B.; Springstubbe, S.; Wüllner, U.; Scheller, D.; et al. PDON: Parkinson’s disease ontology for representation and modeling of the Parkinson’s disease knowledge domain. Theor. Biol. Med. Model. 2015, 12, 1–17. [Google Scholar] [CrossRef]
- Duncan, W.D.; Thyvalikakath, T.; Haendel, M.; Torniai, C.; Hernandez, P.; Song, M.; Acharya, A.; Caplan, D.J.; Schleyer, T.; Ruttenberg, A. Structuring, reuse and analysis of electronic dental data using the Oral Health and Disease Ontology. J. Biomed. Semant. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Malhotra, A.; Younesi, E.; Gündel, M.; Müller, B.; Heneka, M.T.; Hofmann-Apitius, M. ADO: A disease ontology representing the domain knowledge specific to Alzheimer’s disease. Alzheimer’s Dement. 2013, 10, 238–246. [Google Scholar] [CrossRef]
- Dramé, K.; Diallo, G.; Delva, F.; Dartigues, J.F.; Mouillet, E.; Salamon, R.; Mougin, F. Reuse of termino-ontological resources and text corpora for building a multilingual domain ontology: An application to Alzheimer’s disease. J. Biomed. Informatics 2014, 48, 171–182. [Google Scholar] [CrossRef]
- Henry, V.; Moszer, I.; Dameron, O.; Vila Xicota, L.; Dubois, B.; Potier, M.C.; Hofmann-Apitius, M.; Colliot, O.; INSIGHT-preAD Study Group. Converting disease maps into heavyweight ontologies: General methodology and application to Alzheimer’s disease. Database 2021, 2021, baab004. [Google Scholar] [CrossRef]
- Alzheimer Disease Relevance Ontology by Process (AD-DROP). Available online: https://bioportal.bioontology.org/ontologies/AD-DROP (accessed on 16 January 2023).
- Uschold, M.; Gruninger, M. Ontologies: Principles, methods and applications. Knowl. Eng. Rev. 1996, 11, 93–136. [Google Scholar] [CrossRef]
- Arp, R.; Smith, B.; Spear, A.D. Building Ontologies with Basic Formal Ontology; Mit Press: Cambridge, MA, USA, 2015. [Google Scholar] [CrossRef]
- Musen, M.A. The Protege Project: A Look Back and a Look Forward. AI Matters 2015, 1, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, M.R.; Tudorache, T.; Musen, M.A. A systematic analysis of term reuse and term overlap across biomedical ontologies. Semantic Web 2017, 8, 853–871. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ong, E.; Schaub, J.; Dowd, F.; O’Toole, J.F.; Siapos, A.; Mooney, S.D. OPMI: The Ontology of Precision Medicine and Investigation and its Support for Clinical Data and Metadata Representation and Analysis. In Proceedings of the 10th International Conference on Biomedical Ontology (ICBO), Buffalo, NY, USA, 30 July–2 August 2019; pp. 1–10. [Google Scholar]
- Ceusters, W. An information artifact ontology perspective on data collections and associated representational artifacts. Stud. Heal. Technol. Inform. 2012, 180, 68–72. [Google Scholar]
- Glimm, B.; Horrocks, I.; Motik, B.; Stoilos, G.; Wang, Z. HermiT: An OWL 2 Reasoner. J. Autom. Reason. 2014, 53, 245–269. [Google Scholar] [CrossRef]
- Lazarova, S. Alzheimer’s Disease Ontology for Diagnosis and Preclinical Classification. Zenodo 2023. [Google Scholar] [CrossRef]
- Tan, H.; Adlemo, A.; Tarasov, V.; Johansson, M.E. Evaluation of an Application Ontology; JOWO: Bolzano, Italy, 2017. [Google Scholar]
- Brooke, J. SUS: A ‘Quick and Dirty’ Usability Scale. Usability Eval. Ind. 1996, 189, 4–7. [Google Scholar]
- Casellas, N. Ontology Evaluation through Usability Measures. In On the Move to Meaningful Internet Systems: OTM 2009 Workshops; OTM 2009; Lecture Notes in Computer Science; Meersman, R., Herrero, P., Dillon, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 5872, pp. 594–603. [Google Scholar] [CrossRef]
- Ma, X.; Fu, L.; West, P.; Fox, P. Ontology Usability Scale: Context-aware Metrics for the Effectiveness, Efficiency and Satisfaction of Ontology Uses. Data Sci. J. 2018, 17, 10. [Google Scholar] [CrossRef]
- Aisen, P.S.; Cummings, J.; Jack, C.R., Jr.; Morris, J.C.; Sperling, R.; Frölich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimer’s Res. Ther. 2017, 9, 60. [Google Scholar] [CrossRef]
- Persson, K.; Edwin, T.H.; Knapskog, A.-B.; Tangen, G.G.; Selbæk, G.; Engedal, K. Hippocampal Atrophy Subtypes of Alzheimer’s Disease Using Automatic MRI in a Memory Clinic Cohort: Clinical Implications. Dement. Geriatr. Cogn. Disord. 2022, 51, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Lopez, O.L. Mild Cognitive Impairment. Contin. Lifelong Learn. Neurol. 2013, 19, 411–424. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Yaari, R.; Fleisher, A.S.; Tariot, P.N. Updates to Diagnostic Guidelines for Alzheimer’s Disease. Prim. Care Companion CNS Disord. 2011, 13, 01262. [Google Scholar] [CrossRef]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimer’s Dis. 2021, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Leys, D.; Barkhof, F.; Huglo, D.; Weinstein, H.C.; Vermersch, P.; Kuiper, M.; Steinling, M.; Wolters, E.C.; Valk, J. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: Diagnostic value and neuropsychological correlates. J. Neurol. Neurosurg. Psychiatry 1992, 55, 967–972. [Google Scholar] [CrossRef]
- Sheehan, B. Assessment scales in dementia. Ther. Adv. Neurol. Disord. 2012, 5, 349–358. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Weyer, G.; Erzigkeit, H.; Kanowski, S.; Ihl, R.; Hadler, D. Alzheimer’s Disease Assessment Scale: Reliability and Validity in a Multicenter Clinical Trial. Int. Psychogeriatr. 1997, 9, 123–138. [Google Scholar] [CrossRef]
- Hughes, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A New Clinical Scale for the Staging of Dementia. Br. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, R.I.; Kurosaki, T.T.; Harrah, J.C.H.; Chance, J.M.; Filos, R.S. Measurement of Functional Activities in Older Adults in the Community. J. Gerontol. 1982, 37, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Bangor, A.; Kortum, P.; Miller, J. Determining What Individual SUS Scores Mean: Adding an Adjective Rating Scale. J. Usability Stud. 2009, 4, 114–123. [Google Scholar]

| CQs-Based | Scenario-Based |
|---|---|
| Q1: What are the stages within the Alzheimer’s disease spectrum? | SQ1: You are given a dataset containing full range of medical data (demographic data, medical history, csf biomarkers, cognitive assessments and clinical scales, etc.) of patients diagnosed either with mild cognitive impairment (MCI) or with Alzheimer’s disease (AD). However, you notice that the labels with the diagnosis were omitted. Unfortunately, your collaborator is on vacation, and it appears that you have to wait a month before you get the actual labels. You decide to run a preliminary analysis by estimating the diagnosis (MCI or AD) from the rest of the data. Which modalities of the medical data ordinary collected for such patients would be informative for your task to distinguish MCI from AD patients? Why did you choose these modalities? |
| Q2: What is the ATN framework? In what context is it being used? | |
| Q3: What are the pathological hallmarks of Alzheimer’s disease? What methods can we use to check for their presence? | |
| Q4: What are the symptoms of Alzheimer’s disease? What methods can we use to check for their presence? | SQ2: You are interested in the preclinical course of Alzheimer’s disease. You would like to look for pathologic changes that might take place in the brain long before the onset of any symptoms. What assessments and methods would you use to address this task? What assessments are unlikely to be informative in the preclinical stage of Alzheimer’s disease? Motivate your answers. |
| Q5: On the base of what is Alzheimer’s disease diagnosed? | |
| Q6: Name some of the biomarkers for Alzheimer’s disease? |
| Questions | PT1 | PT2 | PT3 | PT4 | PT5 | PT6 | PT7 | PT8 | PT9 | PT10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Your occupation is within the field of: 1. Science; 2. Technology; 3. Engineering; 4. Mathematics; 5. Other | 1; 2; 3 | 5 | 1 | 1 | 3 | 1; 3 | 1; 3 | 1; 2; 3 | 1 | 1 |
| To what extent do you consider yourself familiar with ontologies? From 1 (not at all) to 5 (to a great extent) | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 3 |
| To what extent do you consider yourself familiar with the ontology building tool Protégé? From 1 (not at all) to 5 (to a great extent) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| How familiar are you with Alzheimer’s Disease? From 1 (not at all) to 5 (very familiar) | 1 | 2 | 2 | 3 | 3 | 2 | 2 | 4 | 2 | 3 |
| Have you ever been involved in projects regarding Alzheimer’s Disease? Yes/No | No | No | Yes | No | No | No | No | No | No | No |
| Do you have any previous experience in biomedical research or a related field? Yes/No | No | No | Yes | No | No | No | No | No | No | No |
| Do you have any previous experience with medical data? Yes/No | No | No | Yes | No | No | No | No | Yes | No | Yes |
| Questions | Success | Partial Success | Failure | Omission |
|---|---|---|---|---|
| Q1: What are the stages within the Alzheimer’s disease spectrum? | 100% | 0% | 0% | 0% |
| Q2: What is the ATN framework? In what context is it being used? | 80% | 20% | 0% | 0% |
| Q3: What are the pathological hallmarks of Alzheimer’s disease? What methods can we use to check for their presence? | 50% | 20% | 30% | 0% |
| Q4: What are the symptoms of Alzheimer’s disease? What methods can we use to check for their presence? | 80% | 20% | 0% | 0% |
| Q5: Based on what is Alzheimer’s disease diagnosed? | 70% | 20% | 10% | 0% |
| Q6: Name some of the biomarkers for Alzheimer’s disease. | 70% | 0% | 0% | 30% |
| Applicability task | Success | Partial Success | Failure | Omission |
| SQ1 | 30% | 20% | 50% | 0% |
| SQ2 | 50% | 30% | 0% | 20% |
| Questions | PT1 | PT2 | PT3 | PT4 | PT5 | PT6 | PT7 | PT8 | PT9 | PT10 |
|---|---|---|---|---|---|---|---|---|---|---|
| SUS Total | 67.5 | 75.0 | 70.0 | 42.5 | 45.0 | 70.0 | 70.0 | 77.5 | 65.0 | 47.5 |
| Adjective Grade | OK | Good | OK | Poor | Poor | OK | OK | Good | OK | Poor |
| Acceptability Grade | M | A | A | NA | NA | A | A | A | M | NA |
| SUS Total | 67.5 | 75.0 | 70.0 | 42.5 | 45.0 | 70.0 | 70.0 | 77.5 | 65.0 | 47.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarova, S.; Petrova-Antonova, D.; Kunchev, T. Ontology-Driven Knowledge Sharing in Alzheimer’s Disease Research. Information 2023, 14, 188. https://doi.org/10.3390/info14030188
Lazarova S, Petrova-Antonova D, Kunchev T. Ontology-Driven Knowledge Sharing in Alzheimer’s Disease Research. Information. 2023; 14(3):188. https://doi.org/10.3390/info14030188
Chicago/Turabian StyleLazarova, Sophia, Dessislava Petrova-Antonova, and Todor Kunchev. 2023. "Ontology-Driven Knowledge Sharing in Alzheimer’s Disease Research" Information 14, no. 3: 188. https://doi.org/10.3390/info14030188
APA StyleLazarova, S., Petrova-Antonova, D., & Kunchev, T. (2023). Ontology-Driven Knowledge Sharing in Alzheimer’s Disease Research. Information, 14(3), 188. https://doi.org/10.3390/info14030188







