Comparing Worldwide, National, and Independent Notifications about Adverse Drug Reactions Due to COVID-19 Vaccines
Abstract
:1. Introduction
2. Related Work
3. Background
3.1. Guide to Data Reading
- An adverse event is any adverse episode that may appear after the administration of a vaccine, but which does not necessarily have a causal relationship with the vaccine;
- An adverse reaction is a response to a vaccine that is noxious and unintended. In order to distinguish between adverse events and adverse reactions, we must study potential causalities related to the vaccine;
- An undesirable effect is an unintended effect related to the properties of a vaccine, observed in a number of people, that is not necessarily harmful.
3.2. Signal Detection and Management
3.3. Causality Assessment
- Related to the event, i.e., the causal connection between the event and vaccine is considered possible;
- Unrelated to the event, i.e., other elements and factors can explain the adverse reaction;
- Indeterminate, i.e., the temporal association is valid, but the collected data are not enough to confirm causality;
- Unclassifiable, i.e., all reports that lack sufficient information and for which further investigation is required.
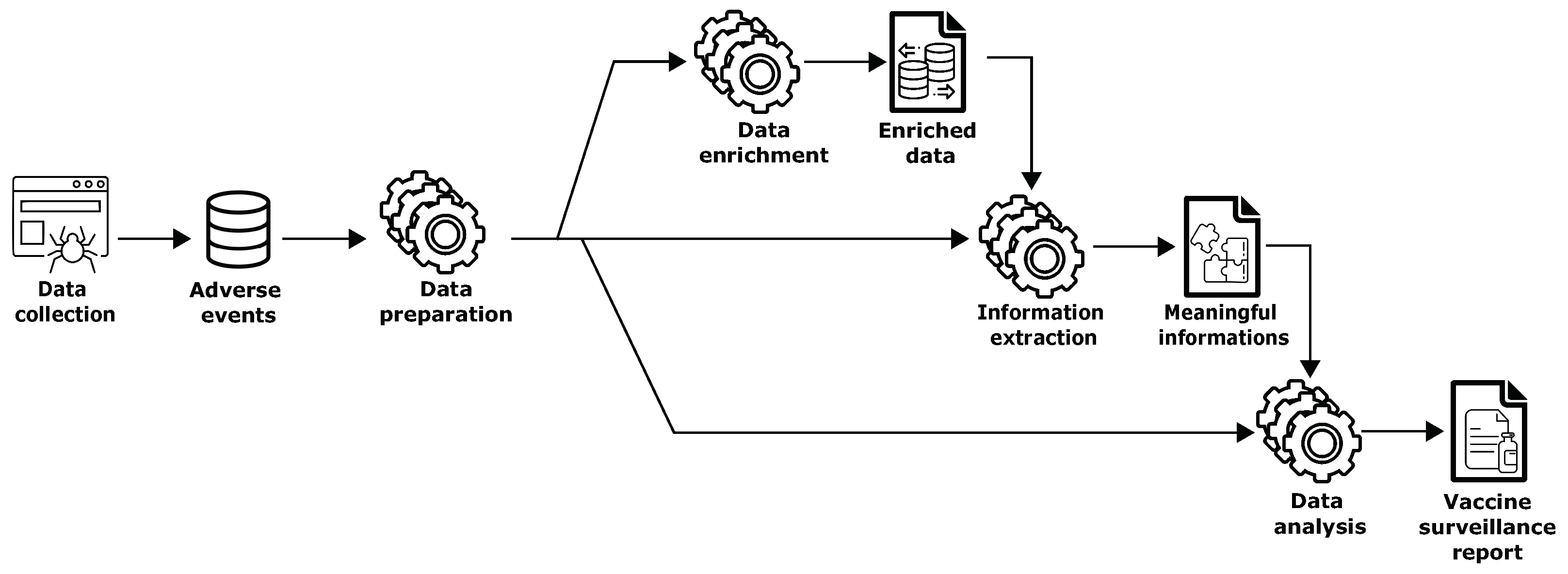
4. Methodology
- Retrieving the information about (i.e., typology of medical product such as Comirnaty, Spikevax, etc.), (i.e., the patient’s age), (i.e., the patient’s sex), and (i.e., the information about the suspected ADRs/) (lines 3–6).
- Creating a pharmacovigilance dataset to make it suitable for analysis (line 7).
- Generating the statistical associations between medicinal products and adverse events, i.e., drug–event pairs, using disproportionality analysis methods (line 9). More details are given in Section 5.3.
- If the lower bound of the 95% confidence interval of signals of disproportionate reporting (SDRs) generated at the previous step is greater than or equal to one, the adverse reactions require further evaluation (lines 11–12).
| Algorithm 1 Adverse events reports processing |
 |
5. Experimental Results
5.1. Pharmacovigilance: Global Overview
5.1.1. VigiBase
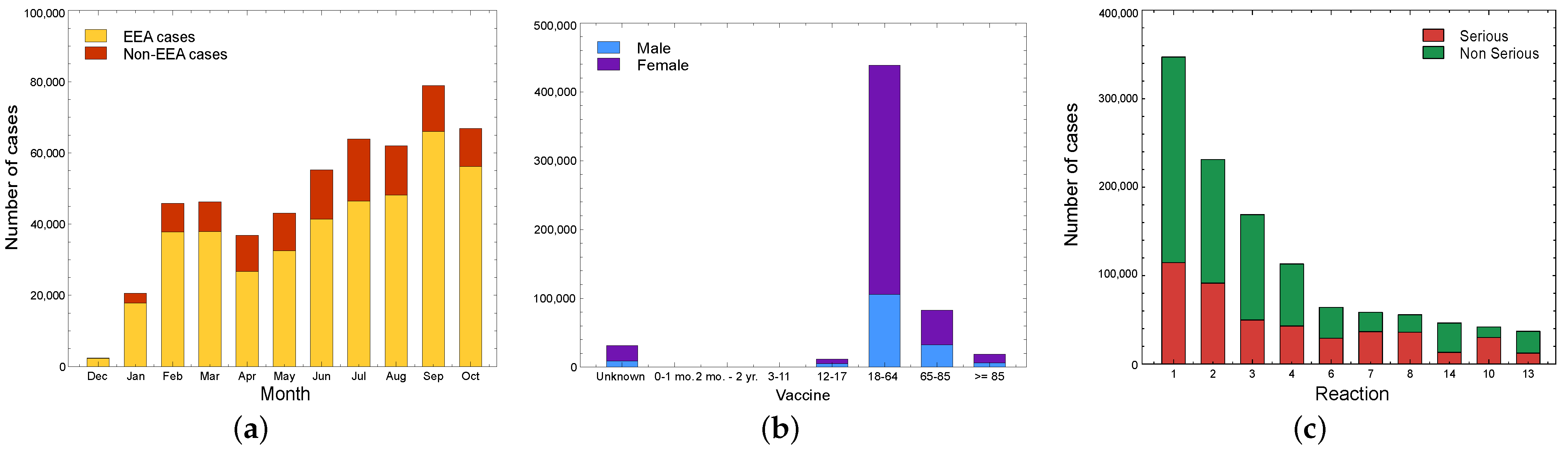
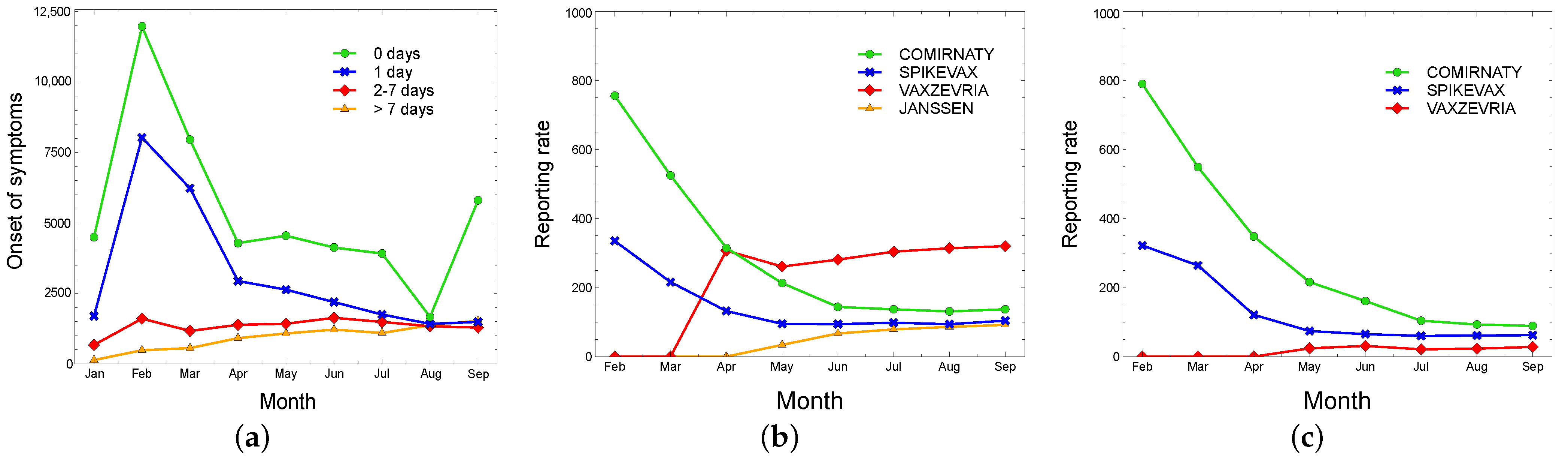
5.1.2. Comirnaty (Pfizer/BioNTech)
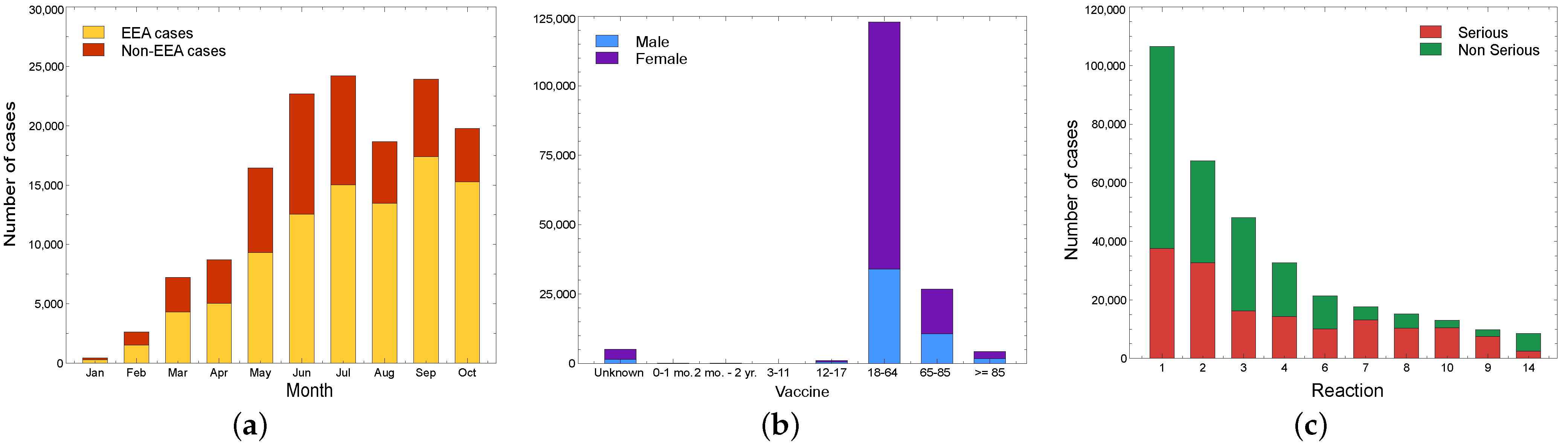
5.1.3. Spikevax (Moderna)
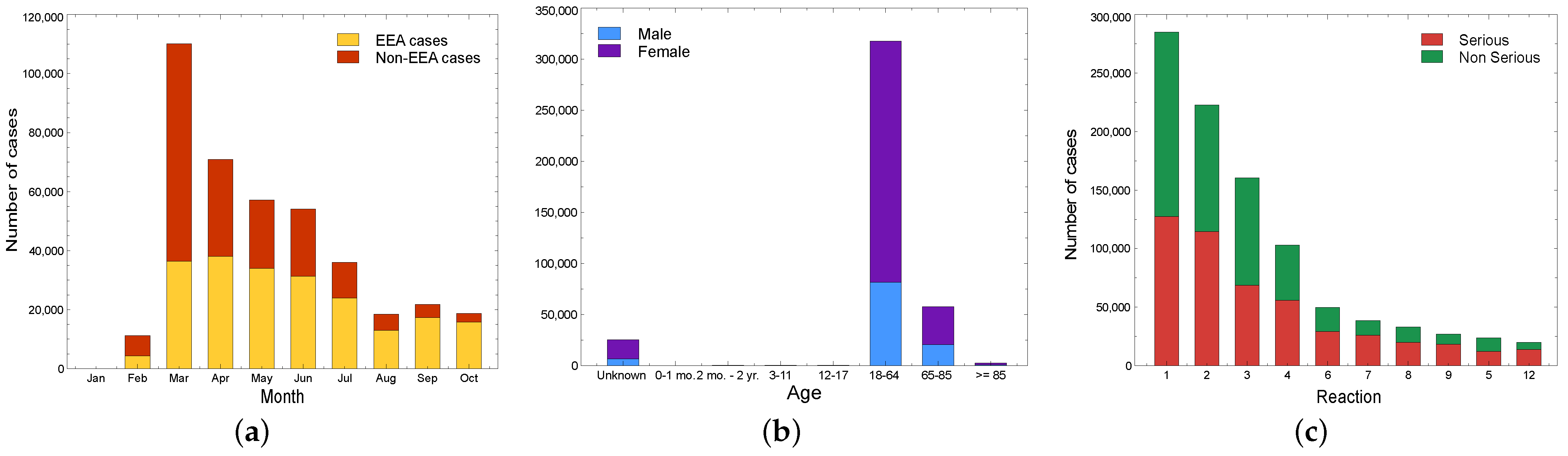
5.1.4. Vaxzevria (AstraZeneca)
5.1.5. Janssen (Janssen-Cilag)
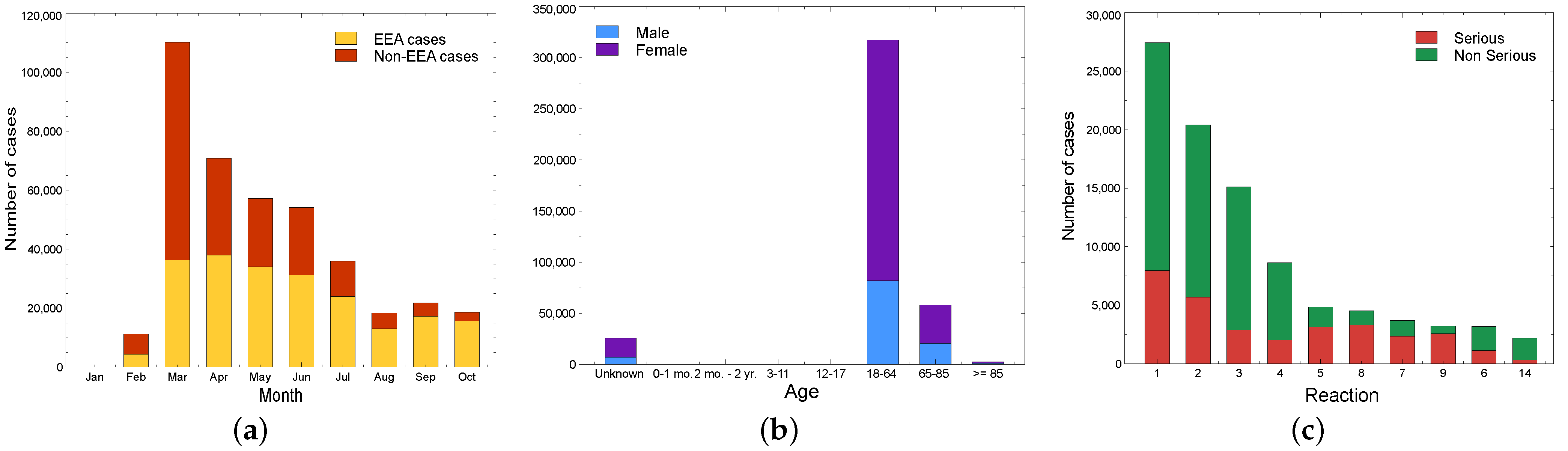
5.2. Pharmacovigilance: Italy Overview
5.2.1. AIFA
- One report concerns a 79-year-old man with a history of high blood pressure, surgery for triple aortocoronary bypass, and pacemaker implantation;
- Two reports concern a 46-year-old man and a 32-year-old woman, who died 12 days after the administration of the 1st dose of Vaxzevria vaccine as a result of thrombotic events and concomitant thrombocytopenia;
- Two reports refer to two patients with respiratory symptoms and positive swabs, 45 and 35 days after completion of the vaccine cycle, respectively, who died from complications of interstitial pneumonia. Both patients had clinical conditions and therapies consistent with a state of immunosuppression;
- One report refers to a fragile patient who experienced fever and vomiting after administration of the first dose of vaccine and died 2 days later;
- Three reports refer to three patients over 80 years old with various diseases, who died after completing the vaccination cycle (in two cases 3 weeks before and in one case 39 days before the fatal event);
- Three reports involve three patients who died from complications of a thrombotic event associated with thrombocytopenia;
- One report involves one patient who died from complications of thrombotic thrombocytopenic purpura;
- Two reports refer to two patients aged 76 and 80 years with various diseases who died after completing the vaccination cycle;
- One death is not described in the AIFA reports.
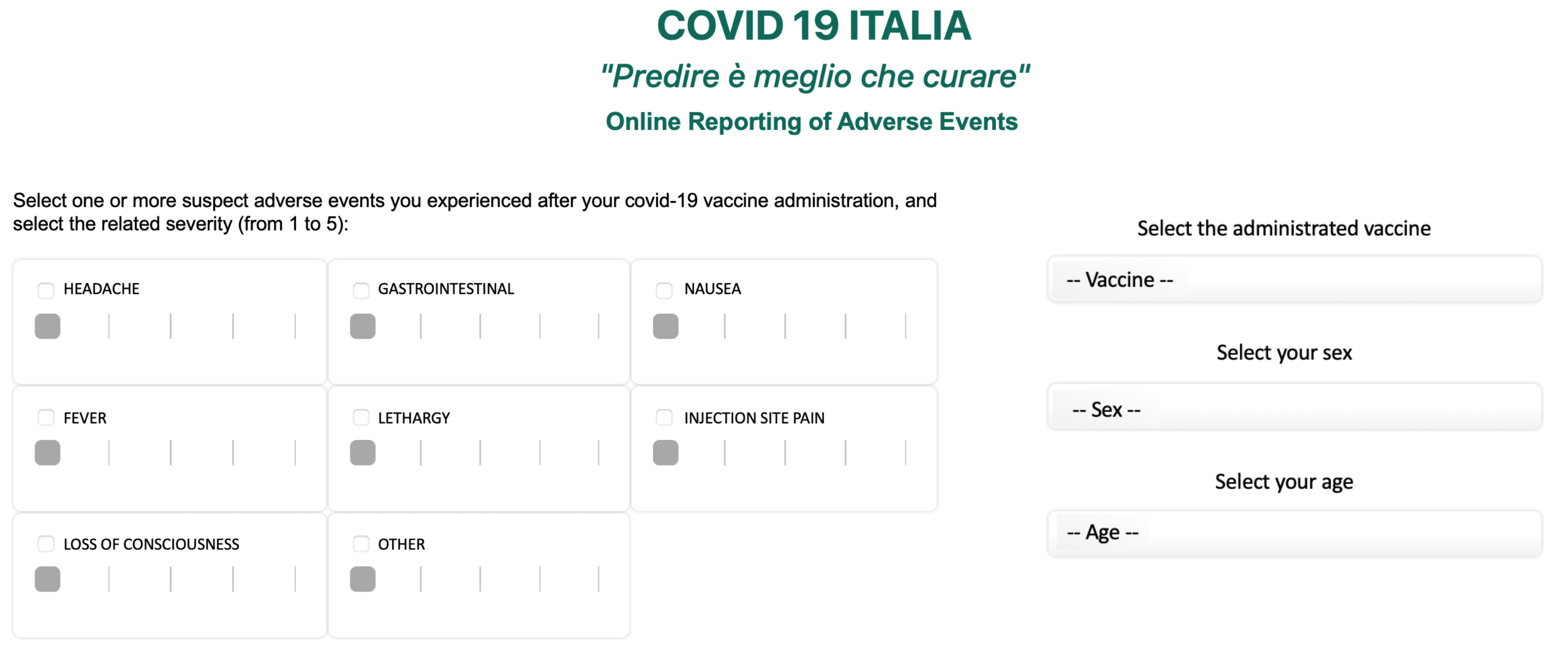
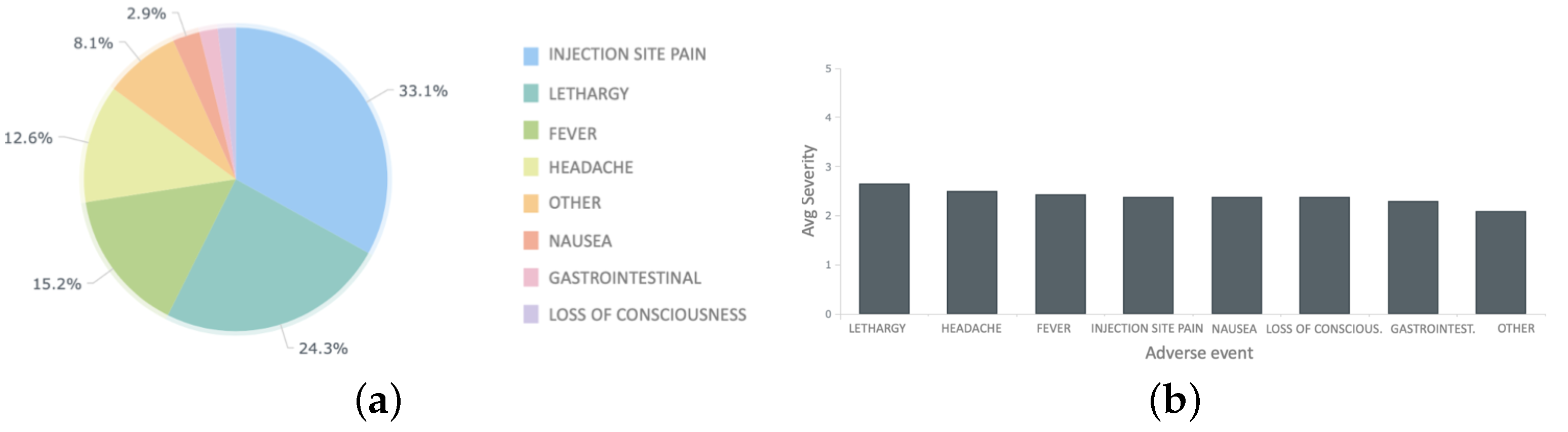
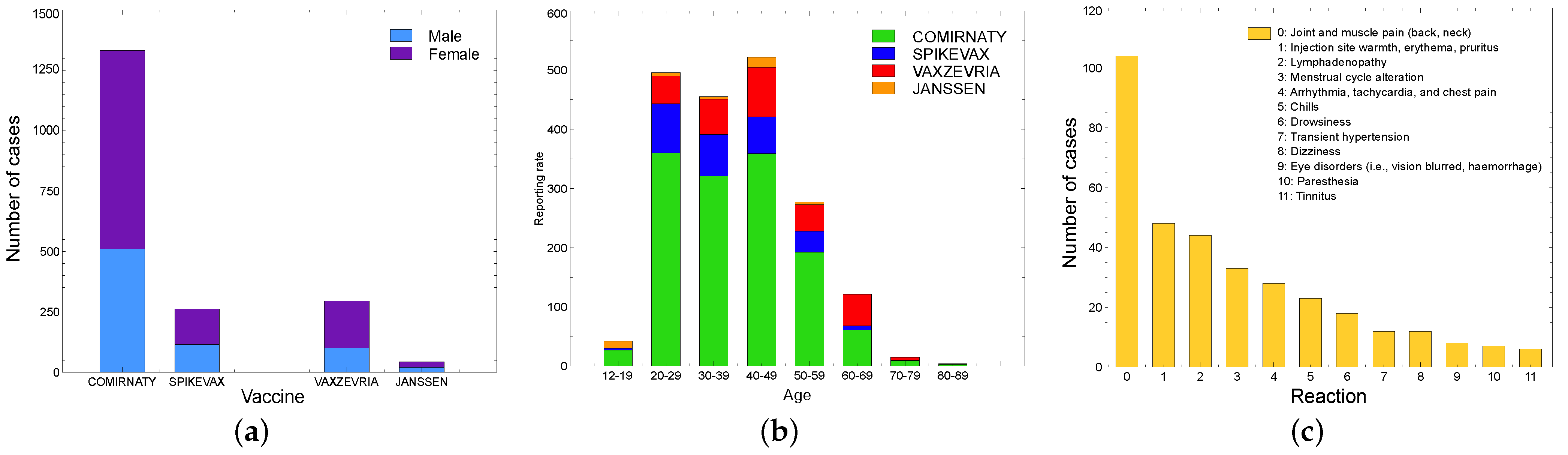
5.2.2. Independent Monitoring Platform
5.3. Disproportionality Analysis
- The value indicates the number of individual cases with the suspect medicinal product involving an adverse event ;
- The value indicates the number of individual cases related to the suspect medicinal product , involving any other adverse events apart from the ;
- The value indicates the number of individual cases involving event in relation to any other medicinal products apart from the ;
- The value indicates the number of individual cases involving any other adverse events apart from the and any other medicinal products apart from the .
- “Neoplasm benign, malignant, and unspecified”, “Renal and urinary disorders”, “Blood and lymphatic system disorders”, and “Vascular disorders” for the Comirnaty vaccine;
- “Social circumstances”, “Hepatobiliary disorders”, “Endocrine disorders”, and “Renal and urinary disorders”, for the Spikevax vaccine;
- “Nervous system disorders”, “Musculoskeletal and connective tissue disorders”, and “Gastrointestinal disorders” for the Vaxzevria vaccine;
- “Social circumstances”, “Renal and urinary disorders”, “Product issues”, and “Cardiac disorders” for the Janssen vaccine.
5.4. Comparative Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belcastro, L.; Marozzo, F.; Talia, D. Programming models and systems for big data analysis. Int. J. Parallel Emergent Distrib. Syst. 2019, 34, 632–652. [Google Scholar] [CrossRef]
- Luo, J.; Wu, M.; Gopukumar, D.; Zhao, Y. Big data application in biomedical research and health care: A literature review. Biomed. Inform. Insights 2016, 8, BII-S31559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashyap, H.; Ahmed, H.A.; Hoque, N.; Roy, S.; Bhattacharyya, D.K. Big data analytics in bioinformatics: A machine learning perspective. arXiv 2015, arXiv:1506.05101. [Google Scholar]
- Ghani, N.A.; Hamid, S.; Hashem, I.A.T.; Ahmed, E. Social media big data analytics: A survey. Comput. Hum. Behav. 2019, 101, 417–428. [Google Scholar] [CrossRef]
- Cesario, E.; Marozzo, F.; Talia, D.; Trunfio, P. SMA4TD: A social media analysis methodology for trajectory discovery in large-scale events. Online Soc. Netw. Media 2017, 3, 49–62. [Google Scholar] [CrossRef]
- Branda, F.; Marozzo, F.; Talia, D. Ticket Sales Prediction and Dynamic Pricing Strategies in Public Transport. Big Data Cogn. Comput. 2020, 4, 36. [Google Scholar] [CrossRef]
- Tosi, D. Cell phone big data to compute mobility scenarios for future smart cities. Int. J. Data Sci. Anal. 2017, 4, 265–284. [Google Scholar] [CrossRef] [Green Version]
- Tosi, D.; Marzorati, S.; La Rosa, M.; Dondossola, G.; Terruggia, R. Big data from cellular networks: How to estimate energy demand at real-time. In Proceedings of the 2015 IEEE International Conference on Data Science and Advanced Analytics, Houston, TX, USA, 17–19 December 2015. [Google Scholar]
- Tosi, D.; Campi, A. How data analytics and big data can help scientists in managing COVID-19 diffusion: Modeling study to predict the COVID-19 diffusion in Italy and the Lombardy region. J. Med. Internet Res. 2020, 22, e21081. [Google Scholar] [CrossRef]
- Tosi, D.; Campi, A.S. How schools affected the covid-19 pandemic in Italy: Data analysis for Lombardy region, Campania region, and Emilia region. Future Internet 2021, 13, 109. [Google Scholar] [CrossRef]
- Tosi, D.; Verde, A.; Verde, M. Clarification of misleading perceptions of COVID-19 fatality and testing rates in Italy: Data analysis. J. Med. Internet Res. 2020, 22, e19825. [Google Scholar] [CrossRef]
- Cinelli, M.; Quattrociocchi, W.; Galeazzi, A.; Valensise, C.M.; Brugnoli, E.; Schmidt, A.L.; Zola, P.; Zollo, F.; Scala, A. The COVID-19 social media infodemic. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Khillan, R.; Mishra, Y.; Khurana, S. The safety profile of COVID-19 vaccinations in the United States. Am. J. Infect. Control 2022, 50, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Beatty, A.L.; Peyser, N.D.; Butcher, X.E.; Cocohoba, J.M.; Lin, F.; Olgin, J.E.; Pletcher, M.J.; Marcus, G.M. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw. Open 2021, 4, e2140364. [Google Scholar] [CrossRef] [PubMed]
- Boekel, L.; Kummer, L.Y.; van Dam, K.P.; Hooijberg, F.; van Kempen, Z.; Vogelzang, E.H.; Wieske, L.; Eftimov, F.; van Vollenhoven, R.; Kuijpers, T.W.; et al. Adverse events after first COVID-19 vaccination in patients with autoimmune diseases. Lancet Rheumatol. 2021, 3, e542–e545. [Google Scholar] [CrossRef]
- Fu, W.; Sivajohan, B.; McClymont, E.; Albert, A.; Elwood, C.; Ogilvie, G.; Money, D. Systematic review of the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating individuals and their infants. Int. J. Gynecol. Obstet. 2022, 156, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Lian, A.T.; Du, J.; Tang, L. Using a Machine Learning Approach to Monitor COVID-19 Vaccine Adverse Events (VAE) from Twitter Data. Vaccines 2022, 10, 103. [Google Scholar] [CrossRef]
- Glossary—WHO Vaccine Safety Basics. Available online: https://vaccine-safety-training.org/glossary.html (accessed on 3 December 2021).
- Shimabukuro, T.T.; Nguyen, M.; Martin, D.; DeStefano, F. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine 2015, 33, 4398–4405. [Google Scholar] [CrossRef] [Green Version]
- COVID-19 Brighton Collaboration. Available online: https://brightoncollaboration.us/covid-19/ (accessed on 30 October 2021).
- Ghosh, D.; Skinner, M.; Ferguson, L.R. The role of the Therapeutic Goods Administration and the Medicine and Medical Devices Safety Authority in evaluating complementary and alternative medicines in Australia and New Zealand. Toxicology 2006, 221, 88–94. [Google Scholar] [CrossRef]
- Lindquist, M. VigiBase, the WHO global ICSR database system: Basic facts. Drug Inf. J. 2008, 42, 409–419. [Google Scholar] [CrossRef]
- Postigo, R.; Brosch, S.; Slattery, J.; van Haren, A.; Dogné, J.M.; Kurz, X.; Candore, G.; Domergue, F.; Arlett, P. EudraVigilance medicines safety database: Publicly accessible data for research and public health protection. Drug Saf. 2018, 41, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Italian Medicines Agency (AIFA). Available online: https://www.aifa.gov.it/en/farmacovigilanza-vaccini-covid-19 (accessed on 30 October 2021).
- COVID19 ITALIA: “Predire è Meglio che Curare”. Available online: http://www.covid19-italy.it/ (accessed on 30 October 2021).
- Medical Dictionary for Regulatory Activities (MedDRA). Available online: https://www.meddra.org/ (accessed on 10 November 2021).
- EudraVigilance—European Database of Suspected Adverse Drug Reaction Reports. Available online: https://www.adrreports.eu/en/index.html (accessed on 27 November 2021).
- AIFA—COVID-19 Vaccine Surveillance Report. Available online: https://www.aifa.gov.it/en/rapporti-su-sorveglianza-dei-vaccini-covid-19 (accessed on 27 September 2021).
- COVID 19 ITALIA “Predire è Meglio che Curare”—Segnalazioni. Available online: http://www.covid19-italy.it/segnalazioni-covid19/segnala.html (accessed on 3 December 2021).
- Predire è Meglio che Curare. Available online: https://www.facebook.com/prediremegliochecurare (accessed on 20 November 2021).
- Zorych, I.; Madigan, D.; Ryan, P.; Bate, A. Disproportionality methods for pharmacovigilance in longitudinal observational databases. Stat. Methods Med. Res. 2013, 22, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.J.; Waller, P.C.; Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef] [PubMed]

| ID | Type of Reaction | Value |
|---|---|---|
| 1 | General disorders and administration site conditions | 1,377,111 |
| 2 | Nervous system disorders | 974,508 |
| 3 | Musculoskeletal and connective tissue disorders | 660,524 |
| 4 | Gastrointestinal disorders | 464,713 |
| 5 | Investigations | 311,319 |
| 6 | Skin and subcutaneous tissue disorders | 310,449 |
| 7 | Respiratory, thoracic, and mediastinal disorders | 240,202 |
| 8 | Infections and infestations | 156,200 |
| 9 | Vascular disorders | 122,603 |
| 10 | Cardiac disorders | 113,225 |
| 11 | Injury, poisoning, and procedural complications | 111,467 |
| 12 | Psychiatric disorders | 107,102 |
| 13 | Blood and lymphatic system disorders | 91,518 |
| 14 | Reproductive system and breast disorders | 90,911 |
| 15 | Eye disorders | 83,206 |
| 16 | Ear and labyrinth disorders | 75,838 |
| 17 | Metabolism and nutrition disorders | 51,461 |
| 18 | Immune system disorders | 32,182 |
| 19 | Surgical and medical procedures | 22,157 |
| 20 | Renal and urinary disorders | 18,321 |
| 21 | Social circumstances | 15,970 |
| 22 | Pregnancy, puerperium, and perinatal conditions | 5181 |
| 23 | Hepatobiliary disorders | 4571 |
| 24 | Product issues | 3698 |
| 25 | Neoplasms benign, malignant, and unspecified | 3403 |
| 26 | Endocrine disorders | 3164 |
| Total | - | 5,451,004 |
| ID | Fatal | Not Recovered/ Not Resolved | Recovered/ Resolved | Recovered/ Resolved with Sequelae | Recovering/ Resolving | Unknown |
|---|---|---|---|---|---|---|
| 1 | 4182 | 86,342 | 160,390 | 2937 | 79,478 | 63,717 |
| 2 | 1579 | 64,359 | 94,775 | 3,828 | 48,971 | 33,666 |
| 3 | 184 | 48,029 | 70,029 | 1656 | 36,974 | 21,787 |
| 4 | 592 | 26,819 | 49,529 | 1274 | 25,469 | 15,131 |
| 6 | 126 | 19,017 | 22,063 | 721 | 13,777 | 11,500 |
| 7 | 1636 | 16,445 | 17,857 | 1044 | 13,022 | 11,722 |
| 8 | 1585 | 12,308 | 11,153 | 738 | 10,132 | 20,796 |
| 14 | 5 | 22,361 | 11,381 | 446 | 6566 | 9525 |
| 10 | 2160 | 11,385 | 11,903 | 1122 | 9013 | 7949 |
| 13 | 211 | 13,152 | 10,411 | 354 | 8710 | 4807 |
| Total | 12,260 | 295,680 | 437,177 | 12,644 | 234,389 | 187,844 |
| ID | Fatal | Not Recovered/ Not Resolved | Recovered/ Resolved | Recovered/ Resolved with Sequelae | Recovering/ Resolving | Unknown |
|---|---|---|---|---|---|---|
| 1 | 3030 | 32,197 | 47,674 | 625 | 25,405 | 18,297 |
| 2 | 840 | 18,297 | 27,008 | 612 | 12,000 | 11,034 |
| 3 | 179 | 14,649 | 19,250 | 289 | 10,255 | 6186 |
| 4 | 329 | 7717 | 14,333 | 226 | 6296 | 4979 |
| 6 | 79 | 6990 | 6990 | 129 | 3907 | 4208 |
| 7 | 940 | 5525 | 4764 | 146 | 2218 | 4586 |
| 8 | 816 | 4586 | 3571 | 101 | 1968 | 4840 |
| 10 | 926 | 3714 | 3538 | 188 | 1994 | 2915 |
| 9 | 330 | 2905 | 2909 | 100 | 1235 | 2419 |
| 14 | 7 | 3872 | 2214 | 72 | 1182 | 1566 |
| Total | 7476 | 100,452 | 132,251 | 2488 | 66,460 | 61,030 |
| ID | Fatal | Not Recovered/ Not Resolved | Recovered/ Resolved | Recovered/ Resolved with Sequelae | Recovering/ Resolving | Unknown |
|---|---|---|---|---|---|---|
| 1 | 1527 | 70,937 | 149,024 | 3720 | 80,254 | 50,431 |
| 2 | 998 | 59,459 | 96,760 | 4247 | 52,759 | 34,657 |
| 3 | 34,657 | 46,840 | 66,890 | 2111 | 39,215 | 18,157 |
| 4 | 334 | 22,075 | 48,513 | 1398 | 24,145 | 14,306 |
| 6 | 51 | 14,786 | 16,666 | 621 | 11,509 | 9796 |
| 7 | 806 | 11,317 | 10,964 | 719 | 9339 | 7923 |
| 8 | 437 | 7282 | 10,357 | 655 | 8029 | 7080 |
| 9 | 450 | 7292 | 7325 | 527 | 6426 | 5683 |
| 5 | 157 | 5071 | 9907 | 272 | 4711 | 4237 |
| 12 | 60 | 5427 | 7153 | 304 | 4145 | 4,134 |
| Total | 39,477 | 250,486 | 423,559 | 14,574 | 240,532 | 156,404 |
| ID | Fatal | Not Recovered/ Not Resolved | Recovered/ Resolved | Recovered/ Resolved with Sequelae | Recovering/ Resolving | Unknown |
|---|---|---|---|---|---|---|
| 1 | 519 | 9106 | 12,308 | 82 | 8527 | 5520 |
| 2 | 211 | 6611 | 7544 | 105 | 4799 | 2403 |
| 3 | 44 | 5282 | 5592 | 38 | 4064 | 1354 |
| 4 | 79 | 2171 | 3773 | 19 | 2087 | 835 |
| 5 | 108 | 1836 | 1537 | 3 | 562 | 891 |
| 8 | 151 | 701 | 749 | 22 | 552 | 2414 |
| 7 | 251 | 1448 | 839 | 16 | 626 | 667 |
| 9 | 148 | 1258 | 611 | 18 | 387 | 842 |
| 6 | 8 | 1183 | 930 | 15 | 629 | 494 |
| 14 | 6 | 1156 | 484 | 8 | 230 | 345 |
| Total | 1525 | 30,752 | 34,367 | 326 | 22,463 | 15,765 |
| Period | Fatal Cases | Reporting Rate | ||||||
|---|---|---|---|---|---|---|---|---|
| C | S | V | J | C | S | V | J | |
| 27 December 2020–26 January 2021 | 13 | - | - | - | 0.8% | - | - | - |
| As of 26 February | 40 | - | - | - | 0.97% | - | - | - |
| As of 26 March | 76 | 12 | 12 | - | 1.1% | 2.8% | 0.7% | - |
| As of 26 April | 150 | 39 | 34 | - | 1.17% | 3.05% | 0.85% | - |
| As of 26 May | 213 | 58 | 53 | 4 | 0.96% | 1.99% | 0.79% | 0.79% |
| As of 26 June | 262 | 75 | 72 | 14 | 0.75% | 1.58% | 0.84% | 1.15% |
| As of 26 July | 307 | 86 | 88 | 17 | 0.66% | 1.30% | 0.78% | 1.28% |
| As of 26 August | 345 | 92 | 94 | 24 | 0.64% | 1.05% | 0.78% | 1.68% |
| As of 26 September | 391 | 96 | 98 | 23 | 0.65% | 0.91% | 0.81% | 1.56% |
| Period | Reports | Events | Reporting Rate | ||
|---|---|---|---|---|---|
| S | NS | S | NS | ||
| 27 December 2020–6 January 2021 | 7337 | 7.3% | 92.4% | 34 | 434 |
| As of 26 February 2021 | 30,015 | 6.1% | 93.6% | 44 | 683 |
| As of 26 March 2021 | 46,237 | 7.1% | 92.7% | 36 | 473 |
| As of 26 April 2021 | 56,110 | 8.6% | 91.0% | 27 | 282 |
| As of 26 May 2021 | 66,258 | 10.4% | 90.0% | 21 | 183 |
| As of 26 June 2021 | 76,206 | 11.9% | 87.9% | 18 | 135 |
| As of 26 July 2021 | 84,322 | 12.8% | 87.1% | 16 | 111 |
| As of 26 August 2021 | 91,360 | 13.8% | 86.1% | 13 | 111 |
| As of 26 September 2021 | 101,110 | 14.4% | 85.4% | 17 | 103 |
| Adverse Event of Interest (AE) | All Other Adverse Events | |
|---|---|---|
| Vaccine of interest (VI) | ||
| Comparator vaccine(s) |
| Type of Reaction | Comirnaty | Spikevax | Vaxzevria | Janssen |
|---|---|---|---|---|
| General disorders and administration site conditions | 0.96 (95% CI: 0.96–0.97) | 1.03 (95% CI: 1.03–1.04) | 1.02 (95% CI: 1.02–1.03) | 1.02 (95% CI: 1.00–1.03) |
| Nervous system disorders | 0.87 (95% CI: 0.86–0.87) | 0.91 (95% CI: 0.90–0.92) | 1.20 (95% CI: 1.20–1.21) | 1.07 (95% CI: 1.06–1.09) |
| Musculoskeletal and connective tissue disorders | 0.88 (95% CI: 0.87–0.88) | 0.89 (95% CI: 0.88–0.90) | 1.19 (95% CI: 1.18–1.20) | 1.10 (95% CI: 1.08–1.12) |
| Gastrointestinal disorders | 0.91 (95% CI: 0.91–0.92) | 0.93 (95% CI: 0.92–0.94) | 1.15 (95% CI: 1.14–1.16) | 0.95 (95% CI: 0.93–0.97) |
| Investigations | 1.37 (95% CI: 1.34–1.39) | 0.98 (95% CI: 0.96–1.00) | 0.75 (95% CI: 0.74–0.77) | 0.65 (95% CI: 0.62–0.68) |
| Skin and subcutaneous tissue disorders | 0.98 (95% CI: 0.97–0.99) | 1.15 (95% CI: 1.14–1.17) | 0.95 (95% CI: 0.94–0.96) | 0.98 (95% CI: 0.95–1.01) |
| Respiratory, thoracic, and mediastinal disorders | 1.12 (95% CI: 1.11–1.14) | 1.11 (95% CI: 1.09–1.13) | 0.82 (95% CI: 0.81–0.83) | 1.08 (95% CI: 1.05–1.12) |
| Infections and infestations | 1.25 (95% CI: 1.23–1.26) | 1.05 (95% CI: 1.03–1.07) | 0.76 (95% CI: 0.75–0.77) | 0.97 (95% CI: 0.94–1.01) |
| Vascular disorders | 1.39 (95% CI: 1.37–1.41) | 0.95 (95% CI: 0.93–0.97) | 0.73 (95% CI: 0.72–0.74) | 0.82 (95% CI: 0.78–0.86) |
| Cardiac disorders | 1.32 (95% CI: 1.31–1.34) | 0.91 (95% CI: 0.89–0.93) | 0.75 (95% CI: 0.74–0.76) | 1.15 (95% CI: 1.11–1.19) |
| Injury, poisoning, and procedural complications | 1.16 (95% CI: 1.14–1.18) | 1.30 (95% CI: 1.27–1.34) | 0.76 (95% CI: 0.75–0.78) | 0.69 (95% CI: 0.65–0.73) |
| Psychiatric disorders | 1.19 (95% CI: 1.17–1.21) | 1.16 (95% CI: 1.13–1.19) | 0.78 (95% CI: 0.77–0.80) | 0.80 (95% CI: 0.75–0.84) |
| Blood and lymphatic system disorders | 1.40 (95% CI: 1.38–1.43) | 0.92 (95% CI: 0.90–0.94) | 0.72 (95% CI: 0.71–0.74) | 0.90 (95% CI: 0.87–0.94) |
| Reproductive system and breast disorders | 1.25 (95% CI: 1.24–1.27) | 1.08 (95% CI: 1.06–1.10) | 0.74 (95% CI: 0.73–0.75) | 1.01 (95% CI: 0.98–1.05) |
| Eye disorders | 1.24 (95% CI: 1.22–1.27) | 1.08 (95% CI: 1.05–1.11) | 0.79 (95% CI: 0.77–0.81) | 0.70 (95% CI: 0.65–0.74) |
| Ear and labyrinth disorders | 1.17 (95% CI: 1.15–1.20) | 0.97 (95% CI: 0.94–1.00) | 0.89 (95% CI: 0.87–0.91) | 0.73 (95% CI: 0.69–0.78) |
| Metabolism and nutrition disorders | 1.15 (95% CI: 1.12–1.19) | 1.63 (95% CI: 1.58–1.69) | 0.62 (95% CI: 0.60–0.64) | 0.45 (95% CI: 0.42–0.49) |
| Immune system disorders | 1.02 (95% CI: 0.99–1.04) | 0.90 (95% CI: 0.87–0.93) | 1.09 (95% CI: 1.07–1.12) | 0.61 (95% CI: 0.57–0.66) |
| Surgical and medical procedures | 1.00 (95% CI: 0.96–1.03) | 1.44 (95% CI: 1.37–1.50) | 0.81 (95% CI: 0.78–0.84) | 1.03 (95% CI: 0.94–1.13) |
| Renal and urinary disorders | 1.48 (95% CI: 1.42–1.55) | 1.73 (95% CI: 1.64–1.82) | 0.37 (95% CI: 0.35–0.39) | 1.44 (95% CI: 1.30–1.58) |
| Social circumstances | 1.01 (95% CI: 0.96–1.06) | 2.09 (95% CI: 1.97–2.21) | 0.53 (95% CI: 0.50–0.56) | 1.55 (95% CI: 1.38–1.73) |
| Pregnancy, puerperium, and perinatal conditions | 1.22 (95% CI: 1.15–1.30) | 1.57 (95% CI: 1.45–1.70) | 0.60 (95% CI: 0.55–0.64) | 0.96 (95% CI: 0.81–1.15) |
| Hepatobiliary disorders | 1.31 (95% CI: 1.21–1.40) | 1.88 (95% CI: 1.72–2.05) | 0.49 (95% CI: 0.45–0.53) | 0.68 (95% CI: 0.53–0.86) |
| Product issues | 0.81 (95% CI: 0.68–0.97) | 1.21 (95% CI: 0.95–1.53) | 1.02 (95% CI: 0.85–1.22) | 1.65 (95% CI: 1.13–2.40) |
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 1.49 (95% CI: 1.37–1.62) | 1.29 (95% CI: 1.15–1.45) | 0.58 (95% CI: 0.52–0.63) | 0.56 (95% CI: 0.41–0.76) |
| Endocrine disorders | 1.22 (95% CI: 1.13–1.32) | 1.81 (95% CI: 1.65–1.99) | 0.56 (95% CI: 0.51–0.61) | 0.64 (95% CI: 0.49–0.83) |
| Vaccine | Suspected-AE | SAE | NSAE |
|---|---|---|---|
| Comirnaty | 1,363,522 | 586,726 | 776,796 |
| Vaxzevria | 1,082,926 | 562,208 | 520,718 |
| Spikevax | 398,352 | 194,269 | 204,083 |
| Janssen | 103,925 | 38,184 | 65,741 |
| Vaccine | Male | Female | Unknown |
|---|---|---|---|
| Comirnaty | 333,325 | 1,010,541 | 19,656 |
| Vaxzevria | 257,784 | 800,993 | 24,149 |
| Spikevax | 112,749 | 282,554 | 3049 |
| Janssen | 39,212 | 63,712 | 1001 |
| Vaccine | 0–1 Months | 2 Months–2 Years | 3–11 Years | 12–17 Years | 18–64 Years | 65–85 Years | ≥85 Years | Unknown |
|---|---|---|---|---|---|---|---|---|
| Comirnaty | 410 | 834 | 270 | 25,661 | 1,045,338 | 176,219 | 36,535 | 78,255 |
| Vaxzevria | 669 | 783 | 608 | 680 | 866,919 | 134,324 | 5541 | 73,402 |
| Spikevax | 93 | 182 | 29 | 2041 | 309,083 | 64,441 | 9585 | 12,898 |
| Janssen | 9 | 20 | 6 | 165 | 89,292 | 6672 | 713 | 7048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branda, F.; Tosi, D. Comparing Worldwide, National, and Independent Notifications about Adverse Drug Reactions Due to COVID-19 Vaccines. Information 2022, 13, 329. https://doi.org/10.3390/info13070329
Branda F, Tosi D. Comparing Worldwide, National, and Independent Notifications about Adverse Drug Reactions Due to COVID-19 Vaccines. Information. 2022; 13(7):329. https://doi.org/10.3390/info13070329
Chicago/Turabian StyleBranda, Francesco, and Davide Tosi. 2022. "Comparing Worldwide, National, and Independent Notifications about Adverse Drug Reactions Due to COVID-19 Vaccines" Information 13, no. 7: 329. https://doi.org/10.3390/info13070329
APA StyleBranda, F., & Tosi, D. (2022). Comparing Worldwide, National, and Independent Notifications about Adverse Drug Reactions Due to COVID-19 Vaccines. Information, 13(7), 329. https://doi.org/10.3390/info13070329







