Paper-Based Flexible Electrode Using Chemically-Modified Graphene and Functionalized Multiwalled Carbon Nanotube Composites for Electrophysiological Signal Sensing
Abstract
:1. Introduction
2. Materials and Methods
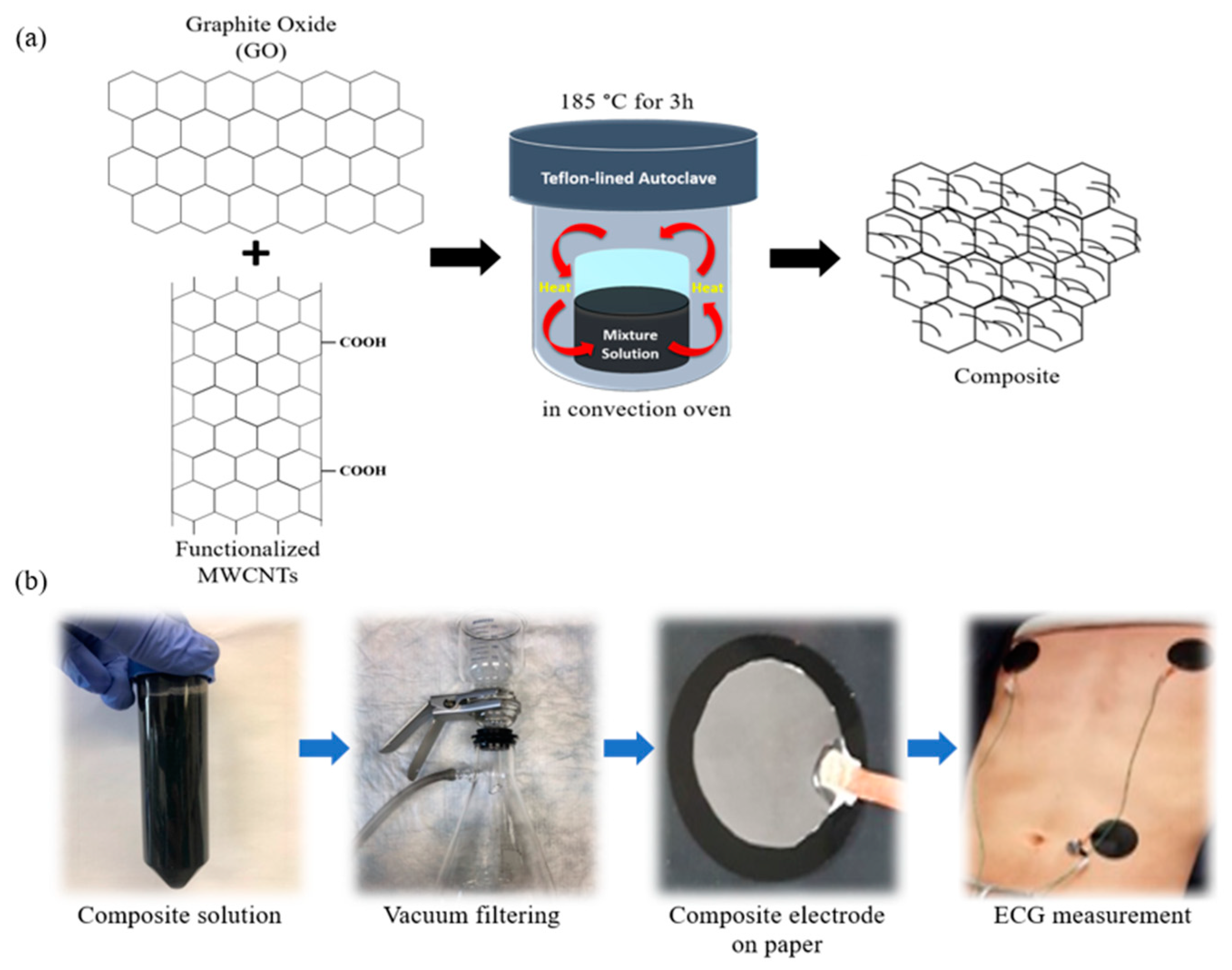
2.1. Electrode Fabrications
2.2. Physical and Electrical Characterization of Composites and Fabricated Electrodes
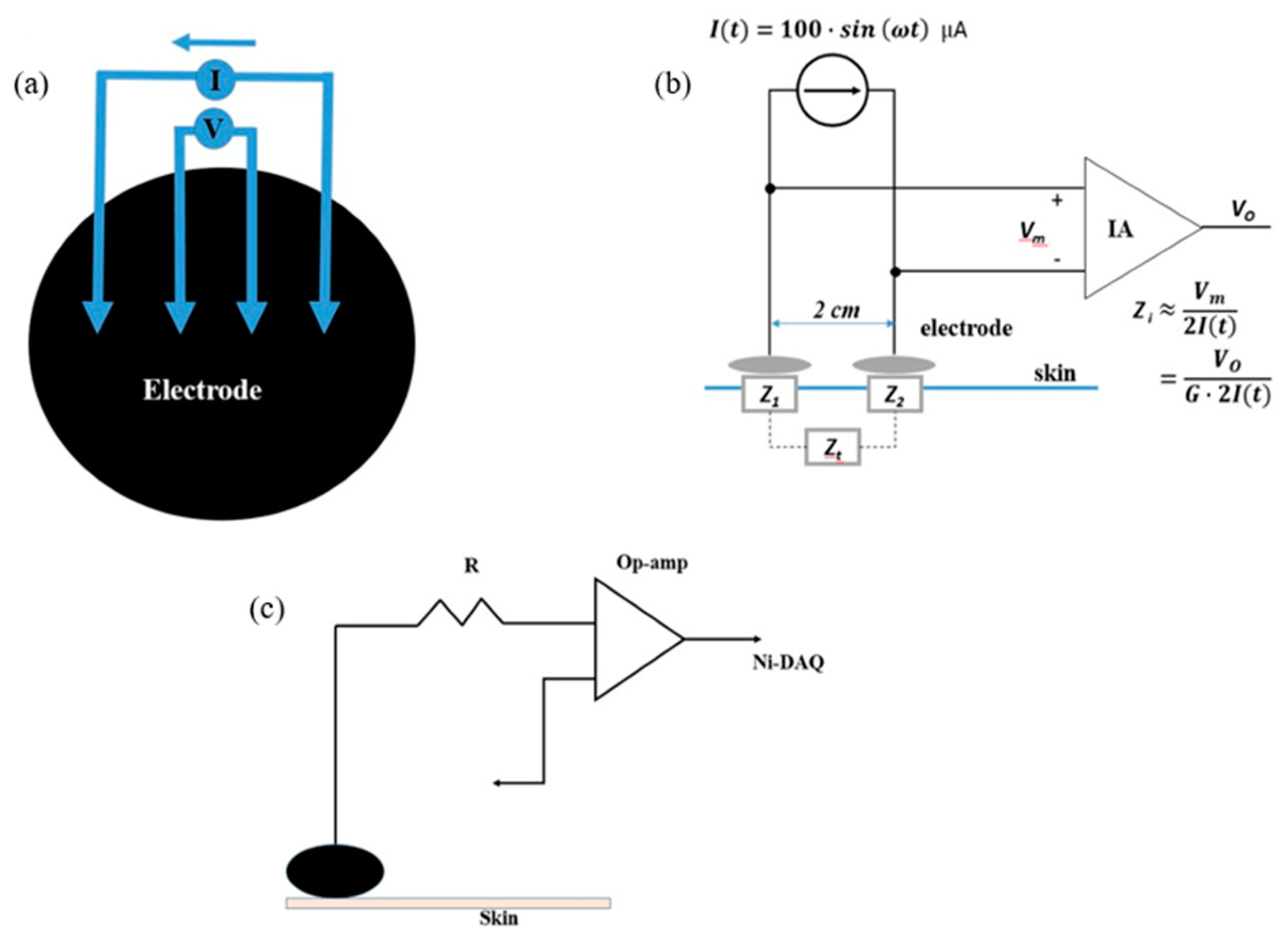
2.3. Skin–Electrode Impedance Measurement System and DC Offset
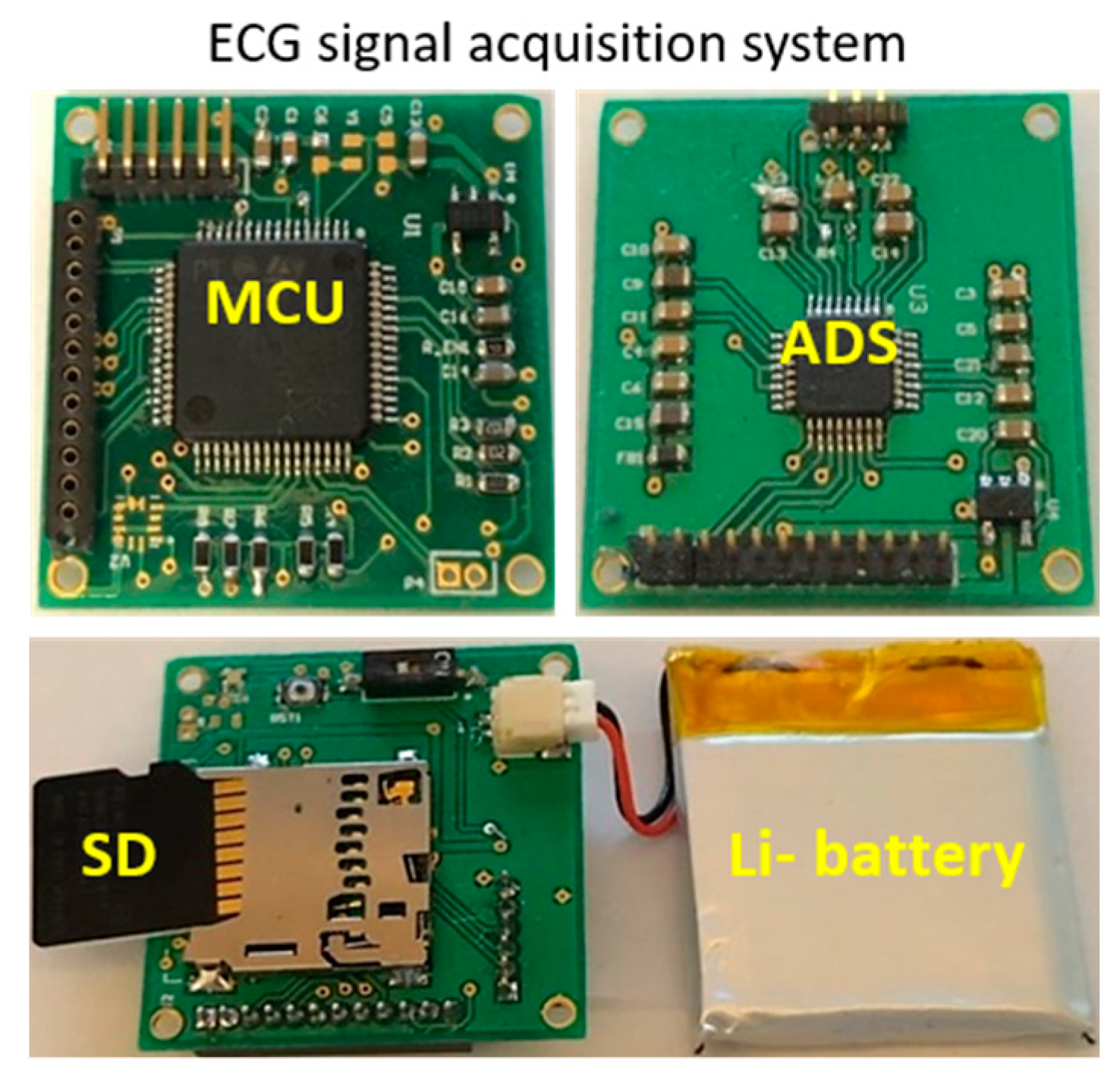
2.4. ECG Signal Measurement and Signal Processing
3. Results
3.1. Physical Characterization of Developed Composites and Electrodes
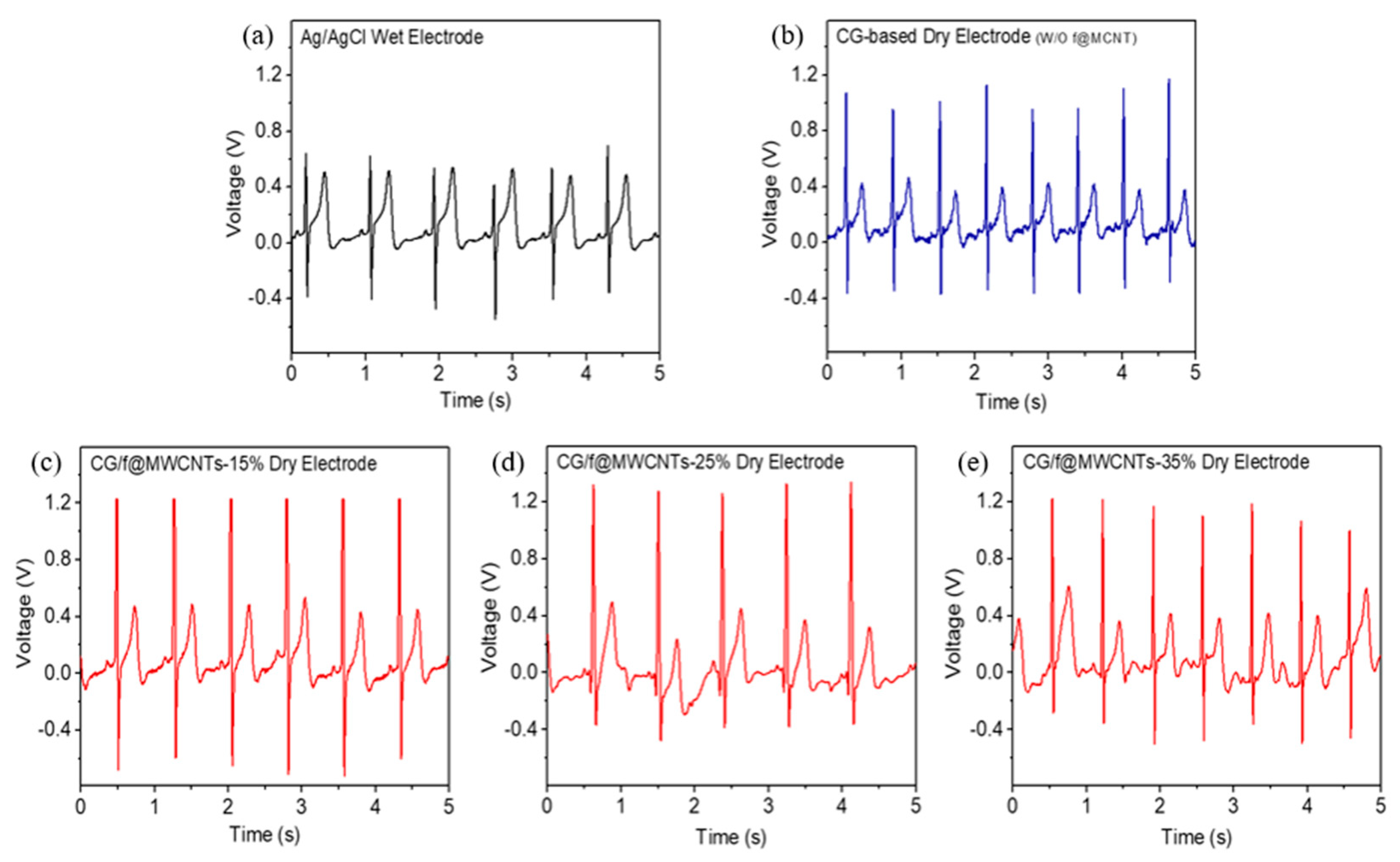
3.2. ECG Signal Measurement Using Different Electrodes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.H.; Hwang, J.-Y.; Hwang, H.R.; Kim, H.S.; Lee, J.H.; Seo, J.W.; Ueon, S.S.; Lee, S.H. Simple and cost-effective method of highly conductive and elastic carbon nanotube polydimethylsiloxane composite for wearable electronics. Sci. Rep. 2018, 8, 1375. [Google Scholar] [CrossRef] [PubMed]
- Chlaihawi, A.A.; Narakathu, B.B.; Emamian, S.; Bazuin, B.J.; Atashbar, M.Z. Development of printed and flexible dry ECG electrodes. Sen. Bio-Sens. Res. 2018, 20, 9–15. [Google Scholar] [CrossRef]
- Xu, S.; Dai, M.; Xu, C.; Chen, C.; Tang, M.; Shi, X.; Dong, X. Performance evaluation of five types of Ag/AgCl bio-electrodes for cerebral electrical impedance tomography. Ann. Biomed. Eng. 2011, 39, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; An, L.H.; Choi, J.M. Flexible polymeric dry electrodes for the long-term monitoring of ECG. Sens. Act. A Phys. 2008, 143, 423–429. [Google Scholar] [CrossRef]
- Hossain, M.F.; Barman, S.C.; Park, J.Y. Seed-mediated growth of platinum nanoparticles anchored on chemically modified graphene and cationic polyelectrolyte composites for electrochemical multi-sensing applications. Sens. Act. B Chem. 2019, 282, 780–789. [Google Scholar] [CrossRef]
- Das, P.S.; Hossain, M.F.; Park, J.Y. Chemically reduced graphene oxide-based dry electrodes as touch sensor for electrocardiograph measurement. Microelectron. Eng. 2017, 180, 45–51. [Google Scholar] [CrossRef]
- Peng, H.; Liu, J.; Dong, Y.; Yang, B.; Chen, X.; Yang, C. Parylene-based flexible dry electrode for bioptential recording. Sens. Actuator B Chem. 2016, 231, 1–11. [Google Scholar] [CrossRef]
- Lee, J.W.; Yun, K.S. ECG Monitoring garment using conductive carbon paste for reduced motion artifacts. Polymers 2017, 9, 439. [Google Scholar]
- Liu, B.; Luo, Z.; Zhang, W.; Tu, Q.; Jin, X. Carbon nanotube-based self-adhesive polymer electrodes for wireless long-term recording of electrocardiogram signals. J. Biomater. Sci. Polym. Ed. 2016, 27, 1899–1908. [Google Scholar] [CrossRef]
- Peng, H.; Liu, J.; Tian, H.; Xu, B.; Dong, Y.; Yang, B.; Chen, X.; Yang, C. Flexible dry electrode based on carbon nanotube/polymer hybrid micropillars for biopotential recording. Sens. Actuator A Phys. 2015, 235, 48–56. [Google Scholar] [CrossRef]
- Hossain, M.F.; Das, P.S.; Park, J.Y. Development of high performance electrochemical and physical biosensors based on chemically modified graphene nanostructured electrodes. J. Electrochem. Soc. 2017, 164, B391–B396. [Google Scholar] [CrossRef]
- Bihar, E.; Roberts, T.; Ismailova, E.; Saadaoui, M.; Isik, M.; Sanchez-Sanchez, A.; Mecerreyes, D.; Herve, T.; De Graaf, J.B.; Malliaras, G.G. Fully printed electrodes on stretchable textiles for long-term electrophysiology. Adv. Mater. Techonol. 2017, 2, 1600251. [Google Scholar] [CrossRef]
- Takamatsu, S.; Lonjaret, T.; Crisp, D.; Badier, J.M.; Malliaras, G.G.; Ismailova, E. Direct patterning of organi conductors on knitted textiles for long-term electrocardiography. Sci. Rep. 2015, 5, 15003. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Park, J.; Sohn, J.; Cho, D.; Jeon, S. Bioinspired, highly stretchable, and conductive dry adhesives based on 1D–2D hybrid carbon nanocomposites for all-in-one ECG electrodes. ACS Nano 2016, 10, 4770–4778. [Google Scholar] [CrossRef]
- Lee, S.M.; Byeon, H.J.; Lee, J.H.; Baek, D.H.; Lee, K.H.; Hong, J.S.; Lee, S. Self-adhesive epidermal carbon nanotube electronics for tether-free long-term continuous recording of biosignals. Sci. Rep. 2014, 4, 6074. [Google Scholar] [CrossRef]
- Yapici, M.K.; Alkhidir, T.; Samad, Y.A.; Liao, K. Graphene-Clad textile electrodes for electrocardiogram monitoring. Sens. Actuator B Chem. 2015, 221, 1469–1474. [Google Scholar] [CrossRef]
- Celik, N.; Manivannan, N.; Strudwick, A.; Balachandran, W. Graphene-Enabled Electrodes for Electrocardiogram Monitoring. Nanomaterials 2016, 2016, 156. [Google Scholar] [CrossRef]
- Lou, C.; Li, R.; Li, Z.; Liang, T.; Wei, Z.; Run, M.; Yan, X.; Liu, X. Flexible graphene electrodes for prolonged dynamic ECG monitoring. Sensors 2016, 16, 1833. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Sprinkle, M.; Ruan, M.; Hu, Y.; Hankinson, J.; Rubio-Roy, M.; Zhang, B.; Wu, X.; Berger, C.; Heer, W.A. Scalable templated growth of graphene nanoribbons on SiC. Nat. Nanotechnol. 2010, 5, 727–731. [Google Scholar] [CrossRef]
- Hossain, M.F.; Park, J.Y. Novel enzymatic glucose biosensor based on distributed electrodes covered with a solvothermal synthesized graphene material and platinum nanoparticles. RSC Adv. 2016, 6, 74453–74461. [Google Scholar] [CrossRef]
- Mahony, C.O.; Pini, F.; Blake, A.; Webster, C.; Brien, J.O.; McCarthy, K.G. Microneedle-based electrodes with integrated through-silicon via for biopotential recording. Sens. Actuator A Phys. 2012, 186, 130–136. [Google Scholar]
- Ruffini, G.; Dunne, S.; Fuentemilla, L.; Grau, C.; Farres, E.; Marco-Pallares, J.; Watts, P.C.P.; Silva, S.R.P. First human trials of a dry electrophysiology sensor using a carbon nanotube array interface. Sens. Actuator A Phys. 2008, 144, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.C.; Moon, J.H.; Baek, D.H.; Lee, J.H.; Choi, Y.Y.; Hong, J.S.; Lee, S.H. CNT/PDMS composite flexible dry electrodes for long-term ECG monitoring. IEEE Trans. Biomed. Eng. 2012, 59, 1472–1479. [Google Scholar] [CrossRef]
- Kang, B.C.; Ha, T.J. Wearable carbon nanotube based dry-electrodes for electrophysiological sensors. Jpn. J. Appl. Phys. 2018, 57, 05GD02. [Google Scholar] [CrossRef]
- Mani, V.; Chen, S.M.; Lou, B.S. Three dimensional graphene oxide-carbon nanotubes and graphene-carbon nanotubes hybrids. Int. J. Electrochem. Sci. 2013, 8, 11641–11660. [Google Scholar]
- Zhao, M.Q.; Zhang, Q.; Huang, J.Q.; Tian, G.L.; Chen, T.C.; Qian, W.Z.; Wei, F. Towards high purity graphene/single-walled carbon nanotube hybrids with improved electrochemical capacitive performance. Carbon 2013, 54, 403. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.A.P.; Asiri, A.M.; Abu-Zied, A.M. Green synthesis of thermally stable Ag-rGO-CNT nano composite with high sensing activity. Compos. Part B Eng. 2016, 86, 27–35. [Google Scholar] [CrossRef]
- Yang, W.; Chen, Y.; Wang, J.; Peng, T.; Xu, J.; Yang, B.; Tang, K. Reduced graphene oxide/carbon nanotube composites as electrochemical energy storage electrode applications. Nanoscale Res. Lett. 2018, 13, 181. [Google Scholar] [CrossRef]
- Ponnamma, D.; Sadasivuni, K.K.; Strankowski, M.; Guob, Q.; Thomas, S. Synergistic effect of multi walled carbon nanotubes and reduced graphene oxides in natural rubber for sensing application. Soft Matter 2013, 9, 10343–10353. [Google Scholar] [CrossRef]
- Huang, J.R.; Her, S.C.; Yang, X.X.; Zhi, M.N. Synthesis and characterization of multi-walled carbon nanotube/graphene nanoplatelet hybrid film for flexible strain sensors. Nanomaterials 2018, 8, 786. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, L.; Liu, T. Strategies for the hybridization of cnts with graphene. in: Graphene-carbon nanotube hybrids for energy and environmental applications. Springer Br. Mol. Sci 2017, 21–51. [Google Scholar]
- Cheng, Q.; Tang, J.; Ma, J.; Zhang, H.; Shinya, N.; Qin, L.C. Graphene and carbon nanotube composite electrodes for supercapacitors with ultra-high energy density. Phys. Chem. Chem. Phys. 2011, 13, 17615–17624. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.K.; Sah, A.N.; Pradhan, A.; Bhattacharya, S. Poly-L-Lysine functionalized MWCNT-rGO nanosheets based 3-d hybrid structure for femtomolar level cholesterol detection using cantilever based sensing platform. Sci. Rep. 2019, 9, 3686. [Google Scholar] [CrossRef]
- Zeng, X.; Yu, S.; Ye, L.; Li, M.; Pan, Z.; Sun, R.; Xu, J. Encapsulating carbon nanotubes with SiO2: A strategy for applying them in polymer nanocomposites with high mechanical strength and electrical insulation. J. Mater. Chem. C 2015, 3, 187. [Google Scholar] [CrossRef]
- Ramli, A.B.; Ahmad, P.A. Correlation analysis for abnormal ECG signal features extraction In Proceeding of the 4th National Conference on Telecommunication Technology, Shah Alam, Malaysia, 14–15 January 2003.
- Achilli, A.; Bonfiglio, B. Design and characterization of screen-printed textile electrodes for ECG monitoring. IEEE Sens. J. 2018, 18, 4097–4107. [Google Scholar] [CrossRef]
- Mathias, D.N.; Park, J.; Kim, E.; Joung, Y.H. Development of a novel noncontact ecg electrode by mems fabrication process. Trans. Electr. Electron. Mater. 2016, 17, 150–154. [Google Scholar] [CrossRef]
- Faiella, G.; Musto, P.; Di Florio, G.; Buosciolo, A.; D’Orazio, L.; Antonucci, V.; Giordano, M. Monitoring the dispersion process of SWNTs in aqueous solutions by UV-Vis and Raman spectroscopies. J. Nanosci. Nanotechnol. 2009, 9, 6026–6033. [Google Scholar] [CrossRef]
- Zhao, H.W.; Hu, L.C.; Chang, C.R. Theoretical studies of “stabilizing” behavior about carbon nanotubes under the electrostatic force. J. Nanosci. Nanotechnol. 2013, 13, 3133–3135. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Kim, H.S.; Kim, J.H.; Shin, U.S.; Lee, S.H. Carbon nanotube nanocomposites with highly enhanced strength and conductivity for flexible electric circuits. Langmuir 2015, 31, 7844–7851. [Google Scholar] [CrossRef]
- Zan, X.; Zheng, F.; Jin, W.; Fei, X.; Huo, F.; Duan, H. Freestanding graphene paper decorated with 2D-assembly of Au@Pt nanoparticles as flexible biosensors to monitor live cell secretion of nitric oxide. Biosens. Bioelectron. 2013, 49, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ilbasmis-Tamer, S.; Ciftci, H.; Turk, M.; Degim, T.; Tamer, U. Multiwalled Carbon Nanotube-Chitosan Scaffold: Cytotoxic, Apoptoti c, and Necrotic Effects on Chondrocyte Cell Lines. Curr. Pharm. Biotechnol. 2017, 18, 327–335. [Google Scholar] [CrossRef] [PubMed]



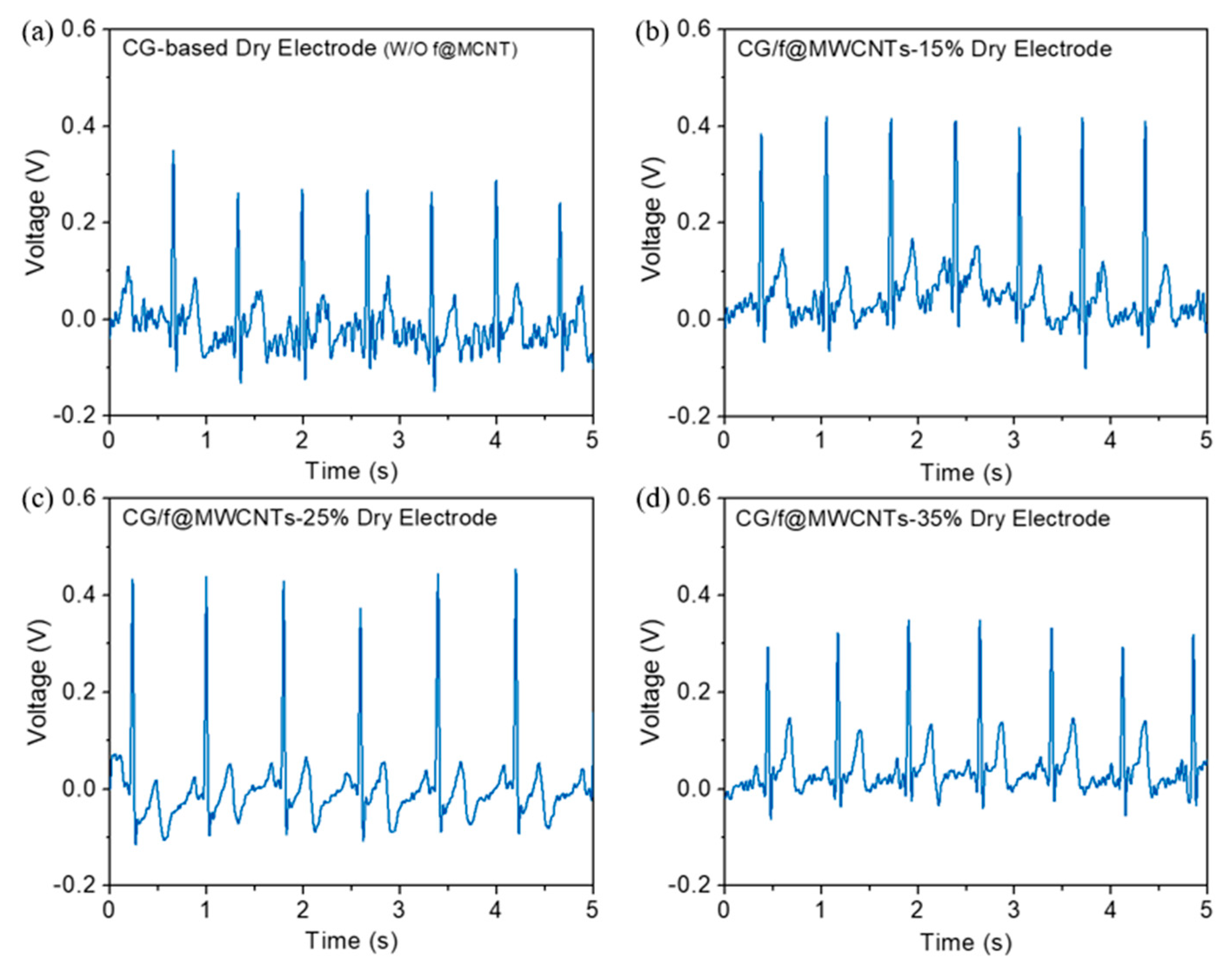
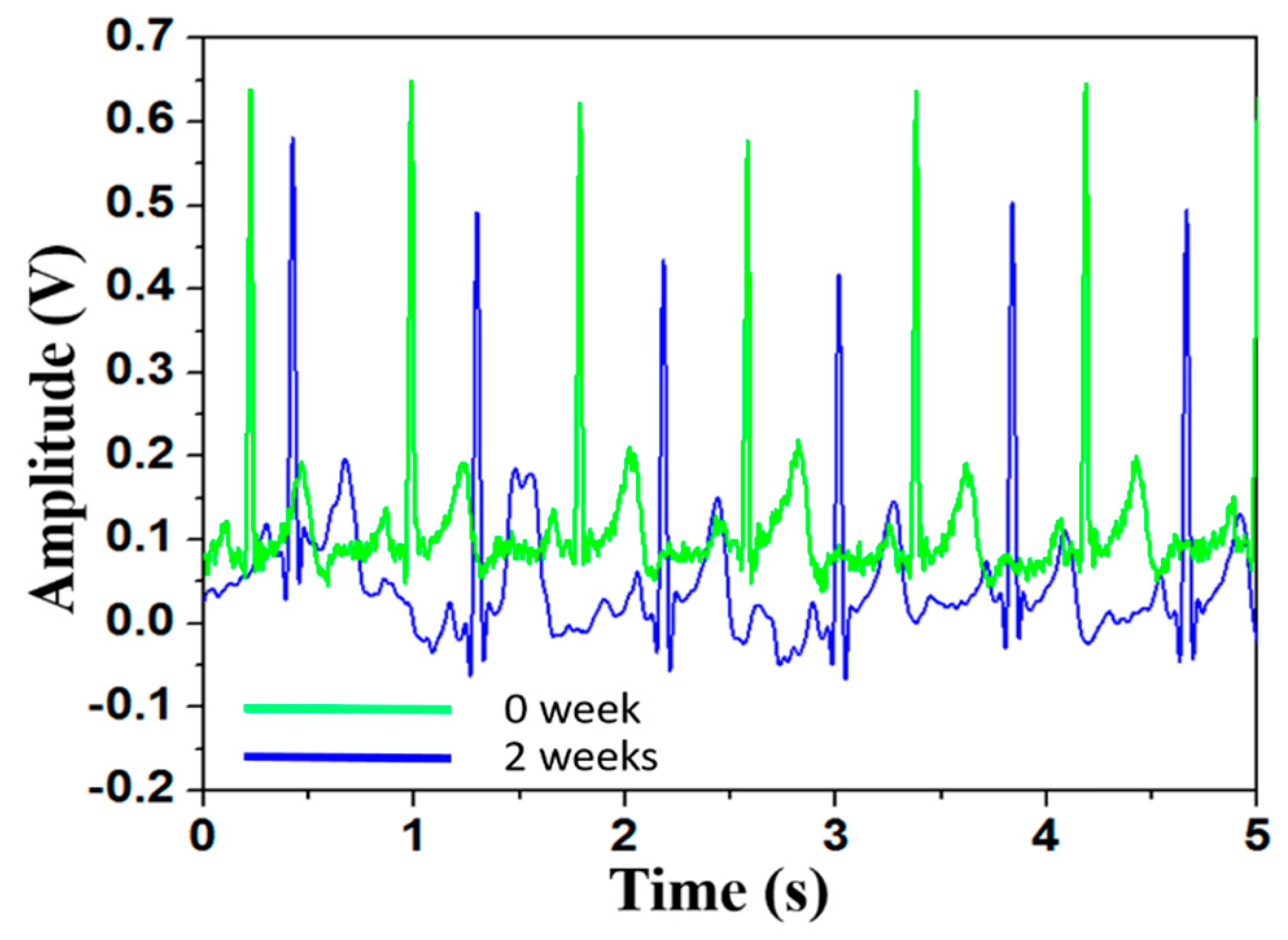

| Sample No. | Sample Name | DC Offset Voltage |
|---|---|---|
| 1 | Ag/AgCl | 11.738 mV |
| 2 | CG | 10.264 mV |
| 3 | CG/f@MWCNTs-15% | 11.120 mV |
| 4 | CG/f@MWCNTs-25% | 11.824 mV |
| 5 | CG/f@MWCNTs-35% | 12.956 mV |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.F.; Heo, J.S.; Nelson, J.; Kim, I. Paper-Based Flexible Electrode Using Chemically-Modified Graphene and Functionalized Multiwalled Carbon Nanotube Composites for Electrophysiological Signal Sensing. Information 2019, 10, 325. https://doi.org/10.3390/info10100325
Hossain MF, Heo JS, Nelson J, Kim I. Paper-Based Flexible Electrode Using Chemically-Modified Graphene and Functionalized Multiwalled Carbon Nanotube Composites for Electrophysiological Signal Sensing. Information. 2019; 10(10):325. https://doi.org/10.3390/info10100325
Chicago/Turabian StyleHossain, Md Faruk, Jae Sang Heo, John Nelson, and Insoo Kim. 2019. "Paper-Based Flexible Electrode Using Chemically-Modified Graphene and Functionalized Multiwalled Carbon Nanotube Composites for Electrophysiological Signal Sensing" Information 10, no. 10: 325. https://doi.org/10.3390/info10100325
APA StyleHossain, M. F., Heo, J. S., Nelson, J., & Kim, I. (2019). Paper-Based Flexible Electrode Using Chemically-Modified Graphene and Functionalized Multiwalled Carbon Nanotube Composites for Electrophysiological Signal Sensing. Information, 10(10), 325. https://doi.org/10.3390/info10100325






