Efficient Free Fatty Acid Reduction in Palm Oil Mill Effluent (POME) for Biodiesel Production: Challenges and Optimization Strategies
Abstract
1. Introduction
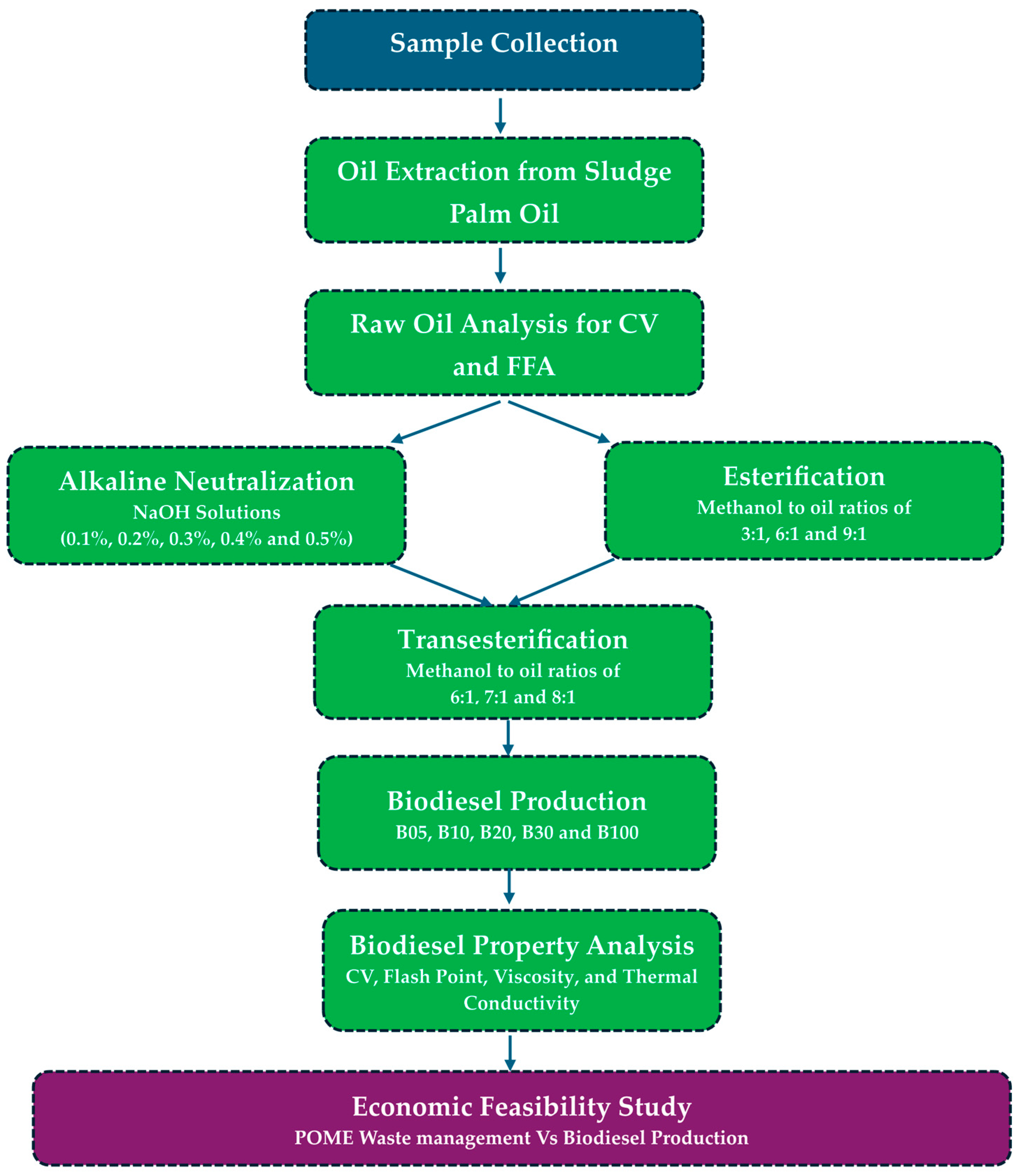
2. Methodology
2.1. Sample Collection
2.2. Oil Extraction from Sludge Palm Oil
2.3. Measurement of Calorific Value of Raw Oil
2.4. Analysis of Sludge Palm Oil Free Fatty Acid Content
2.5. Alkaline Neutralization Procedure
2.6. Esterification
2.7. Transesterification
2.8. Blended Biodiesel Preparation
2.9. Biodiesel Property Analysis Procedure
2.9.1. Calorific Value (CV) Determination
2.9.2. Flash Point Measurement
2.9.3. Density and Viscosity Measurements
2.9.4. Thermal Conductivity Measurement
2.10. Cost Benefit and Sensitive Analysis of POME Management vs. Biodiesel Production
- POME Treatment Cost: A cost of USD 0.50 per m3 of POME was assumed in the base case, with variations of ±10%, ±20%, and ±30%.
- Biodiesel Selling Price: The base price for biodiesel was assumed to be USD 2.00 per liter, with variations of ±10%, ±20%, and ±30%.
- Biodiesel Production Cost per Liter: The base production cost was assumed to be USD 1.50 per liter, with variations of ±10%, ±20%, and ±30%.
3. Results and Discussion
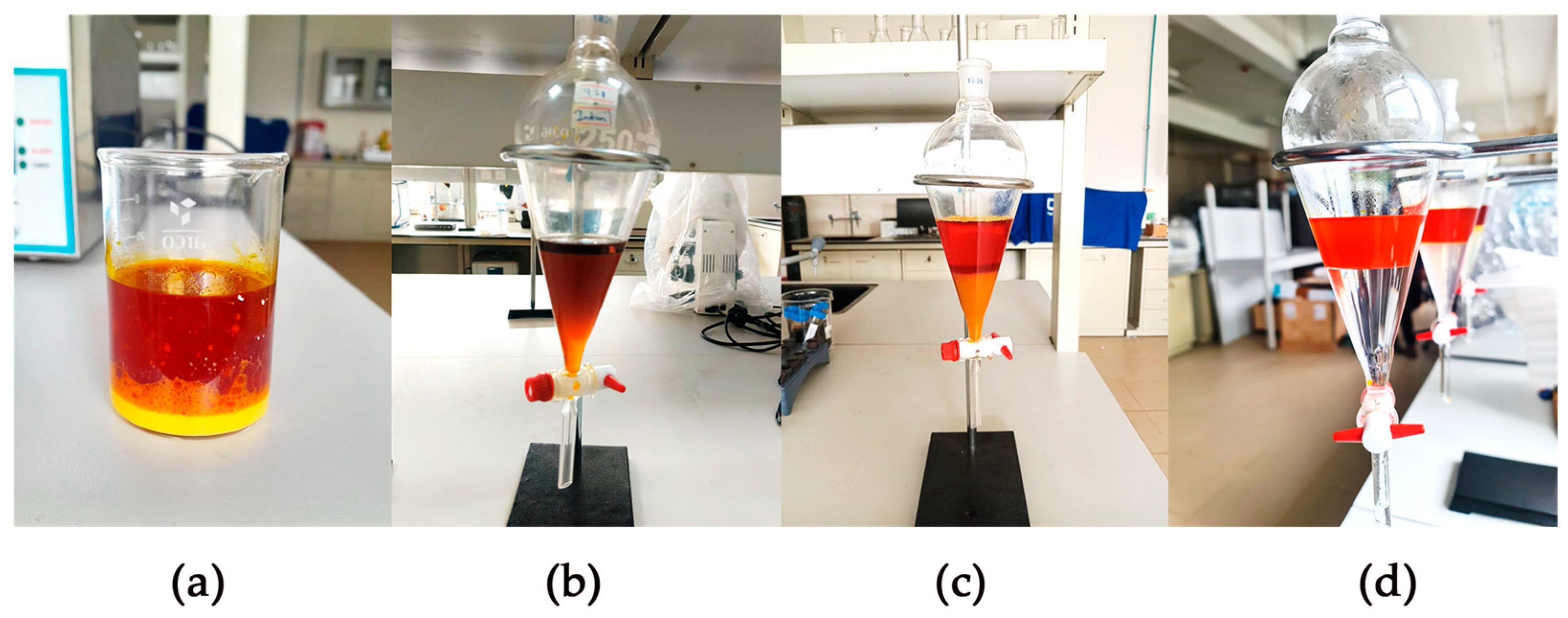
3.1. Centrifugation of Sludge Palm Oil
3.2. Initial FFA Content of Raw Sludge Palm Oil
3.3. Alkaline Neutralization Results
3.4. Transesterification of Alkaline Neutralized Sludge Palm Oil
3.5. Esterification of Sludge Palm Oil
3.6. Cost Comparison of Alkaline Neutralization and Esterification
3.7. Transesterification of Esterified Sludge Palm Oil
3.8. Biodiesel Property Analysis
3.8.1. Calorific Value
3.8.2. Flash Point
3.8.3. Viscosity
3.8.4. Density
3.8.5. Thermal Conductivity (λ Lambda) of Various Biodiesel Blends
3.9. Cost–Benefit Analysis of POME Management vs. Biodiesel Production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASTM | American Society for Testing and Materials |
| B05 | Biodiesel Blend (5% biodiesel, 95% diesel) |
| B10 | Biodiesel Blend (10% biodiesel, 90% diesel) |
| B100 | Biodiesel (100% biodiesel) |
| B20 | Biodiesel Blend (20% biodiesel, 80% diesel) |
| B30 | Biodiesel Blend (30% biodiesel, 70% diesel) |
| BOD | Biochemical Oxygen Demand |
| Cetane Number | Measure of Ignition Quality of Diesel Fuel |
| COD | Chemical Oxygen Demand |
| CPO | Crude Palm Oil |
| EN | European Standards |
| EN 14214 | European Standard for Biodiesel Fuel (Biodiesel from Vegetable Oils) |
| FAMEs | Fatty Acid Methyl Esters |
| FFAs | Free Fatty Acids |
| KOH | Potassium Hydroxide |
| NaOH | Sodium Hydroxide |
| PKO | Palm Kernel Oil |
| POME | Palm Oil Mill Effluent |
| SPO | Sludge Palm Oil |
References
- Nadeesha, S.; Weerasinghe, T.K. Effects of Oil Palm Cultivation on the Properties of the Soil in Some Selected Areas of Nagoda Divisional Secratariat in the Galle District, Sri Lanka. Int. J. Agric. 2016, 3, 114–118. [Google Scholar]
- Bulugahapitiya, D.U.H.; Palihakkara, I.R.; Blasuriya, A. Impact of Organic and Chemical Fertilizer Combinations on Growth, Yield, and Soil Carbon in in Oil Palm Cultivation at Talgaswella Estate, Sri Lanka. Trop. Agric. Res. Ext. 2024, 27, 16–25. [Google Scholar] [CrossRef]
- Kurnia, J.C.; Jangam, S.V.; Akhtar, S.; Sasmito, A.P.; Mujumdar, A.S. Advances in Biofuel Production from Oil Palm and Palm Oil Processing Wastes: A Review. Biofuel Res. J. 2016, 3, 332–346. [Google Scholar] [CrossRef]
- Liyanagama, N.L.; Nugawela, R.C.W.M.R.A.; Attanayake, D.P.S.T.G. The Impact of Oil Palm and Rubber Plantations to the Microenvironment. In Proceedings of the 15th Agricultural Research Symposium (2016); Wayamba University of Sri Lanka: Kuliyapitiya, Sri Lanka, 2016; pp. 537–541. [Google Scholar]
- Thilakarathna, S.C.; Prasad, D.M.; Ramyajith, K.K.; Ariyarathna, M.A.; Ranaraja, C.D.; Arachchige, U.S. Environmental and Social Impacts of Palm Oil Industry in Sri Lanka. J. Res. Technol. Eng. 2020, 1, 13–23. [Google Scholar]
- Sindayikengera, S.; Karikurubu, J.F.; Manirakiza, J.; Ndayikengurukiye, D.; Baseka, M.; Nsabiyumva, P.; Niyukuri, J. Technological Impact on the Quality of Palm Oil from Burundi: Elaeis guineensis, Variety of Dura and Tenera. Food Nutr. Sci. 2024, 15, 759–769. [Google Scholar] [CrossRef]
- Wondi, M.H.; Haris, N.I.N.; Shamsudin, R.; Yunus, R.; Mohd Ali, M.; Iswardi, A.H. Development and Testing of an Oil Palm (Elaeis guineensis Jacq.) Fruit Digester Process for Kernel Free in Crude Palm Oil Production. Ind. Crops Prod. 2024, 208, 117755. [Google Scholar] [CrossRef]
- Mohammad, S.; Baidurah, S.; Kobayashi, T.; Ismail, N.; Leh, C.P. Palm Oil Mill Effluent Treatment Processes—A Review. Processes 2021, 9, 739. [Google Scholar] [CrossRef]
- Bandara, W.M.K.R.T.W.; Basnayake, B.M.L.A.; Shayan, M.N.M.; Illangakoon, I.M.V.U.; Nimanthika, N.A.S.; Thanushka, K.H.; Satoh, H. Feasibility Study for the Treatment of Palm Oil Mill Effluent (POME) Using Electrocoagulation Method in Sri Lanka. Desalin. Water Treat. 2025, 321, 101054. [Google Scholar] [CrossRef]
- Akhbari, A.; Kutty, P.K.; Chuen, O.C.; Ibrahim, S. A Study of Palm Oil Mill Processing and Environmental Assessment of Palm Oil Mill Effluent Treatment. Environ. Eng. Res. 2019, 25, 212–221. [Google Scholar] [CrossRef]
- Nta, S.A.; Udom, I.J.; Udo, S.O. Investigation of Palm Oil Mill Effluent Pollution Impact on Groundwater Quality and Agricultural Soils. Asian J. Environ. Ecol. 2020, 12, 28–36. [Google Scholar] [CrossRef]
- Rathnasekara, R.T.A.J.K.L.; Wjethunga, I.B.; Wijesekara, E.R.J.M.D.D.P.; Amarasinghe, A.M.P.C.; Nilmalgoda, E.P.R.H.H.W. Sustainable Management of Fish Gut Waste Through Transesterification. Int. J. Latest Technol. Eng. Manag. Appl. Sci. 2024, 13, 84–91. [Google Scholar] [CrossRef]
- Wan Osman, W.N.A.; Rosli, M.H.; Mazli, W.N.A.; Samsuri, S. Comparative Review of Biodiesel Production and Purification. Carbon Capture Sci. Technol. 2024, 13, 100264. [Google Scholar] [CrossRef]
- Imam, S.S.; Sani, S.; Mujahid, M.; Adnan, R. Valuable Resources Recovery from Palm Oil Mill Effluent (POME): A Short Review on Sustainable Wealth Reclamation. Waste Manag. Bull. 2025, 3, 1–16. [Google Scholar] [CrossRef]
- Nigatu Gebremariam, S.; Mario Marchetti, J. Norway Biodiesel Production Technologies: Review. AIMS Energy 2017, 5, 425–457. [Google Scholar] [CrossRef]
- Tavizón-Pozos, J.A.; Chavez-Esquivel, G.; Suárez-Toriello, V.A.; Santolalla-Vargas, C.E.; Luévano-Rivas, O.A.; Valdés-Martínez, O.U.; Talavera-López, A.; Rodriguez, J.A. State of Art of Alkaline Earth Metal Oxides Catalysts Used in the Transesterification of Oils for Biodiesel Production. Energies 2021, 14, 1031. [Google Scholar] [CrossRef]
- Da Conceicao Lobato Do Nascimento, W.; De Sousa Sousa, N.; Alberto Lira Júnior, C.; Dos Santos Martins, J.; Pereira Maciel, A. Study of Heterogeneous Catalysts in Transesterification for Biosiesel Production. Rev. Multidiscip. Nordeste Min. 2025, 2, 1–16. [Google Scholar] [CrossRef]
- ASTM D6751-20a; D02 Committee ASTM International. Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM: West Conshohocken, PA, USA, 2023. [CrossRef]
- Murmu, R.; Sutar, H.; Patra, S. Experimental Investigation and Process Optimization of Biodiesel Production from Kusum Oil Using Taguchi Method. Adv. Chem. Eng. Sci. 2017, 7, 464–476. [Google Scholar] [CrossRef]
- Casas, A.; Pérez, Á.; Ramos, M.J. Effects of Diacetinmonoglycerides and Triacetin on Biodiesel Quality. Energies 2023, 16, 6146. [Google Scholar] [CrossRef]
- Acaroglu, M.; Demirbas, A. Relationships between Viscosity and Density Measurements of Biodiesel Fuels. Energy Sour. Part A Recovery Util. Environ. Eff. 2007, 29, 705–712. [Google Scholar] [CrossRef]
- Osman, S.; Stefaniu, A. Density, Viscosity, and Distillation Temperatures of Binary Blends of Diesel Fuel Mixed with Oxygenated Components at Different Temperatures. Sustainability 2023, 15, 15460. [Google Scholar] [CrossRef]
- Linganiso, E.C.; Tlhaole, B.; Magagula, L.P.; Dziike, S.; Linganiso, L.Z.; Motaung, T.E.; Moloto, N.; Tetana, Z.N. Biodiesel Production from Waste Oils: A South African Outlook. Sustainability 2022, 14, 1983. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Makertihartha, I.G.B.N.; Indarto, A.; Prakoso, T.; Soerawidjaja, T.H. A Review of Biodiesel Cold Flow Properties and Its Improvement Methods: Towards Sustainable Biodiesel Application. Energies 2024, 17, 4543. [Google Scholar] [CrossRef]
- Mićić, R.; Tomić, M.; Martinović, F.; Kiss, F.; Simikić, M.; Aleksic, A. Reduction of Free Fatty Acids in Waste Oil for Biodiesel Production by Glycerolysis: Investigation and Optimization of Process Parameters. Green Process. Synth. 2019, 8, 15–23. [Google Scholar] [CrossRef]
- Berlanga-Del Pozo, M.; Gallardo-Guerrero, L.; Gandul-Rojas, B. Influence of Alkaline Treatment on Structural Modifications of Chlorophyll Pigments in NaOH—Treated Table Olives Preserved without Fermentation. Foods 2020, 9, 701. [Google Scholar] [CrossRef]
- Wang, B.; Wang, B.; Shukla, S.K.; Wang, R. Enabling Catalysts for Biodiesel Production via Transesterification. Catalysts 2023, 13, 740. [Google Scholar] [CrossRef]
- Ahmed, R.; Huddersman, K. Review of Biodiesel Production by the Esterification of Wastewater Containing Fats Oils and Grease (FOGs). J. Ind. Eng. Chem. 2022, 110, 1–14. [Google Scholar] [CrossRef]
- ASTM D5865/D5865M-19; D05 Committee ASTM International. Test Method for Gross Calorific Value of Coal and Coke. ASTM: West Conshohocken, PA, USA, 2019. [CrossRef]
- Rusdi, H.O.; Kusumaningrum, I.K.; Nareswari, T.J.; Fauziah, P.N.; Maharani, R.N.; Natasya, S. Separation and Determination of Free Fatty Acids in Corn Oil and Palm Oil by Liquid-Liquid Extraction and Acidi-Alkalimetric Titration. Walisongo J. Chem. 2024, 7, 98–106. [Google Scholar] [CrossRef]
- Uçar, B.; Gholami, Z.; Svobodová, K.; Hradecká, I.; Hönig, V. A Comprehensive Study for Determination of Free Fatty Acids in Selected Biological Materials: A Review. Foods 2024, 13, 1891. [Google Scholar] [CrossRef] [PubMed]
- Aworanti, O.A.; Ajani, A.O.; Agarry, S.E.; Babatunde, K.A.; Akinwunmi, O.D. Effect of Process Variables on the Transesterification Process of Palm Oil Sludge to Biodiesel. Biotechnol. J. Int. 2019, 23, 1–14. [Google Scholar] [CrossRef]
- Brahma, S.; Nath, B.; Basumatary, B.; Das, B.; Saikia, P.; Patir, K.; Basumatary, S. Biodiesel Production from Mixed Oils: A Sustainable Approach towards Industrial Biofuel Production. Chem. Eng. J. Adv. 2022, 10, 100284. [Google Scholar] [CrossRef]
- Musa, I.A. The Effects of Alcohol to Oil Molar Ratios and the Type of Alcohol on Biodiesel Production Using Transesterification Process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef]
- ASTM D93-20; D02 Committee ASTM International. Test Methods for Flash Point by Pensky-Martens Closed Cup Tester. ASTM: West Conshohocken, PA, USA, 2020. [CrossRef]
- ASTM D396-21; D02 Committee ASTM International. Specification for Fuel Oils. ASTM: West Conshohocken, PA, USA, 2021. [CrossRef]
- Sánchez-Rodríguez, G.; Domenzaín-González, J.; Verónico-Sánchez, F.J.; Pérez-López, H.I.; Zúñiga-Moreno, A.; Elizalde-Solis, O. Density and Viscosity in Biodiesel + Diesel Mixtures from Recycled Feedstocks. Appl. Sci. 2025, 15, 3812. [Google Scholar] [CrossRef]
- ASTM D7896-19; D15 Committee ASTM International. Test Method for Thermal Conductivity, Thermal Diffusivity and Volumetric Heat Capacity of Engine Coolants and Related Fluids by Transient Hot Wire Liquid Thermal Conductivity Method. ASTM: West Conshohocken, PA, USA, 2019. [CrossRef]
- ASTM E1952-17; E37 Committee ASTM International. Test Method for Thermal Conductivity and Thermal Diffusivity by Modulated Temperature Differential Scanning Calorimetry. ASTM: West Conshohocken, PA, USA, 2017. [CrossRef]


| Parameter | 0.1% NaOH | 0.2% NaOH | 0.3% NaOH | 0.4% NaOH | 0.5% NaOH |
|---|---|---|---|---|---|
| NaOH Concentration | 0.10% | 0.20% | 0.30% | 0.40% | 0.50% |
| NaOH-to-Oil Ratio | 1:3 | 1:3 | 1:3 | 1:3 | 1:3 |
| Reaction Time | 30 and 120 min | 30 and 120 min | 30 and 120 min | 30 and 120 min | 30 and 120 min |
| Temperature | 60 °C | 60 °C | 60 °C | 60 °C | 60 °C |
| Centrifugation Speed | Centrifugation Time | Initial Oil Weight (g) | Final Oil Weight (g) | Oil Recovery (w/w%) |
|---|---|---|---|---|
| 4500 RPM | 15 min | 50 | 48.2 | 96.40% |
| NaOH Concentration | Reaction Time | Initial FFA (%) | Final FFA (%) | Final Oil Weight (%) | Soap Weight (%) |
|---|---|---|---|---|---|
| 0.10% | 30 min | 10.09% | 8.84% | 84.64% | 14.71% |
| 0.10% | 120 min | 10.09% | 7.91% | 84.41% | 15.35% |
| 0.20% | 30 min | 10.09% | 7.58% | 83.37% | 16.62% |
| 0.20% | 120 min | 10.09% | 7.04% | 81.87% | 18.12% |
| 0.30% | 30 min | 10.09% | 5.03% | 77.70% | 22.29% |
| 0.30% | 120 min | 10.09% | 3.86% | 71.44% | 28.55% |
| 0.40% | 30 min | 10.09% | 3.87% | 69.34% | 30.65% |
| 0.40% | 120 min | 10.09% | 3.62% | 68.75% | 31.24% |
| 0.50% | 30 min | 10.09% | 3.53% | 66.85% | 33.14% |
| 0.50% | 120 min | 10.09% | 3.48% | 64.89% | 35.10% |
| Methanol-to-Oil Ratio | Methanol (mL) | Oil (mL) | Ester Yield (w/w) | Water Yield (w/w) | Initial FFA Content (%) | Final FFA Content (%) |
|---|---|---|---|---|---|---|
| 3:1 | 7.42 mL | 60 mL | 94.22% | 5.77% | 10.09% | 8.60% |
| 6:1 | 14.83 mL | 60 mL | 86.34% | 13.65% | 10.09% | 2.26% |
| 9:1 | 22.24 mL | 60 mL | 85.71% | 14.28% | 10.09% | 1.69% |
| Parameter | Alkaline Neutralization | Esterification |
|---|---|---|
| Initial FFA Content | 10.09% | 10.09% |
| Method for FFA Reduction | NaOH treatment (analytical grade, ≥98%) | Methanol (analytical grade, ≥99%) and H2SO4 (reagent grade, ≥98%) for esterification |
| Best FFA Reduction Achieved | 0.5% NaOH for 120 min | 9:1 methanol-to-oil ratio |
| FFA Reduction Efficiency | FFA reduced to 3.48% | FFA content reduced to 1.69% |
| Amount of Chemicals | 0.005 kg of NaOH per liter of oil at 0.5% concentration | 0.13 L of methanol per liter of oil at 9:1 ratio |
| Cost per Liter of Chemicals | USD 0.002 per liter of oil (0.005 kg × USD 400 per ton of NaOH) | USD 0.065 per liter of oil (0.13 L × USD 500 per ton of methanol) |
| USD 0.002 per liter of oil (0.01 kg × USD 200 per ton of H2SO4) | ||
| Energy Costs | USD 0.01 to USD 0.02 per liter for heating | USD 0.02 to USD 0.03 per liter for heating |
| Total Estimated Chemical and Energy Cost | USD 0.012 per liter | USD 0.087 per liter |
| Processing Time | 30 min to 120 min | Varies, but generally longer than alkaline neutralization |
| Required Additional Processes | Requires esterification or transesterification to produce biodiesel | Can be followed by transesterification for biodiesel |
| Net Benefit of FFA Reduction | Lower initial cost but requires further processing to achieve biodiesel | More effective in reducing FFA, improving biodiesel yield |
| Methanol-to-Oil Ratio | Oil (mL) | Methanol (mL) | NaOH (g) | Biodiesel Yield (g) | Glycerol Yield (g) | Biodiesel Yield (w/w%) |
|---|---|---|---|---|---|---|
| 6:1 | 100.00 | 25.00 | 1.00 | 54.45 | 39.42 | 58.00 |
| 7:1 | 100.00 | 28.88 | 1.00 | 63.42 | 27.18 | 70.00 |
| 8:1 | 80.00 | 26.20 | 0.80 | 61.53 | 24.82 | 71.25 |
| Property | Biodiesel (B100) | Biodiesel (B05) | Biodiesel (B10) | Biodiesel (B20) | Biodiesel (B30) |
|---|---|---|---|---|---|
| Calorific Value (MJ/kg) | 40.21 | 46.24 | 46.025 | 45.187 | 44.708 |
| Flash Point (°C) | 180.5 | 79.5 | 79.5 | 82.5 | 82.5 |
| Temperature (°C) | 20 | 25 | 30 | 35 | 40 |
|---|---|---|---|---|---|
| B05 Density (g/cm3) | 0.8423 | 0.8386 | 0.8351 | 0.8316 | 0.8282 |
| B05 Kinematic Viscosity (mm2/s) | 6.9603 | 6.0685 | 5.3230 | 4.7026 | 4.1589 |
| B10 Density (g/cm3) | 0.8437 | 0.8399 | 0.8364 | 0.8330 | 0.8295 |
| B10 Kinematic Viscosity (mm2/s) | 6.8030 | 5.9257 | 5.1933 | 4.5892 | 4.0556 |
| B20 Density (g/cm3) | 0.8471 | 0.8433 | 0.8398 | 0.8363 | 0.8328 |
| B20 Kinematic Viscosity (mm2/s) | 6.8376 | 5.9569 | 5.2238 | 4.616 | 4.0814 |
| B30 Density (g/cm3) | 0.8472 | 0.8434 | 0.8399 | 0.8363 | 0.8328 |
| B30 Kinematic Viscosity (mm2/s) | 6.5822 | 5.7548 | 5.0615 | 4.4872 | 3.9788 |
| Parameter | POME Management | Biodiesel Production (Esterification + Transesterification) |
|---|---|---|
| POME Volume (Daily) | 250 m3 | 250 m3 |
| Oil Recovery Efficiency | N/A | 96.40% of POME recovered as oil |
| Oil Extracted (Daily) | N/A | 240.92 m3 (96.40% of 250 m3) |
| Biodiesel Yield | N/A | 70% of extracted oil converted into biodiesel |
| Biodiesel Produced (Daily) | N/A | 168.64 m3 (or 168,640 L) |
| Cost of POME Treatment (Per m3) | USD 0.50 | N/A |
| Total Cost of POME Treatment (Daily) | USD 125 | N/A |
| Total Cost of POME Treatment (Annual) | USD 45,625 | N/A |
| Cost of Biodiesel Production (per liter) | N/A | USD 1.50 per liter |
| Daily Production Cost for Biodiesel | N/A | USD 252,960 (168,640 L × USD 1.50 per liter) |
| Annual Production Cost for Biodiesel | N/A | USD 92,378,400 (252,960 × 365 days) |
| Market Price of Biodiesel | N/A | USD 2.00 per liter |
| Daily Revenue | N/A | USD 337,280 (168,640 L × USD 2.00 per liter) |
| Annual Revenue | N/A | USD 337,280,000 (337,280 × 365 days) |
| Net Profit/Loss | −USD 45,625 | USD 244,901,600 (revenue—production cost) |
| Environmental Benefits | None | Reduces environmental impact by converting waste into fuel |
| Byproducts | N/A | Glycerol, which can be used or sold |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamara, I.; Nilmalgoda, H.; Wimalasiri, E. Efficient Free Fatty Acid Reduction in Palm Oil Mill Effluent (POME) for Biodiesel Production: Challenges and Optimization Strategies. Challenges 2025, 16, 28. https://doi.org/10.3390/challe16020028
Chamara I, Nilmalgoda H, Wimalasiri E. Efficient Free Fatty Acid Reduction in Palm Oil Mill Effluent (POME) for Biodiesel Production: Challenges and Optimization Strategies. Challenges. 2025; 16(2):28. https://doi.org/10.3390/challe16020028
Chicago/Turabian StyleChamara, Indunil, Helitha Nilmalgoda, and Eranga Wimalasiri. 2025. "Efficient Free Fatty Acid Reduction in Palm Oil Mill Effluent (POME) for Biodiesel Production: Challenges and Optimization Strategies" Challenges 16, no. 2: 28. https://doi.org/10.3390/challe16020028
APA StyleChamara, I., Nilmalgoda, H., & Wimalasiri, E. (2025). Efficient Free Fatty Acid Reduction in Palm Oil Mill Effluent (POME) for Biodiesel Production: Challenges and Optimization Strategies. Challenges, 16(2), 28. https://doi.org/10.3390/challe16020028







