Preventing the Next Pandemic through a Planetary Health Approach: A Focus on Key Drivers of Zoonosis
Abstract
1. Introduction
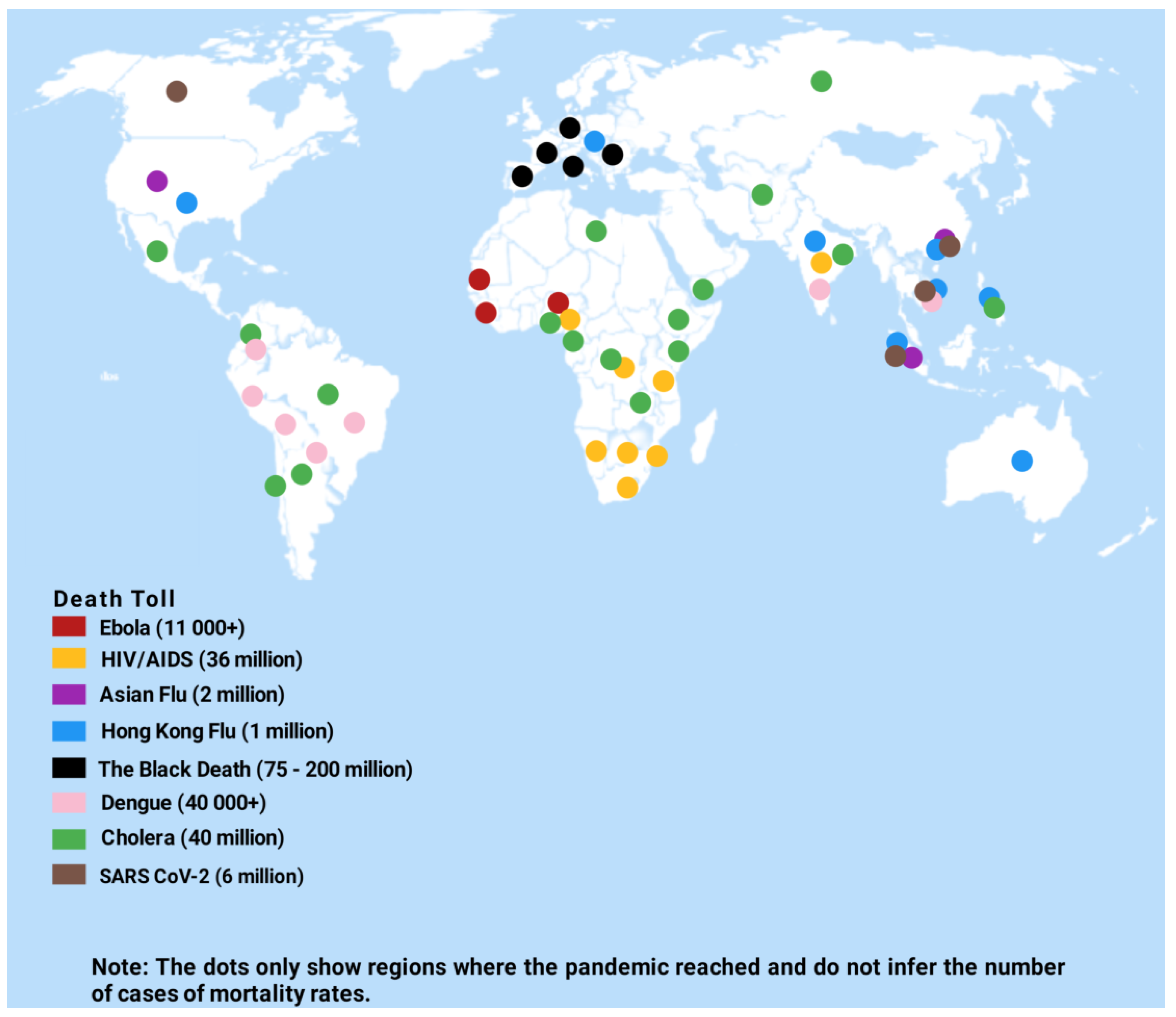
2. Epidemiology of Zoonoses
3. Brief Classification of Zoonoses Based on Aetiological Agents, Transmission Cycles, and Reservoir Hosts
- Bacterial, e.g., tuberculosis and brucellosis;
- Viral, e.g., rabies and dengue;
- Fungal, e.g., histoplasmosis and cryptococcosis;
- Rickettsial and chromydial, e.g., scrub typhus and ornithosis;
- Parasitic, e.g., toxoplasmosis, schistosomiasis, cysticercosis, and trichinellosis.
- Sylvatic Cycle: transmission that involves non-human primates such as monkeys and mosquitoes; whereby the mosquito species transmit the disease from non-human primates to humans, notably those working in the jungle, e.g., monkeypox.
- Synanthropic cycle: transmission that involves synanthropes—species whose population is highly dependent on variables influenced by humans—such as rodents, lizards, and birds, e.g., tularemia and plague [26].
- Human Cycle: transmission that involves man to man or man to animals, e.g., human tuberculosis and giardiasis.
- Anthropozoonoses: these are diseases of domestic and wild animals that occur naturally independent of man and get transmitted from animal to human, e.g., Rabies, Tularemia, Rift Valley fever, and Leptospirosis.
- Zooanthroponoses: these are diseases of pathogens that are normal reservoirs in a human host and can be transmitted to other vertebrates, e.g., human tuberculosis and amoebiasis [27].
- Amphixenoses: these are diseases of pathogens whose reservoir hosts can be both humans and lower vertebrates, and thus diseases can be transmitted in both directions, e.g., Staphylococcal and Streptococcal diseases [28].
4. Biodiversity Loss and Zoonoses
5. Climate Change and Zoonoses
6. Population Mobility and Zoonoses
7. Wildlife Consumption and Zoonoses
8. Addressing Zoonoses via a Planetary Health Approach: Justification for Action
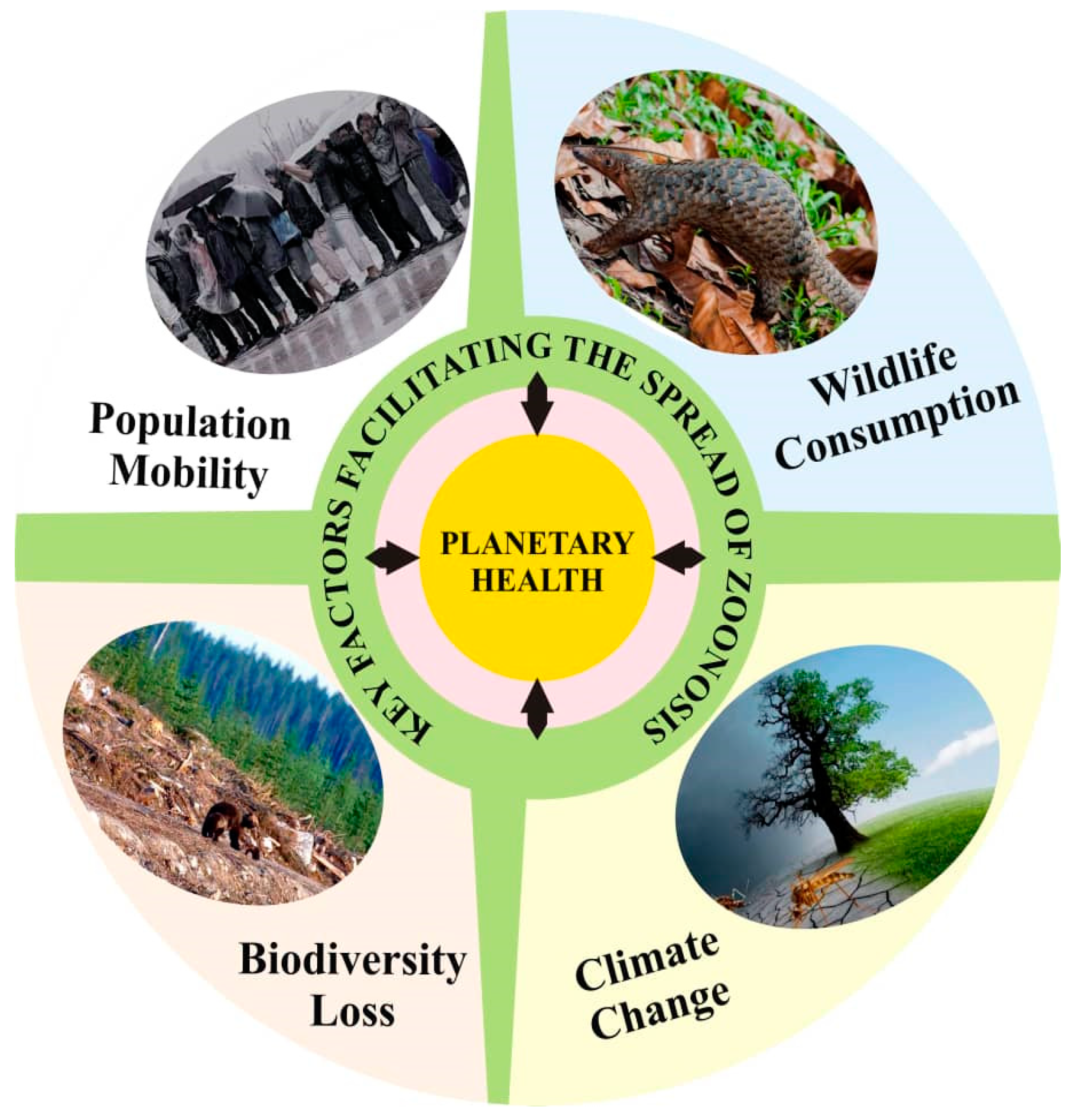
9. Planetary Health and Drivers of Zoonoses
10. Recommendations: Planetary Health as a Panacea for Zoonoses
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McNamara, T.; Richt, J.A.; Glickman, L. A Critical Needs Assessment for Research in Companion Animals and Livestock Following the Pandemic of COVID-19 in Humans. Vector Borne Zoonotic Dis. 2020, 20, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.; Nichols, E. Neglected wild life: Parasitic Biodiversity as a Conservation Target. Int. J. Parasitol. Parasites Wildl. 2013, 2, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Plowright, R.K.; Reaser, J.K.; Locke, H.; Woodley, S.J.; Patz, J.A.; Becker, D.J.; Oppler, G.; Hudson, P.J.; Tabor, G.M. Land Use-Induced Spillover: A Call to Action to Safeguard Environmental, Animal, and Human Health. Lancet Planet. Heal. 2021, 5, e237–e245. [Google Scholar] [CrossRef]
- Taylor, L.; Latham, S.; Woolhouse, M.E. Risk Factors for Human Disease Emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.; Hyatt, A.D. Anthropogenic Environmental Change and the Emergence of Infectious Diseases in Wildlife. Acta Trop. 2001, 78, 103–116. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Zoonotic Disease: Emerging Public Health Threats in the Region. Available online: http://www.emro.who.int/about-who/rc61/zoonotic-diseases.html (accessed on 27 April 2022).
- Bernstein, A.S.; Ando, A.W.; Lock-Temzelides, T.; Vale, M.M.; Li, B.V.; Li, H.; Busch, J.; Chapman, C.A.; Kinnaird, M.; Nowak, K.; et al. The Costs and Benefits of Primary Prevention of Zoonotic Pandemics. Sci. Adv. 2022, 8, eabl4183. [Google Scholar] [CrossRef]
- Lloyd-Smith, J.O.; George, D.; Pepin, K.M.; Pitzer, V.E.; Pulliam, J.R.; Dobson, A.P.; Hudson, P.J.; Grenfell, B.T. Epidemic Dynamics at the Human-Animal Interface. Science 2009, 326, 1362–1367. [Google Scholar] [CrossRef]
- Saadi, A.A.; Moussiaux, N.; Marcotty, T.; Thys, S.; Sahibi, H. Using Qualitative Approaches to Explore the Challenges of Integrated Programmes for Zoonosis Control in Developing Countries: Example of Hydatidosis Control in Morocco. Zoonoses Public Health 2021, 68, 393–401. [Google Scholar] [CrossRef]
- Meslin, F.X. Public Health Impact of Zoonoses and International Approaches for their Detection and Containment. Vet. Ital. 2008, 44, 583–590. [Google Scholar]
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Marco, M.D.; Breit, N.; Olival, K.J.; Daszak, P. Global Hotspots and Correlates of Emerging Zoonotic Diseases. Nat. Commun. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Singh, B.B.; Gajadhar, A.A. Role of India’s Wildlife in the Emergence and Re-Emergence of Zoonotic Pathogens, Risk Factors and Public Health Implications. Acta Trop. 2014, 138, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Aleem, M.A.; Khan, M.S.I.; Hossain, M.E.; Ghosh, S.; Rahman, M.Z. Major Zoonotic Diseases of Public Health Importance in Bangladesh. Vet. Med. Sci. 2021, 7, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Boschiroli, M.L.; Foulongne, V.; O’Callaghan, D. Brucellosis: A Worldwide Zoonosis. Curr. Opin. Microbiol. 2001, 4, 58–64. [Google Scholar] [CrossRef]
- Naicker, P.R. The Impact of Climate Change and other Factors on Zoonotic Diseases. Arch. Clin. Microbiol. 2011, 2. [Google Scholar] [CrossRef]
- Newcomb, J.; Harrington, T.; Aldrich, S. The Economic Impact of Selected Infectious Disease Outbreaks; Bio Economic Research Associates: Cambridge, UK, 2011. [Google Scholar]
- Kumar, A. Ebola Virus Altered Innate and Adaptive Immune Response Signalling Pathways: Implications for Novel Therapeutic Approaches. Infect. Disord. Drug Targets 2016, 16, 79–94. [Google Scholar] [CrossRef]
- Coltart, C.E.; Lindsey, B.; Ghinai, I.; Johnson, A.M.; Heymann, D.L. The Ebola Outbreak, 2013–2016: Old Lessons for New Epidemics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160297. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Outbreaks Chronology: Ebola Virus Disease. Available online: https://www.cdc.gov/vhf/ebola/outbreaks/history/chronology-replaced.html (accessed on 28 April 2022).
- Yoder, J.S.; Harral, C.; Beach, M.J.; Centers for Disease Control and Prevention (CDC). Giardiasis Surveillance—United States, 2006–2008. MMWR Surveill. Summ. 2010, 59, 15–25. [Google Scholar]
- Medscape. Campylobacter Infections. Available online: https://emedicine.medscape.com/article/213720-overview (accessed on 27 April 2022).
- Peiris, J.S.; Yuen, K.Y.; Osterhaus, A.D.; Stohrm, K. The Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003, 349, 2431–2441. [Google Scholar] [CrossRef]
- World Health Organisation. Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 28 April 2022).
- Centers for Disease Control and Prevention (CDC). Commercial Laboratory Seroprevalence Surveys. Available online: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/commercial-lab-surveys.html (accessed on 27 April 2022).
- Ludovico, D.; Luca, B.; Paola, P.; Alessandro, F.; Vincenzo, C.; Giuseppe, C.; Laura, R. Synanthropic Birds and Parasites. Avian Dis. 2013, 57, 756–758. [Google Scholar] [CrossRef]
- Ali, M.M.; Amber, N.B.; Gregory, C.G. Reverse Zoonotic Disease Transmission (Zooanthroponosis): A Systematic Review of Seldom-Documented Human Biological Threats to Animals. PLoS ONE 2014, 9, e89055. [Google Scholar] [CrossRef]
- Chomel, B.B. Zoonoses. Encycl. Microbiol. 2009, 820–829. [Google Scholar] [CrossRef]
- Hassan, R.; Scholes, R.J.; Ash, N. Ecosystems and Human Well-Being: Current State and Trends; The Millennium Ecosystem Assessment Series; Island Press: Washington, DC, USA, 2005; Chapter 4; Volume 1, pp. 77–122. [Google Scholar]
- Tajudeen, Y.A.; Oladunjoye, I.O.; Adebayo, A.O.; Adebisi, Y.A. The Need to Adopt Planetary Health Approach in Understanding the Potential Influence of Climate Change and Biodiversity Loss on Zoonotic Diseases Outbreaks. Public Health Pract. 2021, 2, 100095. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Keesing, F. Biodiversity and Disease Risk: The Case of Lyme Disease. Conserv. Biol. 2000, 14, 722–728. [Google Scholar] [CrossRef]
- Keesing, F.; Ostfeld, R.S. Dilution Effects in Disease Ecology. Ecol. Lett. 2021, 24, 2490–2505. [Google Scholar] [CrossRef] [PubMed]
- Civitello, D.J.; Cohen, J.; Fatima, H.; Halstead, N.T.; Liriano, J.; McMahon, T.A.; Ortega, N.C.; Saucer, E.J.; Sehgal, T.; Young, S.; et al. Biodiversity Inhibits Parasites: Broad Evidence for Dilution Effect. Proc. Natl. Acad. Sci. USA 2015, 112, 8867–8871. [Google Scholar] [CrossRef]
- Allan, B.F.; Langerhans, R.B.; Ryberg, W.A.; Landesman, W.J.; Griffin, N.W.; Katz, R.S.; Oberle, B.J.; Schutzenhofer, M.R.; Smyth, K.N.; de St Maurice, A.; et al. Ecological Correlates of Risk and Incidence of West Nile Virus in the United States. Oecologia 2009, 158, 699–708. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Godsey, M.S.; King, R.J.; Guptill, S.C. Avian Diversity and West Nile Virus: Testing Associations between Biodiversity and Infectious Disease Risk. Proc. Biol. Sci. 2006, 273, 109–117. [Google Scholar] [CrossRef]
- Swaddle, J.P.; Calos, S.E. Increased Avian Diversity is Associated with Lower Incidence of Human West Nile Infection: Observation of the Dilution Effect. PLoS ONE 2008, 3, e2488. [Google Scholar] [CrossRef]
- Suzan, G.; Marce, E.; Giermakowski, J.T.; Mills, J.N.; Ceballos, G.; Ostfeld, R.S.; Armien, B.; Pascale, J.M.; Yates, T.L. Experimental Evidence for Reduced Rodent Diversity Causing Increased Hantavirus Prevalence. PLoS ONE 2009, 4, e5461. [Google Scholar] [CrossRef]
- Rupasinghe, R.; Chomel, B.B.; Martinez-Lopez, B. Climate Change and Zoonoses: A Review of the Current Status, Knowledge Gaps, and Future Trends. Acta Trop. 2022, 226, 106225. [Google Scholar] [CrossRef] [PubMed]
- Tajudeen, Y.A.; Oladunjoye, I.O. Climate Change—An Emblematic Driver of Vector-Borne Diseases: Holistic View as A Way Forward. Glob. Biosecurity 2021, 3. [Google Scholar] [CrossRef]
- Ari, T.B.; Gershunov, A.; Gage, K.L.; Snäll, T.; Ettestad, P.; Kausrud, K.L.; Stenseth, N.C. Human Plague in the USA: The Importance of Regional and Local Climate. Biol. Lett. 2008, 4, 737–740. [Google Scholar] [CrossRef]
- Anyamba, A.; Chretien, J.P.; Britch, S.C.; Soebiyanto, R.P.; Small, J.L.; Jepsen, R.; Forshey, B.M.; Sanchez, J.L.; Smith, R.D.; Harris, R.; et al. Global Disease Outbreaks Associated with the 2015–2016 El-Niño Event. Sci. Rep. 2019, 9, 1930. [Google Scholar] [CrossRef] [PubMed]
- Anyamba, A.; Chretien, J.P.; Small, J.; Tucker, C.J.; Formenty, P.B.; Richardson, J.H.; Britch, S.C.; Schnabel, D.C.; Erickson, R.L.; Linthicum, K.J. Prediction of A Rift Valley Fever Outbreak. Proc. Natl. Acad. Sci. USA 2009, 106, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Hjelle, B.; Glass, G.E. Outbreak of Hantavirus Infection in the Four Corners Region of The United States in The Wake of 1997–1998 El Niño-Southern Oscillation. J. Infect. Dis. 2000, 181, 1569–1573. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; World Health Organization: Geneva, Switzerland, 2009.
- Summary for Policymakers. In Climate Change 2021: The Physical Science Basis: Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 3–22. [Google Scholar]
- Morens, D.M.; Fauci, A.S. Emerging Infectious Diseases: Threats to Human Health and Global Stability. PLoS Pathog. 2013, 9, e1003467. [Google Scholar] [CrossRef]
- Brian, H.B.; Jonna, A.K.M. Detection of Emerging Zoonotic Pathogens: An Integrated One Health Approach. Annu. Rev. Anim. Biosci. 2018, 15, 121–139. [Google Scholar] [CrossRef]
- Machovina, B.; Feeley, K.J.; Ripple, W.J. Biodiversity Conservation: The Key is Reducing Meat Consumption. Sci. Total Environ. 2015, 536, 419–431. [Google Scholar] [CrossRef]
- Croser, E.L.; Marsh, G.A. The Changing Face of The Henipaviruses. Vet. Microbiol. 2013, 167, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; He, B.; Huang, S.Y.; Wei, F.; Zhu, X.Q. Severe Fever with Thrombocytopenia Syndrome, An Emerging Tick-Borne Zoonosis. Lancet Infect. Dis. 2014, 14, 763–772. [Google Scholar] [CrossRef]
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent Insights into Emerging Coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.J.; Grais, R.F.; Bharti, N.; Conlan, A.J.K.; Bjørnstad, O.N.; Wolfson, L.J.; Guerin, P.J.; Djibo, A.; Grenfell, B.T. The Dynamics of Measles in Sub-Saharan Africa. Nature 2008, 451, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.J.; Djibo, A.; Grais, R.F.; Bharti, N.; Grenfell, B.T.; Bjørnstad, O.N. Rural-Urban Gradient in Seasonal Forcing of Measles Transmission in Niger. Proc. Biol. Sci. 2010, 277, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Grenfell, B.T.; Bjørnstad, O.N.; Kappey, J. Travelling Waves and Spatial Hierarchies in Measles Epidemics. Nature 2001, 414, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Arthur, R.F.; Gurley, E.S.; Salje, H.; Bloomfield, L.S.; Jones, J.H. Contact Structure, Mobility, Environmental Impact and Behaviour: The Importance of Social Forces to Infectious Disease Dynamics and Disease Ecology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160454. [Google Scholar] [CrossRef]
- Padmanabha, H.; Fabio, C.; Camilo, R.; Andres, B.; Salua, O.; Jairo, M.; James, H.J.; Diuk-Wasser, M.A. Human Social Behavior and Demography Drive Patterns of Fine-Scale Dengue Transmission in Endemic Areas of Colombia. PLoS ONE 2015, 10, e0144451. [Google Scholar] [CrossRef]
- Silk, M.J.; Fefferman, N.H. The Role of Social Structure and Dynamics in the Maintenance of Endemic Disease. Behav. Ecol. Sociobiol. 2021, 75, 122. [Google Scholar] [CrossRef]
- Stoddard, S.T.; Morrison, A.C.; Vazquez-Prokopec, G.M.; Paz Soldan, V.; Kochel, T.J.; Kitron, U.; Elder, J.P.; Scott, T.W. The Role of Human Movement in the Transmission of Vector-Borne Pathogens. PLoS Negl. Trop. Dis. 2009, 3, e481. [Google Scholar] [CrossRef]
- Nielsen, M.R.; Meilby, H.; Smith-Hall, C.; Pouliot, M.; Treue, T. The Importance of Wild Meat in the Global South. Ecol. Economics. 2018, 146, 696–705. [Google Scholar] [CrossRef]
- Hilderink, M.H.; de Winter, I.I. No Need to Beat around the Bushmeat—The Role of Wildlife Trade and Conservation Initiatives in the Emergence of Zoonotic Diseases. Heliyon 2021, 7, e07692. [Google Scholar] [CrossRef] [PubMed]
- Lauren, C.; Jasmin, W.; Fiona, M.; Stephan, F.; Hunter, D.; Julia, E.F.; Juanita, G.; Yuhan, L.; Lola, N.; Evi, P.; et al. Impacts of Taking, Trade and Consumption of Terrestrial Migratory Species for Wild Meat; Secretariat of the Convention on Migratory Species (CMS): Bonn, Germany, 2021. [Google Scholar]
- Swift, L.; Hunter, P.R.; Lees, A.C.; Bell, D.J. Wildlife Trade and the Emergence of Infectious Diseases. EcoHealth 2007, 4, 25. [Google Scholar] [CrossRef]
- Magouras, I.; Brookes, V.J.; Jori, F.; Martin, A.; Pfeiffer, D.U.; Dürr, S. Emerging Zoonotic Diseases: Should We Rethink the Animal–Human Interface? Front. Vet. Sci. 2020, 7, 582743. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; Wan, X.T.; Jin, Y.H.; Zhang, W. Concept of scientific wildlife conservation and its dissemination. Zool. Res. 2016, 37, 270–274. [Google Scholar] [CrossRef]
- Whitmee, S.; Haines, A.; Beyrer, C.; Boltz, F.; Capon, A.G.; de Souza Dias, B.F.; Ezeh, A.; Frumkin, H.; Gong, P.; Head, P.; et al. Safeguarding human health in the Anthropocene epoch: Report of the Rockefeller Foundation-Lancet Commission on Planetary health. Lancet 2015, 386, 1973–2028. [Google Scholar] [CrossRef]
- Pettan-Brewer, C.; Martins, A.F.; de Abreu, D.P.B.; Brandão, A.P.D.; Barbosa, D.S.; Figueroa, D.P.; Cediel, N.; Kahn, L.H.; Brandespim, D.F.; Velasquez, J.C.C.; et al. From the Approach to the Concept: One Health in Latin America-Experiences and Perspectives in Brazil, Chile, and Colombia. Front. Public Health 2021, 9, 687110. [Google Scholar] [CrossRef]
- Tajudeen, Y.A.; Oladunjoye, I.O.; Mustapha, M.O.; Mustapha, S.T.; Ajide-Bamigboye, N.T. Tackling the Global Health Threat of Arboviruses: An Appraisal of the Three Holistic Approaches to Health. Health Promot. Perspect. 2021, 11, 371–381. [Google Scholar] [CrossRef]
- Pongsiri, M.J.; Bickersteth, S.; Colón, C.; DeFries, R.; Dhaliwal, M.; Georgeson, L.; Haines, A.; Linou, N.; Murray, V.; Naeem, S.; et al. Planetary Health: From Concept to Decisive Action. Lancet Planet. Health 2019, 3, 402–404. [Google Scholar] [CrossRef]
- Baquero, O.S.; Benavidez Fernández, M.N.; Acero Aguilar, M. From Modern Planetary Health to Decolonial Promotion of One Health of Peripheries. Front. Public Health 2021, 9, 637897. [Google Scholar] [CrossRef]
- Brooks, D.; Hoberg, E.; Boeger, W. The Stockholm Paradigm: Climate Change and Emerging Disease; University of Chicago Press: Chicago, IL, USA, 2019; p. 400. [Google Scholar]
- Purse, B.V.; Mellor, P.S.; Rogers, D.J.; Samuel, A.R.; Mertens, P.P.C.; Baylis, M. Climate Change and the Recent Emergence of Bluetongue in Europe. Nat. Rev. Microbiol. 2005, 3, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.H.; Shaw, G.M.; De Cock, K.M.; Sharp, P.M. AIDS as A Zoonosis: Scientific and Public Health Implications. Science 2000, 287, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Preventing Emerging Infectious Diseases: A Strategy for the 21st Century. Overview of the Updated CDC Plan. MMWR Recomm. Rep. 1998, 47, 1–14. [Google Scholar]
- Aronson, D. UN Environment Programme. Unite Human, Animal and Environmental Health to Prevent the Next Pandemic–UN Report. Available online: https://www.unep.org/news-and-stories/press-release/unite-human-animal-and-environmental-health-prevent-next-pandemic-un (accessed on 27 April 2022).
- Lee, V.J.; Aguilera, X.; Heymann, D.; Wilder-Smith, A.; Bausch, D.G.; Briand, S.; Bruschke, C.; Carmo, E.H.; Cleghorn, S.; Dandona, L.; et al. Preparedness for emerging epidemic threats: A Lancet Infectious Diseases Commission. Lancet Infect. Dis. 2020, 20, 17–19. [Google Scholar] [CrossRef]
- IPBES Workshop Report on Biodiversity and Pandemics of the Intergovernmental Platform on Biodiversity and Ecosystem Services; Daszak, P., das Neves, C., Amuasi, J., Hayman, D., Kuiken, T., Roche, B., Zambrana-Torrelio, C., Buss, P., Dundarova, H., Feferholtz, Y., et al., Eds.; IPBES Secretariat: Bonn, Germany, 2020. [Google Scholar]
- Rossi, J.; Garner, S.A. Industrial Farm Animal Production: A Comprehensive Moral Critique. J. Agric. Environ. Ethics. 2014, 27, 479–522. [Google Scholar] [CrossRef]
- Wegner, G.I.; Murray, K.A.; Springmann, M.; Muller, A.; Sokolow, S.H.; Saylors, K.; Morens, D.M. Averting Wildlife-Borne Infectious Disease Epidemics Requires a Focus on Socio-Ecological Drivers and A Redesign of the Global Food System. EClinicalMedicine 2022, 47, 101386. [Google Scholar] [CrossRef] [PubMed]
- Kutz, S.; Tomaselli, M. “Two-eyed Seeing” Supports Wildlife Health. Science 2019, 364, 1135–1137. [Google Scholar] [CrossRef]
- Prescott, S.L.; Logan, A.C. Planetary Health: From the Wellspring of Holistic Medicine to Personal and Public Health Imperative. Explore 2019, 15, 98–106. [Google Scholar] [CrossRef]
- Buse, C.G.; Gislason, M.; Reynolds, A.; Ziolo, M. Enough Tough Talk! It’s Time for the Tough Action(s) to Promote Local to Global Planetary Health. Int. J. Health Promot. Education 2021, 59, 271–275. [Google Scholar] [CrossRef]
- Drivers of Zoonotic Diseases. In Sustaining Global Surveillance and Response to Emerging Zoonotic Diseases; Keusch, G.T., Pappaioanou, M., Gonzalez, M.C., Scott, K.A., Tsai, P., Eds.; National Academies Press: Washington, DC, USA, 2009; pp. 77–114. [Google Scholar]
- Milbank, C.; Vira, B. Wildmeat Consumption and Zoonotic Spillover: Contextualizing Disease Emergence and Policy Responses. Lancet Planet. Health 2022, 6, e439–e448. [Google Scholar] [CrossRef]
- Tasker, A.; Braam, D. Positioning Zoonotic Disease Research in Forced Migration: A Systematic Literature Review of Theoretical Frameworks and Approaches. PLoS ONE 2021, 16, e025474. [Google Scholar] [CrossRef] [PubMed]
- Behera, M.R.; Behera, D.; Satpathy, S.K. Planetary Health and the Role of Community Health Workers. J. Fam. Med. Prim. Care 2020, 9, 3183–3188. [Google Scholar] [CrossRef] [PubMed]
- Tajudeen, Y.A.; Oladipo, H.J.; Yusuf, R.O.; Oladunjoye, I.O.; Adebayo, A.O.; Ahmed, A.F.; El-Sherbini, M.S. The Need to Prioritize Prevention of Viral Spillover in the Anthropopandemicene: A Message to Global Health Researchers and Policymakers. Challenges 2022, 13, 35. [Google Scholar] [CrossRef]
- Tajudeen, Y.A.; Oladunjoye, I.O.; Bajinka, O.; Oladipo, H.J. Zoonotic Spillover in an Era of Rapid Deforestation of Tropical Areas and Unprecedented Wildlife Trafficking: Into the Wild. Challenges 2022, 13, 41. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajudeen, Y.A.; Oladipo, H.J.; Oladunjoye, I.O.; Mustapha, M.O.; Mustapha, S.T.; Abdullahi, A.A.; Yusuf, R.O.; Abimbola, S.O.; Adebayo, A.O.; Ikebuaso, J.G.; et al. Preventing the Next Pandemic through a Planetary Health Approach: A Focus on Key Drivers of Zoonosis. Challenges 2022, 13, 50. https://doi.org/10.3390/challe13020050
Tajudeen YA, Oladipo HJ, Oladunjoye IO, Mustapha MO, Mustapha ST, Abdullahi AA, Yusuf RO, Abimbola SO, Adebayo AO, Ikebuaso JG, et al. Preventing the Next Pandemic through a Planetary Health Approach: A Focus on Key Drivers of Zoonosis. Challenges. 2022; 13(2):50. https://doi.org/10.3390/challe13020050
Chicago/Turabian StyleTajudeen, Yusuf Amuda, Habeebullah Jayeola Oladipo, Iyiola Olatunji Oladunjoye, Mutiat Oluwakemi Mustapha, Sheriff Taye Mustapha, Adam Aberi Abdullahi, Rashidat Onyinoyi Yusuf, Samuel Olushola Abimbola, Aminat Olaitan Adebayo, Joy Ginika Ikebuaso, and et al. 2022. "Preventing the Next Pandemic through a Planetary Health Approach: A Focus on Key Drivers of Zoonosis" Challenges 13, no. 2: 50. https://doi.org/10.3390/challe13020050
APA StyleTajudeen, Y. A., Oladipo, H. J., Oladunjoye, I. O., Mustapha, M. O., Mustapha, S. T., Abdullahi, A. A., Yusuf, R. O., Abimbola, S. O., Adebayo, A. O., Ikebuaso, J. G., Adesuyi, D. S., Okereke, B., Omotosho, A. O., Ahmed, A. F., & El-Sherbini, M. S. (2022). Preventing the Next Pandemic through a Planetary Health Approach: A Focus on Key Drivers of Zoonosis. Challenges, 13(2), 50. https://doi.org/10.3390/challe13020050





