Abstract
Newborns defined as being of “low birth weight” (LBW) or “small for gestational age” (SGA) are global health issues of concern because they are vulnerable to mortality and morbidity. Prenatal exposures may contribute to LBW/SGA. In this review, we searched peer-reviewed scientific literature to determine what location-based hazards have been linked with LBW/SGA in the industrialized nations of Canada and the USA. After selecting studies based on inclusion/exclusion criteria, we entered relevant details in to an evidence table. We classified and summarized 159 articles based on type of environment (built = 108, natural = 10, and social = 41) and general category of environmental variables studied (e.g., air pollution, chemical, water contamination, waste site, agriculture, vegetation, race, SES, etc.). We linked the geographic study areas by province/state to political boundaries in a GIS to map the distributions and frequencies of the studies. We compared them to maps of LBW percentages and ubiquitous environmental hazards, including land use, industrial activity and air pollution. More studies had been completed in USA states than Canadian provinces, but the number has been increasing in both countries from 1992 to 2018. Our geographic inquiry demonstrated a novel, spatially-focused review framework to promote understanding of the human ‘habitat’ of shared environmental exposures that have been associated with LBW/SGA.
1. Introduction
An underlying premise of environmental health and epidemiology involves place—where one lives and where one starts out in life, even during in utero development, ultimately determines lifelong health [1,2]. The embryo and fetus are susceptible to toxicant exposure and other environmental influences on the mother during crucial stages of pregnancy [3,4,5,6], which may lead to babies being born too small, or too early. Because they are important markers of infant survival, development, and future health, newborns that are too small are a serious source of emotional and economic stress on society—hundreds of millions of dollars are spent on specialized equipment and treatments within the first several years of life [7,8]. The Barker hypothesis [9] evolved from studies on low birth weight (as well as premature birth and intrauterine growth restriction) that found significant associations with adult hypertension, coronary heart disease, and non-insulin-dependent diabetes [10,11,12]. The suspected exposures associated with these birth outcomes are widespread, thus heightening the importance of early life health impacts.
The World Health Organization identifies babies born too small as an issue of global health concern, and one that is to be monitored under Sustainable Developmental Goal (SDG) 3 to “ensure healthy lives and promote wellbeing for all at all ages” (www.who.int/sdg/targets). The definitions include:
- Small for gestational age (SGA), which are infants born with a birth weight <10th percentile of a reference population for sex-based gestational age (22 to 42 weeks gestation); and
- Low birth weight at term (LBWT), which are infants born with a birth weight <2500 g, and may or may not be at full term (37–42 weeks gestation) [13,14,15]. Figure 1 graphically defines SGA and LBW at term.
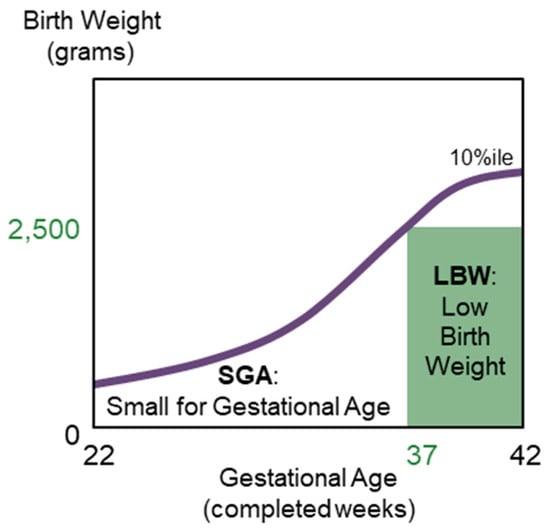 Figure 1. The set of birth weight–for–gestational age standards below the 10th percentile birth weights describes small for gestational age (SGA) in the purple curve; low birth weight at term (LBWT) is a subset of SGA in the green shaded rectangle.
Figure 1. The set of birth weight–for–gestational age standards below the 10th percentile birth weights describes small for gestational age (SGA) in the purple curve; low birth weight at term (LBWT) is a subset of SGA in the green shaded rectangle.
SGA and LBWT are not homogeneous pregnancy outcomes because they may consist of both infants born too early (known as preterm birth) or too small, (typically due to fetal growth restriction) [13,16]. The etiologies are multifactorial, where the most important maternal risk factors are tobacco smoking, nutrition, pre-pregnancy weight, ethnic origin, short maternal stature, and pre-existing health conditions [16,17,18,19]. Other risks include genetic and constitutional, demographic and psychosocial (e.g., socioeconomic status (SES) and stress), obstetric, antenatal care, and toxic exposures.
Globally, the rate of SGA in low- and middle-income countries is around 27% of all live births (varying between 1.2% to 41.5% in Sahelian countries of Africa and south Asia): in 2010, 32.4 million babies were SGA [20]. LBW (all gestational ages) occurred in 15% of all births, mostly in low- and middle-income countries (mostly south Asia) [21]. Of 18 million low-birthweight babies, 10.6 million were born at term. In the United States of America (USA) in 2005, SGA was 10% [22] and LBW was 8.2% [23]. In Canada in 2005, SGA was 8.4% [24] and LBW was 6.0% [25]. Although Canada is lower than the world and U.S., disorders related to short gestation and low birth weight are consistently ranked 2nd out of the 71 leading causes of infant death [26], and their prevalence has been increasing since 2000 [24].
Figure 2 shows the geographic distribution of LBW by Canadian province and U.S. state for the years 2005 and 2016 (values for SGA unavailable). The above nationwide 2005 statistics are relevant for Figure 2a, where it can be observed that Alberta (AB), Ontario (ON), and Nunavut (NU) are higher than Canada overall, and the majority of the southern and eastern states (n = 27) are higher than USA overall.
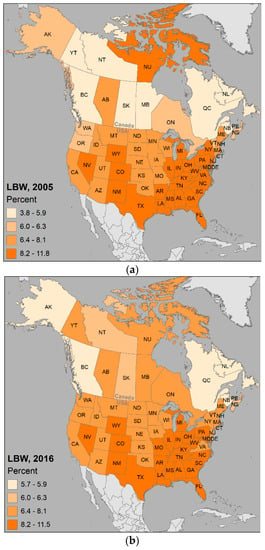
Figure 2.
Percentage births considered low birth weight (LBW (all gestational ages)) in Canada and the USA for: (a) the year 2005; (b) the year 2016.
Given that many areas are close to or exceeding the overall national percentages, and are increasing over time as indicated by the higher number of provinces and states above 6.4 % in Figure 2, it is valuable from a public health perspective to understand the patterns and processes involved in being born too small.
SGA/LBW and their association with the environment necessitate an interdisciplinary research approach with integration of knowledge from medicine and geography. Medical geography is a holistic investigation of health using concepts and methodologies from geography, which also encompasses the social, physical, and biological sciences [27].
Informed by the earlier work of May—who stated that to understand disease as a biological expression of maladjustment, an ecological (i.e., ecosystem-based) study must involve the environment, the host, and the culture [28]—Meade proposed the triangle of human ecology as the framework for the state of human health [27,29]. Meade’s vertices are therefore anchored to:
- Habitat—the natural, social, and built environments where people live.
- Population—people (hosts) as biological organisms structured by age, gender, and genetics.
- Behavior—visible part of culture including beliefs, social organization, and technology.
These three points influence each other and the state of health, as can be seen when modelling and summarizing what is known about neonatal outcomes and maternal exposure to outdoor pollution (Figure 3). The primary population consists of pregnant mothers and their defining individual characteristics of varied ages, pre-existing health conditions and genetic makeup, with the location of where they live and work depending on their social and economic behaviors (i.e., nutritional status, access to quality health services). More research is needed that focuses on the lesser-studied habitat vertex, more specifically, the outdoor environment, since much less attention has been given to integrating ecological factors for understanding disease [27]. The location aspect of habitat (i.e., geography)—where mothers live, where industry and services are situated, where demographic groups congregate, and for many scales—is important to clinicians and specialists in environmental health, and to exposure assessment, epidemiologists, biostatisticians, and health analysts.
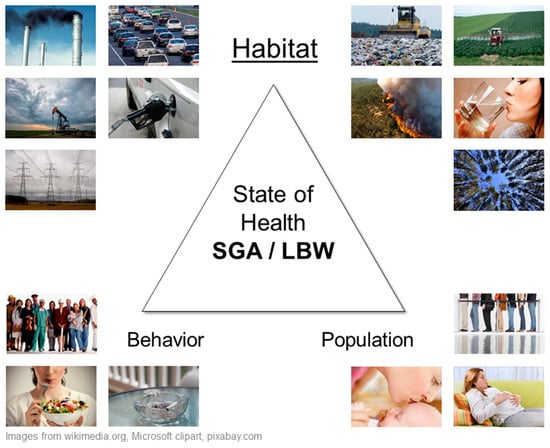
Figure 3.
Meade’s triangle of human ecology for maternal exposures and small for gestational age (SGA) and low birth weight (LBW).
Geography and environmental health are inextricably linked. Environmental health, as defined by the World Health Organization, “comprises those aspects of human health and disease that are determined by factors in the environment, and includes both the direct pathological effects of chemicals, radiation and some biological agents, and the effects (often indirect) on health and wellbeing of the broad physical, psychological, social and aesthetic environment, which includes housing, urban development, land use and transport” [30]. Environmental human health is implicit in the all-encompassing planetary health, “formally defined by the in vivo Planetary Health network as the interdependent vitality of all natural and anthropogenic ecosystems (social, political and otherwise)” [31,32]. These concepts are not new—Hippocrates, the father of medicine, c. 460–c. 370 BC, understood the important interconnections of environment and health, in his “Airs, Waters, and Places” [33]. Hazards in those airs, waters, and places comprise the chemical, physical, and biological aspects that insult human health [27]. Many hazards have been known for centuries (e.g., lead, radiation, microorganisms), but they are only effective in altering health if an individual is exposed to them.
Exposure is the occurrence of a person coming into contact (via air, water, or skin) with a dose (requisite amount) of a toxicant (substance that produces a health effect) and may be isolated, repeated, or continual [34]. The health outcome can only occur if a person is exposed to the integral dose of a hazard for the crucial amount of time. These ideas are directly applicable to being born too small; the system can be simplified as follows:
Hazard (environment) → Exposure (prenatal) → Outcome (SGA/LBW)
The measure of the total environmental exposures of an individual in a lifetime, and how those exposures relate to health, contribute to the human exposome. Evaluating the impact of the exposome is a concept of planetary health, and illuminating the exposures may contribute to understanding disease prevention [32]. This interdependence between human health and place brings us full-circle to early-life location-based exposures on pregnant mothers that may lead to really small newborns.
Mechanisms that trigger adverse birth outcomes, such as being born too small, among mothers exposed to hazards and pollutants are not well understood, but are suspected to include inflammation, direct toxic effects on the placenta and the fetus, interruption of oxygen-hemoglobin interaction, and damage to DNA [35,36,37]. Environmental associations differ among SGA and LBW, enhanced by temporal variations in exposures, personal characteristics (mothers’ health, nutrition, and demographics) and external factors such as region and socioeconomic status (SES), [3,4,38].
Reviewing the published literature allows us to identify where information gaps exist, and also to determine whether the prevalence of the problem matches the number of existing published studies. This review serves to highlight environmental hazards, specifically, the shared exposures of the outdoor environment that have been associated with LBW and/or SGA newborns in Canada and the USA. Mapping the results will characterize where and how much LBW/SGA has been studied in the majority of industrialized North America and what and where the environmental factors are found to be important. The interested reader may use the maps as guides to what and where potential research gaps warrant further medical geographic inquiry.
2. Methods
2.1. Data Sources
Following the methodology proposed by Arksey and O’Malley [39], we searched bibliographic databases (PubMed, Web of Science, Scopus, Google Scholar, Taylor and Francis, and environmental health journal websites) to identify English-language, peer-reviewed, original research articles on outdoor environment and really small newborns. The Venn diagram in Figure 4 displays the search keywords that were used for the health outcome: (low) birth weight, small for gestational age; environmental variable: air pollution, agriculture (herbicide, pesticide, fertilizer), lead, mine, natural gas, road, traffic, (power) transmission, waste, water (contamination), socioeconomic, greenness; and any geographic extent within Canada or the USA (we read titles, abstracts and methods sections to ascertain the study country). We limited the study years to between 1990 (geographic-type analyses were rare prior) and 2018 (current year).
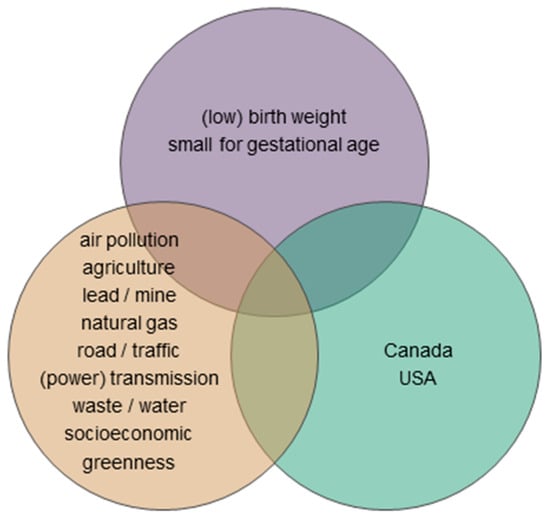
Figure 4.
The keywords used in the literature search for studies associating outdoor environment and really small newborns are grouped by topic: health outcome (top), environmental variable (left), and geographic extent (right).
2.2. Study Selection and Data Extraction
We entered the articles with abstracts including both ABO and any environmental variable keywords in to Mendeley reference manager (www.mendeley.com), and tagged to identify 1 = North American and 2 = ABO. We read full articles that met the inclusion/exclusion criteria—must be Canada/USA, LBW/birth weight/SGA, and outdoor environment—and extracted the following data to a spreadsheet, formatted as the evidence table: year; study identifier; health outcome; detailed variable(s); and geography. To aid in mapping, we standardized the geography to the province or state level using the abbreviations shown in Appendix A (Table A1), regardless of whether the study was in a city, county/region, or larger administrative unit. We classified the variables in to general categories similar to the keywords, and then further generalized the environment as built, social, natural, or none. We summarized frequency statistics for the various studies. Then, we replicated records where there was more than one state or province involved in the study (e.g., a study on BC, Alberta, Manitoba, and Ontario [40] was copied to four rows in the table, one for each province) and generated a pivot table for each category or environment so that we could reliably map these for all locations.
2.3. Mapping
Using ArcGIS 10.6 [41], we joined the pivot table to the map of political boundaries provided by the Commission for Environmental Cooperation (CEC) [42] and created choropleth maps using four categories for the number of studies from all the selected articles—1, 2, 3, and 4 or more—labeled hereafter as frequency maps. We also mapped land use, pollution release transfer reporting (PRTR) industrial facilities [42], and satellite-based particulate matter [43]. To identify future research opportunities, these maps are compared with the 2005 and 2016 LBW percentages in Figure 2. Similarly, we visualized the frequency of studies on the built, natural, social environment, as well as those for studies related to air pollution, agriculture, chemical, vegetation, and individual factors.
3. Results and Discussion
The number of articles we selected for inclusion are documented in Figure 5. From the 159 included studies, associations were examined for built (n = 108), natural (n = 10), and social (n = 41) environmental variables.
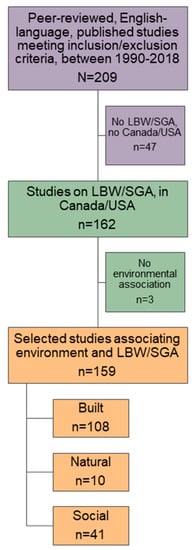
Figure 5.
Flow diagram documenting the selection of published studies within Canada and the USA, between 1990 and 2018, for examining associations of the outdoor environment with low birth weight (LBW), birth weight (BW), and small for gestational age (SGA).
3.1. Outcomes and Variables
Table A2 lists all 159 studies selected for inclusion. The environmental hazards were identified as the following general categories of variables (from most-to-least frequent): air pollution (n = 53), SES (n = 17), chemical (n = 16), race (n = 11), individual (n = 10), water contamination (n = 9), waste site (n = 8), vegetation (n = 8), agriculture (n = 6), roads (n = 3), urban-rural (n = 3), food (n = 2), mining (n = 2), neighborhood (n = 2), weather (n = 2), immigration (n = 2), alcohol (n = 1), noise (n = 1), power (n = 1), transmission lines (n = 1), health care (n = 1). Note that we also included articles that studied birth weight (BW; n = 38) and intrauterine growth restriction (IUGR; n = 4) because they are interrelated with LBW (n = 72) and SGA (n = 27). There were also studies on both LBW and SGA (n = 18). Figure 6 shows how published research has increased over time from 1992 to 2018, with a peak in the year 2012. Individual states (n = 110) had more studies than Canadian provinces (n = 32), while all of USA (n = 8) and all of Canada (n = 8) were equal, with one study that included both countries.
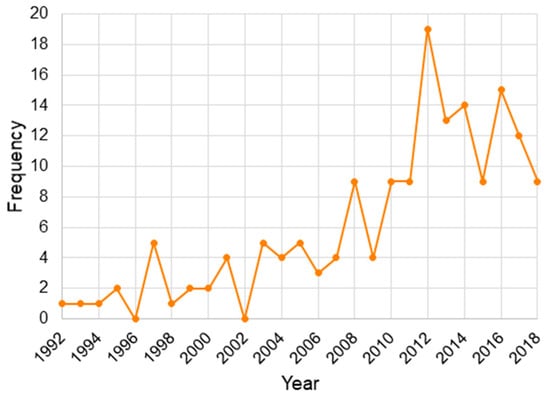
Figure 6.
Yearly distribution of the selected studies, published on associations of the outdoor environment with low birth weight (LBW), birth weight, and small for gestational age (SGA), within Canada and the USA.
3.2. Spatial Associations
The following maps summarize findings from the included studies. Figure 7 maps locations and frequencies of the selected studies across North America; the distribution shows that LBW/SGA research has been conducted in six provinces and 41 states. Upon visually comparing Figure 7 with Figure 2 percentages, we observe that, despite the efforts, there are many regions with LBW and very low numbers of studies on the topic.
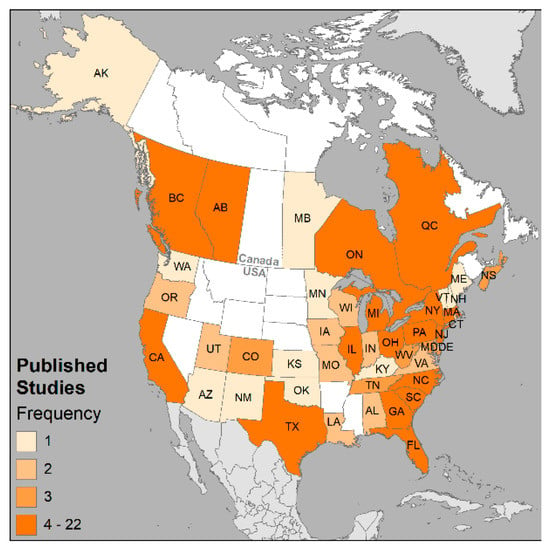
Figure 7.
Geographic distribution and number of studies in provinces/states for the 159 candidate studies across Canada and the USA. Frequency classes standardized across all maps to intuit where the health issue is of interest (1), emerging (2), concern (3), or potential problem (4 or more).
The distributions of the types of environment (built, natural, and social) are shown in Figure 8. Figure 9 displays the most frequently studied categories.
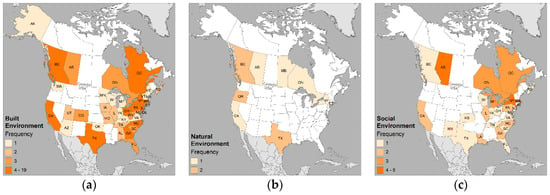
Figure 8.
Geographic distribution of published studies by environment: (a) built; (b) natural; and (c) social. Frequency classes standardized across all maps to intuit where the health issue is of interest (1), emerging (2), concern (3), or potential problem (4 or more).
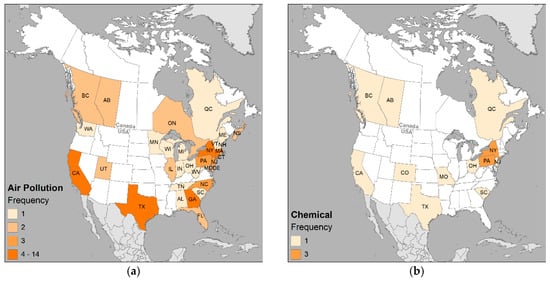
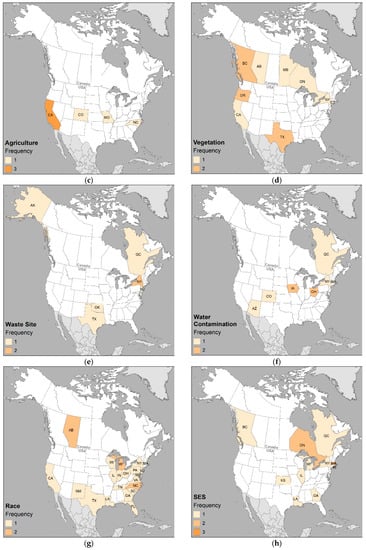
Figure 9.
Geographic distribution of the most frequently published categories of (a) air pollution; (b) chemical; (c) agriculture; (d) vegetation; (e) waste site; (f) water contamination; (g) race; and (h) SES. Frequency classes standardized across all maps to intuit where the health issue is of interest (1), emerging (2), concern (3), or potential problem (4 or more).
For comparison purposes, the major land use classes, industrial facilities, and particulate matter distributions are mapped in Figure 10. Visual assessment highlights that the states and provinces having higher percentages of LBW in Figure 2 coincide with the same areas having relatively more proportions of urban, agriculture, industry, and PM2.5. Inspection of the distribution of studies in Figure 7 through Figure 10 with Figure 4 shows there are clearly areas requiring future research, especially Canada’s northern territories and the states bordering the Mississippi River.
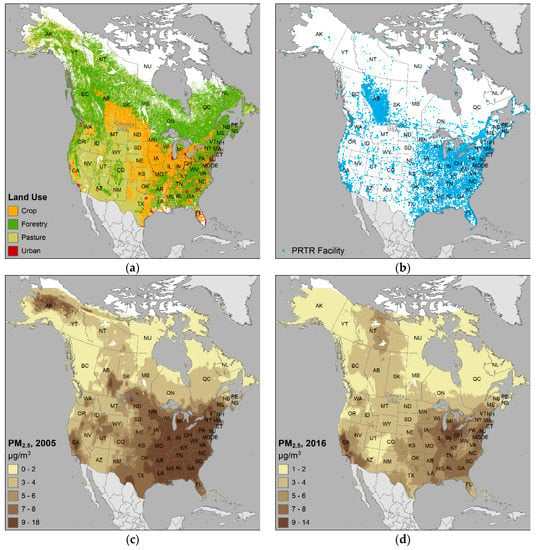
Figure 10.
Selected environmental variables of interest in the SGA/LBW studies for Canada and USA of: (a) land use classes; (b) industrial facilities in pollutant release transfer reporting (PRTR); (c) common air pollutant – particulate matter particles with aerodynamic diameter ≤ 2.5 μm, (PM2.5) for 2005; and (d) PM2.5 for 2016.
3.3. Environmental Variables
The cumulative evidence suggested associations among outdoor environmental hazards and LBW/SGA in Canada and the USA. Most of the studies found that LBW/SGA varied with air pollution gases and/or particles depending on the trimester/gestation. Anthropogenic air pollution originates from industrial/traffic emissions and includes gaseous components—sulfur dioxide (SO2), carbon monoxide (CO), nitrogen oxide (NO), nitrogen dioxide (NO2), ozone (O3)—and particulate matter (PM)—PM2.5 particles with aerodynamic diameter ≤ 2.5 μm and PM10 particles ≤ 10 μm. Electromagnetic frequencies from powerlines was not found to be important, nor was proximity to gas stations, but proximity to roads and waste sites were. The strength of association in the studies varied greatly and had limitations due to sampling, spatial resolution, availability of confounding factors, and inability to quantify duration and intensity of exposures.
Many of the previous studies linked individual or small subsets of factors; however, all factors can be modelled as vertices of the triangle of human ecology, synthesizing the complex disease ecology and advancing hypotheses [27]. As Table A2 exemplifies, the majority of air pollutants under investigation consisted of traffic-related air contaminants. A handful of studies targeted agricultural activities, heavy metals and/or industrial activities. More research is needed on assessing the spatial relationships of the actual chemicals involved in those industrial activities, especially the known or suspected developmental toxicants. Similarly, the combined effect of multipollutant exposures are still relatively unknown. Water contamination was another challenging variable, and King et al. [44] stressed the importance of household rather than distribution system sampling, making it difficult to efficiently study at a population level. Socioeconomic inequalities in LBW showed strong associations, and was larger in the United States than Canada, likely due to differing health care systems [45].
3.4. Exposure Assessment
Note that only English-language, peer-reviewed journal articles were selected; other literature sources have not been included here. Missing publications in other languages causes a conceptual bias, as they contribute to the overall understanding of birth weight and the environment; here, the geographic attention provides an up-to-date review on the predominantly English-publishing countries of Canada and the USA. The focus on shared sources of exposures from the outdoor environment allowed the researchers to incorporate spatial methods (i.e., GIS) in their studies, which was advantageous, especially because they facilitated several steps in exposure assessment [46]. GIS can define epidemiologic study populations, identify source and potential routes of exposure, estimate environmental levels of target contaminants, and estimate personal exposure. The studies reviewed here applied the spatial methods of coincidence, proximity, and surface predictions to identify and estimate exposures at different scales. Postal code/zip code and county-level geography was helpful for understanding broad population patterns, but it will be worthwhile for future studies to analyze all scales with greater detail. Woodruff et al. [47] hypothesized that geographic scale was important in adverse birth outcome studies, proposing that smaller scales are useful to better understand biological mechanisms and apply to local policies, and larger scales are useful to look at population-level factors and apply to regional policy. For many of the studies, the proximity measures would benefit from increased resolution as well. An increasing number of studies are incorporating land-use regression modelling, a promising method for advancing the knowledge of exposures assessment. Analyses should also more fully integrate the socioeconomic and maternal/paternal factors, improve methods for quantifying duration and intensity of exposure, and adjust for residential mobility [35,48,49,50]. As previous non-spatial reviews have also stated, biological mechanisms still remain to be fully understood.
3.5. Protective Variables
Overall, the studies contribute to the evolving evidence that maternal exposure during pregnancy to varying levels of ambient air pollutants is associated with LBW/SGA. An interesting finding is the increase in studies on protective exposures, such as greenness—natural environments promote resiliency and prevent disease, further supporting the concept of planetary health.
4. Conclusions
We compiled previous spatial research on the outdoor environment and really small newborns, and through the use of maps, we presented the parameters that help with understanding how important the ambient environment is and the correspondingly valuable question of location. Such a spatially-focused review, to our knowledge, has not been seen in the literature, and we hope we have provided a useful framework for other countries to better understand environmental associations with the important global health issue of LBW and SGA newborns. North American researchers may consult these maps to aid in understanding their particular study areas.
It is hoped that our review and maps may assist healthcare professionals, in Hippocrates-style, by providing them with what location-based variables may be associated with their patients’ health issues, as well as informing the public that where they live is as important to their current and future family health as what they eat and do. Our focus on environmental associations was not able to account for nutrition, maternal health, or occupation, but those studies conversely rarely accounted for outdoor exposures. Each contributes pieces to the exposome puzzle. Medical researchers are provided with more motivation for studying which components of outdoor environmental exposures may cause reduction in neonatal weight, a condition that, if prevented, will diminish future adverse health, such as adult cardiac disease, diabetes, and other non-communicable diseases that require a strong healthy start in life. Policy makers and planners (health, urban, transportation, industrial) may use this information for mitigating developments to reduce environmental effects on places where pregnant mothers (and everyone else) live. For example, existing land use may need to be altered over time depending on the proximity of industrial activities and residential areas.
May this research add to the many needed arguments for reducing the most widespread source of hazardous exposures—outdoor environmental pollution—in the places where one lives and starts out in life, to promote a more positive state of planetary health for all.
Funding
This research was funded by CIHR/NSERC Funding Reference Number (FRN) 127789 entitled “Spatial data mining exploring co-location of adverse birth outcomes and environmental variables.”
Acknowledgments
Research was part of the Data Mining and Neonatal Outcomes (DoMiNO) interdisciplinary collaborative project (https://sites.google.com/a/ualberta.ca/domino/home/team-members); DoMiNO team members included Aelicks N., Aziz K., Buka I., Bellinger C., Chandra S., Demers P., Erickson A., Jabbar S., Hystad P., Kumar M., Nielsen C., Phipps E., Serrano-Lomelin J., Shah P., Stieb D., Villeneuve P., Wine O., Yuan Y., Zaiane O., and Osornio-Vargas A.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Abbreviations for Canadian provinces and USA states.
Table A1.
Abbreviations for Canadian provinces and USA states.
| Country | Province/State | Abbreviation |
|---|---|---|
| Canada | Alberta | AB |
| British Columbia | BC | |
| Manitoba | MB | |
| New Brunswick | NB | |
| Newfoundland and Labrador | NL | |
| Northwest Territories | NT | |
| Nova Scotia | NS | |
| Nunavut | NU | |
| Ontario | ON | |
| Prince Edward Island | PE | |
| Quebec | QC | |
| Saskatchewan | SK | |
| Yukon Territory | YT | |
| USA | Alabama | AL |
| Alaska | AK | |
| Arizona | AZ | |
| Arkansas | AR | |
| California | CA | |
| Colorado | CO | |
| Connecticut | CT | |
| Delaware | DE | |
| District of Columbia | DC | |
| Florida | FL | |
| Georgia | GA | |
| Hawaii | HI | |
| Idaho | ID | |
| Illinois | IL | |
| Indiana | IN | |
| Iowa | IA | |
| Kansas | KS | |
| Kentucky | KY | |
| Louisiana | LA | |
| Maine | ME | |
| Maryland | MD | |
| Massachusetts | MA | |
| Michigan | MI | |
| Minnesota | MN | |
| Mississippi | MS | |
| Missouri | MO | |
| Montana | MT | |
| Nebraska | NE | |
| Nevada | NV | |
| New Hampshire | NH | |
| New Jersey | NJ | |
| New Mexico | NM | |
| New York | NY | |
| North Carolina | NC | |
| North Dakota | ND | |
| Ohio | OH | |
| Oklahoma | OK | |
| Oregon | OR | |
| Pennsylvania | PA | |
| Rhode Island | RI | |
| South Carolina | SC | |
| South Dakota | SD | |
| Tennessee | TN | |
| Texas | TX | |
| Utah | UT | |
| Vermont | VT | |
| Virginia | VA | |
| Washington | WA | |
| West Virginia | WV | |
| Wisconsin | WI | |
| Wyoming | WY |
Appendix B

Table A2.
List of 159 identified studies examining birth outcomes and the environment.
Table A2.
List of 159 identified studies examining birth outcomes and the environment.
| Year | Study | Outcome 1 | Environment | Category | Variable(s) | Geography 2 |
|---|---|---|---|---|---|---|
| 2000 | Xiang et al. 2000 [51] | LBW | built | agriculture | crops | CO |
| 2010 | Fenster et al. 2010 [52] | LBW, BW | built | agriculture | agricultural occupation | CA |
| 2010 | Sathyanarayana et al. 2010 [53] | LBW | built | agriculture | pesticides | NC |
| 2013 | Gemmill et al. 2013 [54] | BW | built | agriculture | methyl bromide | CA |
| 2014 | Almberg et al. 2014 [55] | LBW | built | agriculture | crops | MO |
| 2017 | Larsen et al. 2017 [56] | BW | built | agriculture | pesticides | CA |
| 1999 | Ritz et al. 1999 [57] | LBW | built | air pollution | CO | CA |
| 2000 | Rogers et al. 2000 [58] | LBW | built | air pollution | SO2, TSP | GA, SC |
| 2001 | Maisonet et al. 2001 [59] | LBW | built | air pollution | CO, SO2, PM10 | CT, MA, PA, DC |
| 2001 | Vassilev et al. 2001 [60] | SGA | built | air pollution | polycyclic organic matter | NJ |
| 2003 | Liu et al. 2003 [61] | LBW, IUGR | built | air pollution | CO, NO2, SO2, O3, PM10 | Canada |
| 2004 | Basu et al. 2004 [62] | BW | built | air pollution | PM2.5 | CA |
| 2004 | Lederman et al. 2004 [63] | BW | built | air pollution | urban disaster | NY |
| 2005 | Salam et al. 2005 [64] | LBW, IUGR | built | air pollution | CO, NO2, O3, PM10 | CA |
| 2006 | Dugandzic et al. 2006 [65] | LBW | built | air pollution | PM10, SO2, O3 | NS |
| 2007 | Bell et al. 2007 [66] | BW | built | air pollution | CO, NO2, SO2, PM10, PM2.5 | CT, MA |
| 2007 | Liu et al. 2007 [67] | IUGR | built | air pollution | CO, NO2, SO2, O3, PM2.5 | AB, QC |
| 2007 | Williams et al. 2007 [68] | BW | built | air pollution | Pb, SO2 | TN |
| 2008 | Brauer et al. 2008 [69] | LBW, SGA | built | air pollution | traffic | BC |
| 2008 | Choi et al. 2008 [70] | SGA | built | air pollution | PAHs | NY |
| 2009 | Currie et al. 2009 [71] | LBW | built | air pollution | industrial releases | USA |
| 2010 | Morello-Frosch et al. 2010 [72] | BW | built | air pollution | CO, NO2, SO2, O3, PM10, PM2.5 | CA |
| 2011 | Darrow et al. 2011 [73] | BW | built | air pollution | CO, NO2, SO2, O3, PM10, PM2.5 | GA |
| 2012 | Berrocal et al. 2012 [74] | BW | built | air pollution | PM2.5 | NC |
| 2012 | Ebisu et al. 2012 [75] | LBW | built | air pollution | PM2.5 | CT, DE, MD, MA, NH, NJ, NY, PA, RI, VT, VI, DC, WV |
| 2012 | Geer et al. 2012 [76] | BW | built | air pollution | CO, NO2, SO2, O3, PM10, PM2.5 | TX |
| 2012 | Ghosh et al. 2012 [77] | LBW | built | air pollution | traffic | CA |
| 2012 | Holstius et al. 2012 [78] | BW | built | air pollution | wildfires | CA |
| 2012 | Kloog et al. 2012 [79] | BW | built | air pollution | PM2.5 | MA |
| 2012 | Kumar et al. 2012 [80] | LBW | built | air pollution | CO, NO2, SO2, O3, PM10, PM2.5 | IL |
| 2012 | Le et al. 2012 [81] | SGA | built | air pollution | CO, NO2, SO2, O3, PM10 | MI |
| 2012 | Padula et al. 2012 [82] | LBW | built | air pollution | traffic | CA |
| 2012 | Sathyanarayana et al. 2012 [83] | SGA | built | air pollution | NO2, PM2.5 | WA |
| 2012 | Wilhelm et al. 2012 [84] | LBW | built | air pollution | PM2.5, NO, NO2, PAHs | CA |
| 2013 | Lee et al. 2013 [85] | SGA | built | air pollution | PM10, PM2.5, O3 | PA |
| 2013 | Meng et al. 2013 [86] | LBW | built | air pollution | traffic | ON |
| 2013 | Trasande et al. 2013 [87] | LBW | built | air pollution | CO, NO2, SO2, PM10, PM2.5, Pb, VOCs | USA |
| 2013 | Warren et al. 2013 [88] | LBW | built | air pollution | O3 | TX |
| 2014 | Basu et al. 2014 [89] | LBW | built | air pollution | PM2.5 | CA |
| 2014 | Gray et al. 2014 [90] | BW | built | air pollution | PM10, PM2.5 | NC |
| 2014 | Ha et al. 2014 [91] | LBW | built | air pollution | PM2.5, O3 | FL |
| 2014 | Harris et al. 2014 [92] | LBW | built | air pollution | PM2.5 | CT, ME, MN, NJ, NY, UT, WI |
| 2014 | Hyder et al. 2014 [93] | LBW, SGA | built | air pollution | PM2.5 | CT, MA |
| 2014 | Porter et al. 2014 [94] | LBW | built | air pollution | industrial releases | AL |
| 2014 | Vinikoor-Imler et al. 2014 [95] | LBW, SGA | built | air pollution | PM2.5, O3 | NC |
| 2015 | Coker et al. 2015 [96] | LBW | built | air pollution | PM2.5 | CA |
| 2015 | Poirier et al. 2015 [97] | LBW | built | air pollution | SO2, NO2, benzene, toluene, PM10, PM2.5 | NS |
| 2016 | Coker et al. 2016 [98] | LBW | built | air pollution | NO, NO2, PM2.5 | CA |
| 2016 | Erickson et al. 2016 [99] | BW | built | air pollution | PM2.5, social | BC |
| 2016 | Laurent et al. 2016 [100] | LBW | built | air pollution | PM10, PM2.5 | CA |
| 2016 | Lavigne et al. 2016 [101] | LBW, SGA | built | air pollution | PM2.5, NO2, O3 | ON |
| 2016 | Stieb et al. 2016 [102] | LBW, SGA, BW | built | air pollution | NO2, PM2.5 | Canada |
| 2016 | Tu et al. 2016 [103] | BW | built | air pollution | O3, PM2.5 | GA |
| 2016 | Twum et al. 2016 [104] | LBW | built | air pollution | PM2.5 | GA |
| 2017 | Ha et al. 2017 [105] | LBW, SGA | built | air pollution | 11 criteria air contaminants and PM | CA, DC, DE, FL, UT, IL, IN, MA, MD, NY, OH, TX |
| 2017 | Jedrychowski et al. 2017 [106] | BW | built | air pollution | PM2.5, PAH | NY |
| 2017 | Ng et al. 2017 [107] | LBW | built | air pollution | PM2.5 | CA |
| 2017 | Nielsen et al. 2017 [108] | LBW, SGA | built | air pollution | industrial releases, built | AB |
| 2018 | Gong et al. 2018 [109] | LBW | built | air pollution | industrial releases | TX |
| 2018 | Seabrook et al. 2018 [110] | LBW | built | alcohol | alcohol | ON |
| 1992 | Shaw et al. 1992 [111] | BW | built | chemical | chemical | CA |
| 1997 | Philion et al. 1997 [112] | SGA, IUGR | built | chemical | lead | BC |
| 2004 | Lawson et al. 2004 [113] | BW | built | chemical | occupational TCDD | NJ, MO |
| 2005 | Perera et al. 2005 [114] | BW | built | chemical | ETS, PAH, pesticides | NY |
| 2008 | Wolff et al. 2008 [115] | BW | built | chemical | phenols, phthalates | NY |
| 2010 | Hamm et al. 2010 [116] | BW | built | chemical | perfluorinated acids | AB |
| 2010 | Zhu et al. 2010 [117] | BW | built | chemical | metals: Pb | NY |
| 2012 | Aelion et al. 2012 [118] | BW | built | chemical | metals: As, Pb | SC |
| 2012 | Rauch et al. 2012 [119] | BW | built | chemical | pesticides | OH |
| 2014 | Mckenzie et al. 2014 [120] | LBW | built | chemical | natural gas | CO |
| 2015 | Stacy et al. 2015 [121] | SGA, BW | built | chemical | natural gas | PA |
| 2015 | Thomas et al. 2015 [122] | SGA | built | chemical | metals: Pb, Hg, Cd, As | Canada |
| 2016 | Casey et al. 2016 [123] | SGA, BW | built | chemical | natural gas | PA |
| 2017 | Whitworth et al. 2017 [124] | SGA, BW | built | chemical | natural gas | TX |
| 2018 | Ashley-Martin et al. 2018 [125] | BW | built | chemical | metals: Mn | QC |
| 2018 | Hill et al. 2018 [126] | SGA | built | chemical | natural gas | PA |
| 2008 | Lane et al. 2008 [127] | LBW | built | food | food, social | NY |
| 2016 | Ma et al. 2016 [128] | LBW, BW | built | food | food | SC |
| 2011 | Ahern et al. 2011 [129] | LBW | built | mining | coal | WV |
| 2017 | Ferdosi et al. 2017 [130] | SGA | built | mining | coal | KY, TN, VA, WV |
| 2011 | Vinikoor-Imler et al. 2011 [131] | LBW | built | neighborhood | neighborhood | NC |
| 2012 | Miranda et al. 2012 [132] | LBW, SGA | built | neighborhood | neighborhood | NC |
| 2014 | Gehring et al. 2014 [133] | LBW, BW | built | noise | noise, traffic | BC |
| 2015 | Ha et al. 2015 [134] | LBW | built | power | power plants | FL |
| 2003 | Wilhelm et al. 2003 [135] | LBW | built | roads | roads | CA |
| 2008 | Généreux et al. 2008 [136] | LBW, SGA | built | roads | roads, social | QC |
| 2012 | Miranda et al. 2012 [137] | LBW, SGA | built | roads | roads | NC |
| 2011 | Auger et al. 2011 [138] | LBW, SGA | built | transmission lines | transmission lines | QC |
| 1997 | Larson et al. 1997 [139] | LBW | built | urban-rural | urban | USA |
| 2009 | Auger et al. 2009 [140] | LBW, SGA | built | urban-rural | urban, social | QC |
| 2013 | Kent et al. 2013 [141] | LBW | built | urban-rural | urban, social | AL |
| 1994 | Sosniak et al. 1994 [142] | LBW | built | waste site | waste site | USA |
| 1995 | Goldberg et al. 1995 [143] | LBW, SGA | built | waste site | waste site | QC |
| 1997 | Berry et al. 1997 [144] | BW | built | waste site | waste site | NJ |
| 2003 | Baibergenova et al. 2003 [145] | LBW | built | waste site | waste site | NY |
| 2006 | Gilbreath et al. 2006 [146] | LBW, IUGR | built | waste site | waste site | AK |
| 2011 | Austin et al. 2011 [147] | LBW | built | waste site | waste site | NY |
| 2014 | Thompson et al. 2014 [148] | LBW | built | waste site | waste site | TX |
| 2016 | Claus et al. Henn et al. 2016 [149] | BW | built | waste site | waste site | OK |
| 1997 | Munger et al. 1997 [150] | IUGR | built | water contamination | herbicides | IA |
| 1998 | Gallagher et al. 1998 [151] | LBW | built | water contamination | trihalmethanes | CO |
| 2005 | Hinckley et al. 2005 [152] | LBW, IUGR | built | water contamination | trihalomethane, haloacetic acid | AZ |
| 2008 | Aschengrau et al. 2008 [153] | BW | built | water contamination | tetrachloroethylene | MA |
| 2009 | Ochoa-Acuña et al. 2009 [154] | SGA | built | water contamination | herbicides | IA |
| 2012 | Forand et al. 2012 [155] | LBW | built | water contamination | tetrachloroethylene and trichloroethylene | NY |
| 2012 | Savitz et al. 2012 [156] | LBW, SGA | built | water contamination | perfluorooctanoic acid | OH |
| 2013 | Darrow et al. 2013 [157] | LBW, BW | built | water contamination | perfluorooctanoic acid and perfluorooctane sulfonate | OH |
| 2015 | Ileka-Priouzeau et al. 2015 [158] | SGA | built | water contamination | haloacetaldehydes, haloacetonitriles | QC |
| 2011 | Donovan et al. 2011 [159] | SGA | natural | vegetation | greenness | OR |
| 2013 | Laurent et al. 2013 [160] | BW | natural | vegetation | greenness | CA |
| 2014 | Hystad et al. 2014 [161] | SGA, BW | natural | vegetation | greenness | BC |
| 2016 | Ebisu et al. 2016 [162] | LBW, SGA, BW | natural | vegetation | greenness, built: urban | CT |
| 2017 | Abelt et al. 2017 [163] | LBW, SGA, BW | natural | vegetation | greenness, blue space | NY |
| 2017 | Cusack et al. 2017 [164] | SGA, BW | natural | vegetation | greenness | TX |
| 2017 | Cusack et al. 2017 [165] | BW | natural | vegetation | greenness | OR, TX |
| 2018 | Cusack et al. 2018 [40] | BW | natural | vegetation | greenness | BC, AB, MB, ON |
| 2012 | Lin et al. 2012 [166] | BW | natural | weather | extreme weather | USA |
| 2014 | Thayer et al. 2014 [167] | LBW | natural | weather | UV-vitamin D, social: race | USA |
| 2016 | Savard et al. 2016 [168] | SGA | social | health care | health care | QC |
| 2010 | Urquia et al. 2010 [169] | BW | social | immigration | immigration | ON |
| 2011 | Janevic et al. 2011 [170] | SGA | social | immigration | immigration | NY |
| 1995 | Mclafferty et al. 1995 [171] | LBW | social | individual | social | NY |
| 2001 | Tough et al. 2001 [172] | LBW | social | individual | maternal health | AB |
| 2003 | English et al. 2003 [173] | LBW | social | individual | maternal health | CA |
| 2005 | Lasker et al. 2005 [18] | LBW | social | individual | maternal health | PA |
| 2008 | Grady et al. 2008 [174] | LBW | social | individual | maternal health | NY |
| 2013 | Heaman et al. 2013 [175] | SGA | social | individual | maternal health | Canada |
| 2014 | Aris et al. 2014 [176] | LBW, IUGR | social | individual | endometriosis | QC |
| 2015 | Chen et al. 2015 [177] | LBW, SGA | social | individual | interpregnancy interval | AB |
| 2016 | Shapiro et al. 2016 [178] | SGA | social | individual | individual | Canada |
| 2018 | Jain et al. 2018 [179] | SGA | social | individual | maternal health | NS |
| 1999 | Gorman et al. 1999 [180] | LBW | social | race | race | USA |
| 2004 | Wenman et al. 2004 [181] | LBW | social | race | race | AB |
| 2008 | Vinikoor et al. 2008 [182] | LBW | social | race | race | NC |
| 2009 | Reichman et al. 2009 [183] | BW | social | race | race | CA, TX, MD, MI, NJ, PA, VA, IN, WI, NY, MA, TN, IL, FL, OH, NM |
| 2010 | Grady et al. 2010 [184] | IUGR | social | race | race | MI |
| 2010 | Nepomnyaschy et al. 2010 [185] | LBW | social | race | race | USA |
| 2011 | Anthopolos et al. 2011 [186] | LBW, BW | social | race | race | NC |
| 2011 | Kirby et al. 2011 [187] | LBW | social | race | race | GA, SC |
| 2013 | Wallace et al. 2013 [188] | LBW | social | race | race | LA |
| 2016 | Oster et al. 2016 [189] | LBW | social | race | race | AB |
| 2018 | Shapiro et al. 2018 [190] | SGA | social | race | race | Canada |
| 1993 | Kieffer et al. 1993 [191] | LBW | social | SES | SES | HI |
| 2003 | Krieger et al. 2003 [192] | LBW | social | SES | SES, blood Pb | MA, RI |
| 2006 | Farley et al. 2006 [193] | IUGR | social | SES | SES | LA |
| 2007 | Masi et al. 2007 [194] | BW | social | SES | SES, built | IL |
| 2008 | Zeka et al. 2008 [195] | SGA, BW | social | SES | SES, built | MA |
| 2010 | Young et al. 2010 [196] | BW | social | SES | SES | MA |
| 2012 | Tu et al. 2012 [197] | BW | social | SES | SES | GA |
| 2013 | Auger et al. 2013 [198] | SGA | social | SES | SES | QC |
| 2013 | Legerski et al. 2013 [199] | LBW | social | SES | SES | KS |
| 2013 | Meng et al. 2013 [200] | LBW | social | SES | SES | ON |
| 2015 | Chan et al. 2015 [201] | LBW, SGA | social | SES | SES | Canada |
| 2015 | Shmool et al. 2015 [202] | BW | social | SES | SES, NO2 | NY |
| 2016 | Martinson et al. 2016 [45] | LBW | social | SES | SES | Canada, USA |
| 2017 | Bushnik et al. 2017 [203] | SGA | social | SES | SES | Canada |
| 2017 | MacQuillan et al. 2017 [204] | LBW | social | SES | SES | MI |
| 2018 | Campbell et al. 2018 [205] | LBW | social | SES | SES | ON |
| 2018 | McRae et al. 2018 [206] | SGA | social | SES | SES | BC |
1 Outcomes included low birth weight (LBW), small for gestational age (SGA), birth weight (BW), and intrauterine growth restriction (IUGR). 2 Geography abbreviations are detailed in Table A1.
References
- Barker, D.J. The fetal and infant origins of adult disease. BMJ Br. Med. J. 1990, 301, 1111. [Google Scholar] [CrossRef]
- Aschengrau, A.; Seage III, G.R. Essentials of Epidemiology in Public Health, 3rd ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2014. [Google Scholar]
- Backes, C.H.; Nelin, T.; Gorr, M.W.; Wold, L.E. Early life exposure to air pollution: How bad is it? Toxicol. Lett. 2013, 216, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Stillerman, K.P.; Mattison, D.R.; Giudice, L.C.; Woodruff, T.J. Environmental exposures and adverse pregnancy outcomes: A review of the science. Reprod. Sci. 2008, 15, 631–650. [Google Scholar] [CrossRef] [PubMed]
- Wigle, D.T.; Arbuckle, T.E.; Turner, M.C.; Bérubé, A.; Yang, Q.; Liu, S.; Krewski, D. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J. Toxicol. Environ. Health Part B Crit. Rev. 2008, 11, 373–517. [Google Scholar] [CrossRef] [PubMed]
- Selevan, S.G.; Kimmel, C.A.; Mendola, P. Identifying critical windows of exposure for children’s health. Environ. Health Perspect. 2000, 108, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Canadian Institute for Health Information (CIHI). Too Early, too Small: A Profile of Small Babies across Canada; CIHI: Ottawa, ON, Canada, 2009. [Google Scholar]
- Lim, G.; Tracey, J.; Boom, N.; Karmakar, S.; Wang, J.; Berthelot, J.-M.; Heick, C. CIHI survey: Hospital costs for preterm and small-for-gestational age babies in Canada. Healthc. Q. 2009, 12, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Last, J.M. A Dictionary of Public Health; Oxford Univ. Press: Oxford, UK, 2007; p. 407. [Google Scholar]
- Barker, D.J.P. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition 1997, 13, 807–813. [Google Scholar] [CrossRef]
- Barker, D.J.P. The developmental origins of chronic adult disease. Acta Paediatr. Suppl. 2004, 93, 26–33. [Google Scholar] [CrossRef]
- Barker, D.J.P.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 327, 1077–1081. [Google Scholar] [CrossRef]
- Kramer, M.S. The epidemiology of adverse pregnancy outcomes: An overview. J. Nutr. 2003, 133, 1592S–1596S. [Google Scholar] [CrossRef]
- Kramer, M.S.; Platt, R.W.; Wen, S.W.; Joseph, K.S.; Allen, A.; Abrahamowicz, M.; Blondel, B.; Bréart, G. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 2001, 108, e1–e7. [Google Scholar] [CrossRef]
- De Onis, M.; Habicht, J.P. Anthropometric reference data for international use: Recommendations from a WHO Expert Committee. Am. J. Clin. Nutr. 1996, 64, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F. Low birth weight in the United States. Am. J. Clin. Nutr. 2007, 85, 584S–590S. [Google Scholar] [CrossRef]
- Kramer, M.S. Determinants of low birth weight: Methodological assessment and meta-analysis. Bull. World Health Organ. 1987, 65, 663–737. [Google Scholar] [CrossRef] [PubMed]
- Lasker, J.N.; Coyle, B.; Li, K.; Ortynsky, M. Assessment of risk factors for low birth weight deliveries. Health Care Women Int. 2005, 26, 262–280. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.S.; Shah, V.; on behalf of Knowledge Synthesis Group on Determinants of Preterm/LBW Births. Influence of the maternal birth status on offspring: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2009, 88, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E. Global prevalence of small for gestational age births. Nestle Nutr. Inst. Workshop Ser. 2015, 81, 1–7. [Google Scholar] [CrossRef]
- Lee, A.C.C.; Katz, J.; Blencowe, H.; Cousens, S.; Kozuki, N.; Vogel, J.P.; Adair, L.; Baqui, A.H.; Bhutta, Z.A.; Caulfield, L.E.; et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob. Health 2013, 1, e26–e36. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention (CDC) QuickStats: Percentage of Small-for-Gestational-Age Births, by Race and Hispanic Ethnicity—United States. 2005. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5750a5.htm (accessed on 28 June 2018).
- Center for Disease Control and Prevention (CDC) Percentage of Babies Born Low Birthweight By State, 2005, 2016 [Digital Data]. Available online: https://www.cdc.gov/nchs/pressroom/sosmap/lbw_births/lbw.htm (accessed on 28 June 2018).
- Statistics Canada Table 102-4318—Birth-Related Indicators (Low and High Birth Weight, Small and Large for Gestational Age, Pre-Term Births), by Sex, Three-Year Average, Canada, Provinces, territories, Census Metropolitan Areas and Metropolitan Influence Zones, Occasional. Available online: http://www5.statcan.gc.ca/cansim/a05?lang=eng&id=01024318 (accessed on 9 May 2018).
- Statistics Canada Table 13-10-0404-01 Low Birth Weight Babies, by Province And Territory (Formerly CANSIM 102-4005), 2005 and 2016. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1310040401 (accessed on 9 May 2018).
- Statistics Canada Table 102-0562—Leading Causes of Death, Infants, by Sex, Canada, Annual, 2006–2012 [Digital Data]. Available online: http://www5.statcan.gc.ca/cansim/pick-choisir?lang=eng&searchTypeByValue=1&id=1020562 (accessed on 9 May 2018).
- Meade, M.S.; Emch, M. Medical Geography, 3rd ed.; Guilford Press: New York, NY, USA, 2010. [Google Scholar]
- May, J.M. The ecology of human disease. Ann. N. Y. Acad. Sci. 1958, 84, 789–794. [Google Scholar] [CrossRef]
- Meade, M.S. Medical geography as human ecology: The dimension of population movement. Geogr. Rev. 1977, 64, 379–393. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Environment and Health: The European Charter and Commentary; WHO Regional Publications, European Series No. 35; WHO: Copenhagen, Denmark, 1989. [Google Scholar]
- Prescott, S.; Kozyrskyj, A.; Logan, A.; Campbell, D. Eighth Annual Conference of inVIVO Planetary Health on Transforming Life: Unify Personal, Public, and Planetary Health. Challenges 2018, 9, 36. [Google Scholar] [CrossRef]
- Prescott, S.; Logan, A.; Albrecht, G.; Campbell, D.; Crane, J.; Cunsolo, A.; Holloway, J.; Kozyrskyj, A.; Lowry, C.; Penders, J.; et al. The Canmore Declaration: Statement of Principles for Planetary Health. Challenges 2018, 9, 31. [Google Scholar] [CrossRef]
- Hippocrates. Hippocrates on Airs, Waters, Places; Translated by Francis Adams; Sydenham Society: London, UK, 1849. [Google Scholar]
- Cromley, E.K.; McLafferty, S.L. GIS and Public Health, 2nd ed.; Guilford Press: New York, NY, USA, 2012. [Google Scholar]
- Shah, P.S.; Balkhair, T. Air pollution and birth outcomes: A systematic review. Environ. Int. 2011, 37, 498–516. [Google Scholar] [CrossRef] [PubMed]
- Vadillo-Ortega, F.; Osornio-Vargas, A.; Buxton, M.A.; Sánchez, B.N.; Rojas-Bracho, L.; Viveros-Alcaráz, M.; Castillo-Castrejón, M.; Beltrán-Montoya, J.; Brown, D.G.; O’Neill, M.S. Air pollution, inflammation and preterm birth: A potential mechanistic link. Med. Hypotheses 2014, 82, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Misra, D.P.; Dvonch, J.T.; Krishnakumar, A. Exposures to airborne particulate matter and adverse perinatal outcomes: A biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ. Health Perspect. 2006, 114, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Nieuwenhuijsen, M.J.; Gallus, S.; Cipriani, S.; La Vecchia, C.; Parazzini, F. Ambient particulate matter and preterm birth or birth weight: A review of the literature. Arch. Toxicol. 2010, 84, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Cusack, L.; Sbihi, H.; Larkin, A.; Chow, A.; Brook, J.R.; Moraes, T.; Mandhane, P.J.; Becker, A.B.; Azad, M.B.; Subbarao, P.; et al. Residential green space and pathways to term birth weight in the Canadian Healthy Infant Longitudinal Development (CHILD) Study. Int. J. Health Geogr. 2018, 17, 43. [Google Scholar] [CrossRef]
- Esri ArcGIS Desktop, Release 10.6. 2017. Available online: https://support.esri.com/en/products/desktop/arcgis-desktop/arcmap/10-6 (accessed on 28 June 2018).
- Commission for Environmental Cooperation (CEC) 2010 Political Boundaries, 2005 Land Cover at 250 meters, 2005 Pollution Transfer Release Reporting (PRTR) [Digital Data]. Available online: http://www.cec.org/naatlas (accessed on 7 December 2018).
- van Donkelaar, A.; Martin, R.V. Satellite-Derived PM2.5, 2005 and 2016, at 35% RH (ug/m3), Dust and Sea-Salt Removed, V4.NA.01 [Digital Data]. Available online: http://fizz.phys.dal.ca/~atmos/martin/?page_id=140 (accessed on 7 December 2018).
- King, W.D.; Dodds, L.; Armson, B.A.; Allen, A.C.; Fell, D.B.; Nimrod, C. Exposure assessment in epidemiologic studies of adverse pregnancy outcomes and disinfection byproducts. J. Expo. Anal. Environ. Epidemiol. 2004, 14, 466–472. [Google Scholar] [CrossRef]
- Martinson, M.L.; Reichman, N.E. Socioeconomic inequalities in low birth weight in the United States, the United Kingdom, Canada, and Australia. Am. J. Public Health 2016, 106, 748–754. [Google Scholar] [CrossRef]
- Nuckols, J.R.; Ward, M.H.; Jarup, L. Using Geographic Information Systems for exposure assessment in environmental epidemiology studies. Environ. Health Perspect. 2004, 112, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.J.; Parker, J.D.; Darrow, L.A.; Slama, R.; Bell, M.L.; Choi, H.; Glinianaia, S.; Hoggatt, K.J.; Karr, C.J.; Lobdell, D.T.; et al. Methodological issues in studies of air pollution and reproductive health. Environ. Res. 2009, 109, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bell, E.M.; Caton, A.R.; Druschel, C.M.; Lin, S. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ. Res. 2010, 110, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Belanger, K. Review of research on residential mobility during pregnancy: Consequences for assessment of prenatal environmental exposures. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.L.; Son, J.Y.; Pereira, G.; Leaderer, B.P.; Bell, M.L. Investigating the impact of maternal residential mobility on identifying critical windows of susceptibility to ambient air pollution during pregnancy. Am. J. Epidemiol. 2018, 187, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Nuckols, J.R.; Stallones, L. A geographic information assessment of birth weight and crop production patterns around mother’s residence. Environ. Res. 2000, 82, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Fenster, L.; Coye, M.J. Birthweight of infants born to Hispanic women employed in agriculture. Arch. Environ. Health 2010, 45, 46–52. [Google Scholar] [CrossRef]
- Sathyanarayana, S.; Basso, O.; Karr, C.J.; Lozano, P.; Alavanja, M.; Sandler, D.P.; Hoppin, J.A. Maternal pesticide use and birth weight in the agricultural health study. J. Agromed. 2010, 15, 127–136. [Google Scholar] [CrossRef]
- Gemmill, A.; Gunier, R.B.; Bradman, A.; Eskenazi, B.; Harley, K.G. Residential proximity to methyl bromide use and birth outcomes in an agricultural population in California. Environ. Health Perspect. 2013, 121, 737–743. [Google Scholar] [CrossRef]
- Almberg, K.S.; Turyk, M.; Jones, R.M.; Anderson, R.; Graber, J.; Banda, E.; Waller, L.A.; Gibson, R.; Stayner, L.T. A study of adverse birth outcomes and agricultural land use practices in Missouri. Environ. Res. 2014, 134, 420–426. [Google Scholar] [CrossRef]
- Larsen, A.E.; Gaines, S.D.; Deschênes, O. Agricultural pesticide use and adverse birth outcomes in the San Joaquin Valley of California. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ritz, B.; Yu, F. The effect of ambient carbon monoxide on low birth weight among children born in southern California between 1989 and 1993. Environ. Health Perspect. 1999, 107, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.F.; Thompson, S.J.; Addy, C.L.; McKeown, R.E.; Cowen, D.J.; Decouflé, P. Association of very low birth weight with exposures to environmental sulfur dioxide and total suspended particulates. Am. J. Epidemiol. 2000, 151, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Maisonet, M.; Bush, T.J.; Correa, A.; Jaakkola, J.J. Relation between ambient air pollution and low birth weight in the Northeastern United States. Environ. Health Perspect. 2001, 109 (Suppl. 3), 351–356. [Google Scholar] [CrossRef]
- Vassilev, Z.P.; Robson, M.G.; Klotz, J.B. Associations of polycyclic organic matter in outdoor air with decreased birth weight: A pilot cross-sectional analysis. J. Toxicol. Environ. Health Part A Curr. Issues 2001, 64, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Krewski, D.; Shi, Y.; Chen, Y.; Burnett, R.T. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environ. Health Perspect. 2003, 111, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Woodruff, T.J.; Parker, J.D.; Saulnier, L.; Schoendorf, K.C. Comparing exposure metrics in the relationship between PM2.5 and birth weight in California. J. Expo. Anal. Environ. Epidemiol. 2004, 14, 391–396. [Google Scholar] [CrossRef]
- Lederman, S.A.; Rauh, V.; Weiss, L.; Stein, J.L.; Hoepner, L.A.; Becker, M.; Perera, F.P. The effects of the World Trade Center event on birth outcomes among term deliveries at three Lower Manhattan hospitals. Environ. Health Perspect. 2004, 112, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.T.; Millstein, J.; Li, Y.-F.; Lurmann, F.W.; Margolis, H.G.; Gilliland, F.D. Birth outcomes and prenatal exposure to ozone, carbon monoxide, and particulate matter: Results from the Children’s Health Study. Environ. Health Perspect. 2005, 113, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Dugandzic, R.; Dodds, L.; Stieb, D.; Smith-Doiron, M. The association between low level exposures to ambient air pollution and term low birth weight: A retrospective cohort study. Environ. Health 2006, 5, 1–8. [Google Scholar] [CrossRef]
- Bell, M.L.; Ebisu, K.; Belanger, K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ. Health Perspect. 2007, 115, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Krewski, D.; Shi, Y.; Chen, Y.; Burnett, R.T. Association between maternal exposure to ambient air pollutants during pregnancy and fetal growth restriction. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.L.; Pennock-Román, M.; Suen, H.K.; Magsumbol, M.S.; Ozdenerol, E. Assessing the impact of the local environment on birth outcomes: A case for HLM. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.; Lencar, C.; Tamburic, L.; Koehoorn, M.; Demers, P.; Karr, C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environ. Health Perspect. 2008, 116, 680–686. [Google Scholar] [CrossRef]
- Choi, H.; Rauh, V.; Garfinkel, R.; Tu, Y.; Perera, F.P. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ. Health Perspect. 2008, 116, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Currie, J.; Schmieder, J.F. Fetal exposures to toxic releases and infant health. Am. Econ. Rev. 2009, 99, 177–183. [Google Scholar] [CrossRef]
- Morello-Frosch, R.; Jesdale, B.M.; Sadd, J.L.; Pastor, M. Ambient air pollution exposure and full-term birth weight in California. Environ. Health 2010, 9, 44. [Google Scholar] [CrossRef]
- Darrow, L.A.; Klein, M.; Strickland, M.J.; Mulholland, J.A.; Tolbert, P.E. Ambient air pollution and birth weight in full-term infants in Atlanta, 1994–2004. Environ. Health Perspect. 2011, 119, 731–737. [Google Scholar] [CrossRef]
- Berrocal, V.J.; Gelfand, A.E.; Holland, D.M.; Burke, J.; Miranda, M.L. On the use of a PM2.5 exposure simulator to explain birthweight. Environmentrics 2012, 22, 553–571. [Google Scholar] [CrossRef]
- Ebisu, K.; Bell, M. Airborne PM2.5 chemical components and low birth weight in the Northeastern and Mid-Atlantic Regions of the United States. Environ. Health Perspect. 2012, 1746–1752. [Google Scholar] [CrossRef]
- Geer, L.A.; Weedon, J.; Bell, M.L. Ambient air pollution and term birth weight in Texas from 1998 to 2004. J. Air Waste Manag. Assoc. 2012, 62, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.K.C.; Wilhelm, M.; Su, J.; Goldberg, D.; Cockburn, M.; Jerrett, M.; Ritz, B. Assessing the influence of traffic-related air pollution on risk of term low birth weight on the basis of land-use-based regression models and measures of air toxics. Am. J. Epidemiol. 2012, 175, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Holstius, D.M.; Reid, C.E.; Jesdale, B.M.; Morello-Frosch, R. Birth weight following pregnancy during the 2003 Southern California wildfires. Environ. Health Perspect. 2012, 120, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Kloog, I.; Melly, S.J.; Ridgway, W.L.; Coull, B.A.; Schwartz, J. Using new satellite based exposure methods to study the association between pregnancy PM2.5 exposure, premature birth and birth weight in Massachusetts. Environ. Health 2012, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N. Uncertainty in the relationship between criteria pollutants and low birth weight in Chicago. Atmos. Environ. 2012, 49, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Le, H.Q.; Batterman, S.A.; Wirth, J.J.; Wahl, R.L.; Hoggatt, K.J.; Sadeghnejad, A.; Hultin, M.L.; Depa, M. Air pollutant exposure and preterm and term small-for-gestational-age births in Detroit, Michigan: Long-term trends and associations. Environ. Int. 2012, 44, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Padula, A.M.; Mortimer, K.; Hubbard, A.; Lurmann, F.; Jerrett, M.; Tager, I.B. Exposure to traffic-related air pollution during pregnancy and term low birth weight: Estimation of causal associations in a semiparametric model. Am. J. Epidemiol. 2012, 176, 815–824. [Google Scholar] [CrossRef]
- Sathyanarayana, S.; Zhou, C.; Rudra, C.B.; Gould, T.; Larson, T.; Koenig, J.; Karr, C.J. Prenatal ambient air pollution exposure and small for gestational age birth in the Puget Sound Air Basin. Air Qual. Atmos. Health 2012, 6, 455–463. [Google Scholar] [CrossRef]
- Wilhelm, M.; Ghosh, J.K.; Su, J.; Cockburn, M.; Jerrett, M.; Ritz, B. Traffic-related air toxics and term low birth weight in Los Angeles County, California. Environ. Health Perspect. 2012, 120, 132–138. [Google Scholar] [CrossRef]
- Lee, P.-C.; Roberts, J.M.; Catov, J.M.; Talbott, E.O.; Ritz, B. First trimester exposure to ambient air pollution, pregnancy complications and adverse birth outcomes in Allegheny County, PA. Matern. Child Health J. 2013, 17, 545–555. [Google Scholar] [CrossRef]
- Meng, G.; Hall, G.B.; Thompson, M.E.; Seliske, P. Spatial and environmental impacts on adverse birth outcomes in Ontario. Can. Geogr. 2013, 57, 154–172. [Google Scholar] [CrossRef]
- Trasande, L.; Wong, K.; Roy, A.; Savitz, D.A.; Thurston, G. Exploring prenatal outdoor air pollution, birth outcomes and neonatal health care utilization in a nationally representative sample. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.L.; Fuentes, M.; Herring, A.H.; Langlois, P.H. Air pollution metric analysis while determining susceptible periods of pregnancy for low birth weight. ISRN Obstet. Gynecol. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Harris, M.; Sie, L.; Malig, B.; Broadwin, R.; Green, R. Effects of fine particulate matter and its constituents on low birth weight among full-term infants in California. Environ. Res. 2014, 128, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.C.; Edwards, S.E.; Schultz, B.D.; Miranda, M.L. Assessing the impact of race, social factors and air pollution on birth outcomes: A population-based study. Environ. Health 2014, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Hu, H.; Roussos-Ross, D.; Haidong, K.; Roth, J.; Xu, X. The effects of air pollution on adverse birth outcomes. Environ. Res. 2014, 134, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.; Thompson, W.D.; Fitzgerald, E.; Wartenberg, D. The association of PM2.5 with full term low birth weight at different spatial scales. Environ. Res. 2014, 134, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Hyder, A.; Lee, H.J.; Ebisu, K.; Koutrakis, P.; Belanger, K.; Bell, M.L. PM2.5 exposure and birth outcomes: Use of satellite- and monitor-based data. Epidemiology 2014, 25, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.R.; Kent, S.T.; Su, W.; Beck, H.M.; Gohlke, J.M. Spatiotemporal association between birth outcomes and coke production and steel making facilities in Alabama, USA: A cross-sectional study. Environ. Health A Glob. Access Sci. Source 2014, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vinikoor-Imler, L.C.; Davis, J.A.; Meyer, R.E.; Messer, L.C.; Luben, T.J. Associations between prenatal exposure to air pollution, small for gestational age, and term low birthweight in a state-wide birth cohort. Environ. Res. 2014, 132, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Coker, E.; Ghosh, J.; Jerrett, M.; Gomez-Rubio, V.; Beckerman, B.; Cockburn, M.; Liverani, S.; Su, J.; Li, A.; Kile, M.L.; et al. Modeling spatial effects of PM2.5 on term low birth weight in Los Angeles County. Environ. Res. 2015, 142, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Poirier, A.; Dodds, L.; Dummer, T.; Rainham, D.; Maguire, B.; Johnson, M. Maternal exposure to air pollution and adverse birth outcomes in Halifax, Nova Scotia. J. Occup. Environ. Med. 2015, 57, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Coker, E.; Liverani, S.; Ghosh, J.K.; Jerrett, M.; Beckerman, B.; Li, A.; Ritz, B.; Molitor, J. Multi-pollutant exposure profiles associated with term low birth weight in Los Angeles County. Environ. Int. 2016, 91, 1–13. [Google Scholar] [CrossRef]
- Erickson, A.C.; Ostry, A.; Chan, L.H.M.; Arbour, L. The reduction of birth weight by fine particulate matter and its modification by maternal and neighbourhood-level factors: A multilevel analysis in British Columbia, Canada. Environ. Health 2016, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Laurent, O.; Hu, J.; Li, L.; Kleeman, M.J.; Bartell, S.M.; Cockburn, M.; Escobedo, L.; Wu, J. Low birth weight and air pollution in California: Which sources and components drive the risk? Environ. Int. 2016, 92–93, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, E.; Yasseen, A.S.; Stieb, D.M.; Hystad, P.; van Donkelaar, A.; Martin, R.V.; Brook, J.R.; Crouse, D.L.; Burnett, R.T.; Chen, H.; et al. Ambient air pollution and adverse birth outcomes: Differences by maternal comorbidities. Environ. Res. 2016, 148, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Stieb, D.M.; Chen, L.; Beckerman, B.S.; Jerrett, M.; Crouse, D.L.; Omariba, D.W.; Peters, P.A.; van Donkelaar, A.; Martin, R.V.; Burnett, R.T.; et al. Associations of pregnancy outcomes and PM in a national Canadian study. Environ. Health Perspect. 2016, 124, 243–249. [Google Scholar] [CrossRef]
- Tu, J.; Tu, W.; Tedders, S.H. Spatial variations in the associations of term birth weight with ambient air pollution in Georgia, USA. Environ. Int. 2016, 92–93, 146–156. [Google Scholar] [CrossRef]
- Twum, C.; Zhu, J.; Wei, Y. Maternal exposure to ambient PM2.5 and term low birthweight in the State of Georgia. Int. J. Environ. Health Res. 2016, 26, 92–100. [Google Scholar] [CrossRef]
- Ha, S.; Zhu, Y.; Liu, D.; Sherman, S.; Mendola, P. Ambient temperature and air quality in relation to small for gestational age and term low birthweight. Environ. Res. 2017, 155, 394–400. [Google Scholar] [CrossRef]
- Jedrychowski, W.A.; Majewska, R.; Spengler, J.D.; Camann, D.; Roen, E.L.; Perera, F.P. Prenatal exposure to fine particles and polycyclic aromatic hydrocarbons and birth outcomes: A two-pollutant approach. Int. Arch. Occup. Environ. Health 2017, 90, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.; Malig, B.; Hasheminassab, S.; Sioutas, C.; Basu, R.; Ebisu, K. Source apportionment of fine particulate matter and risk of term low birth weight in California: Exploring modification by region and maternal characteristics. Sci. Total Environ. 2017, 605–606, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.C.; Amrhein, C.G.; Osornio-Vargas, A.R. Mapping outdoor habitat and abnormally small newborns to develop an ambient health hazard index. Int. J. Health Geogr. 2017, 16, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Lin, Y.; Bell, M.L.; Zhan, F.B. Associations between maternal residential proximity to air emissions from industrial facilities and low birth weight in Texas, USA. Environ. Int. 2018, 120, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Seabrook, J.A.; Woods, N.; Clark, A.; De Vrijer, B.; Penava, D.; Gilliland, J. The association between alcohol outlet accessibility and adverse birth outcomes: A retrospective cohort study. J. Neonatal Perinat. Med. 2018, 11, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.M.; Schulman, J.; Frisch, J.D.; Cummins, S.K.; Harris, J.A. Congenital malformations and birthweight in areas with potential environmental contamination. Arch. Environ. Health 1992, 47, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Philion, J.J.; Schmitt, N.; Rowe, J.; Gelpke, P.M. Effect of lead on fetal growth in a Canadian smelter city, 1961–1990. Arch. Environ. Health 1997, 52, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.C.; Schnorr, T.M.; Whelan, E.A.; Deddens, J.A.; Dankovic, D.A.; Piacitelli, L.A.; Sweeney, M.H.; Connally, L.B. Paternal occupational exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin and birth outcomes of offspring: Birth weight, preterm delivery, and birth defects. Environ. Health Perspect. 2004, 112, 1403–1408. [Google Scholar] [CrossRef]
- Perera, F.P.; Rauh, V.; Whyatt, R.M.; Tang, D.; Tsai, W.Y.; Bernert, J.T.; Tu, Y.H.; Andrews, H.; Barr, D.B.; Camann, D.E.; et al. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology 2005, 26, 573–587. [Google Scholar] [CrossRef]
- Wolff, M.S.; Engel, S.M.; Berkowitz, G.S.; Ye, X.; Silva, M.J.; Zhu, C.; Wetmur, J.; Calafat, A.M. Prenatal phenol and phthalate exposures and birth outcomes. Environ. Health Perspect. 2008, 116, 1092–1097. [Google Scholar] [CrossRef]
- Hamm, M.P.; Cherry, N.M.; Chan, E.; Martin, J.W.; Burstyn, I. Maternal exposure to perfluorinated acids and fetal growth. J. Expo. Sci. Environ. Epidemiol. 2010, 20, 589–597. [Google Scholar] [CrossRef]
- Zhu, M.; Fitzgerald, E.F.; Gelberg, K.H.; Lin, S.; Druschel, C.M. Maternal low-level lead exposure and fetal growth. Environ. Health Perspect. 2010, 118, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Aelion, C.M.; Davis, H.T.; Lawson, A.B.; Cai, B.; McDermott, S. Associations of estimated residential soil arsenic and lead concentrations and community-level environmental measures with mother-child health conditions in South Carolina. Health Place 2012, 18, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.A.; Braun, J.M.; Barr, D.B.; Calafat, A.M.; Khoury, J.; Montesano, M.A. Associations of prenatal exposure to organophosphate pesticide metabolites with gestational age and birth weight. Environ. Health Perspect. 2012, 120, 1055–1060. [Google Scholar] [CrossRef]
- Mckenzie, L.M.; Guo, R.; Witter, R.Z.; Savitz, D.A.; Newman, L.S.; Adgate, J.L. Birth outcomes and maternal residential proximity to natural gas development in rural Colorado. Environ. Health Perspect. 2014, 122, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Stacy, S.L.; Brink, L.A.L.; Larkin, J.C.; Sadovsky, Y.; Goldstein, B.D.; Pitt, B.R.; Talbott, E.O. Perinatal outcomes and unconventional natural gas operations in Southwest Pennsylvania. PLoS ONE 2015, 10, e0126425. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Arbuckle, T.E.; Fisher, M.; Fraser, W.D.; Ettinger, A.; King, W. Metals exposure and risk of small-for-gestational age birth in a Canadian birth cohort: The MIREC study. Environ. Res. 2015, 140, 430–439. [Google Scholar] [CrossRef]
- Casey, J.A.; Savitz, D.A.; Rasmussen, S.G.; Ogburn, E.L.; Pollak, J.; Mercer, D.G.; Schwartz, B.S. Unconventional natural gas development and birth outcomes in Pennsylvania, USA. Epidemiology 2016, 27, 163–172. [Google Scholar] [CrossRef]
- Whitworth, K.W.; Marshall, A.K.; Symanski, E. Maternal residential proximity to unconventional gas development and perinatal outcomes among a diverse urban population in Texas. PLoS ONE 2017, 12, e0180966. [Google Scholar] [CrossRef] [PubMed]
- Ashley-Martin, J.; Dodds, L.; Arbuckle, T.E.; Ettinger, A.S.; Shapiro, G.D.; Fisher, M.; Monnier, P.; Morisset, A.S.; Fraser, W.D.; Bouchard, M.F. Maternal and cord blood manganese (Mn) levels and birth weight: The MIREC birth cohort study. Int. J. Hyg. Environ. Health 2018, 221, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.L. Shale gas development and infant health: Evidence from Pennsylvania. J. Health Econ. 2018, 61, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.D.; Keefe, R.H.; Rubinstein, R.; Levandowski, B.A.; Webster, N.; Cibula, D.A.; Boahene, A.K.; Dele-Michael, O.; Carter, D.; Jones, T.; et al. Structural violence, urban retail food markets, and low birth weight. Health Place 2008, 14, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, J.; Hardin, J.W.; Zhao, G.; Liese, A.D. Neighborhood food access and birth outcomes in South Carolina. Matern. Child Health J. 2016, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ahern, M.; Mullett, M.; MacKay, K.; Hamilton, C. Residence in coal-mining areas and low-birth-weight outcomes. Matern. Child Health J. 2011, 15, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Ferdosi, H.; Lamm, S.; Afari-Dwamena, N.; Dissen, E.; Chen, R.; Li, J.; Feinleib, M. Small-for-gestational age prevalence risk factors in central Appalachian states with mountain-top mining. Int. J. Occup. Med. Environ. Health 2017, 31, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Vinikoor-Imler, L.C.; Messer, L.C.; Evenson, K.R.; Laraia, B.A. Neighborhood conditions are associated with maternal health behaviors and pregnancy outcomes. Soc. Sci. Med. 2011, 73, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.L.; Messer, L.C.; Kroeger, G.L. Associations between the quality of the residential built environment and pregnancy outcomes among women in North Carolina. Environ. Health Perspect. 2012, 120, 471–477. [Google Scholar] [CrossRef]
- Gehring, U.; Tamburic, L.; Sbihi, H.; Davies, H.W.; Brauer, M. Impact of noise and air pollution on pregnancy outcomes. Epidemiology 2014, 25, 351–358. [Google Scholar] [CrossRef]
- Ha, S.; Hu, H.; Roth, J.; Kan, H.; Xu, X. Associations between residential proximity to power plants and adverse birth outcomes. Am. J. Epidemiol. 2015, 182, 215–224. [Google Scholar] [CrossRef]
- Wilhelm, M.; Ritz, B. Residential proximity to traffic and adverse birth outcomes in Los Angeles County, California, 1994-1996. Environ. Health Perspect. 2003, 111, 207–216. [Google Scholar] [CrossRef]
- Généreux, M.; Auger, N.; Goneau, M.; Daniel, M. Neighbourhood socioeconomic status, maternal education and adverse birth outcomes among mothers living near highways. J. Epidemiol. Community Health 2008, 62, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.L.; Edwards, S.E.; Chang, H.H.; Auten, R.L. Proximity to roadways and pregnancy outcomes. J. Expo. Sci. Environ. Epidemiol. 2012, 23, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Auger, N.; Joseph, D.; Goneau, M.; Daniel, M. The relationship between residential proximity to extremely low frequency power transmission lines and adverse birth outcomes. J. Epidemiol. Community Health 2011, 65, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.H.; Hart, L.G.; Rosenblatt, R.A. Is non-metropolitan residence a risk factor for poor birth outcome in the U.S.? Soc. Sci. Med. 1997, 45, 171–188. [Google Scholar] [CrossRef]
- Auger, N.; Authier, M.A.; Martinez, J.; Daniel, M. The association between rural-urban continuum, maternal education and adverse birth outcomes in Québec, Canada. J. Rural Health 2009, 25, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.T.; McClure, L.A.; Zaitchik, B.F.; Gohlke, J.M. Area-level risk factors for adverse birth outcomes: Trends in urban and rural settings. BMC Pregnancy Childbirth 2013, 13. [Google Scholar] [CrossRef]
- Sosniak, W.A.; Kaye, W.E.; Gomez, T.M. Data linkage to explore the risk of low birthweight associated with maternal proximity to hazardous waste sites from the National Priorities List. Arch. Environ. Health 1994, 49, 251–255. [Google Scholar] [CrossRef]
- Goldberg, M.S.; Goulet, L.; Riberdy, H.; Bonvalot, Y. Low birth weight and preterm births among infants born to women living near a municipal solid waste landfill site in Montreal, Quebec. Environ. Res. 1995, 69, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.; Bove, F. Birth weight reduction associated with residence near a hazardous waste landfill. Environ. Health Perspect. 1997, 105, 856–861. [Google Scholar] [CrossRef]
- Baibergenova, A.; Kudyakov, R.; Zdeb, M.; Carpenter, D.O. Low birth weight and residential proximity to PCB-contaminated waste sites. Environ. Health Perspect. 2003, 1352–1357. [Google Scholar] [CrossRef]
- Gilbreath, S.; Kass, P.H. Adverse birth outcomes associated with open dumpsites in Alaska Native Villages. Am. J. Epidemiol. 2006, 164, 518–528. [Google Scholar] [CrossRef]
- Austin, A.A.; Fitzgerald, E.F.; Pantea, C.I.; Gensburg, L.J.; Kim, N.K.; Stark, A.D.; Hwang, S. Reproductive outcomes among former Love Canal residents, Niagara Falls, New York. Environ. Res. 2011, 111, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Bissett, W.T.; Sweeney, A.M. Evaluating geostatistical modeling of exceedance probability as the first step in disease cluster investigations: Very low birth weights near toxic Texas sites. Environ. Health A Glob. Access Sci. Source 2014, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Claus Henn, B.; Ettinger, A.S.; Hopkins, M.R.; Jim, R.; Amarasiriwardena, C.; Christiani, D.C.; Coull, B.A.; Bellinger, D.C.; Wright, R.O. Prenatal arsenic exposure and birth outcomes among a population residing near a mining-related superfund site. Environ. Health Perspect. 2016, 124, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Munger, R.; Isacson, P.; Hu, S.; Burns, T.; Hanson, J.; Lynch, C.F.; Cherryholmes, K.; Van Dorpe, P.; Hausler, W.J. Intrauterine growth retardation in Iowa communities with herbicide-contaminated drinking water supplies. Environ. Health Perspect. 1997, 105, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.D.; Nuckols, J.R.; Stallones, L.; Savitzl, D.A. Exposure to trihalomethanes and adverse pregnancy outcomes. Epidemiology 1998, 9, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Hinckley, A.F.; Bachand, A.M.; Reif, J.S. Late pregnancy exposures to disinfection by-products and growth-related birth outcomes. Environ. Health Perspect. 2005, 113, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Aschengrau, A.; Weinberg, J.; Rogers, S.; Gallagher, L.; Winter, M.; Vieira, V.; Webster, T.; Ozonoff, D. Prenatal exposure to tetrachloroethylene-contaminated drinking water and the risk of adverse birth outcomes. Environ. Health Perspect. 2008, 116, 814–820. [Google Scholar] [CrossRef]
- Ochoa-Acuña, H.; Frankenberger, J.; Hahn, L.; Carbajo, C. Drinking-water herbicide exposure in Indiana and prevalence of small-for-gestational-age and preterm delivery. Environ. Health Perspect. 2009, 117, 1619–1624. [Google Scholar] [CrossRef]
- Forand, S.P.; Lewis-Michl, E.L.; Gomez, M.I. Adverse birth outcomes and maternal exposure to trichloroethylene and tetrachloroethylene through soil vapor intrusion in New York State. Environ. Health Perspect. 2012, 120, 616–621. [Google Scholar] [CrossRef]
- Savitz, D.A.; Stein, C.R.; Elston, B.; Wellenius, G.A.; Bartell, S.M.; Shin, H.; Vieira, V.M.; Fletcher, T. Relationship of perfluorooctanoic acid exposure to pregnancy outcome based on birth records in the Mid-Ohio Valley. Environ. Health Perspect. 2012, 120, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Darrow, L.; Stein, C.; Steenland, K. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005-2010. Environ. Health Perspect. 2013, 121, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Ileka-Priouzeau, S.; Campagna, C.; Legay, C.; Deonandan, R.; Rodriguez, M.J.; Levallois, P. Women exposure during pregnancy to haloacetaldehydes and haloacetonitriles in drinking water and risk of small-for-gestational-age neonate. Environ. Res. 2015, 137, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Donovan, G.H.; Michael, Y.L.; Butry, D.T.; Sullivan, A.D.; Chase, J.M. Urban trees and the risk of poor birth outcomes. Health Place 2011, 17, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Laurent, O.; Wu, J.; Li, L.; Milesi, C. Green spaces and pregnancy outcomes in Southern California. Health Place 2013, 24, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Hystad, P.; Davies, H.W.; Frank, L.; Van Loon, J.; Gehring, U.; Tamburic, L.; Brauer, M. Residential greenness and birth outcomes: Evaluating the influence of spatially correlated built-environment factors. Environ. Health Perspect. 2014, 122, 1095–1102. [Google Scholar] [CrossRef]
- Ebisu, K.; Holford, T.R.; Bell, M.L. Association between greenness, urbanicity, and birth weight. Sci. Total Environ. 2016, 542, 750–756. [Google Scholar] [CrossRef]
- Abelt, K.; McLafferty, S. Green streets: Urban green and birth outcomes. Int. J. Environ. Res. Public Health 2017, 14, 771. [Google Scholar] [CrossRef]
- Cusack, L.; Larkin, A.; Carozza, S.; Hystad, P. Associations between residential greenness and birth outcomes across Texas. Environ. Res. 2017, 152, 88–95. [Google Scholar] [CrossRef]
- Cusack, L.; Larkin, A.; Carozza, S.E.; Hystad, P. Associations between multiple green space measures and birth weight across two US cities. Health Place 2017, 47, 36–43. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, T. Examining extreme weather effects on birth weight from the individual effect to spatiotemporal aggregation effects. J. Agric. Biol. Environ. Stat. 2012, 17, 490–507. [Google Scholar] [CrossRef]
- Thayer, Z.M. The vitamin D hypothesis revisited: Race-based disparities in birth outcomes in the United States and ultraviolet light availability. Am. J. Epidemiol. 2014, 179, 947–955. [Google Scholar] [CrossRef]
- Savard, N.; Levallois, P.; Rivest, L.P.; Gingras, S. Association between prenatal care and small for gestational age birth: An ecological study in Quebec, Canada. Health Promot. Chronic Dis. Prev. Can. Res. Policy Pract. 2016, 36, 121–129. [Google Scholar] [CrossRef]
- Urquia, M.L.; Frank, J.W.; Glazier, R.H. From places to flows. International secondary migration and birth outcomes. Soc. Sci. Med. 2010, 71, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Janevic, T.; Savitz, D.A.; Janevic, M. Maternal education and adverse birth outcomes among immigrant women to the United States from Eastern Europe: A test of the healthy migrant hypothesis. Soc. Sci. Med. 2011, 73, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Mclafferty, S.; Tempalski, B. Restructuring and women’s reproductive health: Implications for low birthweight in New York City. Geoforum 1995, 26, 309–323. [Google Scholar] [CrossRef]
- Tough, S.C.; Svenson, L.W.; Johnston, D.W.; Schopflocher, D. Characteristics of preterm delivery and low birthweight among 113,994 infants in Alberta: 1994-1996. Can. J. Public Health 2001, 92, 276–280. [Google Scholar]
- English, P.B.; Kharrazi, M.; Davies, S.; Scalf, R.; Waller, L.; Neutra, R. Changes in the spatial pattern of low birth weight in a southern California county: The role of individual and neighborhood level factors. Soc. Sci. Med. 2003, 56, 2073–2088. [Google Scholar] [CrossRef]
- Grady, S.C.; Ramírez, I.J. Mediating medical risk factors in the residential segregation and low birthweight relationship by race in New York City. Health Place 2008, 14, 661–677. [Google Scholar] [CrossRef]
- Heaman, M.; Kingston, D.; Chalmers, B.; Sauve, R.; Lee, L.; Young, D. Risk factors for preterm birth and small-for-gestational-age births among Canadian women. Paediatr. Perinat. Epidemiol. 2013, 27, 54–61. [Google Scholar] [CrossRef]
- Aris, A. A 12-year cohort study on adverse pregnancy outcomes in Eastern Townships of Canada: Impact of endometriosis. Gynecol. Endocrinol. 2014, 30, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Jhangri, G.S.; Lacasse, M.; Kumar, M.; Chandra, S. Relationship between interpregnancy interval and adverse perinatal and neonatal outcomes in Northern Alberta. J. Obstet. Gynaecol. Can. 2015, 37, 598–605. [Google Scholar] [CrossRef]
- Shapiro, G.D.; Bushnik, T.; Sheppard, A.J.; Kramer, M.S.; Kaufman, J.S.; Yang, S. Missing paternal data and adverse birth outcomes in Canada. Health Reports 2016, 27, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Jain, L.H.; Van Eyk, N.; Woolcott, C.; Kuhle, S. Characteristics and outcomes of adolescent births in Nova Scotia: A retrospective cohort study. J. Obstet. Gynaecol. Can. 2018, 40, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Gorman, B.K. Racial and ethnic variation in low birthweight in the United States: Individual and contextual determinants. Health Place 1999, 5, 195–207. [Google Scholar] [CrossRef]
- Wenman, W.M.; Joffres, M.R.; Tataryn, I.V. Edmonton Perinatal Infections Group A prospective cohort study of pregnancy risk factors and birth outcomes in Aboriginal women. Can. Med. Assoc. J. 2004, 171, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Vinikoor, L.C.; Kaufman, J.S.; MacLehose, R.F.; Laraia, B.A. Effects of racial density and income incongruity on pregnancy outcomes in less segregated communities. Soc. Sci. Med. 2008, 66, 255–259. [Google Scholar] [CrossRef]
- Reichman, N.E.; Teitler, J.O.; Hamilton, E.R. Effects of neighborhood racial composition on birthweight. Health Place 2009, 15, 814–821. [Google Scholar] [CrossRef]
- Grady, S.C. Racial residential segregation impacts on low birth weight using improved neighborhood boundary definitions. Spat. Spatiotempor. Epidemiol. 2010, 1, 239–249. [Google Scholar] [CrossRef]
- Nepomnyaschy, L. Race disparities in low birth weight in the U.S. South and the rest of the nation. Soc. Sci. Med. 2010, 70, 684–691. [Google Scholar] [CrossRef]
- Anthopolos, R.; James, S.A.; Gelfand, A.E.; Miranda, M.L. A spatial measure of neighborhood level racial isolation applied to low birthweight, preterm birth, and birthweight in North Carolina. Spat. Spatiotempor. Epidemiol. 2011, 2, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Kirby, R.S.; Liu, J.; Lawson, A.B.; Choi, J.; Cai, B.; Hossain, M. Spatio-temporal patterning of small area low birth weight incidence and its correlates: A latent spatial structure approach. Spat. Spatiotempor. Epidemiol. 2011, 2, 265–271. [Google Scholar] [CrossRef]
- Wallace, M.; Harville, E.; Theall, K.; Webber, L.; Chen, W.; Berenson, G. Neighborhood poverty, allostatic load, and birth outcomes in African American and white women: Findings from the Bogalusa Heart Study. Health Place 2013, 24, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Oster, R.T.; Toth, E.L. Longitudinal rates and risk factors for adverse birth weight among First Nations pregnancies in Alberta. J. Obstet. Gynaecol. Can. 2016, 38, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.D.; Sheppard, A.J.; Bushnik, T.; Kramer, M.S.; Mashford-Pringle, A.; Kaufman, J.S.; Yang, S. Adverse birth outcomes and infant mortality according to registered First Nations status and First Nations community residence across Canada. Can. J. Public Health 2018, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, E.C.; Alexander, G.R.; Lewis, N.D.; Mor, J. Geographic patterns of low birth weight in Hawaii. Soc. Sci. Med. 1993, 36, 557–564. [Google Scholar] [CrossRef]
- Krieger, N.; Chen, J.T.; Waterman, P.D.; Soobader, M.-J.; Subramanian, S.V.; Carson, R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US). J. Epidemiol. Community Health 2003, 57, 188–199. [Google Scholar] [CrossRef]
- Farley, T.A.; Mason, K.; Rice, J.; Habel, J.D.; Scribner, R.; Cohen, D.A. The relationship between the neighbourhood environment and adverse birth outcomes. Paediatr. Perinat. Epidemiol. 2006, 20, 188–200. [Google Scholar] [CrossRef]
- Masi, C.M.; Hawkley, L.C.; Piotrowski, Z.H.; Pickett, K.E. Neighborhood economic disadvantage, violent crime, group density, and pregnancy outcomes in a diverse, urban population. Soc. Sci. Med. 2007, 65, 2440–2457. [Google Scholar] [CrossRef]
- Zeka, A.; Melly, S.J.; Schwartz, J. The effects of socioeconomic status and indices of physical environment on reduced birth weight and preterm births in Eastern Massachusetts. Environ. Health 2008, 7, 1–12. [Google Scholar] [CrossRef]
- Young, R.L.; Weinberg, J.; Vieira, V.; Aschengrau, A.; Webster, T.F. A multilevel non-hierarchical study of birth weight and socioeconomic status. Int. J. Health Geogr. 2010, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Tu, W.; Tedders, S.H. Spatial variations in the associations of birth weight with socioeconomic, environmental, and behavioral factors in Georgia, USA. Appl. Geogr. 2012, 34, 331–344. [Google Scholar] [CrossRef]
- Auger, N.; Park, A.L.; Daniel, M. Contribution of local area deprivation to cultural-linguistic inequalities in foetal growth restriction: Trends over time in a Canadian metropolitan centre. Health Place 2013, 22, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Legerski, E.M.; Thayn, J.B. The effects of spatial patterns of neighborhood risk factors on adverse birth outcomes. Soc. Sci. J. 2013, 50, 635–645. [Google Scholar] [CrossRef]
- Meng, G.; Thompson, M.E.; Hall, G.B. Pathways of neighbourhood-level socio-economic determinants of adverse birth outcomes. Int. J. Health Geogr. 2013, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Serrano, J.; Chen, L.; Stieb, D.M.; Jerrett, M.; Osornio-Vargas, A. Development of a Canadian socioeconomic status index for the study of health outcomes related to environmental pollution. BMC Public Health 2015, 15, 714. [Google Scholar] [CrossRef]
- Shmool, J.L.C.; Bobb, J.F.; Ito, K.; Elston, B.; Savitz, D.A.; Ross, Z.; Matte, T.D.; Johnson, S.; Dominici, F.; Clougherty, J.E. Area-level socioeconomic deprivation, nitrogen dioxide exposure, and term birth weight in New York City. Environ. Res. 2015, 142, 624–632. [Google Scholar] [CrossRef]
- Bushnik, T.; Yang, S.; Kaufman, J.S.; Kramer, M.S.; Wilkins, R. Socioeconomic disparities in small-forgestational- age birth and preterm birth. Health Reports 2017, 28, 3–10. [Google Scholar] [CrossRef]
- MacQuillan, E.L.; Curtis, A.B.; Baker, K.M.; Paul, R.; Back, Y.O. Using GIS mapping to target public health interventions: Examining birth outcomes across GIS techniques. J. Community Health 2017, 42, 633–638. [Google Scholar] [CrossRef]
- Campbell, E.E.; Gilliland, J.; Dworatzek, P.D.N.; De Vrijer, B.; Penava, D.; Seabrook, J.A. Socioeconomic status and adverse birth outcomes: A population-based Canadian sample. J. Biosoc. Sci. 2018, 50, 102–113. [Google Scholar] [CrossRef]
- McRae, D.N.; Janssen, P.A.; Vedam, S.; Mayhew, M.; Mpofu, D.; Teucher, U.; Muhajarine, N. Reduced prevalence of small-for-gestational-age and preterm birth for women of low socioeconomic position: A population-based cohort study comparing antenatal midwifery and physician models of care. BMJ Open 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).