Genomic Analysis of West Nile Virus Lineage 1 Detected in Mosquitoes during the 2020–2021 Outbreaks in Andalusia, Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Surveillance and Identification
2.2. Molecular Diagnosis of WNV
2.3. Viral Sequencing and Genome Assembly
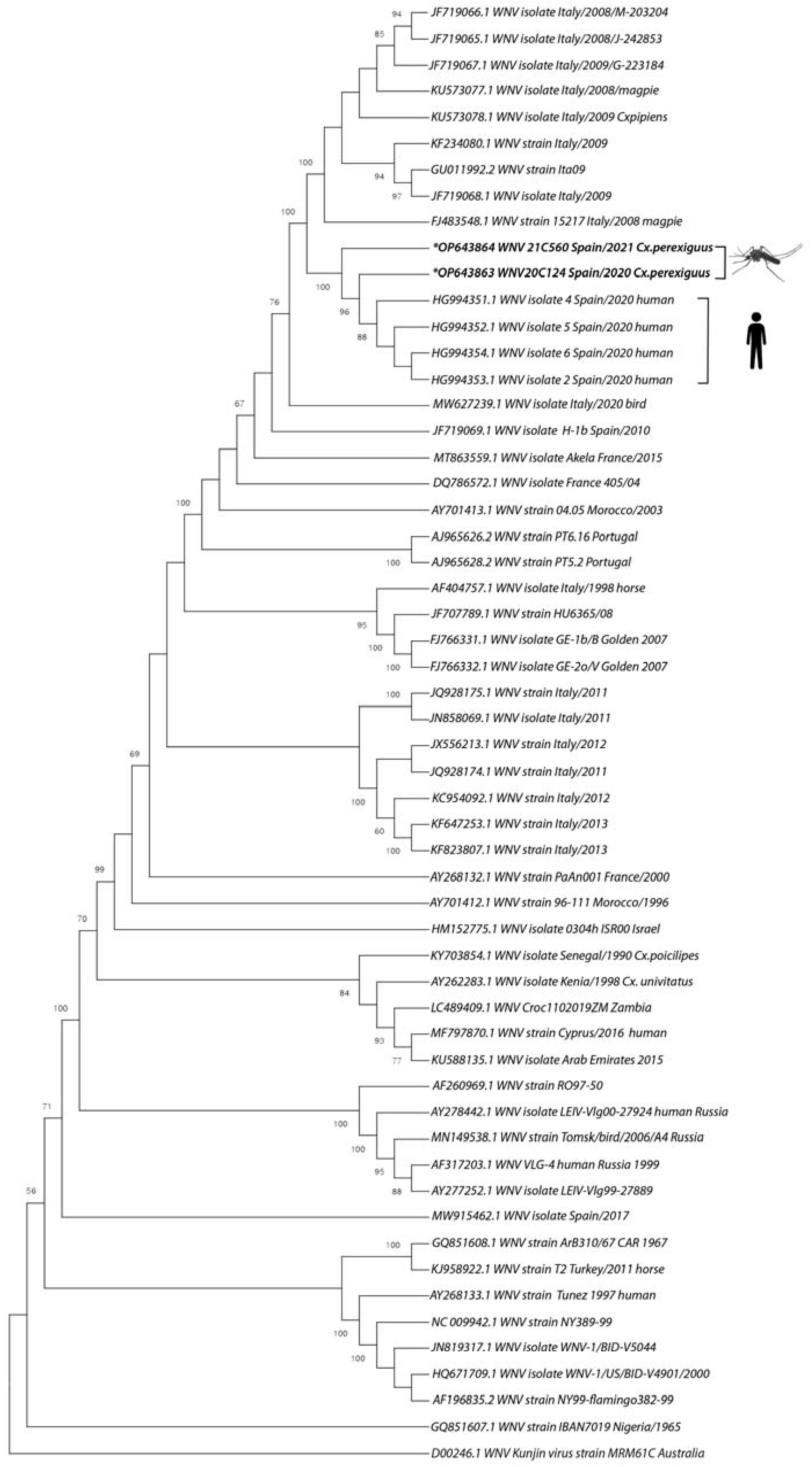
2.4. Phylogenetic Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weissenböck, H.; Hubálek, Z.; Bakonyi, T.; Nowotny, N. Zoonotic Mosquito-Borne Flaviviruses: Worldwide Presence of Agents with Proven Pathogenicity and Potential Candidates of Future Emerging Diseases. Vet. Microbiol. 2010, 140, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Jimenez-Clavero, M.; Leblond, A.; Durand, B.; Nowotny, N.; Leparc-Goffart, I.; Zientara, S.; Jourdain, E.; Lecollinet, S. Flaviviruses in Europe: Complex Circulation Patterns and Their Consequences for the Diagnosis and Control of West Nile Disease. Int. J. Environ. Res. Public Health 2013, 10, 6049–6083. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, E.; Llorente, F.; Jiménez-Clavero, M. Experimental Infections of Wild Birds with West Nile Virus. Viruses 2014, 6, 752–781. [Google Scholar] [CrossRef] [PubMed]
- Murgue, B.; Murri, S.; Triki, H.; Deubel, V.; Zeller, H.G. West Nile in the Mediterranean Basin: 1950–2000. Ann. N. Y. Acad. Sci. 2006, 951, 117–126. [Google Scholar] [CrossRef]
- Rizzoli, A.; Jiménez-Clavero, M.A.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The Challenge of West Nile Virus in Europe: Knowledge Gaps and Research Priorities. Eurosurveillance 2015, 20. [Google Scholar] [CrossRef]
- Barrett, A.D.T. West Nile in Europe: An Increasing Public Health Problem. J. Travel Med. 2018, 25, tay096. [Google Scholar] [CrossRef]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The Global Ecology and Epidemiology of West Nile Virus. BioMed Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef]
- Zehender, G.; Ebranati, E.; Bernini, F.; Presti, A.L.; Rezza, G.; Delogu, M.; Galli, M.; Ciccozzi, M. Phylogeography and Epidemiological History of West Nile Virus Genotype 1a in Europe and the Mediterranean Basin. Infect. Genet. Evol. 2011, 11, 646–653. [Google Scholar] [CrossRef]
- Aguilera-Sepúlveda, P.; Napp, S.; Llorente, F.; Solano-Manrique, C.; Molina-López, R.; Obón, E.; Solé, A.; Jiménez-Clavero, M.Á.; Fernández-Pinero, J.; Busquets, N. West Nile Virus Lineage 2 Spreads Westwards in Europe and Overwinters in North-Eastern Spain (2017–2020). Viruses 2022, 14, 569. [Google Scholar] [CrossRef]
- Figuerola, J.; Angel Jiménez-Clavero, M.; Rojo, G.; Gómez-Tejedor, C.; Soriguer, R. Prevalence of West Nile Virus Neutralizing Antibodies in Colonial Aquatic Birds in Southern Spain. Avian Pathol. 2007, 36, 209–212. [Google Scholar] [CrossRef]
- Figuerola, J.; Soriguer, R.; Rojo, G.; Tejedor, C.G.; Jimenez-Clavero, M.A. Seroconversion in Wild Birds and Local Circulation of West Nile Virus, Spain. Emerg. Infect. Dis. 2007, 13, 1915–1917. [Google Scholar] [CrossRef] [PubMed]
- Höfle, U.; Blanco, J.M.; Crespo, E.; Naranjo, V.; Jiménez-Clavero, M.A.; Sanchez, A.; de la Fuente, J.; Gortazar, C. West Nile Virus in the Endangered Spanish Imperial Eagle. Vet. Microbiol. 2008, 129, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, A.; Sánchez-Seco, M.P.; Ruiz, S.; Molero, F.; Hernández, L.; Moreno, J.; Magallanes, A.; Tejedor, C.G.; Tenorio, A. Putative New Lineage of West Nile Virus, Spain. Emerg. Infect. Dis. 2010, 16, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, A.; Ruiz, S.; Herrero, L.; Moreno, J.; Molero, F.; Magallanes, A.; Sánchez-Seco, M.P.; Figuerola, J.; Tenorio, A. West Nile and Usutu Viruses in Mosquitoes in Spain, 2008–2009. Am. J. Trop. Med. Hyg. 2011, 85, 178–181. [Google Scholar] [CrossRef]
- Jiménez-Clavero, M.A.; Sotelo, E.; Fernandez-Pinero, J.; Llorente, F.; Blanco, J.M.; Rodriguez-Ramos, J.; Perez-Ramirez, E.; Höfle, U. West Nile Virus in Golden Eagles, Spain, 2007. Emerg. Infect. Dis. 2008, 14, 1489–1491. [Google Scholar] [CrossRef]
- Jiménez-Clavero, M.A.; Llorente, F.; Sotelo, E.; Soriguer, R.; Gómez-Tejedor, C.; Figuerola, J. West Nile Virus Serosurveillance in Horses in Doñana, Spain, 2005 to 2008. Vet. Rec. 2010, 167, 379–380. [Google Scholar] [CrossRef]
- Kaptoul, D.; Viladrich, P.F.; Domingo, C.; Niubó, J.; Martínez-Yélamos, S.; De Ory, F.; Tenorio, A. West Nile Virus in Spain: Report of the First Diagnosed Case (in Spain) in a Human with Aseptic Meningitis. Scand. J. Infect. Dis. 2007, 39, 70–71. [Google Scholar] [CrossRef]
- García-Bocanegra, I.; Jaén-Téllez, J.A.; Napp, S.; Arenas-Montes, A.; Fernández-Morente, M.; Fernández-Molera, V.; Arenas, A. West Nile Fever Outbreak in Horses and Humans, Spain, 2010. Emerg. Infect. Dis. 2011, 17, 2397–2399. [Google Scholar] [CrossRef]
- García San Miguel Rodríguez-Alarcón, L.; Fernández-Martínez, B.; Sierra Moros, M.J.; Vázquez, A.; Julián Pachés, P.; García Villacieros, E.; Gómez Martín, M.B.; Figuerola Borras, J.; Lorusso, N.; Ramos Aceitero, J.M.; et al. Unprecedented Increase of West Nile Virus Neuroinvasive Disease, Spain, Summer 2020. Eurosurveillance 2021, 26, 2002010. [Google Scholar] [CrossRef]
- ECD Epidemological Update. Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2021 (accessed on 24 January 2022).
- Aguilera-Sepúlveda, P.; Gómez-Martín, B.; Agüero, M.; Jiménez-Clavero, M.Á.; Fernández-Pinero, J. A New Cluster of West Nile Virus Lineage 1 Isolated from a Northern Goshawk in Spain. Transbounding Emerg. Dis. 2022, 69, 3121–3127. [Google Scholar] [CrossRef]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Schaffner, F.; Angel, G.; Geoffroy, B.; Hervy, J.P.; Rhaiem, A.; Brunhes, J. The Mosquitoes of Europe, an Identification and Training Programme; CD-Rom; IRD Editions: Montpellier, France, 2001. [Google Scholar]
- Figuerola, J.; Jiménez-Clavero, M.Á.; Ruíz-López, M.J.; Llorente, F.; Ruiz, S.; Hoefer, A.; Aguilera-Sepúlveda, P.; Jiménez-Peñuela, J.; García-Ruiz, O.; Herrero, L.; et al. A One Health View of the West Nile Virus Outbreak in Andalusia (Spain) in 2020. Emerg. Microbes Infect. 2022, 11, 2570–2578. [Google Scholar] [CrossRef]
- Vázquez, A.; Herrero, L.; Negredo, A.; Hernández, L.; Sánchez-Seco, M.P.; Tenorio, A. Real Time PCR Assay for Detection of All Known Lineages of West Nile Virus. J. Virol. Methods 2016, 236, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Varona, S.; Monzón, S.; Espinosa-Carrasco, J.; Heuer, M.L.; Underwood, A.; Gabernet, G.; Bot, N.C.; Ewels, P.; Miguel, J.; et al. nf-core/viralrecon: Nf-core/viralrecon v2.4.1—Plastered Magnesium Marmoset. Zenodo 2022. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 24 January 2022).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Lu, J.; Langmead, B. Improved Metagenomic Analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Grubaugh, N.D.; Gangavarapu, K.; Quick, J.; Matteson, N.L.; De Jesus, J.G.; Main, B.J.; Tan, A.L.; Paul, L.M.; Brackney, D.E.; Grewal, S.; et al. An Amplicon-Based Sequencing Framework for Accurately Measuring Intrahost Virus Diversity Using PrimalSeq and IVar. Genome Biol. 2019, 20, 8. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A Program for Annotating and Predicting the Effects of Single Nucleotide Polymorphisms, SnpEff: SNPs in the Genome of Drosophila Melanogaster Strain w 1118; Iso-2; Iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef]
- Cingolani, P.; Patel, V.M.; Coon, M.; Nguyen, T.; Land, S.J.; Ruden, D.M.; Lu, X. Using Drosophila Melanogaster as a Model for Genotoxic Chemical Mutational Studies with a New Program, SnpSift. Front. Gene. 2012, 3, 35. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A Flexible Suite of Utilities for Comparing Genomic Features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- May, F.J.; Davis, C.T.; Tesh, R.B.; Barrett, A.D.T. Phylogeography of West Nile Virus: From the Cradle of Evolution in Africa to Eurasia, Australia, and the Americas. J. Virol. 2011, 85, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- Calistri, P.; Giovannini, A.; Savini, G.; Monaco, F.; Bonfanti, L.; Ceolin, C.; Terregino, C.; Tamba, M.; Cordioli, P.; Lelli, R. West Nile Virus Transmission in 2008 in North-Eastern Italy. Zoonoses Public Health 2010, 57, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, E.; Fernández-Pinero, J.; Llorente, F.; Vázquez, A.; Moreno, A.; Agüero, M.; Cordioli, P.; Tenorio, A.; Jiménez-Clavero, M.Á. Phylogenetic Relationships of Western Mediterranean West Nile Virus Strains (1996–2010) Using Full-Length Genome Sequences: Single or Multiple Introductions? J. Gen. Virol. 2011, 92, 2512–2522. [Google Scholar] [CrossRef]
- Rudolf, I.; Betášová, L.; Blažejová, H.; Venclíková, K.; Straková, P.; Šebesta, O.; Mendel, J.; Bakonyi, T.; Schaffner, F.; Nowotny, N.; et al. West Nile Virus in Overwintering Mosquitoes, Central Europe. Parasites Vectors 2017, 10, 452. [Google Scholar] [CrossRef]
- Casimiro-Soriguer, C.S.; Perez-Florido, J.; Fernandez-Rueda, J.L.; Pedrosa-Corral, I.; Guillot-Sulay, V.; Lorusso, N.; Martinez-Gonzalez, L.J.; Navarro-Marí, J.M.; Dopazo, J.; Sanbonmatsu-Gámez, S. Phylogenetic Analysis of the 2020 West Nile Virus (WNV) Outbreak in Andalusia (Spain). Viruses 2021, 13, 836. [Google Scholar] [CrossRef]

| Sample | 20c124 | 21c560 |
|---|---|---|
| Total reads | 2,225,818 | 2,126,416 |
| Reads host R1 | 865,285 | 920,637 |
| Reads host | 1,730,570 | 1,841,274 |
| %reads host | 77.75 | 86.59 |
| Reads virus | 475,496 | 275,042 |
| %reads virus | 21.36 | 12.93 |
| Unmapped reads | 19,752 | 10,100 |
| %unmaped reads | 0.89 | 0.47 |
| medianDPcoverage virus | 5985 | 3411 |
| Coverage > 10×(%) | 100 | 100 |
| Variants in consensus ×10 | 139 | 144 |
| Missense Variants | 12 | 12 |
| %Ns10× | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-López, M.J.; Muñoz-Chimeno, M.; Figuerola, J.; Gavilán, A.M.; Varona, S.; Cuesta, I.; Martínez-de la Puente, J.; Zaballos, Á.; Molero, F.; Soriguer, R.C.; et al. Genomic Analysis of West Nile Virus Lineage 1 Detected in Mosquitoes during the 2020–2021 Outbreaks in Andalusia, Spain. Viruses 2023, 15, 266. https://doi.org/10.3390/v15020266
Ruiz-López MJ, Muñoz-Chimeno M, Figuerola J, Gavilán AM, Varona S, Cuesta I, Martínez-de la Puente J, Zaballos Á, Molero F, Soriguer RC, et al. Genomic Analysis of West Nile Virus Lineage 1 Detected in Mosquitoes during the 2020–2021 Outbreaks in Andalusia, Spain. Viruses. 2023; 15(2):266. https://doi.org/10.3390/v15020266
Chicago/Turabian StyleRuiz-López, María José, Milagros Muñoz-Chimeno, Jordi Figuerola, Ana M. Gavilán, Sarai Varona, Isabel Cuesta, Josué Martínez-de la Puente, Ángel Zaballos, Francisca Molero, Ramón C. Soriguer, and et al. 2023. "Genomic Analysis of West Nile Virus Lineage 1 Detected in Mosquitoes during the 2020–2021 Outbreaks in Andalusia, Spain" Viruses 15, no. 2: 266. https://doi.org/10.3390/v15020266
APA StyleRuiz-López, M. J., Muñoz-Chimeno, M., Figuerola, J., Gavilán, A. M., Varona, S., Cuesta, I., Martínez-de la Puente, J., Zaballos, Á., Molero, F., Soriguer, R. C., Sánchez-Seco, M. P., Ruiz, S., & Vázquez, A. (2023). Genomic Analysis of West Nile Virus Lineage 1 Detected in Mosquitoes during the 2020–2021 Outbreaks in Andalusia, Spain. Viruses, 15(2), 266. https://doi.org/10.3390/v15020266






