Bulk Chemical and Optical Spectroscopy Characterisations of Dissolved Organic Matter Extracted from the Tropical Coastal Sediment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area, Sample Collection and Sample Pre-Treatment
2.2. Bulk Marine Sediment Analysis
2.3. Extraction of DOM from Marine Sediment
2.4. UV-Vis Spectroscopy Characterization
2.5. Fluorescent Spectroscopy
2.6. Attenuated Total Reflectance Infrared ATR-FTIR Analysis
2.7. Statistical Analysis
3. Results
3.1. Properties of Bulk Marine Sediment
3.2. Elemental Analysis of DOM
3.3. DOC in Extracted DOM
3.4. UV-Visspectroscopy Analysis
3.5. Fluorescent Properties of DOM
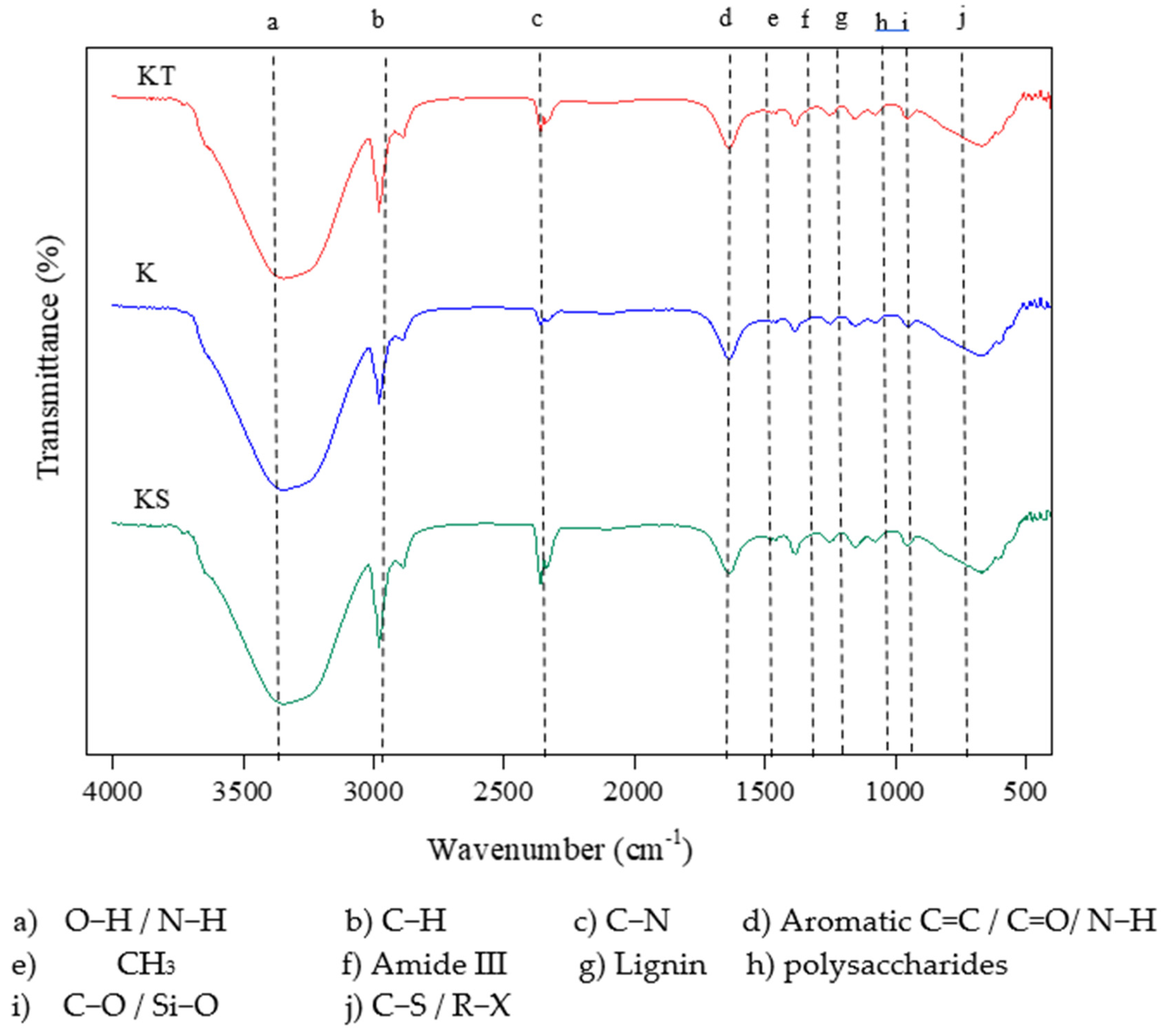
3.6. DOM ATR-FTIR Spectra
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carreira, R.S.; Cordeiro, L.G.M.S.; Bernardes, M.C.; Hatje, V. Distribution and characterization of organic matter using lipid biomarkers: A case study in a pristine tropical bay in NE Brazil. Estuar. Coast. Shelf Sci. 2016, 168, 1–9. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Saubusse, S.; Etcheber, H.; Abril, G.; Relexans, S.; Ibalot, F.; Parlanti, E. New insights into the size distribution of fluorescent dissolved organic matter in estuarine waters. Org. Geochem. 2010, 41, 595–610. [Google Scholar] [CrossRef]
- Kolic, P.E.; Roy, E.D.; White, J.R.; Cook, R.L. Spectroscopic measurements of estuarine dissolved organic matter dynamics during a large-scale Mississippi River flood diversion. Sci. Total Environ. 2014, 485–486, 518–527. [Google Scholar] [CrossRef] [PubMed]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Traversa, A.; D’Orazio, V.; Mezzapesa, G.N.; Bonifacio, E.; Farrag, K.; Senesi, N.; Brunetti, G. Chemical and spectroscopic characteristics of humic acids and dissolved organic matter along two Alfisol profiles. Chemosphere 2014, 111, 184–194. [Google Scholar] [CrossRef]
- Hur, J.; Kim, G. Comparison of the heterogeneity within bulk sediment humic substances from a stream and reservoir via selected operational descriptors. Chemosphere 2009, 75, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.B.; Novotny, E.H.; Bloise, A.C.; de Azevedo, E.R.; Bonagamba, T.J.; Zucchi, M.R.; Santos, V.L.C.S.; Azevedo, A.E.G. Characterization of organic matter in sediment cores of the Todos os Santos Bay, Bahia, Brazil, by elemental analysis and 13C NMR. Mar. Pollut. Bull. 2011, 62, 1883–1890. [Google Scholar] [CrossRef] [Green Version]
- Giovanela, M.; Crespo, J.S.; Antunes, M.; Adamatti, D.S.; Fernandes, A.N.; Barison, A.; Da Silva, C.W.P.; Guégan, R.; Motelica-Heino, M.; Sierra, M.M.D. Chemical and spectroscopic characterization of humic acids extracted from the bottom sediments of a Brazilian subtropical microbasin. J. Mol. Struct. 2010, 981, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Guo, L. Molecular size-dependent abundance and composition of dissolved organic matter in river, lake and sea waters. Water Res. 2017, 117, 115–126. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bünemann, E.K.; Oguejiofor, C.U.; Meier, J.; Gort, G.; Comans, R.; Mäder, P.; Brussaard, L.; de Goede, R. Sensitivity of labile carbon fractions to tillage and organic matter management and their potential as comprehensive soil quality indicators across pedoclimatic conditions in Europe. Ecol. Indic. 2019, 99, 38–50. [Google Scholar] [CrossRef]
- Sarkar, A.; Chakraborty, P.; Nagender Nath, B. Distribution and nature of sedimentary organic matter in a tropical estuary: An indicator of human intervention on environment. Mar. Pollut. Bull. 2016, 102, 176–186. [Google Scholar] [CrossRef]
- Lin, H.; Cai, Y.; Sun, X.; Chen, G.; Huang, B.; Cheng, H.; Chen, M. Sources and mixing behavior of chromophoric dissolved organic matter in the Taiwan Strait. Mar. Chem. 2016, 187, 43–56. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, G.P.; Liu, L.; Zhang, P.Y.; Leng, W.S. Sources, behaviors and degradation of dissolved organic matter in the East China Sea. J. Mar. Syst. 2016, 155, 84–97. [Google Scholar] [CrossRef]
- Liow, G.R.; Lau, W.L.S.; Law, I.K.; Hii, K.S.; Mohammad Noor, N.; Leaw, C.P.; Lim, P.T. Phytoplankton community changes in Kuantan Port (Malaysia), with emphasis on the paralytic-shellfish toxin-producing dinoflagellate Alexandrium tamiyavanichii. Reg. Stud. Mar. Sci. 2019, 26, 100504. [Google Scholar] [CrossRef]
- Santos, E.C.; Celino, J.J.; Santos, V.L.C.S.; Bispo De Souza, J.R. Source and distribution of organic matter in surface sediments from mangroves on the island of Itaparica, Bahia/Brazil. Environ. Monit. Assess. 2013, 185, 9857–9869. [Google Scholar] [CrossRef]
- Yin, Y.; Impellitteri, C.A.; You, S.-J.; Allen, H.E. The importance of organic matter distribution and extract soil:solution ratio on the desorption of heavy metals from soils. Sci. Total Environ. 2002, 287, 107–119. [Google Scholar] [CrossRef]
- Wang, W.; Lai, D.Y.F.; Wang, C.; Pan, T.; Zeng, C. Effects of rice straw incorporation on active soil organic carbon pools in a subtropical paddy field. Soil Tillage Res. 2015, 152, 8–16. [Google Scholar] [CrossRef]
- Wang, S.; Jiao, L.; Yang, S.; Jin, X.; Liang, H.; Wu, F. Organic matter compositions and DOM release from the sediments of the shallow lakes in the middle and lower reaches of Yangtze River region, China. Appl. Geochemistry 2011, 26, 1458–1463. [Google Scholar] [CrossRef]
- Yang, K.; Miao, G.; Wu, W.; Lin, D.; Pan, B.; Wu, F.; Xing, B. Sorption of Cu2+ on humic acids sequentially extracted from a sediment. Chemosphere 2015, 138, 657–663. [Google Scholar] [CrossRef]
- Guigue, J.; Mathieu, O.; Leveque, J.; Mounier, S.; Laffont, R.; Maron, P.A.; Navarro, N.; Chateua, C.; Amiotte-Suchet, P.; Lucas, Y. A comparison of extraction procedures for water-extractable organic matter in soils. Eur. J. Soil Sci. 2014, 65, 520–530. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Asefaw, A.; Xiao, H.; Liu, L.; Wang, D.; Peng, H.; Zeng, G. Characterizing dissolved organic matter in eroded sediments from a loess hilly catchment using fl uorescence EEM-PARAFAC and UV – Visible absorption: Insights from source identi fi cation and carbon cycling. Geoderma 2019, 334, 37–48. [Google Scholar] [CrossRef]
- Kalbitz, K.; Geyer, S.; Geyer, W. A comparative characterization of dissolved organic matter by means of original aqueous samples and isolated humic substances. Chemosphere 2000, 40, 1305–1312. [Google Scholar] [CrossRef]
- He, W.; Lee, J.-H.; Hur, J. Anthropogenic signature of sediment organic matter probed by UV–Visible and fluorescence spectroscopy and the association with heavy metal enrichment. Chemosphere 2016, 150, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Shen, Z.; Chen, J.; Feng, C. Characterization and spacial distribution variability of chromophoric dissolved organic matter (CDOM) in the Yangtze Estuary. Chemosphere 2014, 95, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, S.; Dong, H.; Zhao, Q.; Lu, X.; Shi, J. An analysis of organic matter sources for surface sediments in the central South Yellow Sea, China: Evidence based on macroelements and n-alkanes. Mar. Pollut. Bull. 2014, 88, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.Y.; Tay, J.H.; Suratman, S.; Simoneit, B.R.T.; Mohd Tahir, N. Input of organic matter in Brunei Bay, East Malaysia, as indicated by sedimentary steroids and multivariate statistics. Mar. Pollut. Bull. 2020, 156, 111269. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhang, H.; Yuan, Z.; Sun, Y.; Zhao, M. Terrestrial and marine biomarker estimates of organic matter sources and distributions in surface sediments from the East China Sea shelf. Cont. Shelf Res. 2011, 31, 1106–1115. [Google Scholar] [CrossRef]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Duan, L. Distribution and contamination of heavy metals in surface sediments of the South Yellow Sea. Mar. Pollut. Bull. 2012, 64, 2151–2159. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Shen, Z.; Feng, C.; Zhang, X. Investigation of the interaction between As and Sb species and dissolved organic matter in the Yangtze Estuary, China, using excitation–emission matrices with parallel factor analysis. Environ. Sci. Pollut. Res. 2014, 22, 1819–1830. [Google Scholar] [CrossRef]
- Fong, S.S.; Mohamed, M. Chemical characterization of humic substances occurring in the peats of Sarawak, Malaysia. Org. Geochem. 2007, 38, 967–976. [Google Scholar] [CrossRef]
- He, M.; Shi, Y.; Lin, C. Characterization of humic acids extracted from the sediments of the various rivers and lakes in China. J. Environ. Sci. 2008, 20, 1294–1299. [Google Scholar] [CrossRef]
- Timofeevna Shirshova, L.; Ghabbour, E.A.; Davies, G. Spectroscopic characterization of humic acid fractions isolated from soil using different extraction procedures. Geoderma 2006, 133, 204–216. [Google Scholar] [CrossRef]
- Ogawa, Y.; Okamoto, Y.; Sadaba, R.B.; Kanzaki, M. Sediment organic matter source estimation and ecological classification in the semi-enclosed Batan Bay Estuary, Philippines. Int. J. Sediment Res. 2021, 36, 110–119. [Google Scholar] [CrossRef]
- Li, Z.; Huang, M.; Luo, N.; Wen, J.; Deng, C. Spectroscopic study of the effects of dissolved organic matter compositional changes on availability of cadmium in paddy soil under different water management practices. Chemosphere 2019, 225, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Janzen, H.H.; Campbell, C.A.; Brandt, S.A.; Lafond, G.P.; Townley-Smith, L. Light-Fraction Organic Matter in Soils from Long-term Crop Rotations. Soil Sci. Soc. Am. J. 1992, 56, 1799–1806. [Google Scholar] [CrossRef] [Green Version]
- Xi, M.; Zi, Y.; Wang, Q.; Wang, S.; Cui, G.; Kong, F. Assessment of the content, structure, and source of soil dissolved organic matter in the coastal wetlands of Jiaozhou Bay, China. Phys. Chem. Earth 2018, 103, 35–44. [Google Scholar] [CrossRef]
- Chen, J.; Gu, B.; LeBoeuf, E.J.; Pan, H.; Dai, S. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere 2002, 48, 59–68. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wang, S.; Zhao, H.; Zhang, R. Composition, structural characteristics and indication of water quality of dissolved organic matter in Dongting Lake sediments. Ecol. Eng. 2016, 97, 370–380. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, T.; Zhu, Y.; Qu, X.; He, Z.; Giesy, J.P.; Meng, W. Molecular characterization of macrophyte-derived dissolved organic matters and their implications for lakes. Sci. Total Environ. 2018, 616–617, 602–613. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Schlenger, P.; García-Valverde, M. Monitoring changes in the structure and properties of humic substances following ozonation using UV-Vis, FTIR and1H NMR techniques. Sci. Total Environ. 2016, 541, 623–637. [Google Scholar] [CrossRef]
- Wu, D.; Ren, C.; Jiang, L.; Li, Q.; Zhang, W.; Wu, C. Characteristic of dissolved organic matter polar fractions with variable sources by spectrum technologies: Chemical properties and interaction with phenoxy herbicide. Sci. Total Environ. 2020, 724, 138262. [Google Scholar] [CrossRef]
- Li, Y.; Harir, M.; Lucio, M.; Gonsior, M.; Koch, B.P.; Schmitt-Kopplin, P.; Hertkorn, N. Comprehensive structure-selective characterization of dissolved organic matter by reducing molecular complexity and increasing analytical dimensions. Water Res. 2016, 106, 477–487. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of Specific Ultraviolet Absorbance as an Indicator of the Chemical Composition and Reactivity of Dissolved Organic Carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Wang, M.; Qin, B. Compositional differences of chromophoric dissolved organic matter derived from phytoplankton and macrophytes. Org. Geochem. 2013, 55, 26–37. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Skyllberg, U.; Björn, E.; Green, N.W.; Tang, J.; Wang, D.; Gao, J.; Li, C. Characteristics of dissolved organic matter (DOM) and relationship with dissolved mercury in Xiaoqing River-Laizhou Bay estuary, Bohai Sea, China. Environ. Pollut. 2017, 223, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osburn, C.L.; Stedmon, C.A. Linking the chemical and optical properties of dissolved organic matter in the Baltic–North Sea transition zone to differentiate three allochthonous inputs. Mar. Chem. 2011, 126, 281–294. [Google Scholar] [CrossRef]
- Harvey, E.T.; Kratzer, S.; Andersson, A. Relationships between colored dissolved organic matter and dissolved organic carbon in different coastal gradients of the Baltic Sea. Ambio 2015, 44, 392–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, S.; Zhang, L. Composition, source characteristic and indication of eutrophication of dissolved organic matter in the sediments of Erhai Lake. Environ. Earth Sci. 2015, 74, 3739–3751. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Zhang, L.; Zhao, H.; Jiao, L.; Zhao, Y.; He, X. Composition and spectroscopic characteristics of dissolved organic matter extracted from the sediment of Erhai Lake in China. J. Soils Sediments 2014, 14, 1599–1611. [Google Scholar] [CrossRef]
- Couturier, M.; Nozais, C.; Chaillou, G. Microtidal subterranean estuaries as a source of fresh terrestrial dissolved organic matter to the coastal ocean. Mar. Chem. 2016, 186, 46–57. [Google Scholar] [CrossRef]
- Klučáková, M.; Kalina, M. Composition, particle size, charge, and colloidal stability of pH-fractionated humic acids. J. Soils Sediments 2015, 15, 1900–1908. [Google Scholar] [CrossRef]
- Yang, L.; Han, D.H.; Lee, B.-M.; Hur, J. Characterizing treated wastewaters of different industries using clustered fluorescence EEM–PARAFAC and FT-IR spectroscopy: Implications for downstream impact and source identification. Chemosphere 2015, 127, 222–228. [Google Scholar] [CrossRef]
- Haberhauer, G.; Rafferty, B.; Strebl, F.; Gerzabek, M.H. Comparison of the composition of forest soil litter derived from three different sites at various decompositional stages using FTIR spectroscopy. Geoderma 1998, 83, 331–342. [Google Scholar] [CrossRef]
- Praetzel, L.S.E.; Plenter, N.; Schilling, S.; Schmiedeskamp, M.; Broll, G.; Knorr, K.H. Organic matter and sediment properties determine in-lake variability of sediment CO2 and CH4 production and emissions of a small and shallow lake. Biogeosciences 2020, 17, 5057–5078. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, J.; Zhang, F.; Yu, Y.; Zhang, J. Chemical characterization of humic substances isolated from mangrove swamp sediments: The Qinglan area of Hainan Island, China. Estuar. Coast. Shelf Sci. 2011, 93, 220–227. [Google Scholar] [CrossRef]
- Prasad, M.B.K.; Kumar, A.; Ramanathan, A.L.; Datta, D.K. Sources and dynamics of sedimentary organic matter in Sundarban mangrove estuary from Indo-Gangetic delta. Ecol. Process. 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Li, Y.; Kong, F.; Zhang, J.; Xi, M. Source, structural characteristics and ecological indication of dissolved organic matter extracted from sediments in the primary tributaries of the Dagu River. Ecol. Indic. 2020, 109, 105776. [Google Scholar] [CrossRef]
- Baran, A.; Mierzwa-Hersztek, M.; Gondek, K.; Tarnawski, M.; Szara, M.; Gorczyca, O.; Koniarz, T. The influence of the quantity and quality of sediment organic matter on the potential mobility and toxicity of trace elements in bottom sediment. Environ. Geochem. Health 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osburn, C.L.; Boyd, T.J.; Montgomery, M.T.; Bianchi, T.S.; Coffin, R.B.; Paerl, H.W. Optical Proxies for Terrestrial Dissolved Organic Matter in Estuaries and Coastal Waters. Front. Mar. Sci. 2016, 2, 127. [Google Scholar] [CrossRef] [Green Version]
- Bergamino, L.; Rodríguez-Gallego, L.; Pérez-Parada, A.; Chialanza, M.R.; Amaral, V.; Perez, L.; Scarabino, F.; Lescano, C.; García-Sposito, C.; Costa, S.; et al. Autochthonous organic carbon contributions to the sedimentary pool: A multi-analytical approach in Laguna Garzón. Org. Geochem. 2018, 125, 55–65. [Google Scholar] [CrossRef]
- Bueno, C.; Brugnoli, E.; Bergamino, L.; Muniz, P.; García-Rodríguez, F.; Figueira, R. Anthropogenic and natural variability in the composition of sedimentary organic matter of the urbanised coastal zone of Montevideo (Río de la Plata). Mar. Pollut. Bull. 2018, 126, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Gardade, L.; Khandeparker, L. Sedimentary organic matter composition from tropical ports with distinct geographic and morpho-hydrodynamic characteristics: Evaluation through multiple biochemical markers. Mar. Pollut. Bull. 2020, 160, 111679. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Halimoon, N.; Aris, A.Z.; Zakaria, M.P.; Vaezzadeh, V.; Magam, S.M.; Mustafa, S.; Ali, M.M.; Keshavarzifard, M.; Alkhadher, S.A.A.; et al. Seasonal variability of anthropogenic indices of PAHs in sediment from the Kuala Selangor River, west coast Peninsular Malaysia. Environ. Geochem. Health 2018, 40, 2551–2572. [Google Scholar] [CrossRef]
- Zhou, Y.-W.; Zhao, B.; Peng, Y.-S.; Chen, G.-Z. Influence of mangrove reforestation on heavy metal accumulation and speciation in intertidal sediments. Mar. Pollut. Bull. 2010, 60, 1319–1324. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, Z.; Wu, Y.; Liu, S.; Cui, L.; Zhang, J.; Bay, D. Origins of sediment organic matter and their contributions at three contrasting wetlands in a coastal semi-enclosed ecosystem. Mar. Pollut. Bull. 2019, 139, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Damak, M.; Fourati, R.; Ellech, B.; Kallel, M. Assessment of organic and metallic contamination in the surface sediment of Monastir Bay (Eastern Tunisia): Spatial distribution, potential sources, and ecological risk assessment. Mar. Pollut. Bull. 2019, 149, 110500. [Google Scholar] [CrossRef] [PubMed]
- Habibullah-Al-Mamun, M.; Ahmed, M.K.; Raknuzzaman, M.; Islam, M.S.; Negishi, J.; Nakamichi, S.; Sekine, M.; Tokumura, M.; Masunaga, S. Occurrence and distribution of perfluoroalkyl acids (PFAAs) in surface water and sediment of a tropical coastal area (Bay of Bengal coast, Bangladesh). Sci. Total Environ. 2016, 571, 1089–1104. [Google Scholar] [CrossRef]
- Marziah, Z.; Mahdzir, A.; Musa, M.N.; Jaafar, A.B.; Azhim, A.; Hara, H. Abundance of sulfur-degrading bacteria in a benthic bacterial community of shallow sea sediment in the off-Terengganu coast of the South China Sea. Microbiologyopen 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mathew, J.; Gopinath, A.; Vareed, R.A. Spectroscopic characterization of humic substances isolated from tropical mangrove sediments. Arab. J. Geosci. 2021, 14. [Google Scholar] [CrossRef]
- Lu, C.J.; Benner, R.; Fichot, C.G.; Fukuda, H.; Yamashita, Y.; Ogawa, H. Sources and transformations of dissolved lignin phenols and chromophoric dissolved organic matter in Otsuchi Bay, Japan. Front. Mar. Sci. 2016, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Pempkowiak, J. Limitation of lignin derivatives as biomarkers of land derived organic matter in the coastal marine sediments. Oceanologia 2020, 62, 374–386. [Google Scholar] [CrossRef]
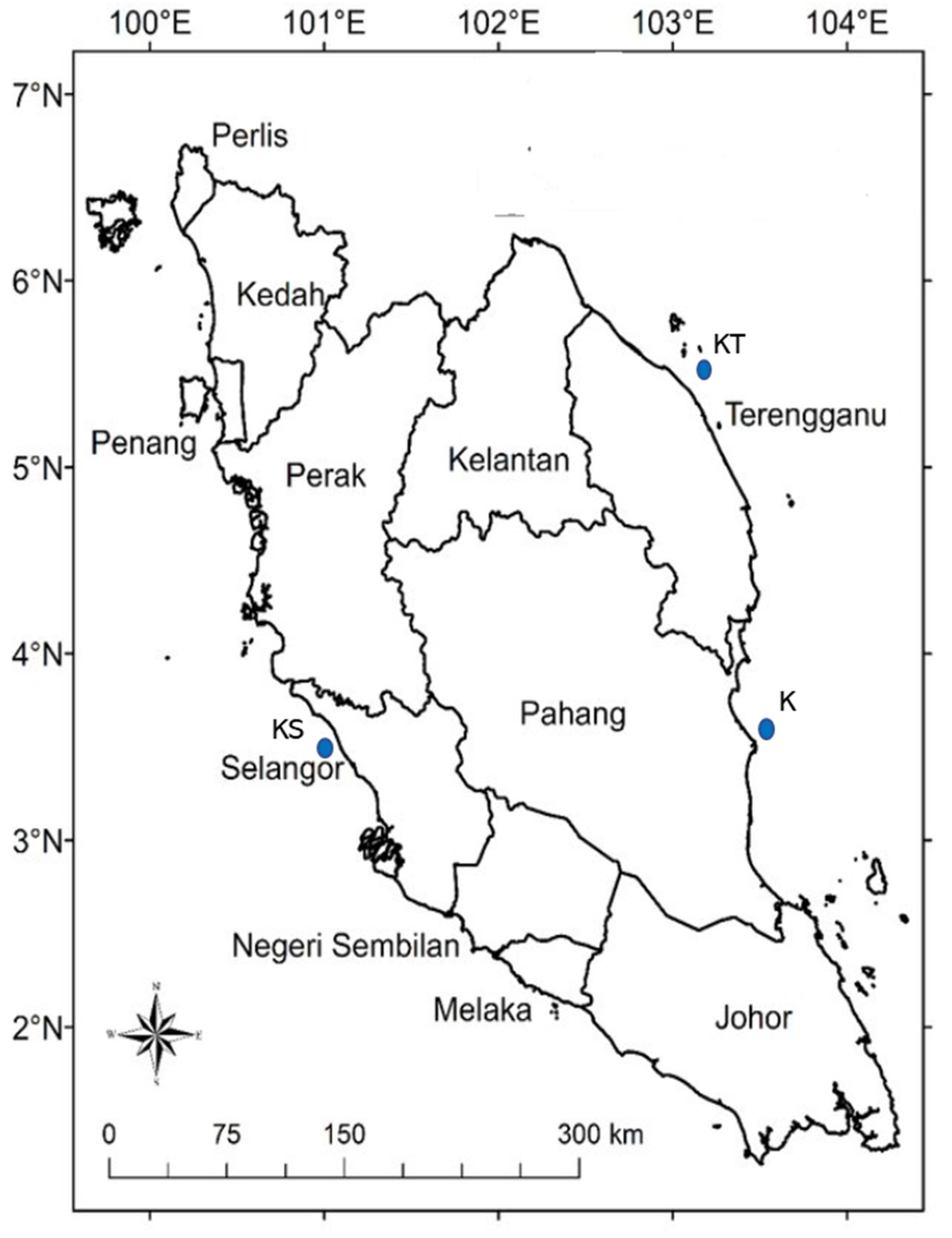
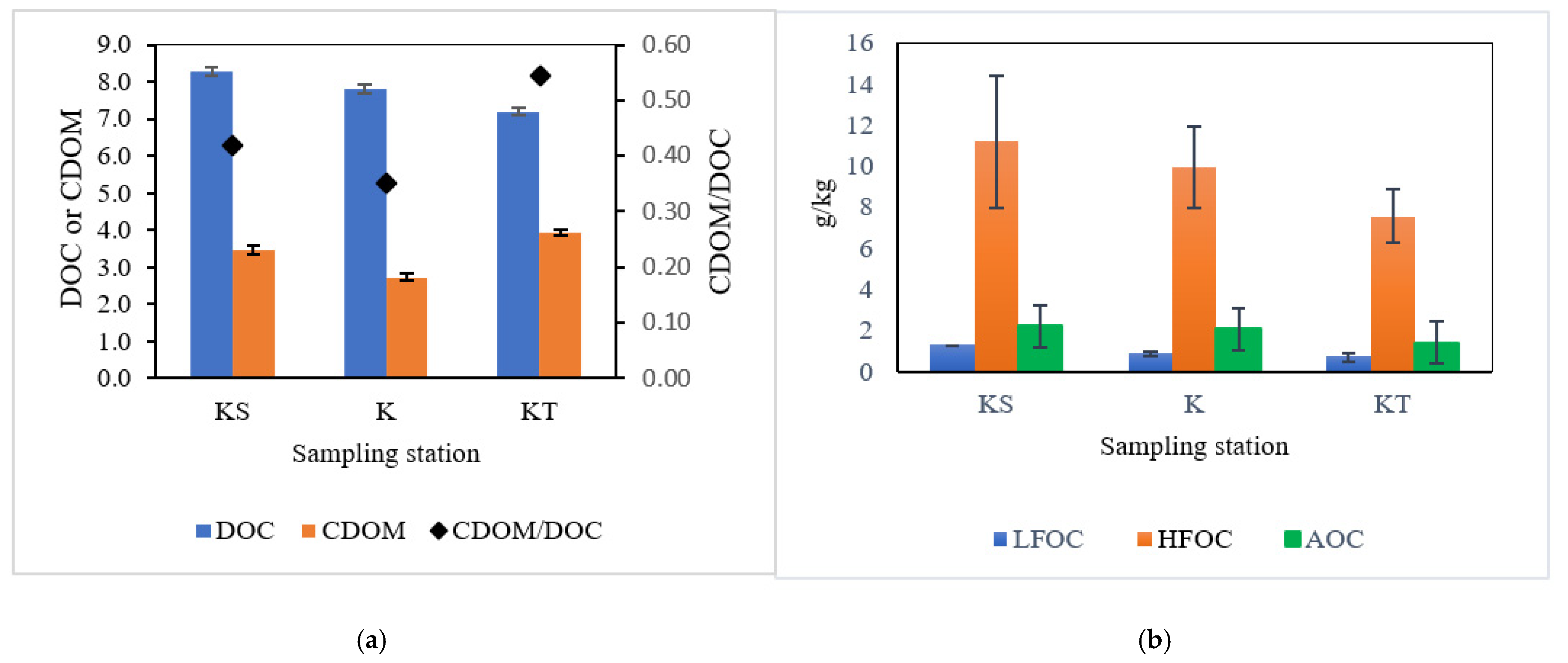

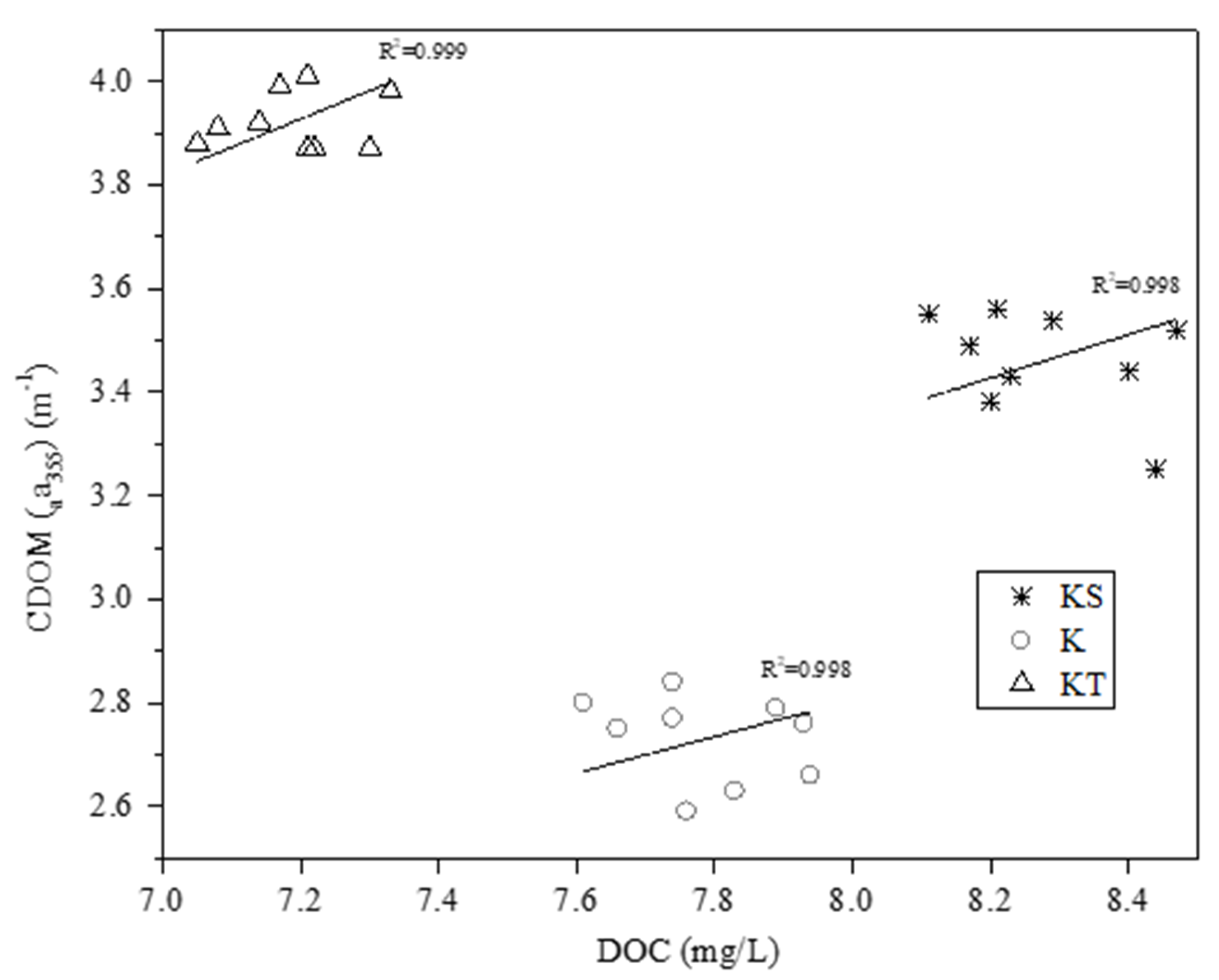

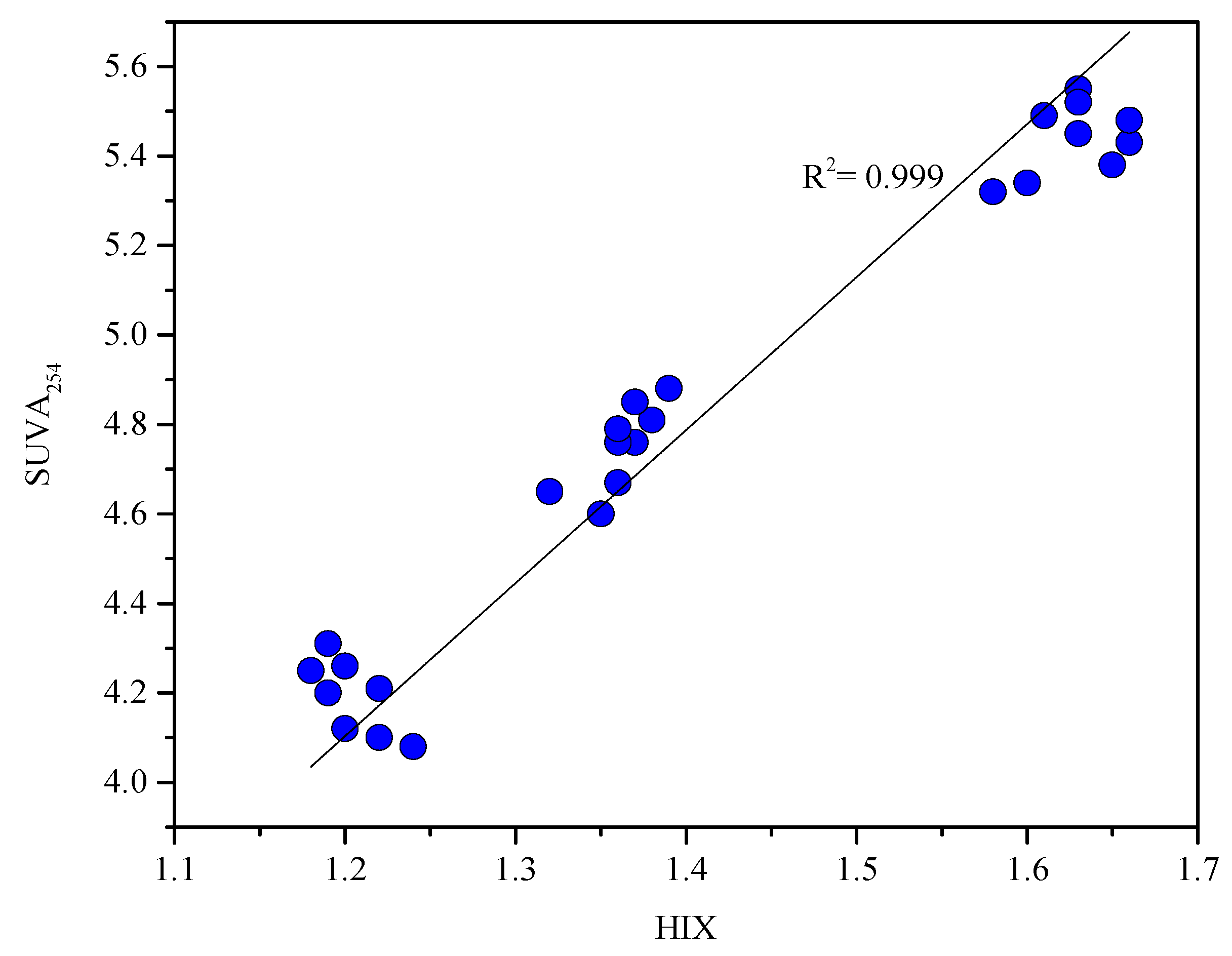
| Station | Longitude | Latitude | Depth of Water Column (m) | Description |
|---|---|---|---|---|
| Kuala Selangor (KS) | 101°18′05.5″ E | 3°13′48.5″ N | 15 m | 3 km from estuary with mangrove forest Fishing village, aquaculture pond |
| Kuala Terengganu (KT) | 103°09′23.2″ E | 5°20′36.9″ N | 15 m | 5 km off Kuala Terengganu river mouth |
| Kuantan (K) | 103°22′39.2″ E | 3°48′28.3″ N | 15 m | 2 km of Kuantan port |
| Station | TOC(g/kg) Mean (±SD) | Grain Distribution % | Sediment Classification USDA | Total Acidic Group (mmol/g) | COOH Group (mmol/g) | Phenolic Group (mmol/g) | pH | ||
|---|---|---|---|---|---|---|---|---|---|
| Clay | Silt | Sand | |||||||
| KS | 1.25 ± 0.73 | 46 | 20 | 34 | Clay | 7.34 | 4.12 | 3.22 | 7.45 |
| KT | 0.83 ± 0.30 | 10 | 24 | 66 | Sandy loam | 6.52 | 3.60 | 2.55 | 7.56 |
| K | 1.08 ± 1.04 | 37 | 31 | 32 | Clay loam | 6.23 | 3.50 | 2.73 | 7.81 |
| Station | C (%) | H (%) | N (%) | O (%) | H/C a | O/C b | C/N c |
|---|---|---|---|---|---|---|---|
| KS | 28.60 | 30.20 | 1.60 | 39.61 | 1.06 | 1.38 | 17.88 |
| KT | 30.40 | 15.01 | 3.52 | 48.64 | 0.49 | 1.58 | 8.69 |
| K | 33.10 | 35.83 | 2.72 | 27.53 | 1.08 | 0.90 | 11.04 |
| Station | UV-Visible Indices | Fluorescent Indices | ||||||
|---|---|---|---|---|---|---|---|---|
| SUVA254 | E2/E3 | E4/E6 | A355 | SR | HIX | FI | BIX | |
| KS | 5.44 ± 0.08 | 8.32 | 4.32 | 3.43 | 0.13 | 1.63 ± 0.03 | 0.95 ± 0.08 | 0.67 ± 0.02 |
| K | 4.75 ± 0.09 | 10.71 | 6.50 | 2.77 | 0.07 | 1.36 ± 0.02 | 1.10 ± 0.07 | 0.62 ± 0.01 |
| KT | 4.19 ± 0.08 | 18.43 | 8.64 | 3.98 | 0.59 | 1.20 ± 0.02 | 1.58 ± 0.09 | 1.27 ± 0.12 |
| SUVA254 | E2/E3 | E4/E6 | A355 | HIX | BIX | FI | |
|---|---|---|---|---|---|---|---|
| SUVA254 | 1 | ||||||
| E2/E3 | −0.926 * | 1 | |||||
| E4/E6 | −0.984 * | 0.946 * | 1 | ||||
| A355 | −0.316 | 0.606 * | 0.339 | 1 | |||
| HIX | 0.979 * | −0.905 * | −0.982 * | −0.235 | 1 | ||
| BIX | −0.878 * | 0.946 * | 0.895 * | 0.627 * | −0.825 * | 1 | |
| FI | −0.904 * | 0.957 * | 0.906 * | 0.597 * | −0.866 * | 0.898 * | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzulkafli, N.F.; Mahdzir, A.; Hara, H. Bulk Chemical and Optical Spectroscopy Characterisations of Dissolved Organic Matter Extracted from the Tropical Coastal Sediment. J. Mar. Sci. Eng. 2021, 9, 997. https://doi.org/10.3390/jmse9090997
Dzulkafli NF, Mahdzir A, Hara H. Bulk Chemical and Optical Spectroscopy Characterisations of Dissolved Organic Matter Extracted from the Tropical Coastal Sediment. Journal of Marine Science and Engineering. 2021; 9(9):997. https://doi.org/10.3390/jmse9090997
Chicago/Turabian StyleDzulkafli, Noor Fazreen, Akbariah Mahdzir, and Hirofumi Hara. 2021. "Bulk Chemical and Optical Spectroscopy Characterisations of Dissolved Organic Matter Extracted from the Tropical Coastal Sediment" Journal of Marine Science and Engineering 9, no. 9: 997. https://doi.org/10.3390/jmse9090997
APA StyleDzulkafli, N. F., Mahdzir, A., & Hara, H. (2021). Bulk Chemical and Optical Spectroscopy Characterisations of Dissolved Organic Matter Extracted from the Tropical Coastal Sediment. Journal of Marine Science and Engineering, 9(9), 997. https://doi.org/10.3390/jmse9090997






