Controlling Factors of Phytoplankton Productivity in Marshes in a Hot Climate with High Seasonal Variation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Chemical and Biological Analyses
2.3. Modeling Primary Production
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erwin, K.L. Wetlands and global climate change: The role of wetland restoration in a changing world. Wetl. Ecol. Manag. 2009, 17, 71–84. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ralph, T.J. Gross primary productivity of phytoplankton and planktonic respiration in inland floodplain wetlands of southeast Australia: Habitat-dependent patterns and regulating processes. Ecol. Res. 2013, 28, 833–843. [Google Scholar] [CrossRef]
- Rojo, C.; Mercedes, M. Sustained primary production with changing phytoplankton assemblages in a semiarid wetland. Hydrobiologia 2010, 639, 55–62. [Google Scholar] [CrossRef]
- Mitsch, W.; Gosselink, J. Wetlands 2015. Available online: https://www.wiley.com/en-am/Wetlands%2C+5th+Edition-p-9781118676820 (accessed on 22 July 2021).
- Mitsch, W.J.; Gosselink, J.G.; Zhang, L.; Anderson, C.J. Wetland Ecosystems; John Wiley & Sons: Hoboken, NY, USA, 2009. [Google Scholar]
- Al-Zaidy, K.J.L.; Parisi, G.; Ali Abed, S.; Salim, M.A. Classification of the Key Functional Diversity of the Marshes of Southern Iraq Marshes. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1294, p. 072021. [Google Scholar] [CrossRef]
- Rodríguez, P.; Vera, M.S.; Pizarro, H. Primary production of phytoplankton and periphyton in two humic lakes of a South American wetland. Limnology 2012, 13, 281–287. [Google Scholar] [CrossRef]
- Waltham, N.J.; Alcott, C.; Barbeau, M.A.; Cebrian, J.; Connolly, R.M.; Deegan, L.A.; Dodds, K.; Gaines, L.A.G.; Gilby, B.L.; Henderson, C.J.; et al. Tidal marsh restoration optimism in a changing climate and urbanizing seascape. Estuaries Coasts 2021, 44, 1681–1690. [Google Scholar] [CrossRef]
- Burford, M.A.; Webster, I.T.; Revill, A.T.; Kenyon, R.A.; Whittle, M.; Curwen, G. Controls on phytoplankton productivity in a Wet–Dry tropical estuary. Estuar. Coast. Shelf Sci. 2012, 113, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Molinari, B.; Stewart-Koster, B.; Malthus, T.J.; Bunn, S.E. Assessing Spatial Variation in Algal Productivity in a Tropical River Floodplain Using Satellite Remote Sensing. Remote Sens. 2021, 13, 1710. [Google Scholar] [CrossRef]
- Zefrehei, A.R.P.; Hedayati, A.; Pourmanafi, S.; Kashkooli, O.B.; Ghorbani, R. Monitoring spatiotemporal variability of water quality parameters Using Landsat imagery in Choghakhor International Wetland during the last 32 years. In Annales de Limnologie-International Journal of Limnology; EDP Sciences: Les Ulis, France, 2020; Volume 56, p. 6. [Google Scholar] [CrossRef]
- Vargas-Lopez, I.A.; Rivera-Monroy, V.H.; Day, J.W.; Whitbeck, J.; Maiti, K.; Madden, C.J.; Trasviña-Castro, A. Assessing chlorophyll a spatiotemporal patterns combining in situ continuous fluorometry measurements and Landsat 8/OLI data across the Barataria Basin (Louisiana, USA). Water 2021, 13, 512. [Google Scholar] [CrossRef]
- Stumpner, E.B.; Bergamaschi, B.A.; Kraus, T.E.C.; Parker, A.E.; Wilkerson, F.P.; Downing, B.D.; Dugdale, R.C.; Murrell, M.C.; Carpenter, K.D.; Orlando, J.L.; et al. Spatial variability of phytoplankton in a shallow tidal freshwater system reveals complex controls on abundance and community structure. Sci. Total Environ. 2020, 700, 134392. [Google Scholar] [CrossRef]
- Richardson, C.J.; Hussain, N.A. Restoring the Garden of Eden: An ecological assessment of the marshes of Iraq. AIBS Bull. 2006, 56, 477–489. [Google Scholar] [CrossRef]
- Hussain, N.A. Biotopes of the Iraqi Marshes; Dhifaf Publishing House: Basra, Iraq, 2014. [Google Scholar]
- Douabul, A.A.Z.; AlMaarofi, S.S. Restoring the Garden of Eden: Negative WSD; ACADEMIA: San Francisco, CA, USA, 2014. [Google Scholar]
- Colombano, D.D.; Litvin, S.Y.; Ziegler, S.L.; Alford, S.B.; Baker, R.; Barbeau, M.A.; Cebrián, J.; Connolly, R.M.; Currin, C.A.; Deegan, L.A.; et al. Climate change implications for tidal marshes and food web linkages to estuarine and coastal nekton. Estuaries Coasts 2021, 44, 1–12. [Google Scholar] [CrossRef]
- Almaarofi, S.S.; Douabul, A. Restoration Assessment: I-Phosphorus and Nitrogen dynamics versus phytoplankton biomass in Al-Hawizeh marshland, Iraq. Int. J. Manag. Sci. Bus. Res. 2013, 2, 31–42. [Google Scholar]
- Ameen, F.; AlMaarofi, S.; Talib, A.; Almansob, A.; Al-Homaidan, A.A. Phytoplankton diversity recovers slowly and cyanobacterial abundance remains high after the reflooding of drained marshes. Hydrobiologia 2019, 843, 79–92. [Google Scholar] [CrossRef]
- Stainton, M.; Capel, M.J.; Armstrong, F.A.J. Chemical Analysis of Fresh Water. 1977. Available online: https://waves-vagues.dfo-mpo.gc.ca/Library/110147.pdf (accessed on 22 July 2021).
- Karadzic, V.; Simic, G.S.; Natic, D.; Rzanicanin, A.; Iric, M.; Gacic, Z. Changes in the phytoplankton community and dominance of Cylindrospermopsis raciborskii (Wolosz.) Subba Raju in a temperate lowland river (Ponjavica, Serbia). Hydrobiologia 2013, 711, 43–60. [Google Scholar] [CrossRef]
- Selvaraj, G.S.D. Estimation of primary productivity (modified light and dark bottle oxygen method). CMFRI Spec. Publ. Mangrove Ecosyst. Man. Assess. Biodivers. 2005, 83, 199–200. [Google Scholar]
- Brivio, P.A.; Giardino, C.; Zilioli, E. Determination of chlorophyll concentration changes in Lake Garda using an image-based radiative transfer code for Landsat TM images. Int. J. Remote Sens. 2001, 22, 487–502. [Google Scholar] [CrossRef]
- Al-thahaibawi, B.M.H. Study of Ecological Characteristics and Biodiversity of Al-Auda Marsh in Maysan Province. Master’s Thesis, University of Baghdad, Baghdad, Iraq, 2014. [Google Scholar]
- Al-Mosewi, T.J.K. Water Quality of Al-Hammar Marsh South Iraq. J. Eng. 2009, 15, 3999–4008. [Google Scholar]
- Tahir, M.A.; Risen, A.K.; Hussain, N.A. Monthly variations in the physical and chemical properties of the restored southern Iraqi marshes. Marsh Bull. 2008, 3, 81–94. [Google Scholar]
- Allo, H.G.I. A Study of the Epipelic Algae in Abu-Zirig Marsh Southern Iraq. Master’s Thesis, University of Baghdad, Baghdad, Iraq, 2006. [Google Scholar]
- Centre of Restoration of the Iraq Marshland (CRIM). Report Shows Spaces Ratio Flooded Areas of the Iraqi Marshes; CRIM: Basrah, Iraq, 2012. [Google Scholar]
- Krah, M.; McCarthy, T.S.; Huntsman-Mapila, P.; Wolski, P.; Annegarn, H.; Sethebe, K. Nutrient budget in the seasonal wetland of the Okavango Delta, Botswana. Wetl. Ecol. Manag. 2006, 14, 253–267. [Google Scholar] [CrossRef]
- Al-Imarah, F.J.M.; Al-Shawi, I.J.M.; Issa, A.M.; Al-Badran, M. Seasonal variation for levels of nutrients in water from Southern Iraqi Marshlands after Rehabilitation 2003. Marsh Bull. 2006, 1, 82–91. [Google Scholar]
- Hoosier, R. Volunteer Stream Monitoring Training Manual. Indiana’s Volunteer Stream Monitoring Program; Natural Resources Education Center: Indianapolis, IN, USA, 2000; Available online: https://www3.nd.edu/~aseriann/Riverwatch_Monitoring_Manual.pdf (accessed on 22 July 2021).
- Al-Mayah, A.A.; Al-Hilli, M.R.; Hassan, F.M. Marsh flora of southern Iraq before desiccation. Univ. Basrah. Mar. Sci. Cent. Publ. B 2014, 195, 252–386. [Google Scholar]
- Felip, M.; Catalan, J. The relationship between phytoplankton biovolume and chlorophyll in a deep oligotrophic lake: Decoupling in their spatial and temporal maxima. J. Plankton Res. 2000, 22, 91–106. [Google Scholar] [CrossRef]
- Lau, S.S.S.; Lane, S.N. Biological and chemical factors influencing shallow lake eutrophication: A long-term study. Sci. Total Environ. 2002, 288, 167–181. [Google Scholar] [CrossRef]
- Hassan, F.M.; Talib, A.H.; Taylor, W.D.; Abdulah, D.S. Phytoplankton primary production in southern Iraqi marshes after restoration. Baghdad Sci. J. 2010, 8, 519–527. [Google Scholar]
- Mahamed, S. Phosphorus and Nitrogen in the Al-Hawizeh Marshes, Southern Iraq. 2008. Available online: https://uwspace.uwaterloo.ca/handle/10012/3880 (accessed on 22 July 2021).
- Frantzich, J.; Sommer, T.; Schreier, B. Physical and biological responses to flow in a tidal freshwater slough complex. San Franc. Estuary Watershed Sci. 2018, 16. [Google Scholar] [CrossRef] [Green Version]
- Al Azad, S.; Jinau, V.J. Spatial Distribution of Dissolved Inorganic Nutrients and Phytoplankton around Kota Kinabalu Wetland, Sabah, Malaysia. Adv. Biol. Chem. 2020, 10, 113–126. [Google Scholar] [CrossRef]
- Zainol, Z.; Akhir, M.F.; Abdullah, S. Hydrodynamics, nutrient concentrations, and phytoplankton biomass in a shallow and restricted coastal lagoon under different tidal and monsoonal environmental drivers. Reg. Stud. Mar. Sci. 2020, 38, 101376. [Google Scholar] [CrossRef]
- Roelke, D.L.; Li, H.-P.; Hayden, N.J.; Miller, C.J.; Davis, S.E.; Quigg, A.; Buyukates, Y. Co-occurring and opposing freshwater inflow effects on phytoplankton biomass, productivity and community composition of Galveston Bay, USA. Mar. Ecol. Prog. Ser. 2013, 477, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, S.J.; Roland, F.; Loverde-Oliveira, S.M.; de Moraes Huszar, V.L. Phytoplankton abundance, biomass and diversity within and between Pantanal wetland habitats. Limnologica 2012, 42, 235–241. [Google Scholar] [CrossRef]
- Aardema, H.M.; Rijkeboer, M.; Lefebvre, A.; Veen, A.; Kromkamp, J.C. High resolution in situ measurements of phytoplankton photosynthesis and abundance in the Dutch North Sea. Ocean Sci. Discuss. 2018, 15, 1267–1285. [Google Scholar] [CrossRef]
- Jiang, Z.; Du, P.; Liu, J.; Chen, Y.; Zhu, Y.; Shou, L.; Zeng, J.; Chen, J. Phytoplankton biomass and size structure in Xiangshan Bay, China: Current state and historical comparison under accelerated eutrophication and warming. Mar. Pollut. Bull. 2019, 142, 119–128. [Google Scholar] [CrossRef]
- Vernet, M.; Richardson, T.L.; Metfies, K.; Nöthig, E.-M.; Peeken, I. Models of plankton community changes during a warm water anomaly in arctic waters show altered trophic pathways with minimal changes in carbon export. Front. Mar. Sci. 2017, 4, 160. [Google Scholar] [CrossRef] [Green Version]
- Villafane, V.E.; Valiñas, M.S.; Cabrerizo, M.J.; Helbling, E.W. Physio-ecological responses of Patagonian coastal marine phytoplankton in a scenario of global change: Role of acidification, nutrients and solar UVR. Mar. Chem. 2015, 177, 411–420. [Google Scholar] [CrossRef]
- Masmoudi, S.; Tastard, E.; Guermazi, W.; Caruso, A.; Morant-Manceau, A.; Ayadi, H. Salinity gradient and nutrients as major structuring factors of the phytoplankton communities in salt marshes. Aquat. Ecol. 2015, 49, 1–19. [Google Scholar] [CrossRef]
- Mu, S.; Li, B.; Yao, J.; Yang, G.; Wan, R.; Xu, X. Monitoring the spatio-temporal dynamics of the wetland vegetation in Poyang Lake by Landsat and MODIS observations. Sci. Total Environ. 2020, 725, 138096. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Jin, P.; Liu, N.; Li, F.; Tong, S.; Hutchins, D.A.; Gao, K. The acclimation process of phytoplankton biomass, carbon fixation and respiration to the combined effects of elevated temperature and pCO2 in the northern South China Sea. Mar. Pollut. Bull. 2017, 118, 213–220. [Google Scholar] [CrossRef] [PubMed]
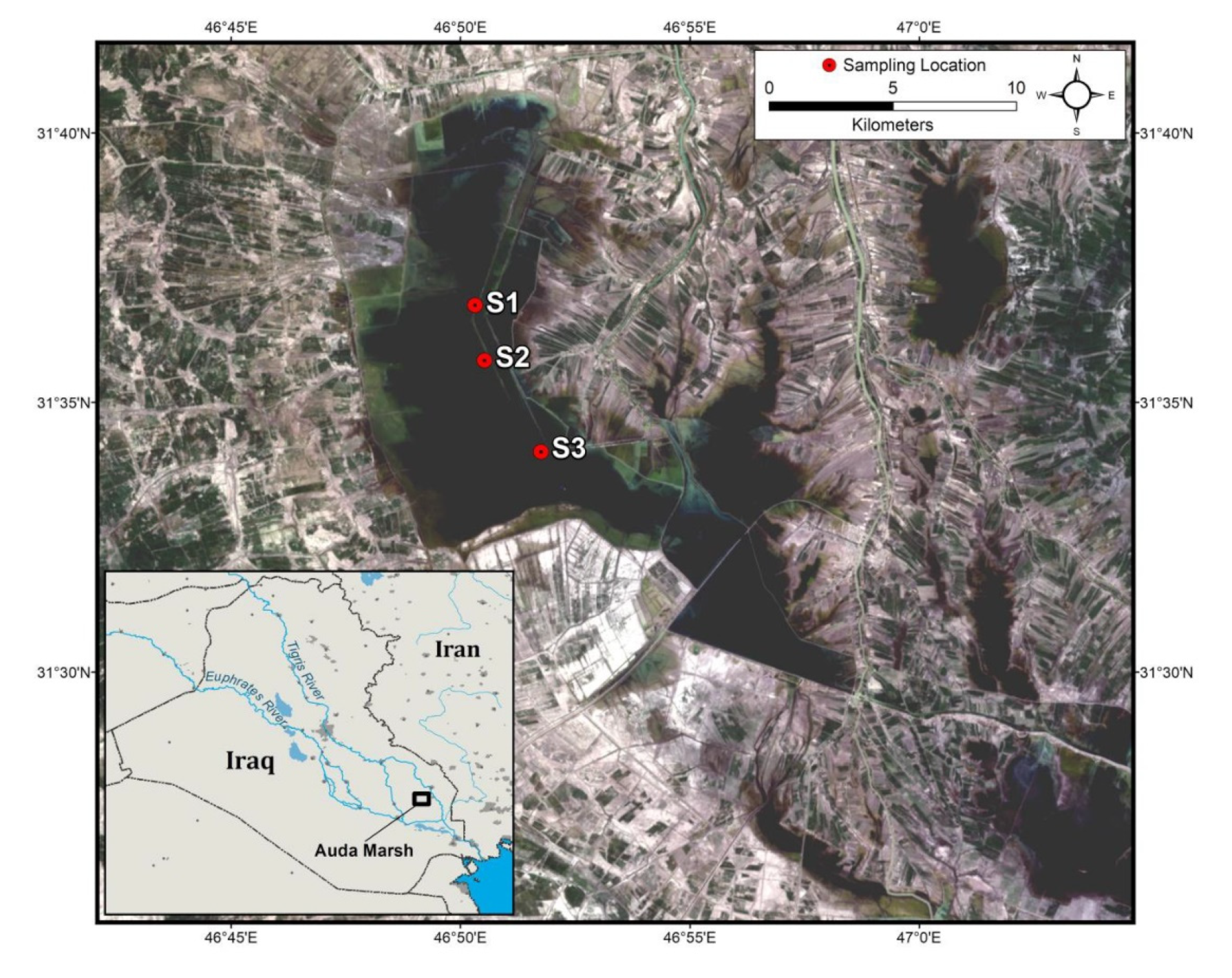
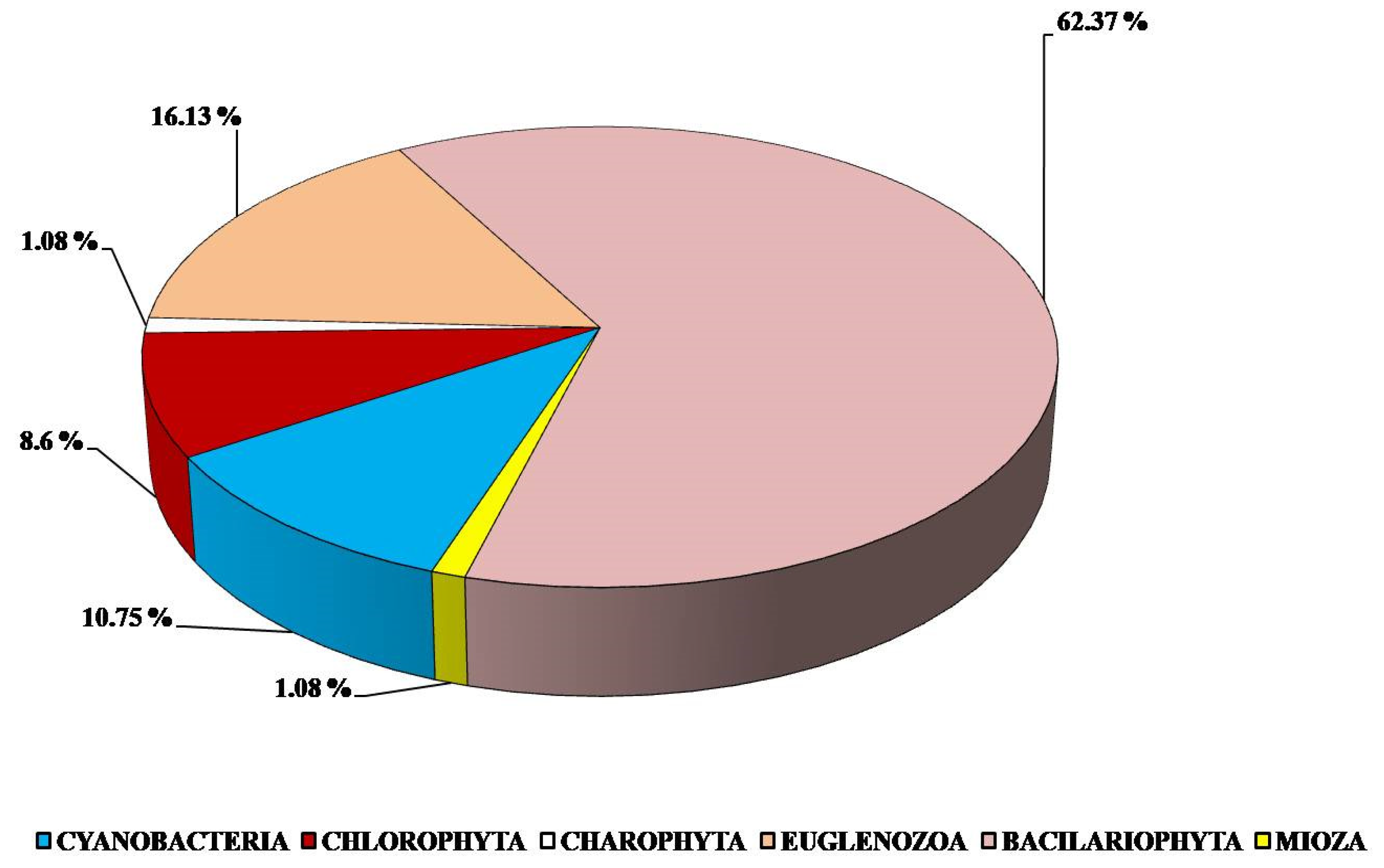
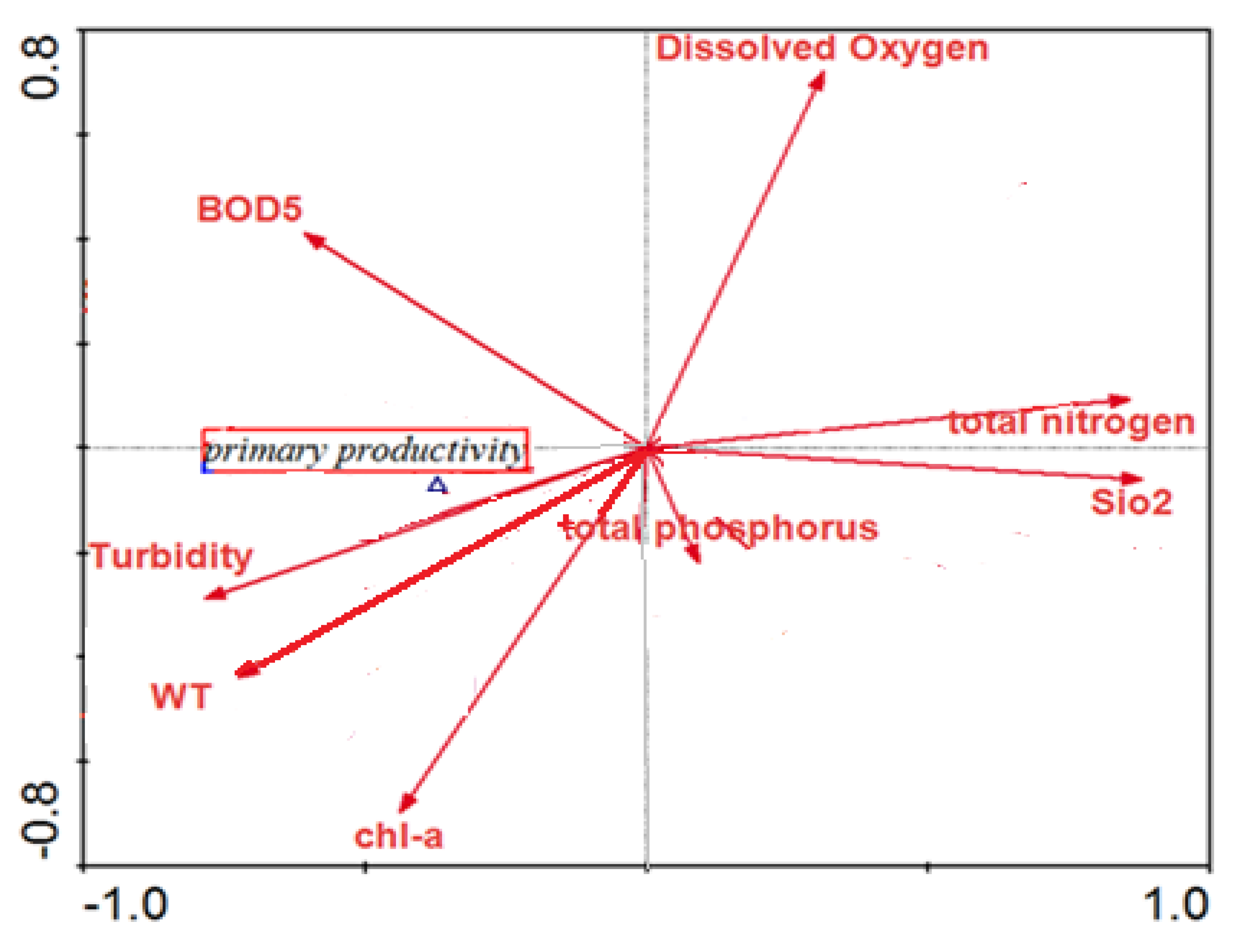
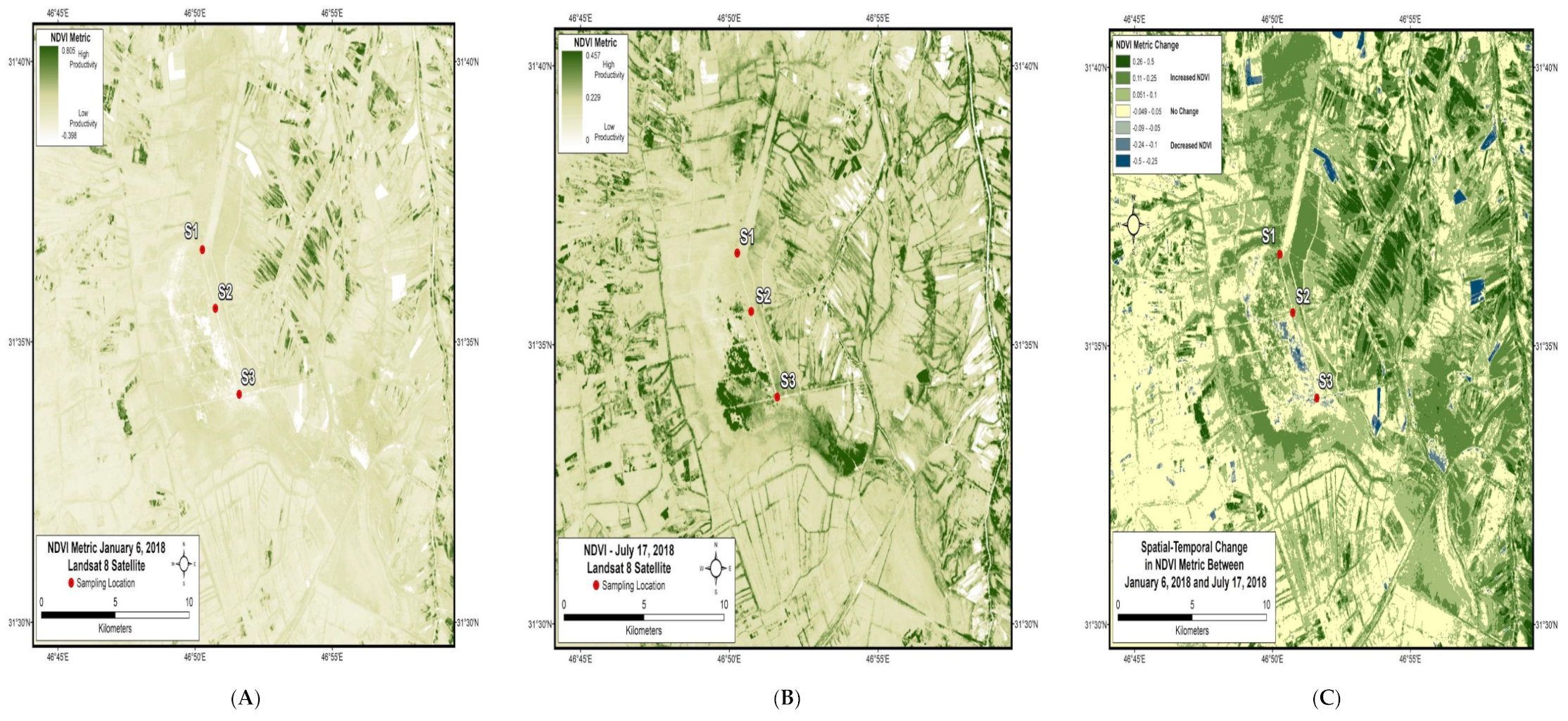
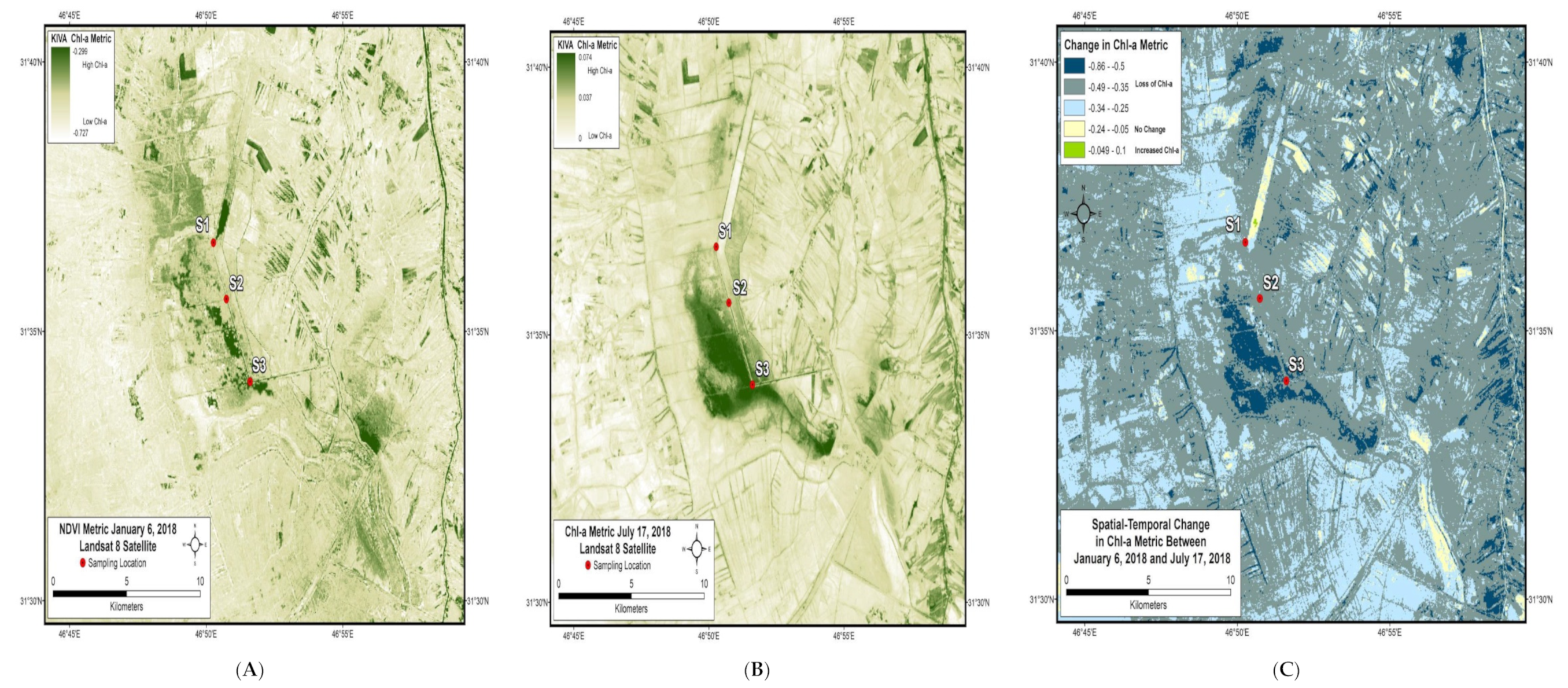
| Site | Depth | Ditching | Vegetation |
|---|---|---|---|
| S1 | 3.3 m | Channels 5–15 m width, represent permanent part of the marsh | Low cover |
| S2 | 2.7 m | One main channel 35 m width, moderate open area | High cover dominated by Phragmites australis |
| S3 | 1.5 m | Large open area | Vegetation consists of Phragmites australis, Typha domingensis and the free-floating plant Ceratophyllum demersum |
| Wet | Dry | |
|---|---|---|
| Temperature (°C) | 15.9 ± 0.5 | 28.1 ± 0.6 |
| Turbidity (NTU) | 26.0 ± 5.1 | 48.4 ± 15.3 |
| DO (mg L−1) | 7.6 ± 0.3 | 4.5 ± 0.5 |
| BOD5 (mg L−1) | 2.1 ± 0.1 | 1.8 ± 0.2 |
| Si (µg L−1) | 26.8 ± 3.1 | 19.3 ± 3.5 |
| TP (µg L−1) | 0.10 ± 0.01 | 0.17 ± 0.01 |
| TN (µg L−1) | 1.5 ± 1.5 | 1.0 ± 0.5 |
| PP (mg C m−3 h−1) | 125 ± 15 | 176 ± 13 |
| Chl-a (µg L−1) | 4.7 ± 1.9 | 9.8 ± 1.5 |
| Temperature | Turbidity | DO | BOD5 | SiO3−2 | TP | TN | PP | |
|---|---|---|---|---|---|---|---|---|
| Temperature | 1 | |||||||
| Turbidity | 0.84 | 1 | ||||||
| DO | −0.82 | −0.71 | 1 | |||||
| BOD5 | 1 | |||||||
| SiO3−2 | −0.68 | −0.71 | 0.65 | 1 | ||||
| TP | 0.55 | 0.63 | −0.62 | 1 | ||||
| TN | 1 | |||||||
| PP | 1 | |||||||
| Chl-a | 0.64 | 0.71 | −0.80 | −0.23 | −0.52 | 0.51 | 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameen, F.; Albueajee, A.I.; Hassan, F.M.; Stephenson, S.L.; Douabul, A.A.Z. Controlling Factors of Phytoplankton Productivity in Marshes in a Hot Climate with High Seasonal Variation. J. Mar. Sci. Eng. 2021, 9, 811. https://doi.org/10.3390/jmse9080811
Ameen F, Albueajee AI, Hassan FM, Stephenson SL, Douabul AAZ. Controlling Factors of Phytoplankton Productivity in Marshes in a Hot Climate with High Seasonal Variation. Journal of Marine Science and Engineering. 2021; 9(8):811. https://doi.org/10.3390/jmse9080811
Chicago/Turabian StyleAmeen, Fuad, Alaa I. Albueajee, Fikrat M. Hassan, Steven L. Stephenson, and Ali A. Z. Douabul. 2021. "Controlling Factors of Phytoplankton Productivity in Marshes in a Hot Climate with High Seasonal Variation" Journal of Marine Science and Engineering 9, no. 8: 811. https://doi.org/10.3390/jmse9080811
APA StyleAmeen, F., Albueajee, A. I., Hassan, F. M., Stephenson, S. L., & Douabul, A. A. Z. (2021). Controlling Factors of Phytoplankton Productivity in Marshes in a Hot Climate with High Seasonal Variation. Journal of Marine Science and Engineering, 9(8), 811. https://doi.org/10.3390/jmse9080811







