Response to Environmental Factors of Spawning Ground in the Pearl River Estuary, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Egg Data
2.2. Environmental Data
2.3. GAM
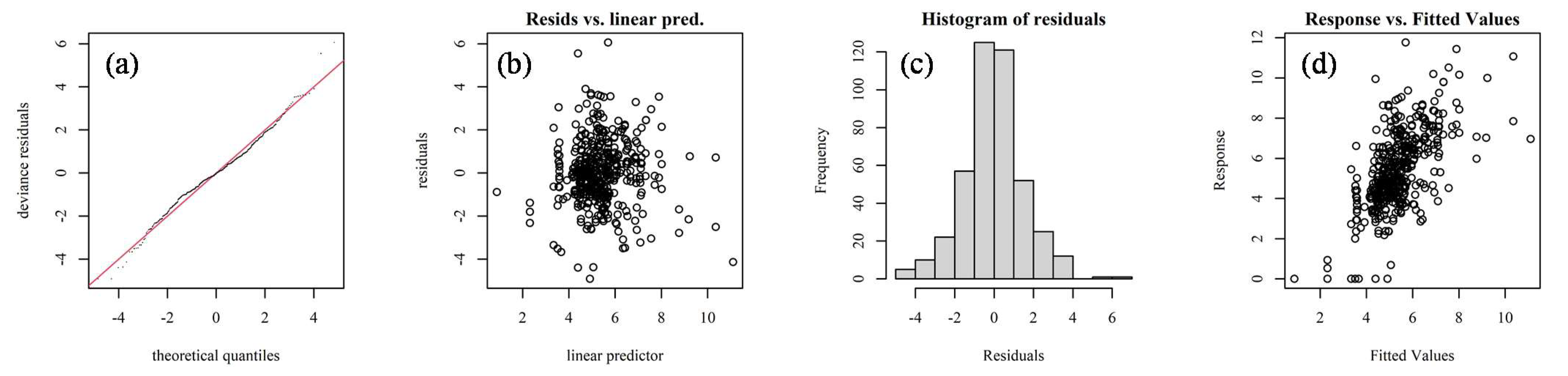
2.4. Model Testing
3. Results
3.1. GAMs Analysis
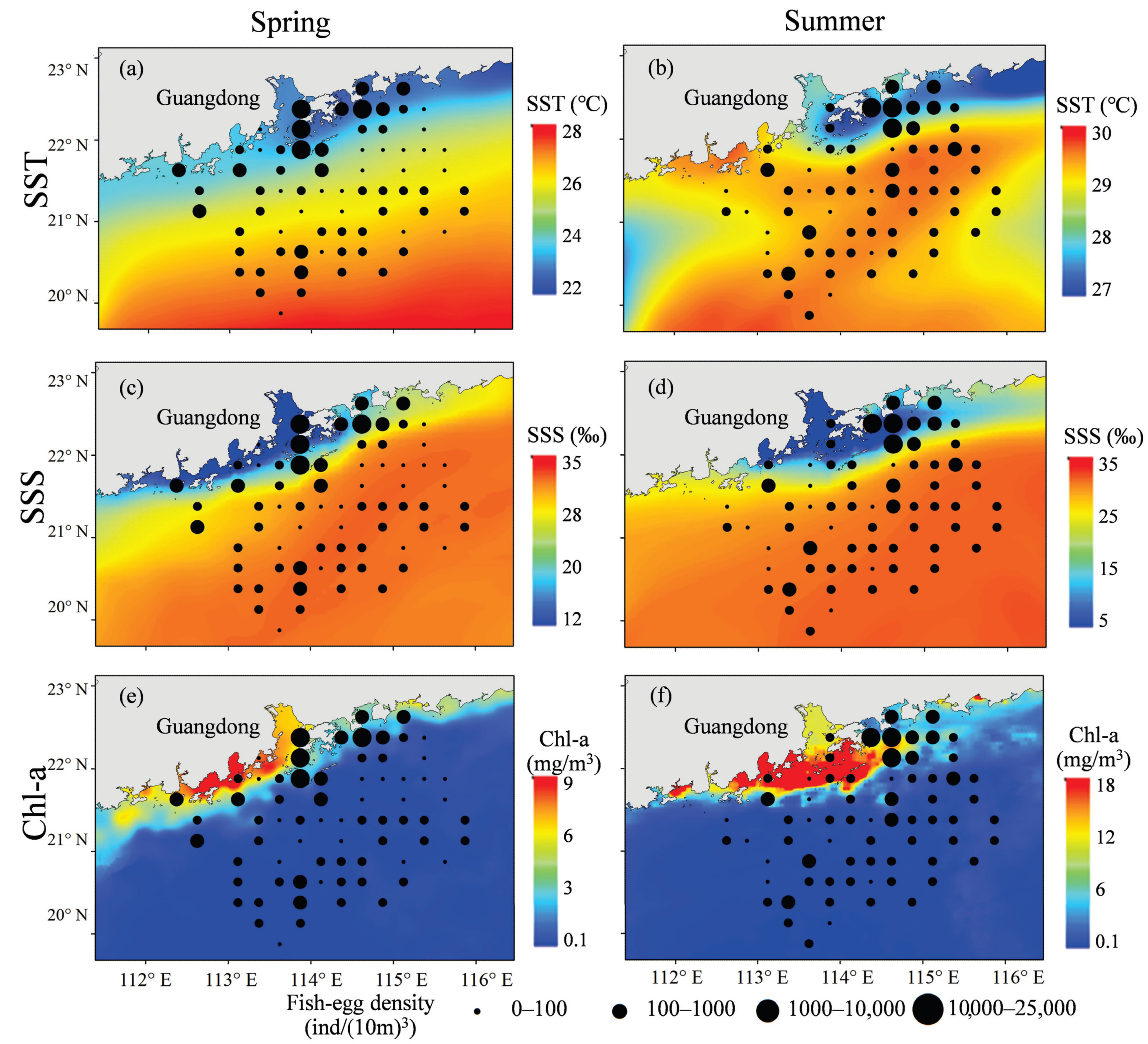
3.2. Spatiotemporal Distribution of Egg Density and SST, SSS, Chl-a
3.3. Spatiotemporal Distribution of Fish Egg Density and Current
4. Discussion
4.1. Relationship between Egg Density and SST, SSS, Chl-a, Depth
4.2. Spatiotemporal Variation of Fish Eggs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yingjie, P. Fisheries Dictionary (Essence); Shanghai Lexicographical Publishing House: Shanghai, China, 2007. [Google Scholar]
- Rui, W.J.; Shan, S. The category composition and abundance of ichthyoplankton in the ecosystem of the Yellow Sea and the East China Sea. Acta Zool. Sin. 2006, 52, 28–44. [Google Scholar]
- Jingrui, W.; Dingyong, Z.; Xiaodong, B.; Xiaobo, N. Species composition and abundance distribution pattern of ichthyoplankton and their relationship with environmental factors in the East China Sea ecosystem. J. Fish. China 2014, 38, 1375–1398. [Google Scholar]
- Nanami, A.; Sato, T.; Kawabata, Y.; Okuyama, J. Spawning aggregation of white-streaked grouper Epinephelus ongus: Spatial distribution and annual variation in the fish density within a spawning ground. PeerJ 2017, 5, e3000. [Google Scholar] [CrossRef]
- Roussel, E.; Crec’hriou, R.; Lenfant, P.; Mader, J.; Planes, S. Relative influences of space, time and environment on coastal ichthyoplankton assemblages along a temperate rocky shore. J. Plankton Res. 2010, 32, 1443–1457. [Google Scholar] [CrossRef]
- Richardson, D.E.; Llopiz, J.K.; Leaman, K.D.; Vertes, P.S.; Muller-Karger, F.E.; Cowen, R.K. Sailfish (Istiophorus platypterus) spawning and larval environment in a Florida Current frontal eddy. Prog. Oceanogr. 2009, 82, 252–264. [Google Scholar] [CrossRef]
- Ospina-Alvarez, A.; Catalán, I.A.; Bernal, M.; Roos, D.; Palomera, I. From egg production to recruits: Connectivity and inter-annual variability in the recruitment patterns of European anchovy in the northwestern Mediterranean. Prog. Oceanogr. 2015, 138, 431–447. [Google Scholar] [CrossRef]
- Porcaro, R.R.; Zani-Teixeira, M.D.L.; Katsuragawa, M.; Namiki, C.; Ohkawara, M.H.; Favero, J.M.D. Spatial and temporal distribution patterns of larval sciaenids in the estuarine system and adjacent continental shelf off Santos, Southeastern Brazil. Braz. J. Oceanogr. 2014, 62, 149–164. [Google Scholar] [CrossRef]
- Coulter, A.A.; Keller, D.; Bailey, E.J.; Goforth, R.R. Predictors of bigheaded carp drifting egg density and spawning activity in an invaded, free-flowing river. J. Great Lakes Res. 2016, 42, 83–89. [Google Scholar] [CrossRef]
- Petereit, C.; Hinrichsen, H.H.; Voss, R.; Kraus, G.; Freese, M.; Clemmesen, C. The influence of different salinity conditions on egg buoyancy and development and yolk sac larval survival and morphometric traits of Baltic Sea sprat (Sprattus sprattus balticus Schneider). Sci. Mar. 2009, 73, 59–72. [Google Scholar] [CrossRef]
- Genner, M.J.; Halliday, N.C.; Simpson, S.D.; Southward, A.J.; Hawkins, S.J.; Sims, D.W. Temperature-driven phenological changes within a marine larval fish assemblage. J. Plankton Res. 2009, 32, 699–708. [Google Scholar] [CrossRef]
- Zenitani, H.; Onishi, Y.; Obata, Y. Spawning grounds of red sea bream in the east Seto Inland Sea. Fish. Sci. 2014, 80, 499–504. [Google Scholar] [CrossRef]
- Bonanno, A.; Zgozi, S.; Cuttitta, A.; El Turki, A.; Di Nieri, A.; Ghmati, H.; Basilone, G.; Aronica, S.; Hamza, M.; Barra, M.; et al. Influence of environmental variability on anchovy early life stages (Engraulis encrasicolus) in two different areas of the Central Mediterranean Sea. Hydrobiologia 2012, 701, 273–287. [Google Scholar] [CrossRef]
- Hinrichsen, H.H.; Lehmann, A.; Petereit, C.; Nissling, A.; Ustups, D.; Bergström, U.; Hüssy, K. Spawning areas of eastern Baltic cod revisited: Using hydrodynamic modelling to reveal spawning habitat suitability, egg survival probability, and connectivity patterns. Prog. Oceanogr. 2016, 143, 13–25. [Google Scholar] [CrossRef]
- Xiaodong, B.; Xiumei, Z.; Tianxiang, G.; Ruijing, W.; Peidong, Z. Category composition and distributional patterns of ichthyoplankton in the Yellow River estuary during spring and summer 2007. J. Fish. Sci. China 2010, 17, 199–211. [Google Scholar]
- Shuang, L.; Jimin, Z.; Yu, L. Species and distribution characteristics of fish eggs and larvae at the Yellow River Estuary. Mar. Sci. Bull. 2011, 30, 662–667. [Google Scholar]
- Xiukai, S.; Aiying, L.; Yanyan, Y.; Jianmin, Y.; Lihua, R.; Lijuan, L.; Guohua, S.; Xiaojing, L. Distributing of ichthyoplankton and the correlation with environmental factors in laizhou bay. Oceanol. Limnol. Sin. 2010, 41, 378–385. [Google Scholar]
- Lu, Y.; Yu, J.; Lin, Z.; Chen, P. Environmental influence on the spatiotemporal variability of spawning grounds in the western Guangdong waters, South China Sea. J. Mar. Sci. Eng. 2020, 8, 607. [Google Scholar] [CrossRef]
- Yuzhang, X.; Rong, W.; Qiang, O.; Hongda, F. Relationship between abundance distribution of fish eggs,larvae and juveniles and environmental factors in the Pearl River Estuary waters in spring. J. Oceanogr. Taiwan Strait 2010, 29, 488–495. [Google Scholar] [CrossRef]
- Stenseth, N.C.; Mysterud, A.; Ottersen, G.; Hurrell, J.W.; Chan, K.-S.; Lima, M. Ecological Effects of Climate Fluctuations. Science 2002, 297, 1292–1296. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R. Generalized Additive Models; Chapman and Hall: London, UK, 1990. [Google Scholar]
- González-Irusta, J.; Wright, P.J. Spawning grounds of whiting (Merlangius merlangus). Fish. Res. 2017, 195, 141–151. [Google Scholar] [CrossRef]
- Loots, C.; Vaz, S.; Koubbi, P.; Planque, B.; Coppin, F.; Verin, Y. Inter-annual variability of North Sea plaice spawning habitat. J. Sea Res. 2010, 64, 427–435. [Google Scholar] [CrossRef]
- Li, K.Z.; Yin, J.Q.; Huang, L.M.; Tan, Y.H. Spatial and temporal variations of mesozooplankton in the Pearl River estuary, China. Estuar. Coast. Shelf Sci. 2006, 67, 543–552. [Google Scholar] [CrossRef]
- Yuan, G.; Zini, L.; Wanling, Y.; Yanyi, Z. Changes of zooplankton community before and after the spring closed season in the Pearl River in 2014—2015. Ecol. Environ. Sci. 2017, 026, 1562–1569. [Google Scholar]
- Fangmin, S.; Xinhui, L.; Qianpu, L.; Yuefei, L.; Jiping, Y.; Jie, L.; Fangcan, C. Spatial patterns of fish diversity and distribution in the Pearl River. Acta Ecol. Sin. 2017, 37, 3182–3192. [Google Scholar]
- Lu, Z.; Gan, J. Controls of seasonal variability of phytoplankton blooms in the Pearl River Estuary. Deep. Sea Res. Part. II Top. Stud. Oceanogr. 2015, 117, 86–96. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, X.; Jiang, Y. Distribution and species composition of fish eggs in Daya Bay. J. Fish. Sci. China/Zhongguo Shuichan Kexue 2010, 17, 543–550. [Google Scholar]
- Ruijing, W.; Feng, Z.; Xiujuan, S.; Shan, S.J.A.E.S. Impacts of variability of habitat factors on species composition of ichthyoplankton and distribution of fish spawning ground in the Changjiang River estuary and its adjacent waters. Acta Ecol. Sin. 2010, 30, 155–165. [Google Scholar] [CrossRef]
- Team, R.C. R development core team. RA Lang Env. Stat Comput. 2013, 55, 275–286. [Google Scholar]
- Müller, D. Estimation of algae concentration in cloud covered scenes using geostatistical methods. In Proceedings of the ENVISAT Symposium, Montreux, Switzerland, 23 April 2007. [Google Scholar]
- Jing, Y.; Qiwei, H.; Huarong, Y.; Pimao, C. Effect assessment of summer fishing moratorium in Daya Bay based on remote sensing data. South China Fish. Sci. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Yanfeng, W.; Qiwei, H.; Jing, Y.; Pimao, C.; Liming, S. Effect assessment of fishery resources proliferation in Zhelin Bay marine ranching in eastern Guangdong. South China Fish. Sci. 2019, 15. [Google Scholar] [CrossRef]
- Wood, S. Generalized Additive Models: An Introduction with R; CRC Press: Boca Raton, FL, USA, 2006; Volume 66, p. 391. [Google Scholar]
- Venables, W.N.; Dichmont, C.M. GLMs, GAMs and GLMMs: An overview of theory for applications in fisheries research. Fish. Res. 2004, 69, 319–337. [Google Scholar] [CrossRef]
- Hilborn, R.; Mangel, M. The Ecological Detective; Princeton University Press: Princeton, NJ, USA, 1997. [Google Scholar]
- Mangel, M.; Quinn, T.J.; Deriso, R.B. Quantitative fish dynamics. Q. Rev. Biol. 1999, 2, 286–287. [Google Scholar]
- Stone, C.J. Additive regression and other nonparametric models. Ann. Stats 1985, 13, 689–705. [Google Scholar] [CrossRef]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Breakthroughs in Statistics: Foundations and Basic Theory; Kotz, S., Johnson, N.L., Eds.; Springer: New York, NY, USA, 1992; pp. 610–624. [Google Scholar]
- Burnham, K.; Anderson, D.; Burnham, P.; Anderson, R. Model Selection and Multimodel Inference: A Practical Information-Theoretical Approach; Springer: New York, NY, USA, 2010. [Google Scholar]
- Shu, Y.; Wang, Q.; Zu, T. Progress on shelf and slope circulation in the northern South China Sea. Sci. China Earth Sci. 2018, 61, 560–571. [Google Scholar] [CrossRef]
- Bacha, M.; Jeyid, M.A.; Vantrepotte, V.; Dessailly, D.; Amara, R. Environmental effects on the spatio-temporal patterns of abundance and distribution ofSardina pilchardusand sardinella off the Mauritanian coast (North-West Africa). Fish. Oceanogr. 2017, 26, 282–298. [Google Scholar] [CrossRef]
- Keefer, M.L.; Moser, M.L.; Boggs, C.T.; Daigle, W.R.; Peery, C.A. Variability in migration timing of adult Pacific lamprey (Lampetra tridentata) in the Columbia River, U.S.A. Environ. Biol. Fishes 2009, 85, 253–264. [Google Scholar] [CrossRef]
- Kimura, S.; Kato, Y.; Kitagawa, T.; Yamaoka, N. Impacts of environmental variability and global warming scenario on Pacific bluefin tuna (Thunnus orientalis) spawning grounds and recruitment habitat. Prog. Oceanogr. 2010, 86, 39–44. [Google Scholar] [CrossRef]
- Jingqi, L. Study on The nature regulative adaptibility of The Hairtail(Trichiurus haumela) in the oceanic environment. Trans. Oceanol. Limnol. 1981, 1, 60–65. [Google Scholar]
- Renzhai, Z. Spawning grounds and periods of chub mackerel in the northern South China Sea. Fish. Sci. Technol. Inf. 1981, 6, 6–9. [Google Scholar]
- Renzhai, Z. Morphology, spawning ground and spawning period of larva and juvenile of Nemipterus aureus in the northern South China Sea. Fish. Res. 1986, 7, 1. [Google Scholar]
- Spies, B.T.; Steele, M.A. Effects of temperature and latitude on larval traits of two estuarine fishes in differing estuary types. Mar. Ecol. Prog. Ser. 2016, 544, 243–255. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Xiao, Y.; Wang, T.; Li, C. Population characteristics of fish eggs and larvae and their relationship with environmental factors in Daya Bay. J. Fish. Sci. China 2019, 26, 14–25. [Google Scholar] [CrossRef]
- Selleslagh, J.; Amara, R. Environmental factors structuring fish composition and assemblages in a small macrotidal estuary (eastern English Channel). Estuar. Coast. Shelf Sci. 2008, 79, 507–517. [Google Scholar] [CrossRef]
- Walsh, C.T.; Reinfelds, I.V.; Ives, M.C.; Gray, C.A.; West, R.J.; van der Meulen, D.E. Environmental influences on the spatial ecology and spawning behaviour of an estuarine-resident fish, Macquaria colonorum. Estuar. Coast. Shelf Sci. 2013, 118, 60–71. [Google Scholar] [CrossRef]
- Fonds, M. Laboratory Observations on the Influence of Temperature and Salinity on Development of the Eggs and Growth of the Larvae of Solea solea (Pisces). Mar. Ecol. Prog. Ser. 1979, 1, 91–99. [Google Scholar] [CrossRef]
- Shane, O.; Hickford, M.; Schiel, D.R. Earthquake-induced habitat migration in a riparian spawning fish has implications for conservation management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 702–712. [Google Scholar]
- Goarant, A.; Petitgas, P.; Bourriau, P. Anchovy (Engraulis encrasicolus) egg density measurements in the Bay of Biscay: Evidence for the spatial variation in egg density with sea surface salinity. Mar. Biol. 2007, 151, 1907–1915. [Google Scholar] [CrossRef][Green Version]
- Jun, Z.; Yongsong, Q.; Zuozhi, C.; Peng, Z.; Kui, Z.; Jiangtao, F.; Guobao, C.; Yancong, C.; Mingshuai, S. Advances in pelagic fishery resources survey and assessment in open South China Sea. South China Fish. Sci. 2018, 14, 118–127. [Google Scholar]
- Suursaar, U.; Raid, T.; Vetemaa, M.; Saat, T. Storm-generated shallow sea turbidity and its influence on spawning and nursery grounds of littoral fish. In Proceedings of the 2015 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Milan, Italy, 26–31 July 2015. [Google Scholar]
- Bakun, A. Patterns in the ocean: Ocean processes and marine population dynamics. Oceanogr. Lit. Rev. 1996, 5, 530. [Google Scholar]
- Hinrichsen, H.-H.; Lehmann, A.; Möllmann, C.; Schmidt, J.O. Dependency of larval fish survival on retention/dispersion in food limited environments: The Baltic Sea as a case study. Fish. Oceanogr. 2003, 12, 425–433. [Google Scholar] [CrossRef]
- Pitchaikani, J.S.; Lipton, A.P. Nutrients and phytoplankton dynamics in the fishing grounds off Tiruchendur coastal waters, Gulf of Mannar, India. Springerplus 2016, 5, 1405. [Google Scholar] [CrossRef]
- Casaucao, A.; González-Ortegón, E.; Jiménez, M.P.; Teles-Machado, A.; Plecha, S.; Peliz, A.J.; Laiz, I. Assessment of the spawning habitat, spatial distribution, and Lagrangian dispersion of the European anchovy (Engraulis encrasicolus) early stages in the Gulf of Cadiz during an apparent anomalous episode in 2016-ScienceDirect. Sci. Total. Environ. 2021, 781, 146530. [Google Scholar] [CrossRef]
- Yu, J.; Hu, Q.; Tang, D.; Chen, P. Environmental effects on the spatiotemporal variability of purpleback flying squid in XishaZhongsha waters, South China Sea. Mar. Ecol. Prog. Ser. 2019, 623, 25–37. [Google Scholar] [CrossRef]
- Wang, D.; Yao, L.; Yu, J.; Chen, P. The Role of Environmental Factors on the Fishery Catch of the Squid Uroteuthis chinensis in the Pearl River Estuary, China. J. Mar. Sci. Eng. 2021, 9, 131. [Google Scholar] [CrossRef]
- Pawelec, A.J.; Sapota, M.R.; Kobos, J. The effect of algal blooms on fish in their inshore nursery grounds in the Gulf of Gdańsk. J. Mar. Biol. Assoc. United Kingd. 2017, 98, 97–104. [Google Scholar] [CrossRef]
- Zeidberg, L.D.; Butler, J.L.; Ramon, D.; Cossio, A.; Stierhoff, K.L.; Henry, A. Estimation of spawning habitats of market squid (Doryteuthis opalescens) from field surveys of eggs off Central and Southern California. Mar. Ecol. 2012, 33, 326–336. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Marine fishery water area map of China (batch 1). China Fish. 2002, 8, 21–22. [Google Scholar]
- Myers, R.A.; Pepin, P. Recruitment variability and oceanographic stability. Fish. Oceanogr. 1994, 3, 246–255. [Google Scholar] [CrossRef]
- Irigoien, X.; Fiksen, Ø.; Cotano, U.; Uriarte, A.; Alvarez, P.; Arrizabalaga, H.; Boyra, G.; Santos, M.; Sagarminaga, Y.; Otheguy, P. Could Biscay Bay Anchovy recruit through a spatial loophole? Prog. Oceanogr. 2007, 74, 132–148. [Google Scholar] [CrossRef]
- Cushing, D.H. Hydrographic containment of a spawning group of plaice in the Southern Bight of the North Sea. Mar. Ecol. Prog. Ser. 1989, 58, 287–297. [Google Scholar] [CrossRef]
- Wong, L.A. A model study of the circulation in the Pearl River Estuary (PRE) and its adjacent coastal waters: 1. Simulations and comparison with observations. J. Geophys. Res. 2003, 108. [Google Scholar] [CrossRef]
- Pan, J.; Gu, Y.; Wang, D. Observations and numerical modeling of the Pearl River plume in summer season. J. Geophys. Res.: Oceans 2014, 119, 2480–2500. [Google Scholar] [CrossRef]
- Ji, X.; Sheng, J.; Tang, L.; Liu, D.; Yang, X. Process study of dry-season circulation in the Pearl River estuary and adjacent coastal waters using a triple-nested coastal circulation model. Atmos. Ocean. 2011, 49, 138–162. [Google Scholar] [CrossRef]
- Yang, Y. Seasonal variation of the Pearl River diluted water and its dynamical cause. Mar. Sci. Bull. 2014, 33, 36–44. [Google Scholar]
- Huayong, X.; Changjian, L.; Dongxiao, W. The upwelling driven by the Zhujiang River runoff in 2006 summer. Haiyang Xuebao 2018, 40, 43–54. [Google Scholar]
- Potter, N.I.C. Movement of larval fishes through the entrance channel of a seasonally open estuary in Western Australia. Estuar. Coast. Shelf Sci. 1992, 35, 213–224. [Google Scholar]
- Harris, S.A.; Cyrus, D.P. Composition, abundance and seasonality of larval fish in richards bay harbour, kwazulu-natal, south africa. South. Afr. J. Aquat. Sci. 1997, 23, 56–78. [Google Scholar] [CrossRef]
- Zhaoli, X.; Hua, C.; Qinghui, C. Distribution patterns of ichthyoplankton in the Oujiang estuary. J. Fish. Sci. China 2008, 32, 733–739. [Google Scholar]
- Suying, O.; Dongxiao, W. A study on the spreading variations and its causes of the Zhujiang River plume. In Proceedings of the 10th China Estuarine and coastal Symposium; China Ocean Press: Beijing, China, 2009; pp. 34–43. [Google Scholar]
- Huijie, X.; Fei, C.; Liya, W.; Jiezhong, C. Zhujiang River Estuarine Circulation Model; Oceanography in China, China Ocean Press: Beijing, China, 2001; Volume 13, pp. 138–151. [Google Scholar]
- Mbaye, B.C.; Brochier, T.; Echevin, V.; Lazar, A.; Lévy, M.; Mason, E.; Gaye, A.T.; Machu, E. DoSardinella auritaspawning seasons match local retention patterns in the Senegalese-Mauritanian upwelling region? Fish. Oceanogr. 2015, 24, 69–89. [Google Scholar] [CrossRef]



| Year | Month | Voyage | Number of Stations |
|---|---|---|---|
| 2014 | April | 1 | 22 |
| May | 2 | 22 | |
| June | 3 | 22 | |
| 2015 | April | 1 | 20 |
| June | 2, 3 | 40 | |
| 2016 | April | 1 | 22 |
| June | 2 | 22 | |
| July | 3 | 22 | |
| 2017 | April | 1 | 24 |
| June | 2 | 24 | |
| July | 3 | 24 |
| Influencing Factors | Unit | Temporal Resolution | Spatial Resolution | Start and End Dates |
|---|---|---|---|---|
| SST | °C | 1 day | 4 km | April 2014–July 2017 |
| Chl-a | mg/m3 | 1 day | 4 km | April 2014–July 2017 |
| SSS | ‰ | 1 month | 1/12° | April 2014–July 2017 |
| Current | / | 1 month | 1/12° | April 2014–July 2017 |
| Depth | m | / | 8.85 m | / |
| Influencing Factors | d.f. | Contribution (%) | Pr(chi) | Pr(F) |
|---|---|---|---|---|
| SST | 9.20 | 14.3 | <0.001 | <0.001 |
| Depth | 3.81 | 9.7 | <0.001 | <0.001 |
| SSS | 8.36 | 8.5 | <0.001 | <0.001 |
| Chl-a | 8.07 | 7.3 | <0.001 | <0.001 |
| Model | AIC | GCV | Adjusted R2 | Deviance Explained (%) | Residual Deviance |
|---|---|---|---|---|---|
| Log(Y+1)~NULL | 1813.34 | 3.91 | 0 | 0 | 1679.5 |
| Log(Y+1)~s(SSS) | 1790.06 | 3.71 | 0.06 | 8.5 | 1537.3 |
| Log(Y+1)~s(SSS) + s(SST) | 1734.37 | 3.26 | 0.20 | 22.8 | 1296.7 |
| Log(Y+1)~s(SSS) + s(SST) + s(Chl-a) | 1705.91 | 3.06 | 0.26 | 30.1 | 1173.6 |
| Log(Y+1)~s(SSS) + s(SST) + s(Chl-a) + s(Depth) | 1654.59 | 2.72 | 0.35 | 39.8 | 993.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Yao, L.; Yu, J.; Chen, P.; Hu, R. Response to Environmental Factors of Spawning Ground in the Pearl River Estuary, China. J. Mar. Sci. Eng. 2021, 9, 763. https://doi.org/10.3390/jmse9070763
Wang D, Yao L, Yu J, Chen P, Hu R. Response to Environmental Factors of Spawning Ground in the Pearl River Estuary, China. Journal of Marine Science and Engineering. 2021; 9(7):763. https://doi.org/10.3390/jmse9070763
Chicago/Turabian StyleWang, Dongliang, Lijun Yao, Jing Yu, Pimao Chen, and Ruirui Hu. 2021. "Response to Environmental Factors of Spawning Ground in the Pearl River Estuary, China" Journal of Marine Science and Engineering 9, no. 7: 763. https://doi.org/10.3390/jmse9070763
APA StyleWang, D., Yao, L., Yu, J., Chen, P., & Hu, R. (2021). Response to Environmental Factors of Spawning Ground in the Pearl River Estuary, China. Journal of Marine Science and Engineering, 9(7), 763. https://doi.org/10.3390/jmse9070763






