Using Drones to Measure Jellyfish Density in Shallow Estuaries
Abstract
1. Introduction
2. Materials and Methods
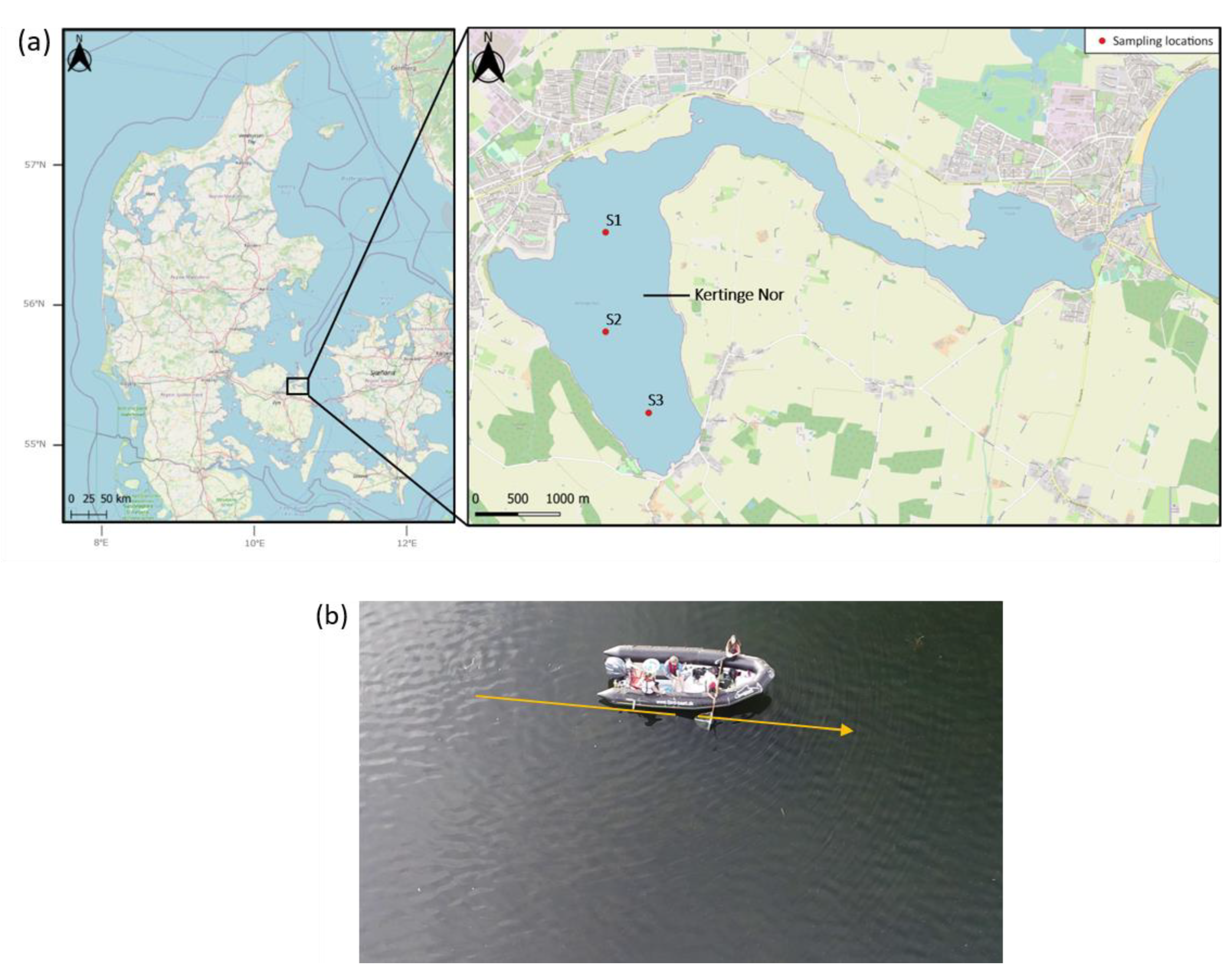
2.1. Study Site and Species
2.2. Protocol for Net Sampling
2.3. Protocol for Drone Sampling
2.4. Analyses of Drone Images
2.5. Statistical Tests
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lynam, C.P.; Lilley, M.K.S.; Bastian, T.; Doyle, T.K.; Beggs, S.E.; Hays, G.C. Have jellyfish in the Irish Sea benefited from climate change and overfishing? Glob. Chang. Biol. 2011, 17, 767–782. [Google Scholar] [CrossRef]
- Condon, R.H.; Graham, W.M.; Duarte, C.M.; Pitt, K.A.; Lucas, C.H.; Haddock, S.H.D.; Sutherland, K.R.; Robinson, K.L.; Dawson, M.N.; Decker, M.B.; et al. Questioning the Rise of Gelatinous Zooplankton in the World’s Oceans. BioScience 2012, 62, 160–169. [Google Scholar] [CrossRef]
- Sanz-Martín, M.; Pitt, K.A.; Condon, R.H.; Lucas, C.H.; Novaes de Santana, C.; Duarte, C.M. Flawed citation practices facilitate the unsubstantiated perception of a global trend toward increased jellyfish blooms. Glob. Ecol. Biogeogr. 2016, 25, 1039–1049. [Google Scholar] [CrossRef]
- Bosch-Belmar, M.; Milisenda, G.; Basso, L.; Doyle, T.K.; Leone, A.; Piraino, S. Jellyfish Impacts on Marine Aquaculture and Fisheries. Rev. Fish. Sci. Aquac. 2021, 29, 242–259. [Google Scholar] [CrossRef]
- Houghton, J.D.R.; Doyle, T.K.; Wilson, M.W.; Davenport, J.; Hays, G.C. Jellyfish aggregations and leatherback turtle foraging patterns in a temperate coastal environment. Ecology 2006, 87, 1967–1972. [Google Scholar] [CrossRef]
- Fleming, N.E.C.; Harrod, C.; Newton, J.; Houghton, J.D.R. Not All Jellyfish Are Equal: Isotopic Evidence for Inter- and Intraspecific Variation in Jellyfish Trophic Ecology. PeerJ 2015, 3, e1110. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.M.; Pagès, F.; Hamner, W.M. A Physical Context for Gelatinous Zooplankton Aggregations: A review. Hydrobiologia 2001, 451, 199–212. [Google Scholar] [CrossRef]
- Olesen, N. Clearance Potential of Jellyfish Aurelia Aurita, and Predation Impact on Zooplankton in a Shallow Cove. Mar. Ecol. Prog. Ser. 1995, 124, 63–72. [Google Scholar] [CrossRef]
- Omori, M.; Ishii, H.; Fujinaga, A. Life History Strategy of Aurelia Aurita (Cnidaria, Scyphomedusae) and Its Impact on the Zooplankton Community of Tokyo Bay. ICES J. Mar. Sci. 1995, 52, 597–603. [Google Scholar] [CrossRef]
- Hansson, L.J.; Moeslund, O.; Kiørboe, T.; Riisgård, H.U. Clearance Rates of Jellyfish and Their Potential Predation Impact on Zooplankton and Fish Larvae in a Neritic Ecosystem (Limfjorden, Denmark). Mar. Ecol. Prog. Ser. 2005, 304, 117–131. [Google Scholar] [CrossRef]
- Doyle, T.K.; Houghton, J.D.R.; Buckley, S.M.; Hays, G.C.; Davenport, J. The Broad-Scale Distribution of Five Jellyfish Species across a Temperate Coastal Environment. Hydrobiologia 2007, 579, 29–39. [Google Scholar] [CrossRef]
- Bastian, T.; Haberlin, D.; Purcell, J.E.; Hays, G.C.; Davenport, J.; McAllen, R.; Doyle, T.K. Large-Scale Sampling Reveals the Spatio-Temporal Distributions of the Jellyfish Aurelia Aurita and Cyanea Capillata in the Irish Sea. Mar. Biol. 2011, 158, 2639–2652. [Google Scholar] [CrossRef]
- Purcell, J.E. Extension of Methods for Jellyfish and Ctenophore Trophic Ecology to Large-Scale Research. Hydrobiologia 2009, 616, 23–50. [Google Scholar] [CrossRef]
- Hosia, A.; Falkenhaug, T.; Baxter, E.J.; Pagès, F. Abundance, Distribution and Diversity of Gelatinous Predators along the Northern Mid-Atlantic Ridge: A Comparison of Different Sampling Methodologies. PLoS ONE 2017, 12, e0187491. [Google Scholar] [CrossRef]
- Brierley, A.S.; Axelsen, B.E.; Buecher, E.; Sparks, C.A.J.; Boyer, H.; Gibbons, M.J. Acoustic Observations of Jellyfish in the Namibian Benguela. Mar. Ecol. Prog. Ser. 2001, 210, 55–66. [Google Scholar] [CrossRef]
- Båmstedt, U.; Kaartvedt, S.; Youngbluth, M. An Evaluation of Acoustic and Video Methods to Estimate the Abundance and Vertical Distribution of Jellyfish. J. Plankton Res. 2003, 25, 1307–1318. [Google Scholar] [CrossRef][Green Version]
- Brierley, A.S.; Boyer, D.C.; Axelsen, B.E.; Lynam, C.P.; Sparks, C.A.J.; Boyer, H.J.; Gibbons, M.J. Towards the Acoustic Estimation of Jellyfish Abundance. Mar. Ecol. Prog. Ser. 2005, 295, 105–111. [Google Scholar] [CrossRef]
- Han, C.-H.; Uye, S.-I. Quantification of the Abundance and Distribution of the Common Jellyfish Aurelia Aurita s.l. with a Dual-Frequency IDentification SONar (DIDSON). J. Plankton Res. 2009, 31, 805–814. [Google Scholar] [CrossRef]
- Graham, W.; Martin, D.; Martin, J. In Situ Quantification and Analysis of Large Jellyfish Using a Novel Video Profiler. Mar. Ecol. Prog. Ser. 2003, 254, 129–140. [Google Scholar] [CrossRef]
- Hoving, H.-J.; Christiansen, S.; Fabrizius, E.; Hauss, H.; Kiko, R.; Linke, P.; Neitzel, P.; Piatkowski, U.; Körtzinger, A. The Pelagic In Situ Observation System (PELAGIOS) to Reveal Biodiversity, Behavior, and Ecology of Elusive Oceanic Fauna. Ocean Sci. 2019, 15, 1327–1340. [Google Scholar] [CrossRef]
- Houghton, J.; Doyle, T.; Davenport, J.; Hays, G. Developing a Simple, Rapid Method for Identifying and Monitoring Jellyfish Aggregations from the Air. Mar. Ecol. Prog. Ser. 2006, 314, 159–170. [Google Scholar] [CrossRef]
- Purcell, J.E.; Brown, E.D.; Stokesbury, K.D.E.; Haldorson, L.H.; Shirley, T.C. Aggregations of the Jellyfish Aurelia Labiata: Abundance, Distribution, Association with Age-0 Walleye Pollock, and Behaviors Promoting Aggregation in Prince William Sound, Alaska, USA. Mar. Ecol. Prog. Ser. 2000, 195, 145–158. [Google Scholar] [CrossRef][Green Version]
- Magome, S.; Yamashita, T.; Kohama, T.; Kaneda, A.; Hayami, Y.; Takahashi, S.; Takeoka, H. Jellyfish Patch Formation Investigated by Aerial Photography and Drifter Experiment. J. Oceanogr. 2007, 63, 761–773. [Google Scholar] [CrossRef]
- Fossette, S.; Gleiss, A.C.; Chalumeau, J.; Bastian, T.; Armstrong, C.D.; Vandenabeele, S.; Karpytchev, M.; Hays, G.C. Current-Oriented Swimming by Jellyfish and Its Role in Bloom Maintenance. Curr. Biol. 2015, 25, 342–347. [Google Scholar] [CrossRef]
- Schaub, J.; Hunt, B.; Pakhomov, E.; Holmes, K.; Lu, Y.; Quayle, L. Using Unmanned Aerial Vehicles (UAVs) to Measure Jellyfish Aggregations. Mar. Ecol. Prog. Ser. 2018, 591, 29–36. [Google Scholar] [CrossRef]
- Raoult, V.; Colefax, A.P.; Allan, B.M.; Cagnazzi, D.; Castelblanco-Martínez, N.; Ierodiaconou, D.; Johnston, D.W.; Landeo-Yauri, S.; Lyons, M.; Pirotta, V.; et al. Operational Protocols for the Use of Drones in Marine Animal Research. Drones 2020, 4, 64. [Google Scholar] [CrossRef]
- Raoult, V.; Gaston, T.F. Rapid Biomass and Size-Frequency Estimates of Edible Jellyfish Populations Using Drones. Fish. Res. 2018, 207, 160–164. [Google Scholar] [CrossRef]
- Rowley, O.C.; Courtney, R.L.; Browning, S.A.; Seymour, J.E. Bay Watch: Using Unmanned Aerial Vehicles (UAV’s) to Survey the Box Jellyfish Chironex Fleckeri. PLoS ONE 2020, 15, e0241410. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, H.J.; Seo, M.H.; Soh, H.Y. Density Estimation of Nemopilema Nomurai (Scyphozoa, Rhizostomeae) Using a Drone. J. Indian Soc. Remote Sens. 2021, 1–6. [Google Scholar] [CrossRef]
- Olesen, N.J.; Frandsen, K.; Riisgård, H.U. Population Dynamics, Growth and Energetics of Jellyfish Aurelia Aurita in a Shallow Fjord. Mar. Ecol. Prog. Ser. 1994, 105, 9–18. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Barth-Jensen, C.; Madsen, C. High Abundance of the Jellyfish Aurelia Aurita Excludes the Invasive Ctenophore Mnemiopsis Leidyi to Establish in a Shallow Cove (Kertinge Nor, Denmark). Aquat. Invasions 2010, 5. [Google Scholar] [CrossRef]
- Goldstein, J.; Riisgård, H.U. Population Dynamics and Factors Controlling Somatic Degrowth of the Common Jellyfish, Aurelia Aurita, in a Temperate Semi-Enclosed Cove (Kertinge Nor, Denmark). Mar. Biol. 2016, 163, 33. [Google Scholar] [CrossRef]
- Lüskow, F. Importance of Environmental Monitoring: Long-Term Record of Jellyfish (Aurelia Aurita) Biomass in a Shallow Semi-Enclosed Cove (Kertinge Nor, Denmark). Reg. Stud. Mar. Sci. 2020, 34, 100998. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Jensen, M.H.; Rask, N.; Caldwell, M.M.; Heldmaier, G.; Jackson, R.B.; Lange, O.L.; Mooney, H.A.; Schulze, E.D.; Sommer, U. Odense Fjord and Kerteminde Fjord/Kertinge Nor. In Ecology of Baltic Coastal Waters; Schiewer, U., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 197, pp. 361–394. [Google Scholar]
- Nielsen, A.S.; Pedersen, A.W.; Riisgård, H.U. Implications of Density Driven Currents for Interaction between Jellyfish (Aurelia Aurita) and Zooplankton in a Danish Fjord. Sarsia 1997, 82, 297–305. [Google Scholar] [CrossRef]
- OpenStreetMap Contributors. 2020. Available online: https://www.openstreetmap.org (accessed on 1 May 2021).
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2020. Available online: http://qgis.osgeo.org (accessed on 1 May 2021).
- Pau, G.; Fuchs, F.; Sklyar, O.; Boutros, M.; Huber, W. EBImage—An R Package for Image Processing with Applications to Cellular Phenotypes. Bioinformatics 2010, 26, 979–981. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2021; Available online: http://www.rstudio.com/ (accessed on 1 May 2021).
- Abramoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Available online: http://localhost/handle/1874/204900 (accessed on 1 May 2021).
- Shkurti, F.; Xu, A.; Meghjani, M.; Gamboa Higuera, J.C.; Girdhar, Y.; Giguère, P.; Dey, B.B.; Li, J.; Kalmbach, A.; Prahacs, C.; et al. Multi-Domain Monitoring of Marine Environments Using a Heterogeneous Robot Team. In Proceedings of the 2012 IEEE/RSJ International Conference on Intelligent Robots and Systems, Algarve, Portugal, 7–12 October 2012; pp. 1747–1753. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Wang, Y.; Zhao, Z.; Liu, J.; Liu, Y.; Sun, C.; Zhou, C. Automatic Fish Population Counting by Machine Vision and a Hybrid Deep Neural Network Model. Animals 2020, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Gauci, A.; Deidun, A.; Abela, J. Automating Jellyfish Species Recognition through Faster Region-Based Convolution Neural Networks. Appl. Sci. 2020, 10, 8257. [Google Scholar] [CrossRef]
- Helm, R.R.; Clark, N.; Harden-Davies, H.; Amon, D.; Girguis, P.; Bordehore, C.; Earle, S.; Gibbons, M.J.; Golbuu, Y.; Haddock, S.H.D.; et al. Protect high seas biodiversity. Science 2021, 372, 1048–1049. [Google Scholar]

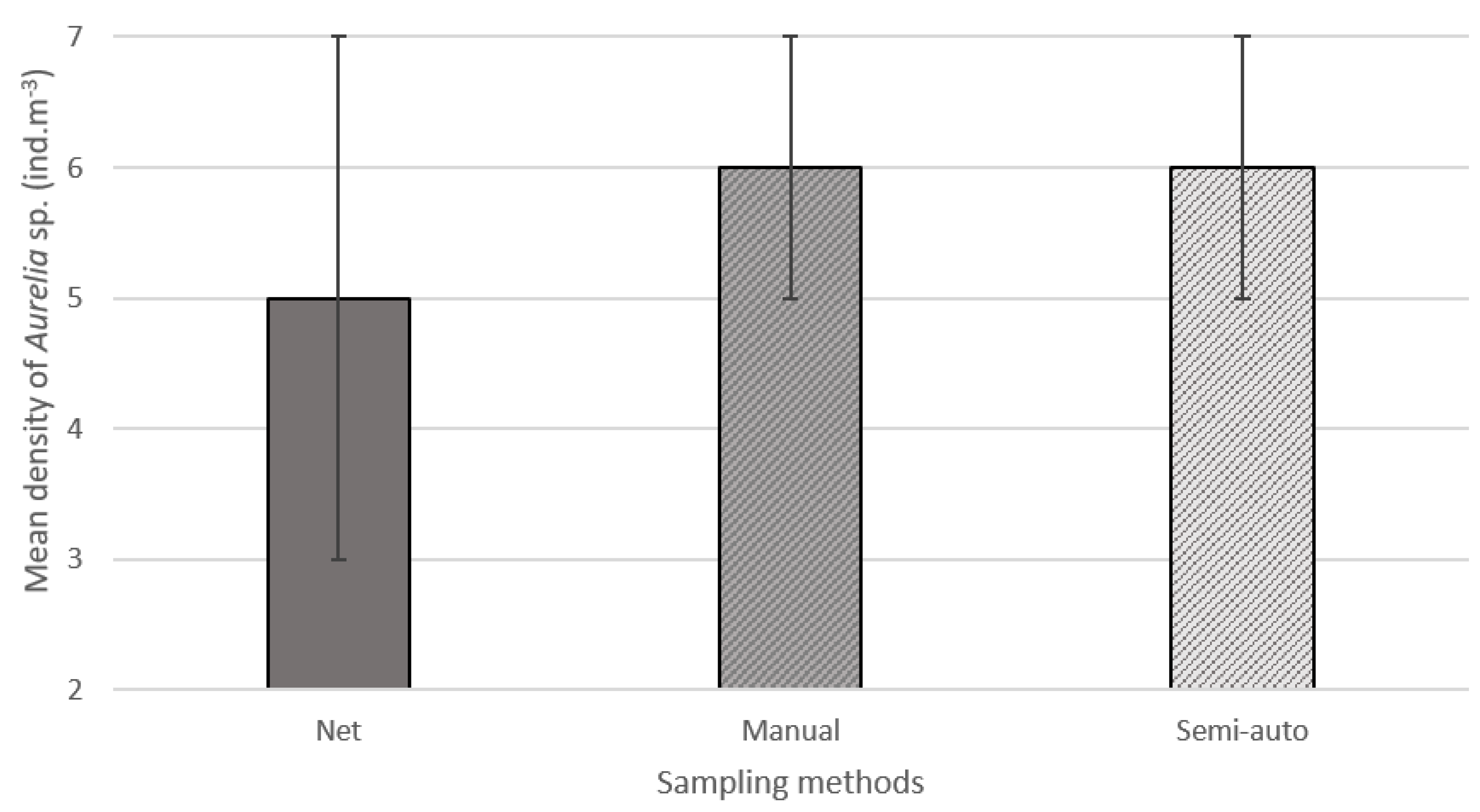
| Station | Manual | Semi-Auto Mean (95% CI) |
|---|---|---|
| 1 | 209 | 206 (190–221) |
| 2 | 212 | 211 (201–220) |
| 3 | 142 | 160 (142–178) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamel, H.; Lhoumeau, S.; Wahlberg, M.; Javidpour, J. Using Drones to Measure Jellyfish Density in Shallow Estuaries. J. Mar. Sci. Eng. 2021, 9, 659. https://doi.org/10.3390/jmse9060659
Hamel H, Lhoumeau S, Wahlberg M, Javidpour J. Using Drones to Measure Jellyfish Density in Shallow Estuaries. Journal of Marine Science and Engineering. 2021; 9(6):659. https://doi.org/10.3390/jmse9060659
Chicago/Turabian StyleHamel, Héloïse, Sébastien Lhoumeau, Magnus Wahlberg, and Jamileh Javidpour. 2021. "Using Drones to Measure Jellyfish Density in Shallow Estuaries" Journal of Marine Science and Engineering 9, no. 6: 659. https://doi.org/10.3390/jmse9060659
APA StyleHamel, H., Lhoumeau, S., Wahlberg, M., & Javidpour, J. (2021). Using Drones to Measure Jellyfish Density in Shallow Estuaries. Journal of Marine Science and Engineering, 9(6), 659. https://doi.org/10.3390/jmse9060659






