Environmental Effects on the Spatiotemporal Variability of Fish Larvae in the Western Guangdong Waters, China
Abstract
1. Introduction
2. Materials and Methods
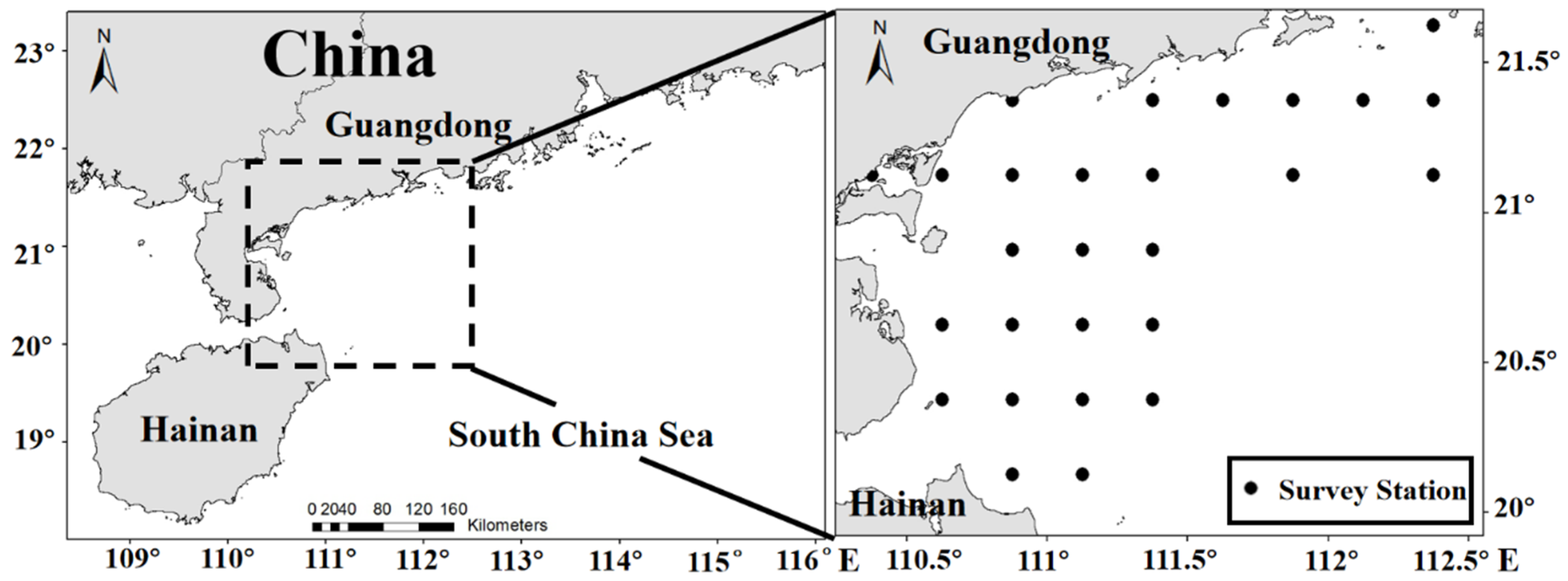
2.1. Fish Larvae Data
2.2. Environmental and Geographic Data
2.3. GAMs Fitting Procedures
2.4. Model Test
2.5. Center of Gravity of Fish Larvae Density
3. Results
3.1. GAMs Analysis
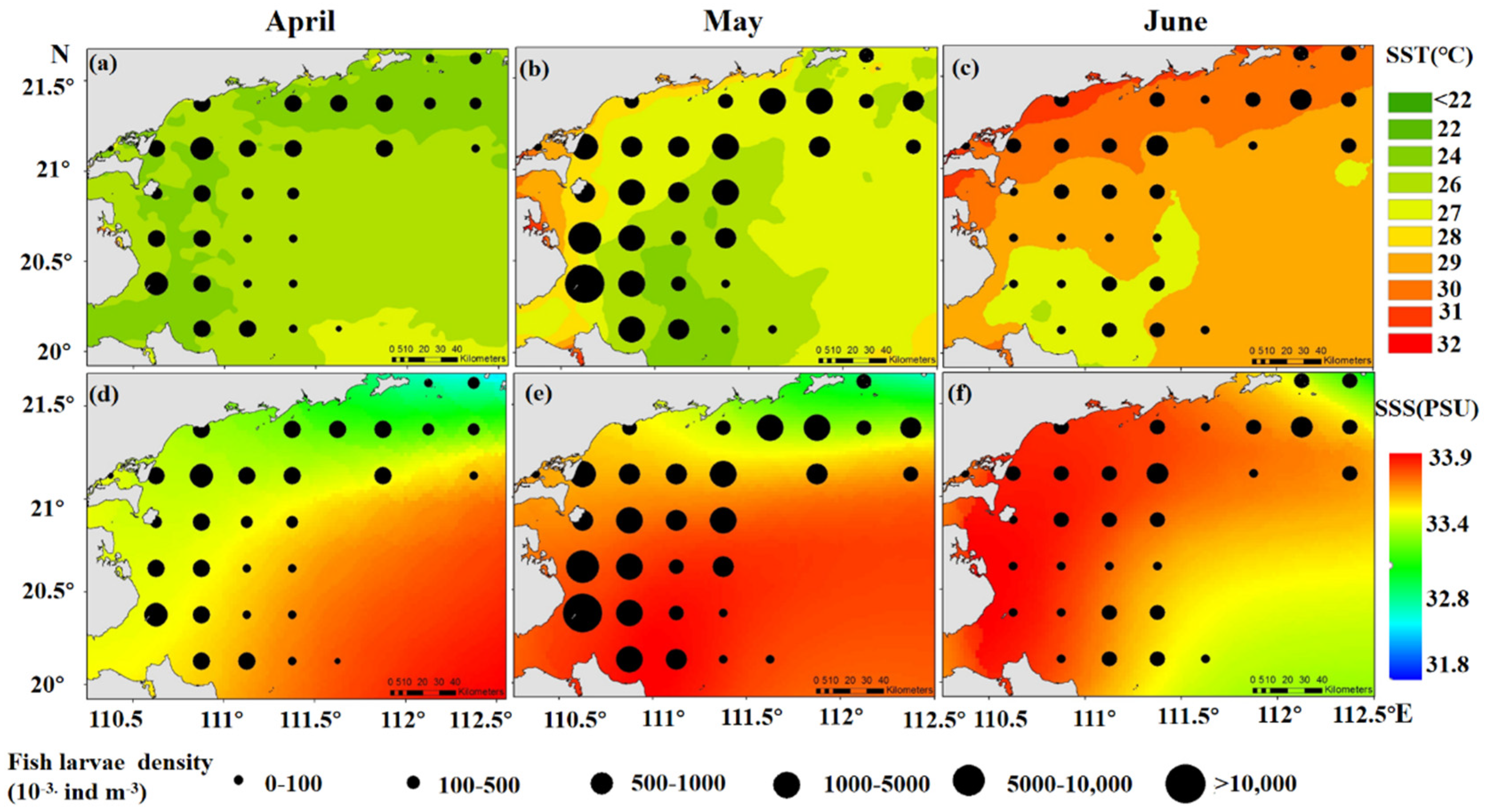
3.2. Relationship between Spatiotemporal Variability of Fish Larvae, SST, and SSS
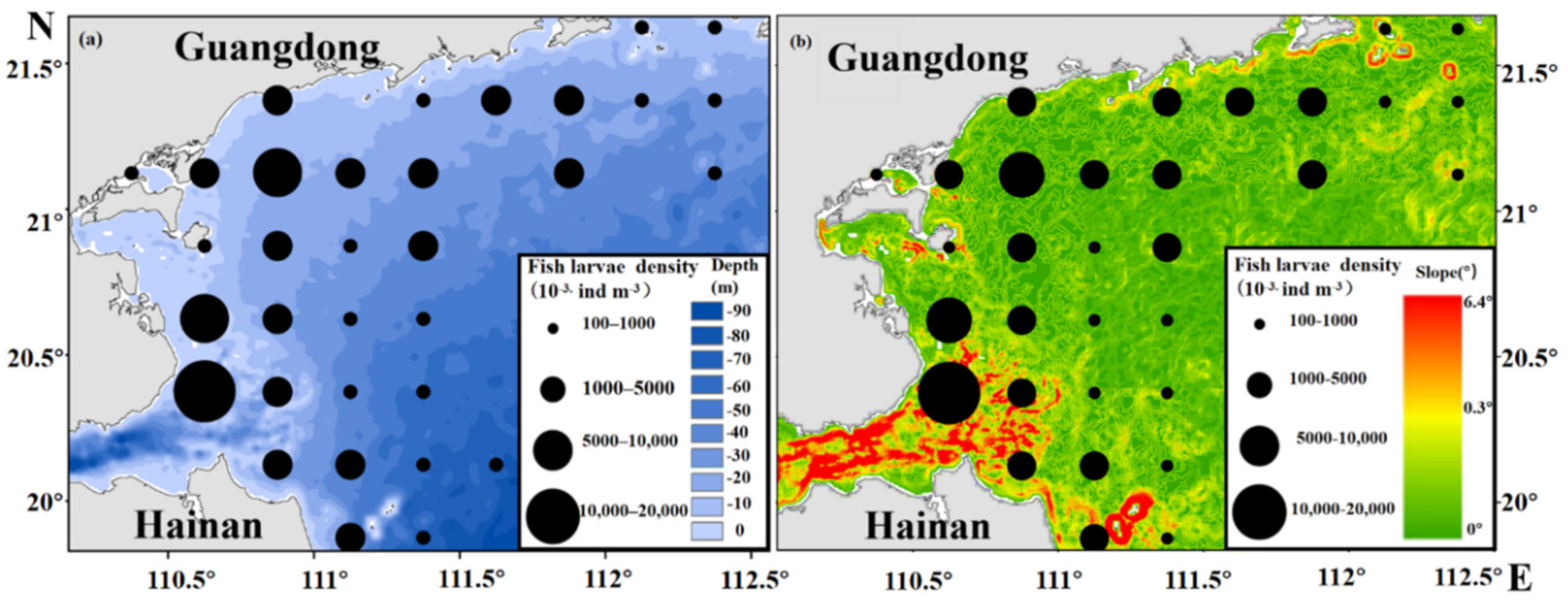
3.3. Relationship between Spatiotemporal Variability of Fish Larvae, Depth and Slope
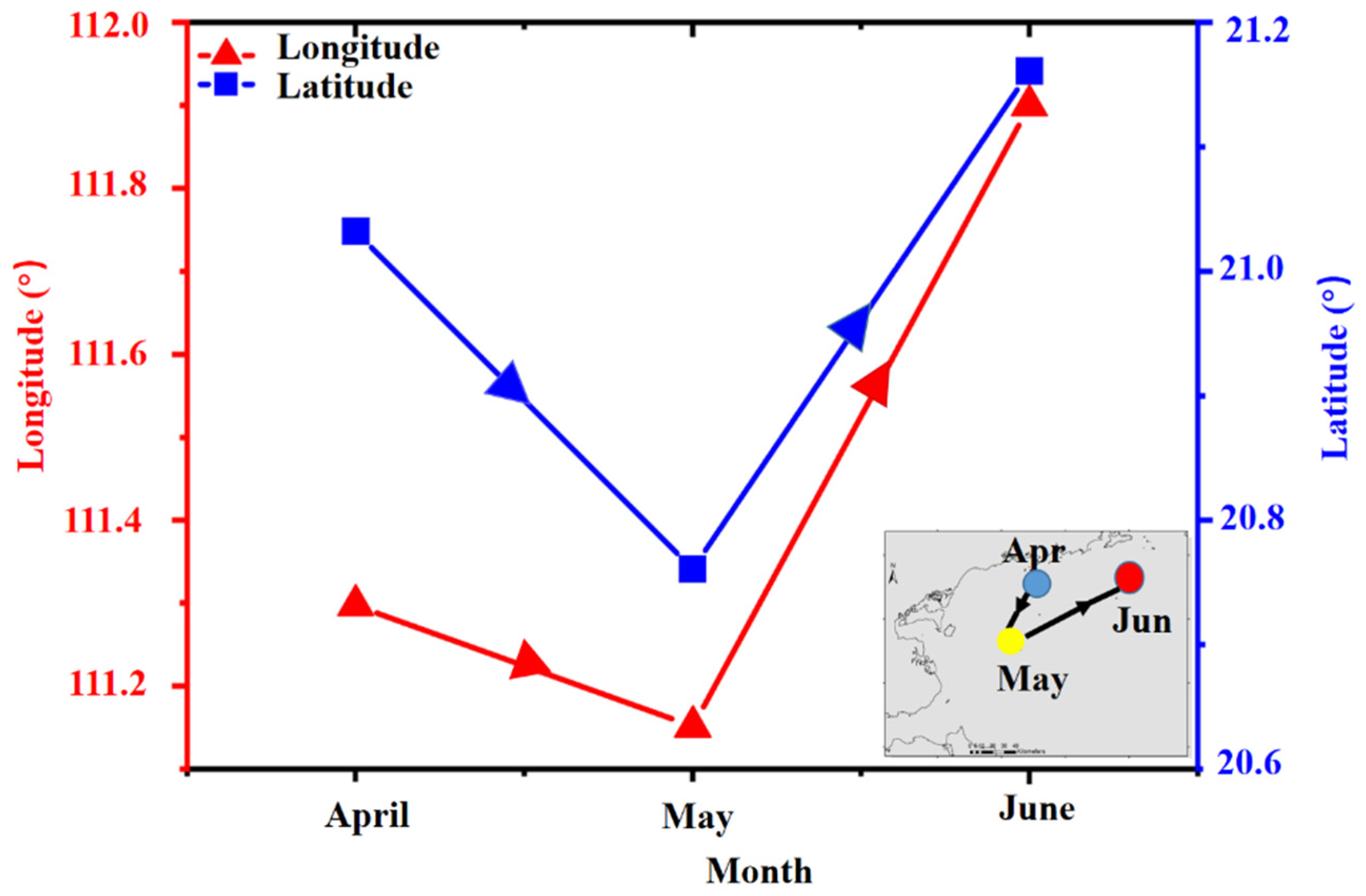
3.4. Spatiotemporal Variability of Center of Gravity (CoG) of Fish Larvae Density
4. Discussion
4.1. Effects of SST and SSS on Fish Larvae
4.2. Effects of Depth, Slope, and Offshore Distance on Fish Larvae
4.3. Effects of Seasonal Upwelling on Fish Larvae
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, H.J.; Xu, S.N.; Liu, Y.; Gong, Y.Y.; Li, C.H. Ecological characteristics of the phytoplankton community structure in the West Guangdong coastal area. Mar. Sci. 2016, 40, 73–82. [Google Scholar]
- Pan, Y.J. Fisheries Dictionary (Essence); Shanghai Lexicographical Press: Shanghai, China, 2007. [Google Scholar]
- Ellis, T.; Nash, R.D.M. Predation by sprat and herring on pelagic fish eggs in a plaice spawning area in the Irish Sea. J. Fish. Biol. 2010, 50, 1195–1202. [Google Scholar] [CrossRef]
- Hovenkamp, F. Growth-dependent mortality of larval plaice Pleuronectes platessa in the North Sea. Mar. Ecol. Prog. Ser. 1992, 82, 95–101. [Google Scholar] [CrossRef]
- Wan, R.J.; Sun, S. The category composition and abundance of ichthyoplankton in the ecosystem of the Yellow Sea and the East China Sea. Acta Zool. Sin. 2006, 52, 28–44. [Google Scholar] [CrossRef]
- Wan, R.J.; Jiang, Y.W. Studies on the Ecology of Eggs and Larvae of Osteichthyes in the Yellow Sea. Mar. Fish. Res. 1998, 19, 60–73. [Google Scholar]
- Zhang, R.Z. Fish Eggs and Larvae in the Coastal Waters of China; Shanghai Science and Technology Press: Shanghai, China, 1985. [Google Scholar]
- Houde, E.D.; Hoyt, R. Fish early life dynamics and recruitment variability. Trans. Am. Fish. Soc. 1987, 2, 17–29. [Google Scholar]
- Chambers, R.C.; Trippel, E.A. Early Life History and Recruitment in Fish Populations, The Netherlands; Springer: Berlin/Heidelberg, Germany, 1997; Volume 17, pp. 469–493. [Google Scholar] [CrossRef]
- Li, L.; Zhong, J.S.; Tang, J.H.; Wu, L.; Xu, C.L. Abundance and distribution of larvae and juveniles of Stolephorus chinensis in the coast of Jiangsu Province and its relationship with environmental factors. J. Shanghai Ocean Univ. 2009, 33–38. [Google Scholar]
- Teodósio, M.A.; Paris, C.B.; Wolanski, E.; Morais, P. Biophysical processes leading to the ingress of temperate fish larvae into estuarine nursery areas: A review. Estuar. Coast. Shelf Sci. 2016, 183, 187–202. [Google Scholar] [CrossRef]
- Baptista, V.; Morais, P.; Cruz, J.; Castanho, S.; Ribeiro, L.; Pousão-Ferreira, P.; Leitão, F.; Wolanski, E.; Teodósio, M.A. Swimming Abilities of Temperate Pelagic Fish Larvae Prove that They May Control Their Dispersion in Coastal Areas. Diversity 2019, 11, 185. [Google Scholar] [CrossRef]
- Hjort, J. Fluctuations in the great fisheries of northern Europe viewed in the light of biological research. Rapp. P.-V. Reun. Cons. Int. Explor. Mer. 1914, 20, 1–227. [Google Scholar]
- Fauchald, P.; Erikstad, K.E.; Skarsfjord, H. Scale-dependent predator-prey Interactions: The Hierarchical spatial distribution of seabirds and prey. Ecology 2000, 81, 773. [Google Scholar] [CrossRef]
- Stenseth, N.C.; Mysterud, A.; Ottersen, G.; Hurrell, J.W.; Chan, K.S.; Lima, M. Ecological effects of climate fluctuations. Science 2002, 297, 1292–1296. [Google Scholar] [CrossRef]
- Dingsor, G.E.; Ciannelli, L.; Chan, K.; Ottersen, G.; Stenseth, N.C. Density dependence and density independence during the early life stages of four marine fish stocks. Ecology 2007, 88, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Hastie, T.; Tibshirani, R. Generalized Additive Models; Chapman and Hall: London, UK, 1990; pp. 587–602. [Google Scholar]
- Guisan, A.; Edwards, T.C.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Yu, J.; Hu, Q.; Tang, D.; Chen, P. Environmental effects on the spatiotemporal variability of purpleback flying squid in Xisha-Zhongsha waters, South China Sea. Mar. Ecol. Prog. 2019, 623, 25–37. [Google Scholar] [CrossRef]
- Wang, Y.F.; Yu, J.; Chen, P.M.; Yu, J.; Liu, Z.N. Relationship between spatial-temporal distribution of light falling-net fishing ground and marine environments. J. Trop. Oceanogr. 2019, 38, 68–76. [Google Scholar] [CrossRef]
- Wan, R.J.; Jiang, Y.W. The species and biological characteristics of the eggs and larvae of Osteichthyes in the Bohai Sea and Yellow Sea. J. Shanghai Fish. Univ. 2000, 9, 290–297. [Google Scholar]
- State Oceanic Administration. Marine Survey Code; Standards Press of China: Beijing, China, 1977. [Google Scholar]
- Yu, J.; Hu, Q.W.; Yuan, H.R.; Chen, P.M. Effect assessment of summer fishing moratorium in Daya Bay based on remote sensing data. South China Fish. Sci. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.F.; Hu, Q.W.; Yu, J.; Chen, P.M.; Shu, L. Effect assessment of fishery resources proliferation in Zhelin Bay marine ranching in eastern Guangdong. South China Fish. Sci. 2019, 15, 12–19. [Google Scholar] [CrossRef]
- Tang, G.A.; Liu, X.J.; Lv, G.N. Principle and Method of Digital Elevation Model and Geoscience Analysis; Science Press: Beijing, China, 2005. [Google Scholar]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Wood, S.N. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Am. Stat. Assoc. 2004, 99, 673–686. [Google Scholar] [CrossRef]
- Venables, W.N.; Dichmont, C.M. GLMs, GAMs and GLMMs: An overview of theory for applications in fisheries research. Fish. Res. 2004, 70, 319–337. [Google Scholar] [CrossRef]
- Stone, C.J. Additive Regression and Other Nonparametric Models. Ann. Stat. 1985, 13, 689–705. [Google Scholar] [CrossRef]
- Shchetinnikov, A.S. Feeding spectrum of squid Sthenoteuthis oualaniensis (Oegopsida) in the eastern Pacific. J. Mar. Biol. Assoc. UK 1992, 72, 849–860. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models. An Introduction with R; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Chen, X.J.; Qian, W.G.; Xu, L.X.; Tian, S.Q. Comparision among annual positions of fishing grounds for Ommastrephe bartrami from 150°E to 165°E in the North Pacific. J. Zhanjiang Ocean. Univ. 2003, 23, 26–32. [Google Scholar] [CrossRef]
- Shih, C.L.; Chen, Y.H.; Hsu, C.C. Modeling the Effect of environmental factors on the ricker stock-recruitment relationship for North Pacific Albacore using generalized additive models. Atmos. Ocean. Sci. 2014, 25, 581–590. [Google Scholar] [CrossRef]
- Mangel, M.; Quinn, T.J.; Deriso, R.B. Quantitative fish dynamics. Q. Rev. Biol. 1999, 2, 286–287. [Google Scholar]
- Liu, J.; Wang, Y.Y.; Guo, B.H.; Zhang, X.H. Construction and validation of flavor model of fermented milk during storage. Food Sci. 2011, 32, 296–300. [Google Scholar]
- Lu, Y.; Yu, J.; Lin, Z.; Chen, P. Environmental Influence on the Spatiotemporal Variability of Spawning Grounds in the Western Guangdong Waters, South China Sea. J. Mar. Sci. Eng. 2020, 8, 607. [Google Scholar] [CrossRef]
- Wan, R.J.; Zeng, D.Y.; Bian, X.D.; Ni, X.B. Species composition and abundance distribution pattern of ichthyoplankton and their relationship with environmental factors in the East China Sea ecosystem. J. Fish. China 2014, 38, 1375–1398. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Bian, X.D.; Shan, X.J.; Jin, X.S.; Guan, L.S. Distribution of the age 0 group Pacific cod (Gadus macrocephalus) in the Yellow Sea and its relationship with environmental factors. J. Fish. China 2018, 42, 870–880. [Google Scholar]
- Chen, G.B.; Li, Y.Z.; Zhao, X.Y.; Chen, Y.Z.; Jin, X.S. Acoustic assessment of commercial fish resources in the northern waters of South China Sea. J. Fish. Sci. China 2005, 4, 445–451. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Z.Z.; Chen, G.B.; Zhang, K.; Xu, Y.W.; Sun, M.S. Biomass and distribution of carangoid fish resources in the offshore South China Sea. South China Fish. Sci. 2016, 12, 38–48. [Google Scholar] [CrossRef]
- Lin, J.Q. Study on the natural regulative adaptibilty of the Hairtail (Trichiurus Haumela) in the oceanic environment. Trans. Oceanol. Limnol. 1981, 1, 58–63. [Google Scholar]
- Loots, C.; Vaz, S.; Koubbi, P.; Planque, B.; Coppin, F.; Verin, Y. Inter-annual variability of North Sea plaice spawning habitat. J. Sea Res. 2010, 64, 427–435. [Google Scholar] [CrossRef]
- Shi, Z.H.; Xie, M.M.; Peng, S.M.; Zhang, C.J.; Gao, Q.X. Effects of temperature stress on activities of digestive enzymes and serum biochemical indices of Pampus argenteus juveniles. Prog. Fish. Sci. 2016, 37, 30–37. [Google Scholar] [CrossRef]
- Fu, C.; Cao, Z.; Fu, S. The influence of temperature and starvation on sesting metabolic rate and spontaneous activity in Juvenile Cyprinus carpio. Chin. Chin. J. Zool. 2012, 47, 85–90. [Google Scholar] [CrossRef]
- Anzhi, S.; Huiming, J.U.; Guiyang, L.I.; Jie, L.I.; Zhaolan, M.O. Expression patterns of hsp90 of Paralichthys olivaceus in response to temperature treatment and Vibrio anguillarum-infection. Prog. Fish. Sci. 2016, 37, 1–8. [Google Scholar] [CrossRef]
- Chai, X.J.; Sun, M.; Xu, Y.J. Effects of temperature and salinity on embryonic development of Nibea japonica. South China Fish. Sci. 2011, 7, 43–49. [Google Scholar] [CrossRef]
- Chen, X.Q.; Ruan, C.X.; Yuan, C.G. Effects of different temperature and food on growth and survival rates of the larvae of Trichogaster trichopterus. J. Fujian Fish. 2008, 2, 9–12. [Google Scholar] [CrossRef]
- Targońska, K.; Arski, D.; Kupren, K.; Palińska-Arska, K.; Mamcarz, A.; Kujawa, R.; Skrzypczak, A.; Furga A-Selezniow, G.Y.; Czarkowski, T.K.; Haku, B.A.; et al. Influence of temperature during four following spawning seasons on the spawning effectiveness of common bream, Abramis brama (L.) under natural and controlled conditions. J. Therm. Biol. 2014, 39, 17–23. [Google Scholar] [CrossRef]
- Yuan, L.Q.; Xie, X.J.; Cao, Z.D.; Luo, Y.P. Effects of temperature on embryonic development of the darkbarbel catfish Pelteobagrus vachelli. Acta Zool. Sin. 2005, 51, 753–757. [Google Scholar] [CrossRef]
- Li, X.Z.; Chang, Y.L.; Kang, Q.J.; Liu, X.C.; Qiao, Z.G. Effects of different temperatures on growth of Glyptosternum maculatum larvae and juveniles. Chin. J. Fish. 2011, 32, 96–99. [Google Scholar] [CrossRef]
- Hou, Y.B.; Xiao, G.H.; Zhang, L.K. Effects of temperature and density on the survival rate of juvenile Brachymystax lenok. Hebei Fish. 2016, 3, 6–7. [Google Scholar] [CrossRef]
- Jiang, M.; Shen, X.Q.; Chen, L.F. Relationship between with abundance distribution of fish eggs, larvae and environmental factors in the Changjiang Estuary and vicinity waters in spring. Mar. Environ. Sci. 2006, 25, 37–39. [Google Scholar]
- Song, C.; Liu, Y.Y.; Lv, Y.; Zhao, F.; Yang, G.; Zhang, T.T.; Zhuang, P. Distribution of Salanx ariakensis Larvae in the Yangtze Estuary and its relationship with environment factors. Mar. Fish. 2015, 37, 318–324. [Google Scholar]
- Qu, H.T.; Li, X.X.; He, Q.; Li, H.Z. Effects of temperature and salinity on hatching rates and larval survival of Epinephelus lanceol. Hebei Fish. 2009, 8, 6–9. [Google Scholar] [CrossRef]
- Song, C.; Wang, Y.T.; Liu, Z.L.; Zhang, H.; Lin, Y.; Jiang, Y.Z.; Li, S.F.; Lin, N. Relationship between environmental factors and distribution of Scomberomorus niphonius eggs, larvae, and juveniles in Xiangshan Bay. J. Fish. Sci. China 2016, 23, 1197–1204. [Google Scholar]
- Zhang, R.Z. On the eggs and larvae of the golden-thread Nemipterus Virgatus (Houttuyn). Acta Zool. Sin. 1980, 2, 39–42. [Google Scholar] [CrossRef]
- Holliday, T.F.G. 4 The effect of salinity on the eggs and larvae of teleosts. Fish Physiol. 1969, 1, 293–311. [Google Scholar] [CrossRef]
- Wang, H.S. Effects of salinity on egg development and growth, larval and juvenile survival rate of Pagrosomus major. J. Fish. Sci. China 2002, 9, 33–38. [Google Scholar] [CrossRef]
- Li, B.; Zhong, Y.B.; Lyu, W.Q. Salinity tolerance of Pseudosciaena crocea during early development. J. Shanghai Ocean Univ. 2012, 21, 204–211. [Google Scholar] [CrossRef]
- Wang, H.S. Effects of salinity on growth survival rate and albinism rate of the larvae and juveniles of Paralichthys Olivaceus. Ocean. Limnol. Sin. 1997, 28, 399–405. [Google Scholar]
- Wang, H.T. Effects of environmental conditions on fertilized eggs and early larva of marine fishes. Mar. Sci. 1998, 22, 50–52. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Gao, Y.J.; Wang, J.P.; Xu, B.Q.; Sun, C.X. Community structure of ichthyoplankton and its relationship with environmental factors in Laizhou Bay. Chin. J. Zool. 2018, 37, 2976–2984. [Google Scholar] [CrossRef]
- Moir, H.J.; Soulsby, C.; Youngson, A.F. Hydraulic and sedimentary characteristics of habitat utilized by Atlantic salmon for spawning in the Girnock Burn, Scotland. Fish. Manag. Ecol. 2010, 5, 241–254. [Google Scholar] [CrossRef]
- Lelievre, S.; Vaz, S.; Martin, C.S.; Loots, C. Delineating recurrent fish spawning habitats in the North Sea. J. Sea Res. 2014, 91, 1–14. [Google Scholar] [CrossRef]
- Chen, G.B.; Li, Y.Z. Distribution of the Carangidae fishes in the continental shelf waters of northern South China Sea. J. Shanghai Fish. Univ. 2003, 2, 146–151. [Google Scholar] [CrossRef]
- Chen, G.B.; Li, Y.Z.; Chen, P.M. Spawning ground of Nemipterus bathybius in northern continental shelf waters of South China Sea. J. Zhanjiang Ocean Univ. 2002, 22, 20–25. [Google Scholar]
- Li, J.S.; Lin, N.; Ling, J.Z. Temporal variation in the composition and abundance of fish larvae and juveniles off the Yangtze River Estuary in spring and summer. J. Fish. Sci. China 2018, 25, 123–131. [Google Scholar] [CrossRef]
- Zhang, J.B.; Huang, Z.Y. An investigation on fish eggs and larvae in sea area around planning Yangjiang Nuclear Plant. J. Trop. Oceanogr. 2003, 22, 78–84. [Google Scholar]
- Gibson, R.N. Flatfishes: Biology and Exploitation. Flatfishes Bio. 2015, 9. [Google Scholar] [CrossRef]
- Teodósio, M.A.; Garrido, S.; Peters, J.; Leitão, F.; Ré, P.; Peliz, A.; Santos, A.M.P. Assessing the impact of environmental forcing on the condition of anchovy larvae in the Cadiz Gulf using nucleic acid and fatty acid-derived indices. Estuar. Coast. Shelf Sci. 2017, 185, 94–106. [Google Scholar] [CrossRef]
- Wolanski, E.; Kingsford, M.J. Oceanographic and behavioural assumptions in models of the fate of coral and coral reef fish larvae. J. R. Soc. Interface 2014, 11, 20140209. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, W.N.; Wang, Y.T. Hydrography and distribution dynamics of larval and juvenile fishes in the coastal waters of the Tanshui River estuary, Taiwan, with reference to estuarine larval transport. Mar. Biol. 1993, 116, 205–217. [Google Scholar] [CrossRef]
- Clark, D.L.; Leis, J.M.; Hay, A.C.; Trnski, T. Swimming ontogeny of larvae of four temperate marine fishes. Mar. Ecol. Prog. Ser. 2005, 292, 287–300. [Google Scholar] [CrossRef]
- Kingsford, M.J.; Leis, J.M.; Shanks, A.; Lindeman, K.C.; Pineda, J. Sensory environments, larval abilities and local self-recruitment. Bull. Mar. Sci. 2002, 70, 309–340. [Google Scholar]
- Liu, X.J.; Gong, J.Y.; Zhou, Q.M.; Tang, G.A. A study of accuracy and algorithms for calculating slope and aspect based on grid digital elevation model(DEM). Acta Geod. Cartogr. Sin. 2004, 3, 258–263. [Google Scholar] [CrossRef]
- Jinming, W.; Huanchao, Y.; Jian, S. Habitat environmental characteristics of Brachymysax lenok tsinlingensis. Acta Hydrobiol. Sin. 2017, 41, 214–219. [Google Scholar] [CrossRef]
- Zheng, S.X. Numerical Simulation of Tide and Tidal Currents in the Qiongzhou Strait Based on FVCOM; Ocean University of China: Qingdao, China, 2016. [Google Scholar]
- Chen, D.S.; Chen, B.; Yan, J.H.; Xu, H.F. The Seasonal variation characteristics of residual currents in the Qiongzhou Strait. Trans. Oceanol. Limnol. 2006, 2, 12–17. [Google Scholar] [CrossRef]
- Li, Z.G. Distribution if Main Species of Stow Net in the South. Yellow Sea based on GAM and Preliminary Study of Characteristics of Ichthyoplankton Assemblages in Haizhou Bay; Ocean University of China: Qingdao, China, 2013. [Google Scholar]
- Guan, B.X.; Yuan, Y.C. Overview of studies on some eddies in the China seas and their adjacent seas. Acta Oceanol. Sin. 2006, 28, 1–16. [Google Scholar] [CrossRef]
- Yang, S.Y.; Bao, X.W.; Chen, C.S.; Chen, F. Analysis on characteristics and mechanism of current system in west coast of Guangdong Province in the summer. Acta Oceanol. Sin. 2003, 6, 1–8. [Google Scholar] [CrossRef]
- Huang, Q.Z.; Li, Y.X. General situations of the current and eddy in the South China Sea. Adv. Earth Sci. 1992, 5, 1–9. [Google Scholar]
- Xu, J.D.; Cai, S.Z.; Xuan, L.L.; Qiu, Y.; Zhu, D.Y. Study on coastal upwelling in eastern Hainan Island and western Guangdong in summer, 2006. Acta Oceanol. Sin. 2013, 35, 11–18. [Google Scholar] [CrossRef]
- Deng, S.; Zhong, H.L.; Wang, M.W.; Yun, F.J. On relation between upwelling off Qionghai and fishery. J. Oceanogr. Taiwan Strait 1995, 1, 51–56. [Google Scholar]
- Gronkjaer, P.; Clemmesen, C.; John, M.S. Nutritional condition and vertical distribution of Baltic cod larvae. J. Fish Biol. 1997, 51, 352–369. [Google Scholar] [CrossRef]
- Fang, R.S.; Zheng, Y.J. A study on the eddy off Diaoyu Island and its relationship to the fishing ground of filefish (Navodon Septentrionalis Gunther). J. Fish. China 1986, 10, 161–176. [Google Scholar]

| Species/Families | Survey Month | Proportion |
|---|---|---|
| Sardinella aurita | Apr/May/Jun | 53.2% |
| Nemipterus virgatus | Apr/May/Jun | 10.4% |
| Anchoviella commersonii | Apr/May/Jun | 8.5% |
| Upeneus bensasi | Apr/May/Jun | 8.0% |
| Carangidae | Apr/May/Jun | 6.0% |
| Factors | VIF |
|---|---|
| Month | 7.611 |
| SSS | 3.092 |
| SST | 7.664 |
| Lon | 2.996 |
| Distance | 1.449 |
| Depth | 2.579 |
| Lat | 4.379 |
| Model Factors | Residual Deviance | Adjusted R2 | AIC | GCV | Deviance Explained (%) |
|---|---|---|---|---|---|
| Log(Y + 1) = NULL | 1701.12 | 0.00 | 1336.43 | 5.93 | 0.0 |
| Log(Y + 1) = s(Month) | 1521.02 | 0.10 | 1307.99 | 5.37 | 10.6 |
| Log(Y + 1) = s(Month) + s(Lon) | 1414.34 | 0.16 | 1290.70 | 5.06 | 16.9 |
| Log(Y + 1) = s(Month) + s(Lon) + s(SSS) | 1294.96 | 0.22 | 1271.01 | 4.73 | 23.9 |
| Log(Y + 1) = s(Month) + s(Lon) + s(SSS) + s(Depth) | 1227.56 | 0.25 | 1260.75 | 4.57 | 27.8 |
| Log(Y + 1) = s(Month) + s(Lon) + s(SSS) + s(Depth) + s(Lat) | 1206.33 | 0.26 | 1260.38 | 4.56 | 28.9 |
| Log(Y + 1) = s(Month) + s(Lon) + s(SSS) + s(Depth) + s(Lat) + s(Distance) | 1135.03 | 0.29 | 1258.24 | 4.55 | 33.3 |
| Log(Y + 1) = s(Month) + s(Lon) + s(SSS) + s(Depth) + s(Lat) + s(Distance) + s(SST) | 1016.41 | 0.34 | 1239.84 | 4.28 | 40.3 |
| Variables | d.f. | Contribution (%) | Pr(F) | Pr(chisq) |
|---|---|---|---|---|
| Month | 1.57 | 10.6 | 1.116 × 10−7 *** | 4.401 × 10−8 *** |
| SSS | 2.88 | 7.0 | 1.654 × 10−5 *** | 9.811 × 10−6 *** |
| SST | 6.14 | 7.0 | 0.0001469 *** | 8.203 × 10−5 *** |
| Lon | 1.95 | 6.3 | 3.16 × 10−5 *** | 2.148 × 10−5 *** |
| Distance | 2.95 | 4.2 | 0.0325 * | 0.02894 * |
| Depth | 2.64 | 3.9 | 0.001677 ** | 0.001399 ** |
| Lat | 2.64 | 1.3 | 0.2094 | 0.2122 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Yao, L.; Zhao, H.; Yu, J.; Lin, Z. Environmental Effects on the Spatiotemporal Variability of Fish Larvae in the Western Guangdong Waters, China. J. Mar. Sci. Eng. 2021, 9, 316. https://doi.org/10.3390/jmse9030316
Feng Y, Yao L, Zhao H, Yu J, Lin Z. Environmental Effects on the Spatiotemporal Variability of Fish Larvae in the Western Guangdong Waters, China. Journal of Marine Science and Engineering. 2021; 9(3):316. https://doi.org/10.3390/jmse9030316
Chicago/Turabian StyleFeng, Yuting, Lijun Yao, Hui Zhao, Jing Yu, and Zhaojin Lin. 2021. "Environmental Effects on the Spatiotemporal Variability of Fish Larvae in the Western Guangdong Waters, China" Journal of Marine Science and Engineering 9, no. 3: 316. https://doi.org/10.3390/jmse9030316
APA StyleFeng, Y., Yao, L., Zhao, H., Yu, J., & Lin, Z. (2021). Environmental Effects on the Spatiotemporal Variability of Fish Larvae in the Western Guangdong Waters, China. Journal of Marine Science and Engineering, 9(3), 316. https://doi.org/10.3390/jmse9030316






