Morphology and Phylogeny of Scrippsiella precaria Montresor & Zingone (Thoracosphaerales, Dinophyceae) from Korean Coastal Waters
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Culture
2.2. Light Microscopy
2.3. Scanning Electron Microscopy (SEM)
2.4. DNA Extraction and Sequencing
2.5. Alignment and Phylogenetic Analyses
3. Results
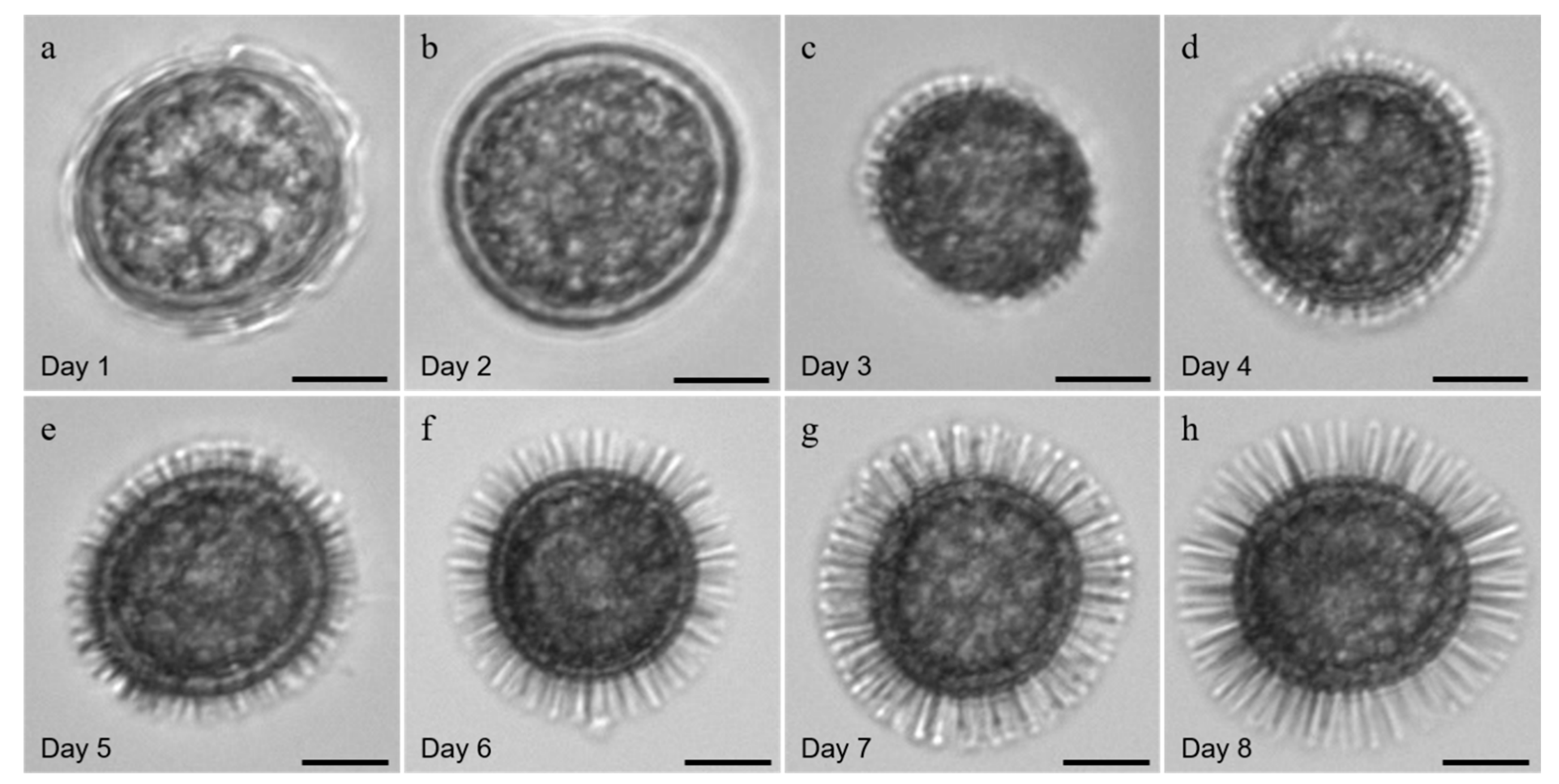
3.1. Morphology of the Resting Cysts of Scrippsiella precaria
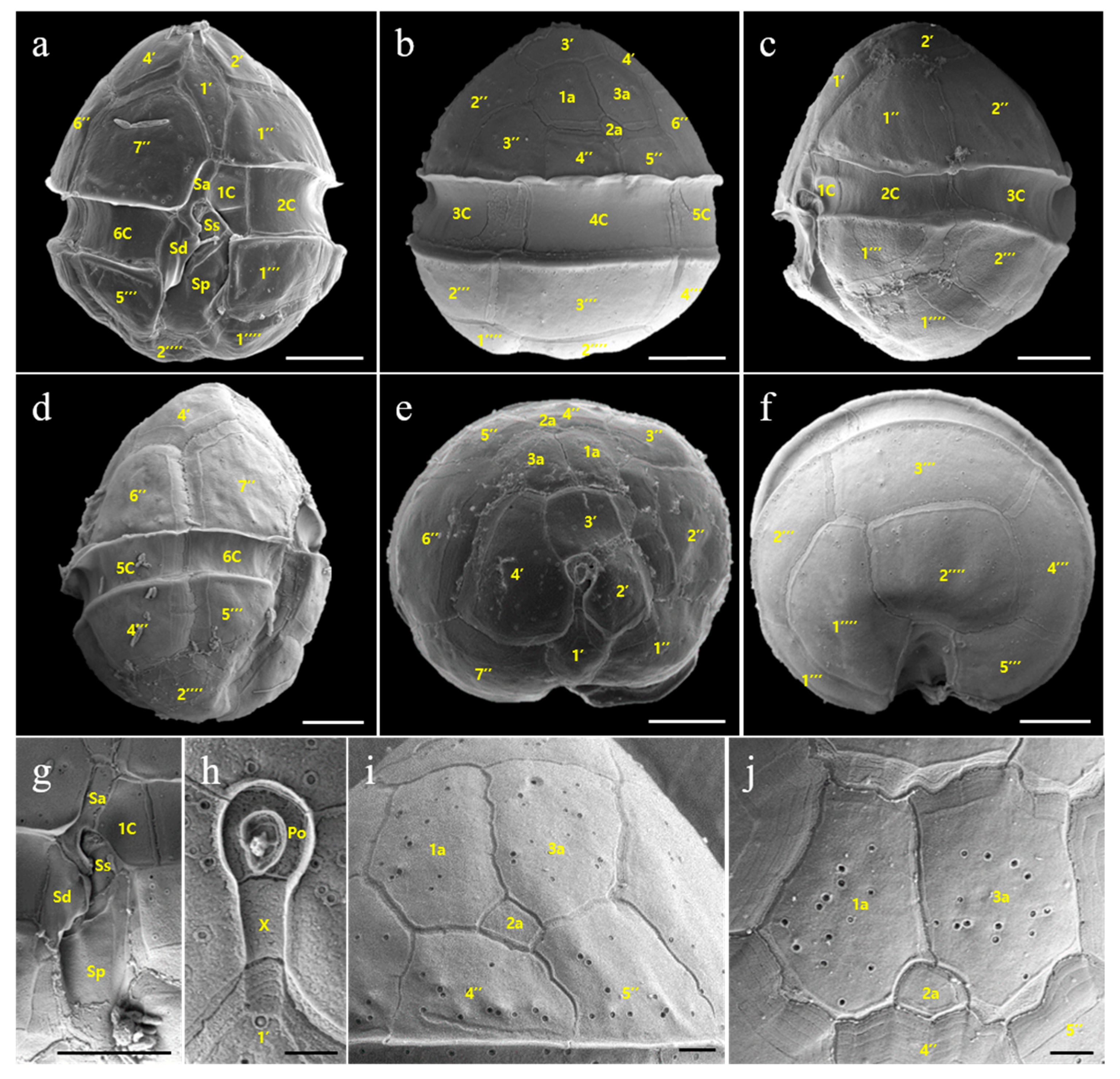
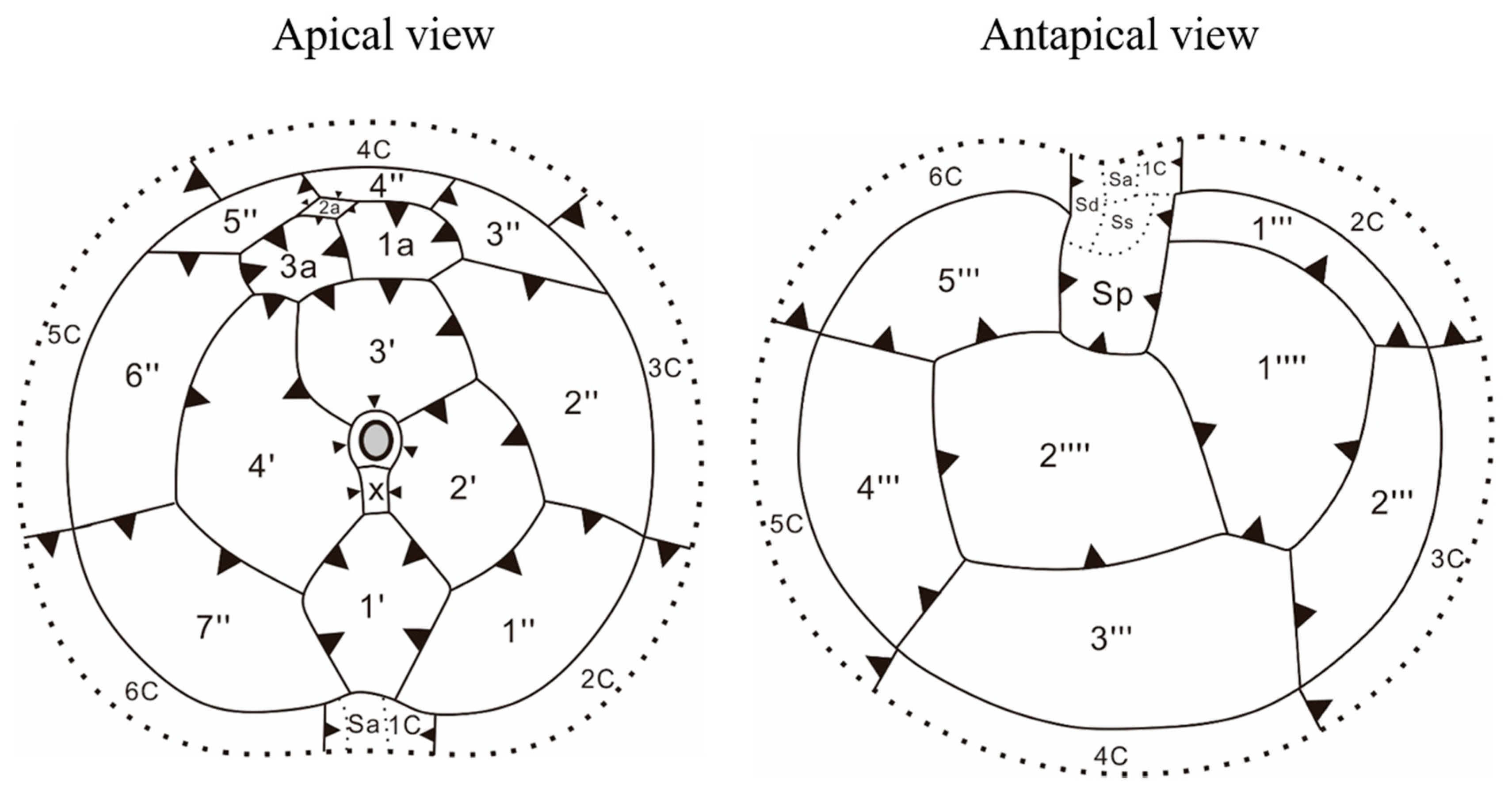
3.2. Morphology of Vegetative Cells of Scrippsiella precaria
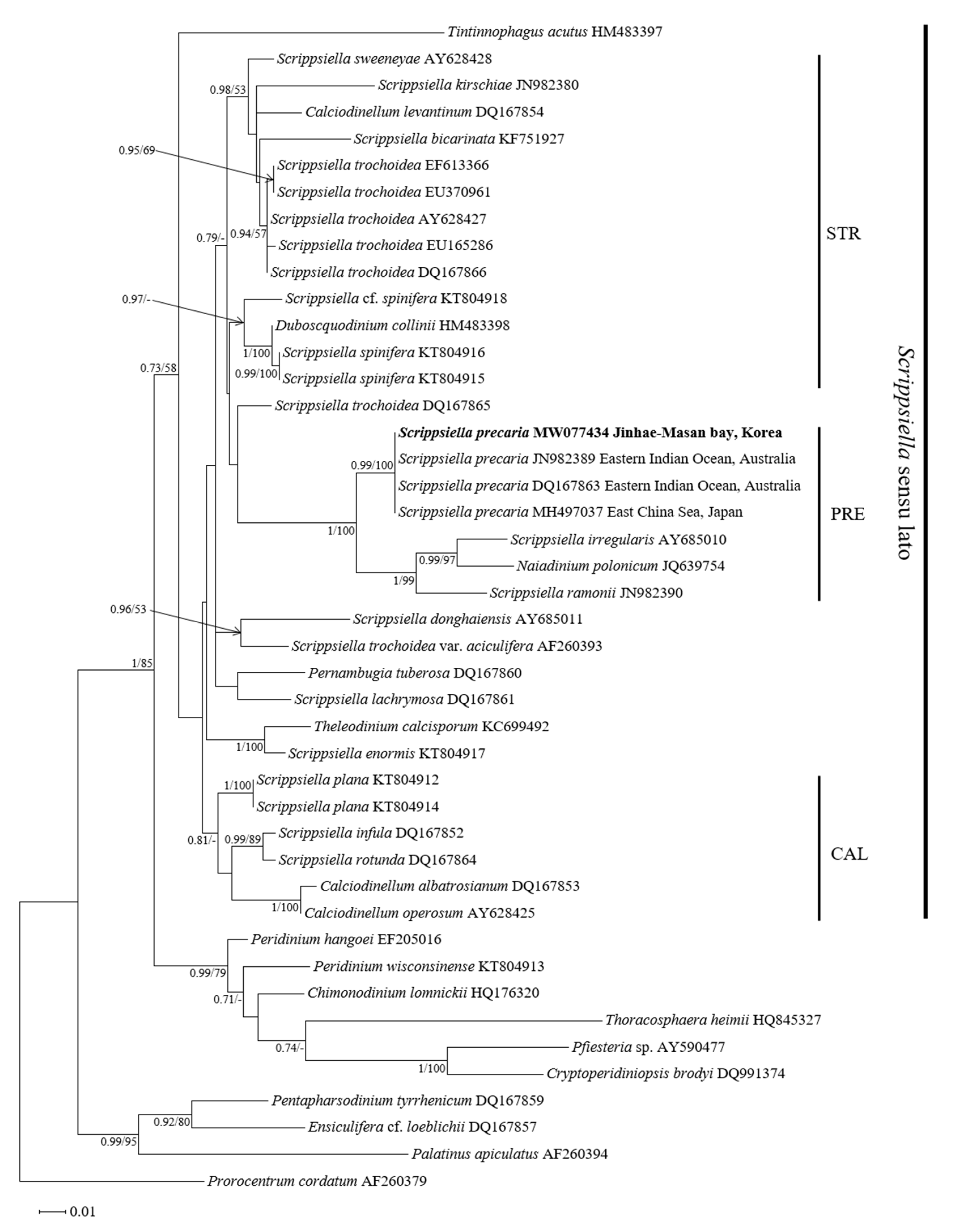
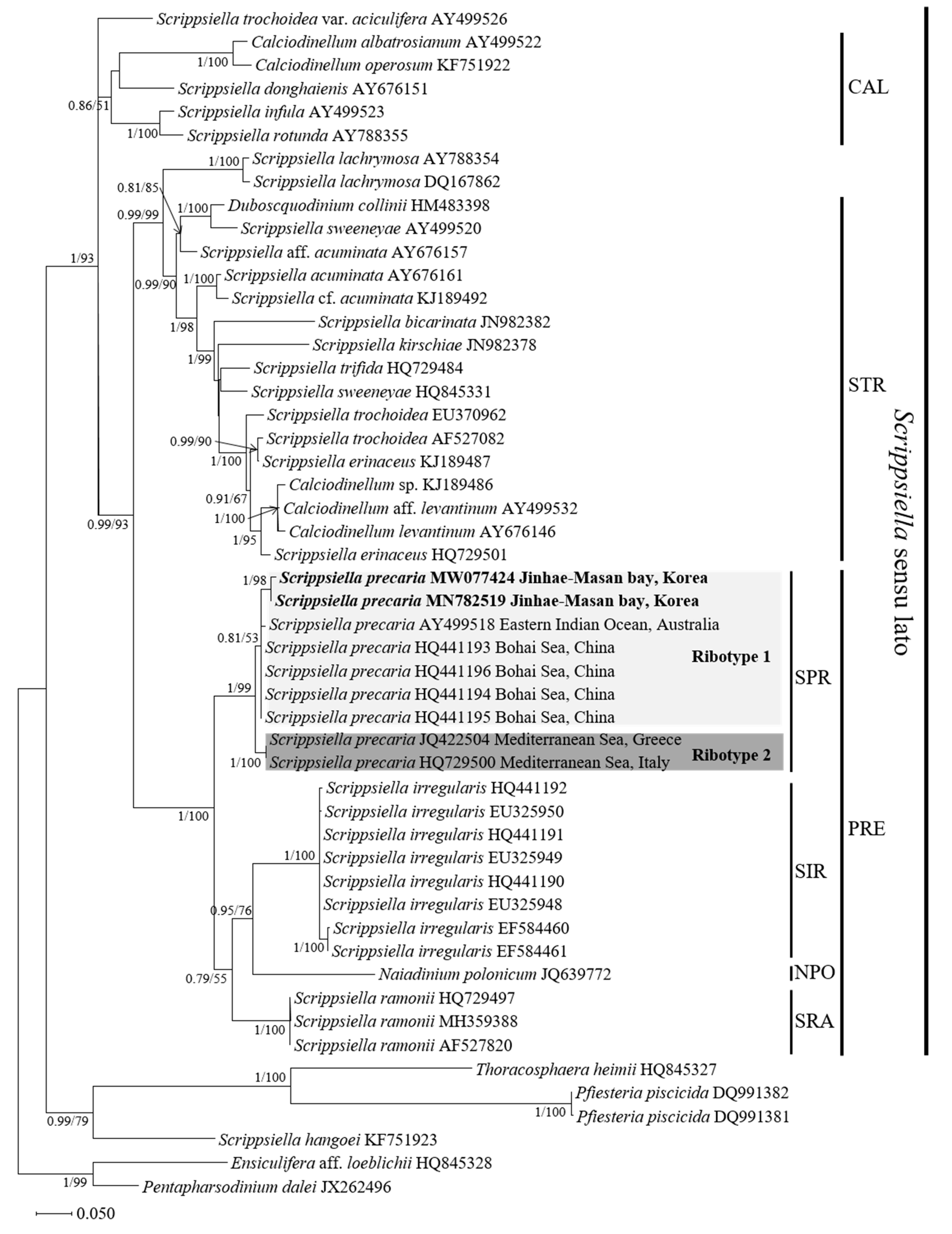
3.3. Molecular Phylogeny of Scrippsiella precaria
4. Discussion
4.1. Morphological Features of Resting Cysts of Scrippsiella precaria
4.2. Morphological Comparisons of Korean Isolates of Scrippsiella precaria with Previously Described Species
4.3. Phylogenetic Position of the Korean Isolates of Scrippsiella precaria
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Balech, E. Two new genera of dinoflagellates from California. Biol. Bull. 1959, 116, 195–203. [Google Scholar] [CrossRef]
- Kretschmann, J.; Zinssmeister, C.; Gottschling, M. Taxonomic clarification of the dinophyte Rhodosphaera erinaceus Kamptner, ≡ Scrippsiella erinaceus comb. nov. (Thoracosphaeraceae, Peridiniales). Syst. Biodivers. 2014, 12, 393–404. [Google Scholar] [CrossRef]
- Kretschmann, J.; Elbrächter, M.; Zinβmeister, C.; Sőhner, S.; Kirsch, M.; Kusber, W.-H.; Gottschling, M. Taxonomic clarification of the dinophyte Peridinium acuminatum Ehrenb., ≡ Scrippsiella acuminata comb. Nov. (Thoracosphaeraceae, Peridiniales). Phytotaxa 2015, 220, 239–256. [Google Scholar] [CrossRef]
- Lewis, J. Cyst-theca relationships in Scrippsiella (DInophyceae) and related Orthoperidinioid Genera. Bot. Mar. 1991, 34, 91–106. [Google Scholar] [CrossRef]
- Zinssmeister, C.; Soehner, S.; Facher, E.; Kirsch, M.; Meier, K.J.S.; Gottschling, M. Catch me if you can: The taxonomic identity of Scrippsiella trochoidea (F.S TEIN) A.R.LOEBL. (Thoracosphaeraceae, Dinophyceae). Syst. Biodivers. 2011, 9, 145–157. [Google Scholar] [CrossRef]
- Guiry, M.D.; Andersen, R.A. Validation of the generic name Symbiodinium (Dinophyceae, Suessiaceae) revisited and the reinstatement of Zooxanthella K.Brandt. Not. Algarum 2018, 58, 1–5. [Google Scholar]
- Craveiro, S.C.; Daugbjerg, N.; Moestrup, O.; Calado, A.J. Studies on Peridinium aciculiferum and Peridinium malmogiense(=Scrippsiella hangoei): Comparison with Chimonodinium lomnickiiand description of Apocalathium gen. nov. (Dinophyceae). Phycologia 2016, 56, 21–35. [Google Scholar] [CrossRef]
- Elbrächter, M.; Gottschling, M.; Hildebrand-Habel, T.; Keupp, H.; Kohring, R.; Lewis, J.; Meier, K.S.; Montresor, M.; Streng, M.; Versteegh, G.J.; et al. Establishing an agenda for calcareous dinoflagellate research (Thoracosphaeraceae, Dinophyceae) including a nomenclatural synopsis of generic names. Taxon 2008, 57, 1289–1303. [Google Scholar] [CrossRef]
- Horiguchi, T.; Chihara, M. Scrippsiella hexapraecingula sp. nov. (Dinophyceae), a Tide Pool Dinoflagellate from the Northwest Pacific. Bot. Mag. 1983, 96, 351–358. [Google Scholar] [CrossRef]
- Horiguchi, T.; Pienaar, R.N. Ultrastructure of a new sand-dwelling dinoflagellate, Scrippsiella arenicola sp. nov. J. Phycol. 1988, 24, 426–438. [Google Scholar]
- Horiguchi, T.; Pienaar, R.N. Validation of Bysmatrum arenicola Horicuchi et pienaar, sp. nov. (Dinophyceae). J. Phycol. 2000, 36, 237. [Google Scholar] [CrossRef]
- Janofske, D. Scrippsiella trochoidea and Scrippsiella regalis, nov. comb. (Peridiniales, Dinophyceae): A comparison. J. Phycol. 2000, 36, 178–189. [Google Scholar] [CrossRef]
- Gu, H.; Sun, J.; Kooistra, W.H.; Zeng, R. Phylogenetic position and morphology of thecae and cysts of Scrippsiella (Dinophyceae) species in the East China Sea. J. Phycoloy 2008, 44, 478–494. [Google Scholar] [CrossRef]
- Gu, H.; Luo, Z.; Liu, T.T.; Lan, D. Morphology and phylogeny of Scrippsiella enormis sp. nov. and S. cf. spinifera (Peridiniales, Dinophyceae) from the China Sea. Phycologia 2013, 52, 182–190. [Google Scholar] [CrossRef]
- Luo, Z.; Mertens, K.N.; Bagheri, S.; Aydin, H.; Takano, Y.; Matsuoka, K.; McCarthy, F.M.G.; Gu, H. Cyst–theca relationship and phylogenetic positions of Scrippsiella plana sp. nov. and S. spinifera (Peridiniales, Dinophyceae). Eur. J. Phycol. 2016, 51, 188–202. [Google Scholar] [CrossRef]
- Matsuoka, K.; Head, M.J. Clarifying cyst–motile stage relationships in dinoflagellates. In Biological and Geological Perspectives of Dinoflagellates; Geological Society: London, UK, 2013; pp. 320–350. [Google Scholar]
- D’Onofrio, G.; Marino, D.; Bianco, L.; Busico, E.; Montresor, M. Toward an assessment on the taxonomy of dinoflagellates that produce calcareous cysts (Calciodinelloideae, Dinophyceae): A morphological and molecular approach. J. Phycol. 1999, 35, 1063–1078. [Google Scholar] [CrossRef]
- Gottschling, M.; Knop, R.; Plotner, J.; Kirsch, M.; Willens, H.; Keupp, H. A molecular phylogeny of Scrippsiella sensu lato (Calciodinellaceae, Dinophyta) with interpretations on morphology and distribution. Eur. J. Phycol. 2005, 40, 207–220. [Google Scholar] [CrossRef]
- Montresor, M.; Sgrosso, S.; Procaccini, G.; Kooistra, W.H. Intraspecific diversity in Scrippsiella trochoidea (Dinopbyceae): Evidence for cryptic species. Phycologia 2003, 42, 56–70. [Google Scholar] [CrossRef]
- Gu, H.; Luo, Z.; Wang, Y.; Lan, D.Z. Diversity, Distribution, and new phylogenetic information of calcareous dinoflagellates from China Sea. J. Syst. Evol. 2011, 49, 126–137. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeong, H.J.; Yun, J.H.; Kim, S.J. Morphological and genetic characterization and the nationwide distribution of the phototrophic dinoflagellate Scrippsiella lachrymose in the Korean waters. Algae 2018, 33, 21–35. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeong, H.J.; Kim, S.J.; Lee, K.H.; Jang, S.H. Scrippsiella masanensis sp. nov. (Thoracosphaerales, Dinophyceae), a phototrophic dinoflagellate from the coastal waters of southern Korea. Phycologia 2019, 58, 287–299. [Google Scholar] [CrossRef]
- Montresor, M.; Zingone, A. Scrippsiella precaria sp. nov. (Dinophyceae), a marine dinoflagellate from the Gulf of Naples. Phycologia 1988, 27, 387–394. [Google Scholar] [CrossRef]
- Montresor, M.; Marino, D. Reproduction and cyst formation in Scrippsiella precaria (Dinophyceae). G. Bot. Ital. 1989, 123, 157–167. [Google Scholar] [CrossRef]
- Ishikawa, A.; Taniguchi, A. Some cysts of the genus Scrippsiella (Dinophyceae) newly found in Japanese waters. Bull. Plankton Soc. Jpn. 1993, 40, 1–7. [Google Scholar]
- Kobayashi, S.; Itakura, S.; IMai, I. Scrippsiella precaria Montresor and Zingone (Dinophyceae) newly found from Hiroshima bay, west Japan. Bull. Plankton Soc. Jpn. 1994, 40, 169–173. [Google Scholar]
- Matsuoka, K.; Fukuyo, Y. Technical Guide for Modern Dinoflagellate Cyst Study; Asian Natural Environmental Science Center, The University of Tokyo, Westpac-Hab/Westpac/Ic: Tokyo, Japan, 2000; p. 29. [Google Scholar]
- Murray, S.A.; Garby, T.; Hoppenrath, M.; Neilan, B.A. Genetic diversity, morphological uniformity and polyketide production in dinoflagellates (Amphidinium, Dinoflagellata). PLoS ONE 2012, 7, e38253. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Horiguchi, T. Molecular phylogenetic study of the heterotophic dinoflagellate genus Protoperidinium (Dinophyceae) inferred from small subunit rRNA gene sequences. Phycol. Res. 2005, 53, 30–42. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jMod-elTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Montresor, M. Scrippsiella ramonii sp. nov. (Peridiniales, Dinophyceae), a marine dinoflagellate producing a calcareous resting cyst. Phycologia 1995, 34, 87–91. [Google Scholar] [CrossRef]
- Head, M.J.; Lewis, J.; de Vernal, A. The cyst of the calcareous dinoflagellate Scrippsiella trifida: Resolving the fossil record of its organic wall with that of Alexandrium tamarense. J. Paleontol. 2006, 80, 1–18. [Google Scholar] [CrossRef]
- Wang, Z.H.; Qi, Y.-Z.; Yang, Y.-F. Cyst formation; an important mechanism for the termination of Scrippsiella trochoidea (Dinophyceae) bloom. J. Plankton Res. 2007, 29, 209–218. [Google Scholar] [CrossRef]
- Shin, H.H.; Jung, S.W.; Jang, M.C.; Kim, Y.O. Effect of pH on the morphology and viability of Scrippsiella trochoidea cysts in the hypoxic zone of a eutrophied area. Harmful Algae 2013, 28, 37–45. [Google Scholar] [CrossRef]
- Attaran-Fariman, G.; Bolch, C.J.S. Scrippsiella irregularis sp. nov. (Dinophyceae), a new dinoflagellate from the southeast coast of Iran. Phycologia 2007, 46, 572–582. [Google Scholar] [CrossRef]
- Netzel, H.; Dürr, G. Dinoflagellate cell Cortex. In Dinoflagellates; Spector, D.L., Ed.; Academic Press Inc. (London) Ltd.: Orlando, FL, USA, 1984; pp. 43–105. [Google Scholar]
- Kretschmann, J.; Žerdoner Čalasan, A.; Gottschling, M. Molecular phylogenetics of dinophytes harboring diatoms as endosymbionts (Kryptoperidiniaceae, Peridiniales), with evolutionaty interpretations and a focus on the identity of Durinskia oculata from Prague. Mol. Phylogenetics Evol. 2018, 118, 392–402. [Google Scholar] [CrossRef]
- Shin, H.H.; Matsuoka, K.; Yoon, Y.H.; Kim, Y.O. Response of dinoflagellate cyst assemblages to salinity changes in Yeoja Bay, Korea. Mar. Micropaleontol. 2010, 77, 15–24. [Google Scholar] [CrossRef]
- Shin, H.H.; Li, Z.; Kim, E.S.; Park, J.W.; Lim, W.A. Which species, Alexandrium catenella (Group I) or A. pacificum (Group IV), is really responsible for past paralytic shellfish poisoning outbreaks in Jinhae-Masan Bay, Korea? Harmful Algae 2017, 68, 31–39. [Google Scholar] [CrossRef]
- Hallegraff, G.M. Transport of toxin dinoflagellates via ships’ ballast water: Bioeconomic risk assessment and efficacy of possible ballast water management strategies. Mar. Ecol. Prog. Ser. 1998, 168, 297–309. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Li, Z.; Kang, N.S.; Gu, H.; Kim, D.; Seo, M.H.; Lee, S.D.; Yun, S.M.; Oh, S.-J.; Shin, H.H. Morphology and Phylogeny of Scrippsiella precaria Montresor & Zingone (Thoracosphaerales, Dinophyceae) from Korean Coastal Waters. J. Mar. Sci. Eng. 2021, 9, 154. https://doi.org/10.3390/jmse9020154
Kim HJ, Li Z, Kang NS, Gu H, Kim D, Seo MH, Lee SD, Yun SM, Oh S-J, Shin HH. Morphology and Phylogeny of Scrippsiella precaria Montresor & Zingone (Thoracosphaerales, Dinophyceae) from Korean Coastal Waters. Journal of Marine Science and Engineering. 2021; 9(2):154. https://doi.org/10.3390/jmse9020154
Chicago/Turabian StyleKim, Hyun Jung, Zhun Li, Nam Seon Kang, Haifeng Gu, Daekyung Kim, Min Ho Seo, Sang Deuk Lee, Suk Min Yun, Seok-Jin Oh, and Hyeon Ho Shin. 2021. "Morphology and Phylogeny of Scrippsiella precaria Montresor & Zingone (Thoracosphaerales, Dinophyceae) from Korean Coastal Waters" Journal of Marine Science and Engineering 9, no. 2: 154. https://doi.org/10.3390/jmse9020154
APA StyleKim, H. J., Li, Z., Kang, N. S., Gu, H., Kim, D., Seo, M. H., Lee, S. D., Yun, S. M., Oh, S.-J., & Shin, H. H. (2021). Morphology and Phylogeny of Scrippsiella precaria Montresor & Zingone (Thoracosphaerales, Dinophyceae) from Korean Coastal Waters. Journal of Marine Science and Engineering, 9(2), 154. https://doi.org/10.3390/jmse9020154









