First Concurrent Measurement of Primary Production in the Yellow Sea, the South Sea of Korea, and the East/Japan Sea, 2018
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Sampling Collection
2.2. Inorganic Nutrients Concentrations
2.3. Chl-a Concentration
2.4. Measurements of Phyoplankton Carbon and Nitrogen Uptake Rate
2.5. Statistical Analysis
3. Results
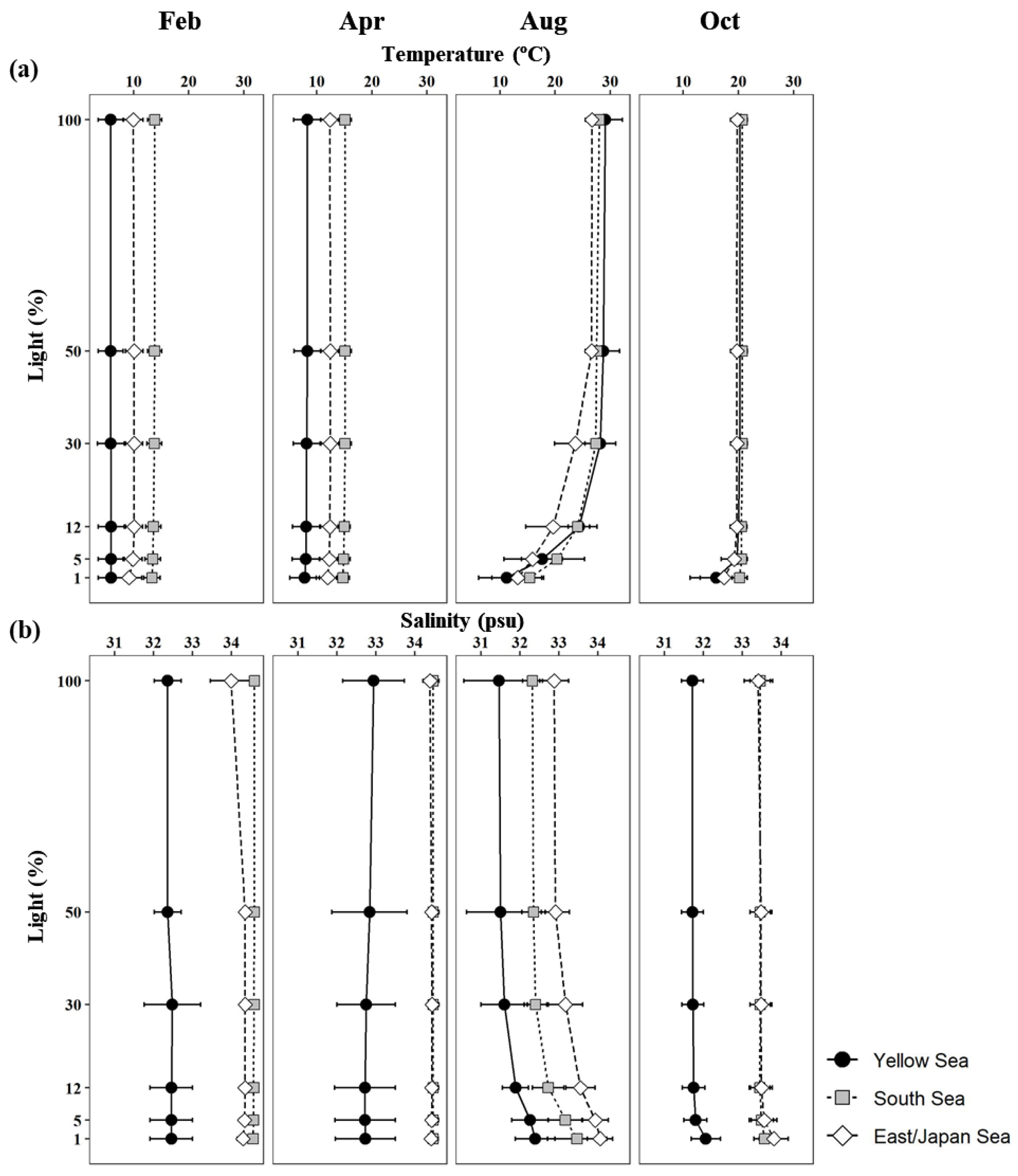
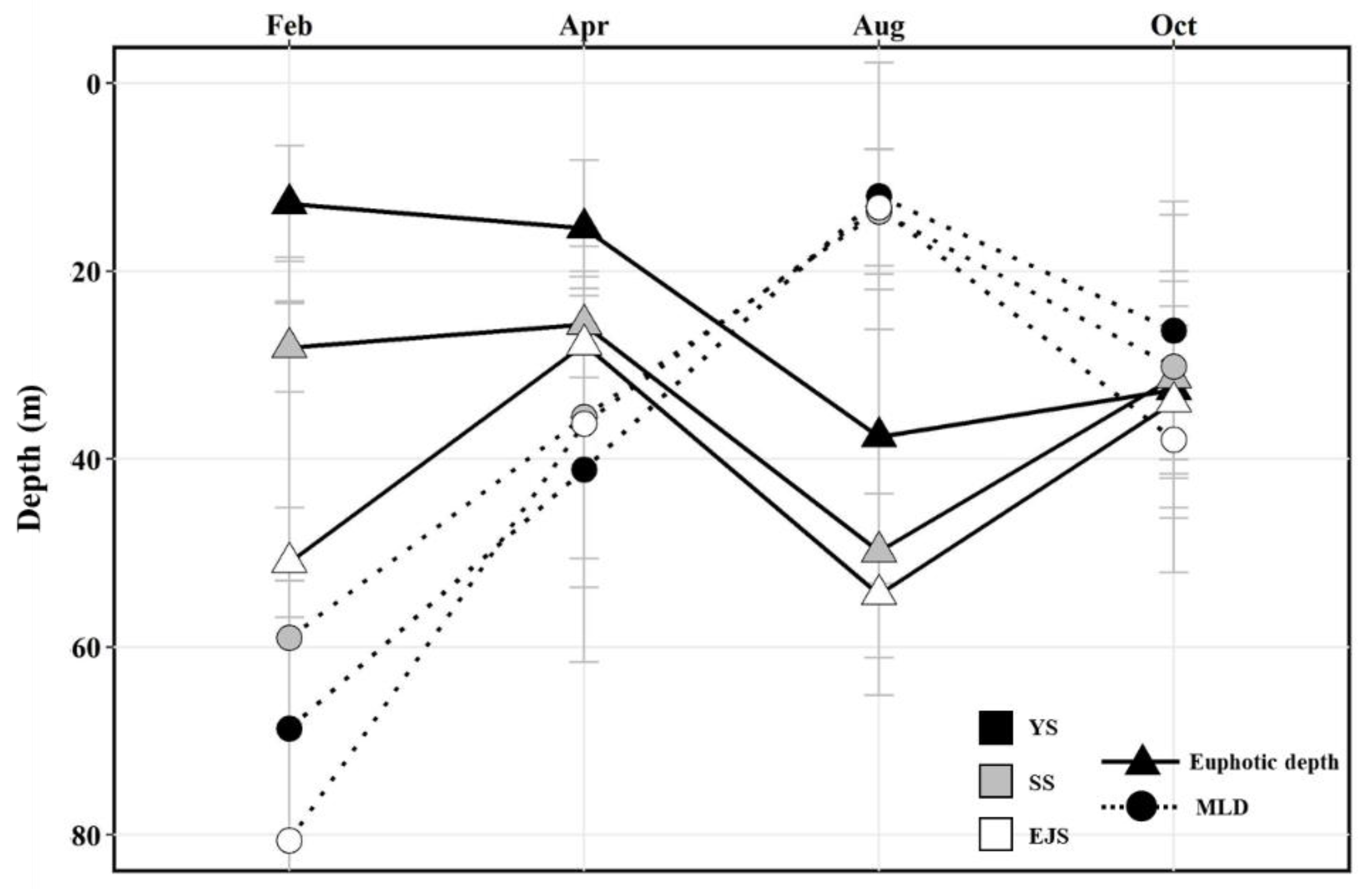
3.1. Physicochemical Environmental Conditions
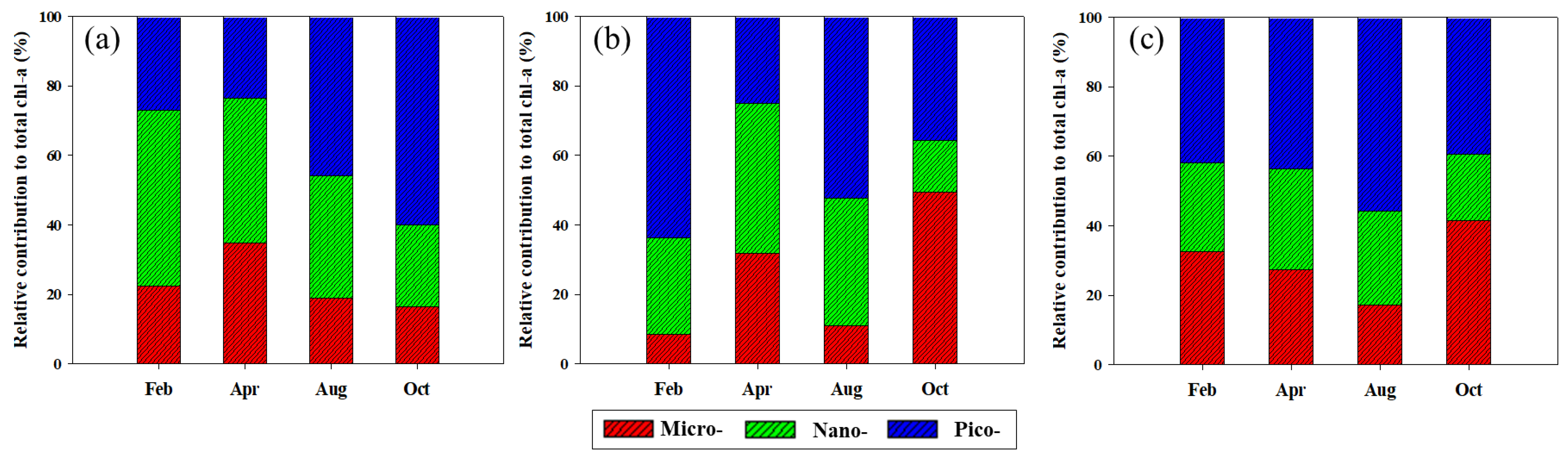
3.2. Concentrations and Size-Fractionated Compositions of chl-a
3.3. POC and PON Concentration
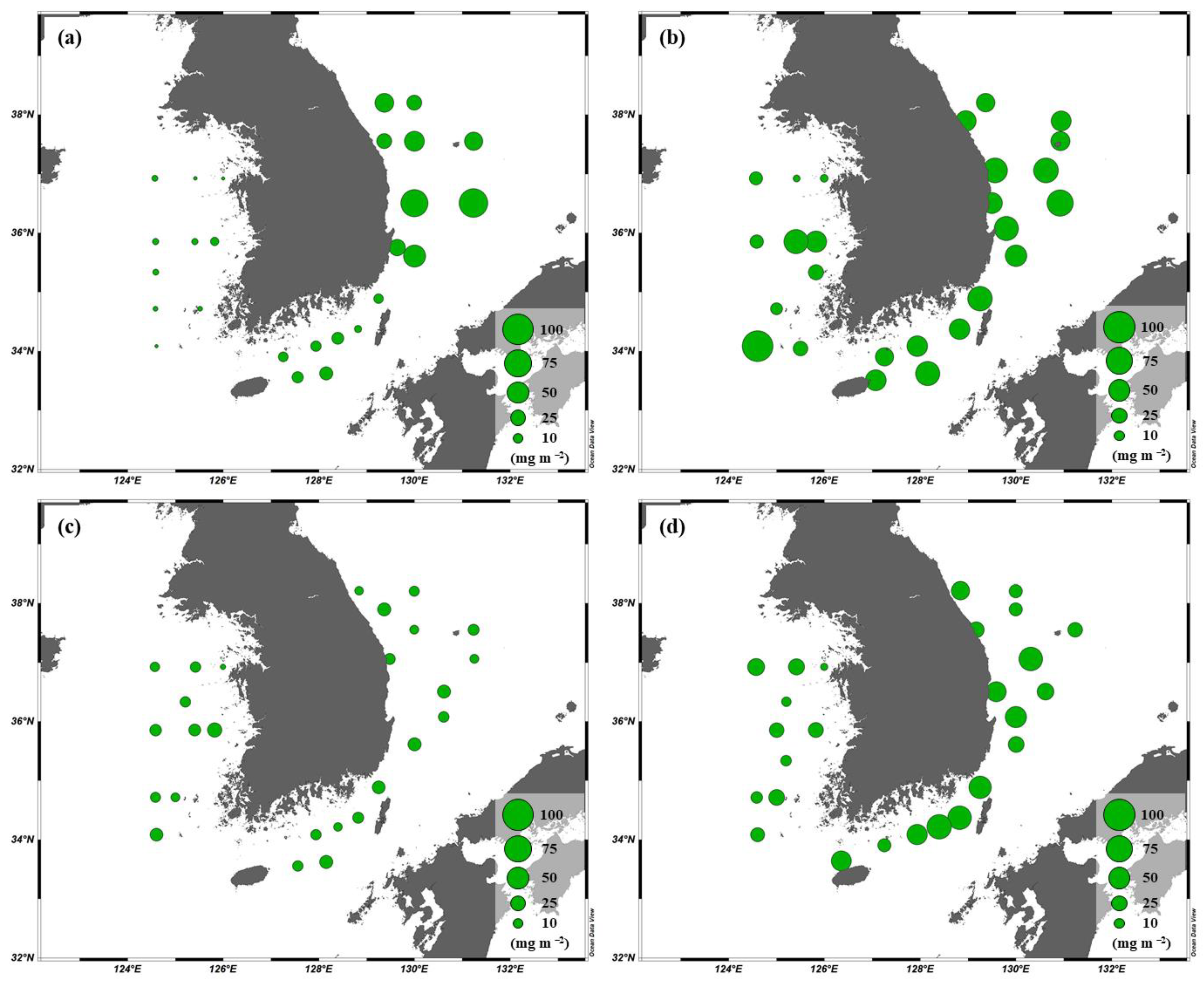
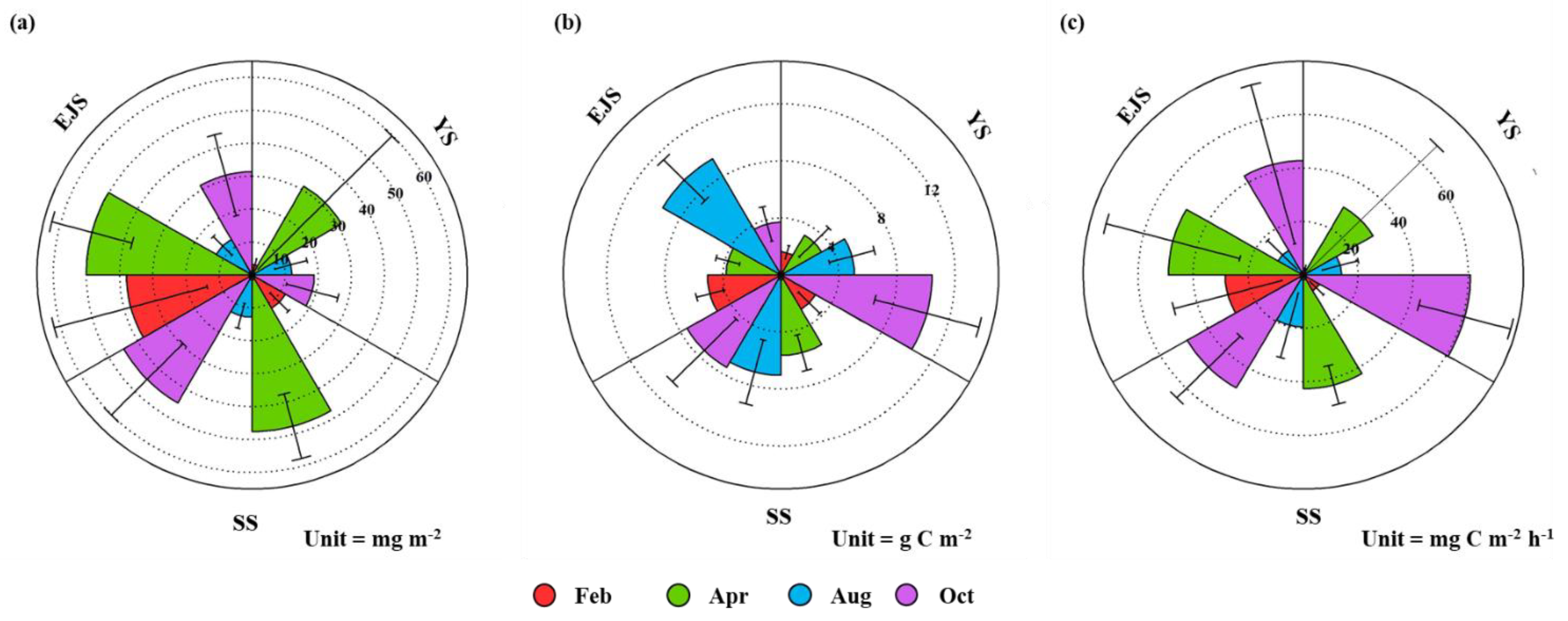
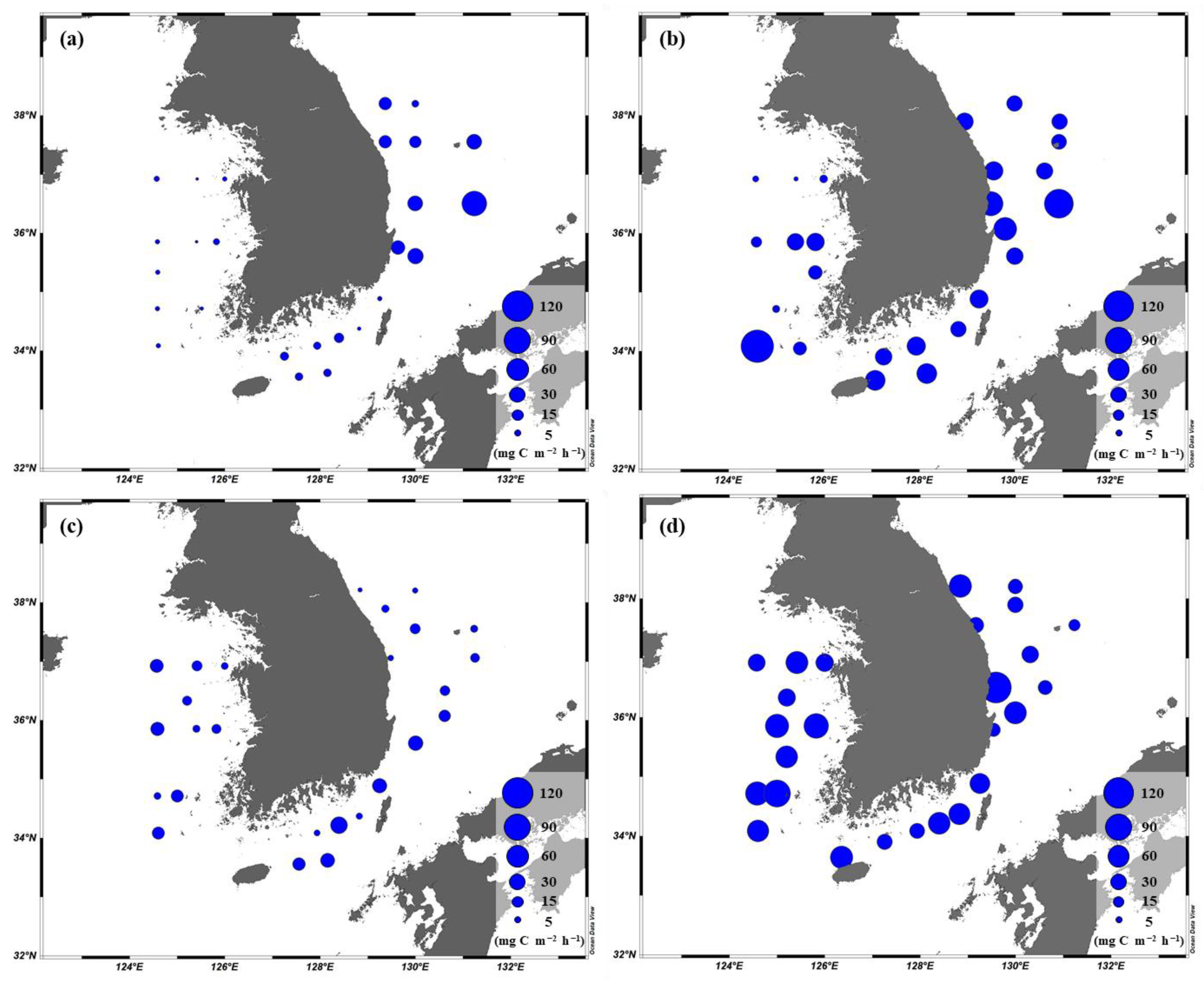
3.4. Primary Production of Phytoplankton
4. Discussion
4.1. Comparisons of Primary Production between This and Previous Studies
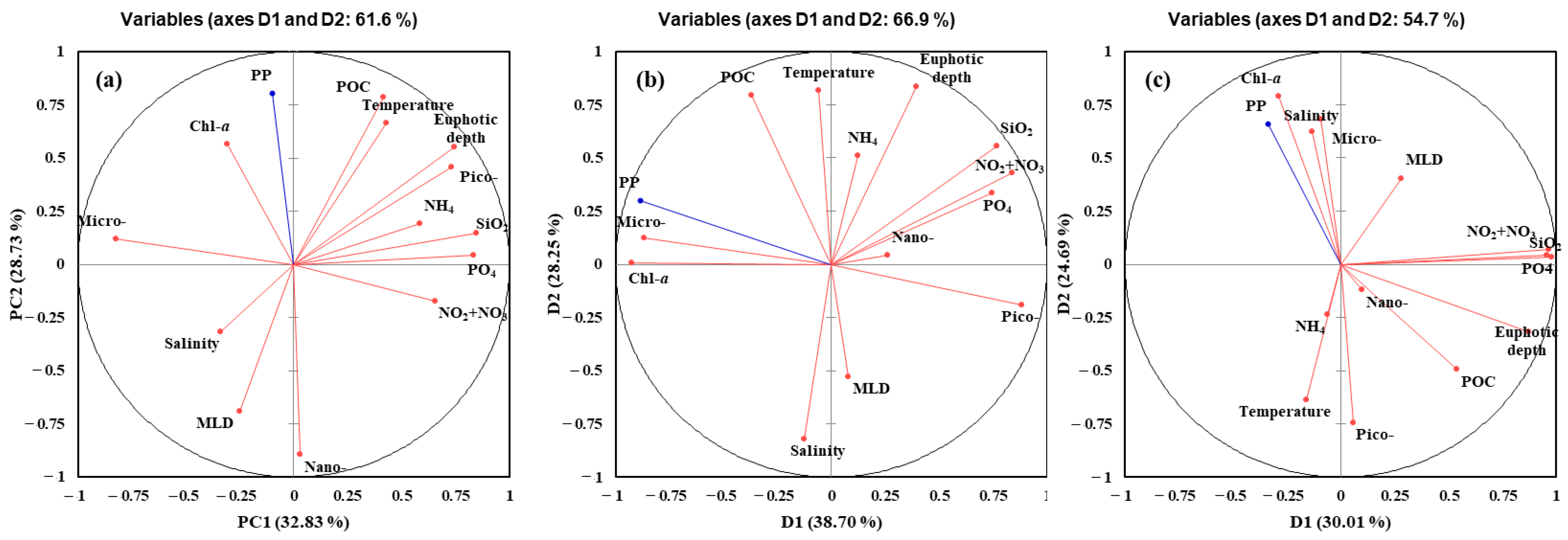
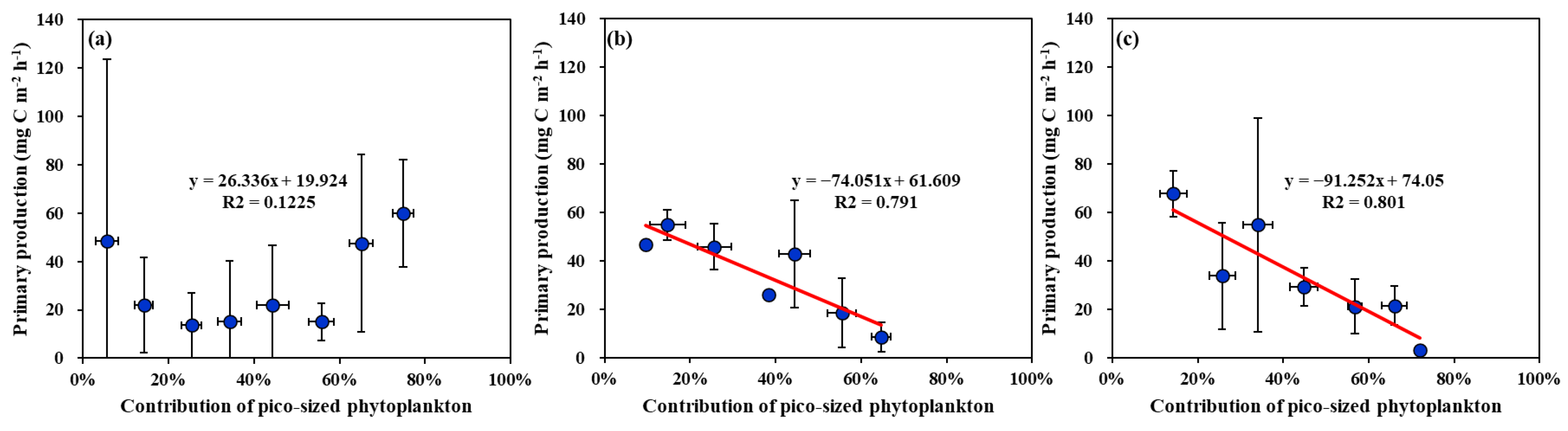
4.2. Main Factors Affecting the Primary Production in the YS, SS, and EJS in 2018
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falkowski, P.G.; Barber, R.T.; Smetacek, V. Biogeochemical controls and feedbacks on ocean primary production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Behrenfeld, M.J.; Randerson, J.T.; McClain, C.R.; Feldman, G.C.; Los, S.O.; Tucker, C.J.; Falkowski, P.G.; Field, C.B.; Frouin, R.; Esaias, W.E.; et al. Biospheric primary production during an ENSO transition. Science 2001, 291, 2594–2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nixon, S.; Thomas, A. On the size of the Peru upwelling ecosystem. Deep. Res. Part I Oceanogr. Res. Pap. 2001, 48, 2521–2528. [Google Scholar] [CrossRef]
- Gong, G.C.; Wen, Y.H.; Wang, B.W.; Liu, G.J. Seasonal variation of chlorophyll a concentration, primary production and environmental conditions in the subtropical East China Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2003, 50, 1219–1236. [Google Scholar] [CrossRef]
- Tremblay, J.É.; Robert, D.; Varela, D.E.; Lovejoy, C.; Darnis, G.; Nelson, R.J.; Sastri, A.R. Current state and trends in Canadian Arctic marine ecosystems: I. Primary production. Clim. Chang. 2012, 115, 161–178. [Google Scholar] [CrossRef] [Green Version]
- Kleppel, G.S.; Burkart, C.A. Egg production and the nutritional environment of Acartia tonsa: The role of food quality in copepod nutrition. ICES J. Mar. Sci. 1995, 52, 297–304. [Google Scholar] [CrossRef]
- Kang, J.J.; Joo, H.T.; Lee, J.H.; Lee, J.H.; Lee, H.W.; Lee, D.; Kang, C.K.; Yun, M.S.; Lee, S.H. Comparison of biochemical compositions of phytoplankton during spring and fall seasons in the northern East/Japan Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2017, 143, 73–81. [Google Scholar] [CrossRef]
- Lee, D.; Son, S.H.; Kim, W.; Park, J.M.; Joo, H.; Lee, S.H. Spatio-temporal variability of the habitat suitability index for chub mackerel (Scomber Japonicus) in the East/Japan Sea and the South Sea of South Korea. Remote Sens. 2018, 10, 938. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Son, S.H.; Lee, C., Il; Kang, C.K.; Lee, S.H. Spatio-temporal variability of the habitat suitability index for the Todarodes pacificus (Japanese Common Squid) around South Korea. Remote Sens. 2019, 11, 2720. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Yun, M.S.; Kim, B.K.; Saitoh, S.I.; Kang, C.K.; Kang, S.H.; Whitledge, T. Latitudinal carbon productivity in the Bering and Chukchi Seas during the summer in 2007. Cont. Shelf Res. 2013, 59, 28–36. [Google Scholar] [CrossRef]
- Kim, B.K.; Joo, H.T.; Song, H.J.; Yang, E.J.; Lee, S.H.; Hahm, D.; Rhee, T.S.; Lee, S.H. Large seasonal variation in phytoplankton production in the Amundsen Sea. Polar Biol. 2015, 38, 319–331. [Google Scholar] [CrossRef]
- Kang, J.J.; Jang, H.K.; Lim, J.H.; Lee, D.; Lee, J.H.; Bae, H.; Lee, C.H.; Kang, C.K.; Lee, S.H. Characteristics of Different Size Phytoplankton for Primary Production and Biochemical Compositions in the Western East/Japan Sea. Front. Microbiol. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Taniguchi, A. Geographical variation of primary production in the Western Pacific Ocean and adjacent seas with reference to the inter-relations between various parameters of primary production. Mem. Fac. Fish. Hokkaido Univ. 1972, 19, 1–33. [Google Scholar]
- Lee, S.H.; Sun Yun, M.; Kyung Kim, B.; Joo, H.T.; Kang, S.H.; Keun Kang, C.; Whitledge, T.E. Contribution of small phytoplankton to total primary production in the Chukchi Sea. Cont. Shelf Res. 2013, 68, 43–50. [Google Scholar] [CrossRef]
- Belkin, I.M. Rapid warming of Large Marine Ecosystems. Prog. Oceanogr. 2009, 81, 207–213. [Google Scholar] [CrossRef]
- Lin, C.; Ning, X.; Su, J.; Lin, Y.; Xu, B. Environmental changes and the responses of the ecosystems of the Yellow Sea during 1976–2000. J. Mar. Syst. 2005, 55, 223–234. [Google Scholar] [CrossRef]
- Chang, P.H.; Cho, C.H.; Ryoo, S.B. Recent changes of mixed layer depth in the East/Japan Sea: 1994-2007. Asia-Pac. J. Atmos. Sci. 2011, 47, 497–501. [Google Scholar] [CrossRef]
- Jin, J.; Liu, S.M.; Ren, J.L.; Liu, C.G.; Zhang, J.; Zhang, G.L.; Huang, D.J. Nutrient dynamics and coupling with phytoplankton species composition during the spring blooms in the Yellow Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2013, 97, 16–32. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kang, D.J.; Lee, T.; Kim, K.R. Long-term trend of CO2 and ocean acidification in the surface water of the Ulleung Basin, the East/Japan Sea inferred from the underway observational data. Biogeosciences 2014, 11, 2443–2454. [Google Scholar] [CrossRef] [Green Version]
- Chiba, S.; Batten, S.; Sasaoka, K.; Sasai, Y.; Sugisaki, H. Influence of the Pacific Decadal Oscillation on phytoplankton phenology and community structure in the western North Pacific. Geophys. Res. Lett. 2012, 39, 2–7. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Emmett Duffy, J.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate change impacts on marine ecosystems. Ann. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Joo, H.T.; Lee, J.H.; Lee, J.H.; Kang, J.J.; Lee, H.W.; Lee, D.; Kang, C.K. Seasonal carbon uptake rates of phytoplankton in the northern East/Japan Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2017, 143, 45–53. [Google Scholar] [CrossRef]
- Wang, J. Study on phytoplankton in the Yellow Sea in spring. Mar. Fisher. Res. 2001, 22, 56–61. [Google Scholar]
- Jang, H.K.; Kang, J.J.; Lee, J.H.; Kim, M.; Ahn, S.H.; Jeong, J.Y.; Yun, M.S.; Han, I.S.; Lee, S.H. Recent Primary Production and Small Phytoplankton Contribution in the Yellow Sea during the Summer in 2016. Ocean Sci. J. 2018, 53, 509–519. [Google Scholar] [CrossRef]
- Lee, S.H.; Son, S.; Dahms, H.U.; Park, J.W.; Lim, J.H.; Noh, J.H.; Kwon, J.I.; Joo, H.T.; Jeong, J.Y.; Kang, C.K. Decadal changes of phytoplankton chlorophyll-a in the East Sea/Sea of Japan. Oceanology 2014, 54, 771–779. [Google Scholar] [CrossRef]
- Joo, H.T.; Son, S.H.; Park, J.W.; Kang, J.J.; Jeong, J.Y.; Lee, C., Il; Kang, C.K.; Lee, S.H. Long-term pattern of primary productivity in the East/Japan sea based on ocean color data derived from MODIS-Aqua. Remote Sens. 2016, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Levitus, S. Climatological Atlas of the World Ocean; NOAA Prof. Paper 13; U.S. Government Printing Office: Washington, DC, USA, 1982; 173p.
- Gardner, W.D.; Chung, S.P.; Richardson, M.J.; Walsh, I.D. The oceanic mixed-layer pump. Deep. Res. Part II 1995, 42, 757–775. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Biological and Chemical Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984. [Google Scholar]
- Dugdale, R.C.; Goering, J.J. Uptake of New and Regenerated Forms of Nitrogen in Primary Productivity. Limnol. Oceanogr. 1967, 12, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Hama, T.; Miyazaki, T.; Ogawa, Y.; Iwakuma, T.; Takahashi, M.; Otsuki, A.; Ichimura, S. Measurement of photosynthetic production of a marine phytoplankton population using as Table 13C isotope. Mar. Biol. 1983, 73, 31–36. [Google Scholar] [CrossRef]
- Garneau, M.È.; Gosselin, M.; Klein, B.; Tremblay, J.É.; Fouilland, E. New and regenerated production during a late summer bloom in an Arctic polynya. Mar. Ecol. Prog. Ser. 2007, 345, 13–26. [Google Scholar] [CrossRef]
- Li, W.K.W.; Irwin, B.D.; Dickie, P.M. Dark fixation of 14C: Variations related to biomass and productivity of phytoplankton and bacteria. Limnol. Oceanogr. 1993, 38, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Gosselin, M.; Levasseur, M.; Wheeler, P.A. Deep Sea Research Part II: Topical Studies in Oceanography—New measurements of phytoplankton and ice algal production in the Arctic Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 1997, 44, 1623–1644. [Google Scholar] [CrossRef]
- Lee, H.W.; Noh, J.H.; Choi, D.H.; Yun, M.; Bhavya, P.S.; Kang, J.J.; Lee, J.H.; Kim, K.W.; Jang, H.K.; Lee, S.H. Picocyanobacterial contribution to the total primary production in the northwestern pacific ocean. Water 2021, 13, 1610. [Google Scholar] [CrossRef]
- Wei, Q.; Yao, Q.; Wang, B.; Wang, H.; Yu, Z. Long-term variation of nutrients in the southern Yellow Sea. Cont. Shelf Res. 2015, 111, 184–196. [Google Scholar] [CrossRef]
- Tao, F.; Daoji, L.; Lihua, Y.; Lei, G.; Lihua, Z. Effects of irradiance and phosphate on growth of nanophytoplankton and picophytoplankton. Acta Ecol. Sin. 2006, 26, 2783–2789. [Google Scholar] [CrossRef]
- Redfeild, A. The influence of organisms on the composition of sea water. Sea 1963, 2, 26–77. [Google Scholar]
- Egge, J.K. Are diatoms poor competitors at low phosphate concentrations? J. Mar. Syst. 1998, 16, 191–198. [Google Scholar] [CrossRef]
- Zhou, M.-j.; Shen, Z.-l.; Yu, R.-c. Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) River. Cont. Shelf Res. 2008, 28, 1483–1489. [Google Scholar] [CrossRef]
- Kang, Y.S.; Choi, J.K.; Chung, K.H.; Park, Y.C. Primary productivity and assimilation umber in the Kyonggi bay and the mid-eastern coast of Yellow Sea. J. Oceanogr. Soc. Korea 1992, 27, 237–246. [Google Scholar]
- Choi, J.K.; Noh, J.H.; Shin, K.S.; Hong, K.H. The early autumn distribution of chlorophyll-a and primary productivity in the Yellow Sea, 1992. The Yellow Sea 1995, 1, 68–80. [Google Scholar]
- Choi, J.K.; Noh, J.H.; Cho, S.H. Temporal and spatial variation of primary production in the Yellow Sea. The present and the future of yellow sea environments. In Proceedings of the Yellow Sea International Symposium, Ansan, Korea, 6–7 November 2003; Korea Ocean R&D Institute: Seoul, Korea, 2003; pp. 103–115. [Google Scholar]
- Son, S.H.; Campbell, J.; Dowell, M.; Yoo, S.; Noh, J. Primary production in the Yellow Sea determined by ocean color remote sensing. Mar. Ecol. Prog. Ser. 2005, 303, 91–103. [Google Scholar] [CrossRef]
- Lee, Y. Phytoplankton Dynamics and Primary Production in the Yellow Sea during Winter and Summer. Unpublished Ph.D. Thsesis, Inha University, Incheon, Korea, August 2012. [Google Scholar]
- Chung, C.S.; Yang, D.B. On the primary productivity in the southern sea of Korea. J. Oceanogr. Soc. Korea 1991, 26, 242–254. [Google Scholar]
- Hama, T.; Shin, K.H.; Handa, N. Spatial variability in the primary productivity in the East China Sea and its adjacent waters. J. Oceanogr. 1997, 53, 41–51. [Google Scholar] [CrossRef]
- Lee Chen, Y.L.; Chen, H.Y. Nitrate-based new production and its relationship to primary production and chemical hydrography in spring and fall in the East China Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2003, 50, 1249–1264. [Google Scholar] [CrossRef]
- Siswanto, E.; Ishizaka, J.; Yokouchi, K. Estimating chlorophyll-a vertical profiles from satellite data and the implication for primary production in the Kuroshio front of the East China Sea. J. Oceanogr. 2005, 61, 575–589. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Ding, Y.P.; Li, T.J.; Xue, B.; Euo, Y.M. Annual variations of chlorophyll a and primary productivity in the East China Sea. Oceanol. Limno. Sin. 2016, 47, 261–268. [Google Scholar] [CrossRef]
- Nagata, H. the Yamato Rise, central Japan Sea. Plankton Bio. Ecol. 1998, 45, 159–170. [Google Scholar]
- Yoshie, N.; Shin, K.H.; Noriki, S. Seasonal variations of primary productivity and assimilation numbers in the western North Pacific. Spec. Rep. Reg. Stud. North-East Eurasia North Pac. Hokkaido Univ. 1999, 1, 49–62. [Google Scholar]
- Sverdrup, H.U. On conditions for the vernal blooming of phytoplankton. ICES J. Mar. Sci. 1953, 18, 287–295. [Google Scholar] [CrossRef]
- Yentsch, C.S. Estimates of “new production” in the mid-North Atlantic. J. Plankton Res. 1990, 12, 717–734. [Google Scholar] [CrossRef]
- Nishikawa, H.; Yasuda, I.; Komatsu, K.; Sasaki, H.; Sasai, Y.; Setou, T.; Shimizu, M. Winter mixed layer depth and spring bloom along the Kuroshio front: Implications for the Japanese sardine stock. Mar. Ecol. Prog. Ser. 2013, 487, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Mayot, N.; D’Ortenzio, F.; Uitz, J.; Gentili, B.; Ras, J.; Vellucci, V.; Golbol, M.; Antoine, D.; Claustre, H. Influence of the Phytoplankton Community Structure on the Spring and Annual Primary Production in the Northwestern Mediterranean Sea. J. Geophys. Res. Ocean. 2017, 122, 9918–9936. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.H.; Jang, C.J.; Oh, I.S.; Park, J.J. Climatology of the mixed layer depth in the East/Japan Sea. J. Mar. Syst. 2012, 96–97, 1–14. [Google Scholar] [CrossRef]
- Bae, H.; Lee, D.; Kang, J.J.; Lee, J.H.; Jo, N.; Kim, K.; Jang, H.K.; Kim, M.J.; Kim, Y.; Kwon, J., Il; et al. Satellite-derived protein concentration of phytoplankton in the Southwestern East/Japan Sea. J. Mar. Sci. Eng. 2021, 9, 189. [Google Scholar] [CrossRef]
- Kang, Y.-S.; Choi, H.-C.; Lim, J.-H.; Jeon, I.-S.; Seo, J.-H. Dynamics of the Phytoplankton Community in the Coastal Waters of Chuksan Harbor, East Sea. Algae 2005, 20, 345–352. [Google Scholar] [CrossRef]
- Zuenko, Y.; Selina, M.; Stonik, I. On conditions of phytoplankton blooms in the coastal waters of the north-western East/Japan Sea. Ocean Sci. J. 2006, 41, 31–41. [Google Scholar] [CrossRef]
- Chang, K.-I.; Zhang, C.-I.; Park, C.; Kang, D.-J.; Ju, S.-J.; Lee, S.-H.; Wimbush, M. (Eds.) Oceanography of the East Sea (Japan Sea), 1st ed.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Kwak, J.H.; Han, E.; Lee, S.H.; Park, H.J.; Kim, K.R.; Kang, C.K. A consistent structure of phytoplankton communities across the warm–cold regions of the water mass on a meridional transect in the East/Japan Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2017, 143, 36–44. [Google Scholar] [CrossRef]
- Chung, C.S.; Shim, J.H.; Park, Y.H.; Park, S.G. Primary productivity and nitrogenous nutrient dynamics in the East Sea of Korea. J. Oceanogr Soc. Korea 1989, 24, 52–61. [Google Scholar]
- Park, J.S.; Kang, C.K.; An, K.H. Community structure and spatial distribution of phytoplankton in the polar front region off the east coast of Korea in summer. Bull. Korean Fish. Soc. 1991, 24, 237–247. [Google Scholar]
- Yamada, K.; Ishizaka, J.; Nagata, H. Spatial and temporal variability of satellite primary production in the Japan Sea from 1998 to 2002. J. Oceanogr. 2005, 61, 857–869. [Google Scholar] [CrossRef]
- Hyun, J.H.; Kim, D.; Shin, C.W.; Noh, J.H.; Yang, E.J.; Mok, J.S.; Kim, S.H.; Kim, H.C.; Yoo, S. Enhanced phytoplankton and bacterioplankton production coupled to coastal upwelling and an anticyclonic eddy in the Ulleung basin. East Sea Aquat. Microb. Ecol. 2009, 54, 45–54. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, S.H.; Park, H.J.; Choy, E.J.; Jeong, H.D.; Kim, K.R.; Kang, C.K. Monthly measured primary and new productivities in the Ulleung Basin as a biological “hot spot” in the East/Japan Sea. Biogeosciences 2013, 10, 4405–4417. [Google Scholar] [CrossRef] [Green Version]
- Kwak, J.H.; Hwang, J.; Choy, E.J.; Park, H.J.; Kang, D.J.; Lee, T.; Chang, K., Il; Kim, K.R.; Kang, C.K. High primary productivity and f-ratio in summer in the Ulleung basin of the East/Japan Sea. Deep. Res. Part I Oceanogr. Res. Pap. 2013, 79, 74–785. [Google Scholar] [CrossRef]
- Joo, H.T.; Son, S.H.; Park, J.W.; Kang, J.J.; Jeong, J.Y.; Kwon, J., Il; Kang, C.K.; Lee, S.H. Small phytoplankton contribution to the total primary production in the highly productive Ulleung Basin in the East/Japan Sea. Deep. Res. Part. II Top. Stud. Oceanogr. 2017, 143, 54–61. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kim, T.W.; Lee, S.H.; Lee, D.; Park, J.; Kim, B.K.; Kim, K.; Jang, H.K.; Bhavya, P.S.; Lee, S.H. Seasonal Variations in the Small Phytoplankton Contribution to the Total Primary Production in the Amundsen Sea, Antarctica. J. Geophys. Res. Ocean. 2019, 124, 8324–8341. [Google Scholar] [CrossRef]
- Agawin, N.S.R.; Duarte, C.M.; Agusti, S. Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnol. Oceanogr. 2000, 45, 591–600. [Google Scholar] [CrossRef]
- Hilligsøe, K.M.; Richardson, K.; Bendtsen, J.; Sørensen, L.L.; Nielsen, T.G.; Lyngsgaard, M.M. Linking phytoplankton community size composition with temperature, plankton food web structure and sea-air CO2 flux. Deep. Res. Part I Oceanogr. Res. Pap. 2011, 58, 826–838. [Google Scholar] [CrossRef]
- Morán, X.A.G.; López-Urrutia, Á.; Calvo-Díaz, A.; LI, W.K.W. Increasing importance of small phytoplankton in a warmer ocean. Glob. Chang. Biol. 2010, 16, 1137–1144. [Google Scholar] [CrossRef]
- Mousing, E.A.; Ellegaard, M.; Richardson, K. Global patterns in phytoplankton community size Structure-evidence for a direct temperature effect. Mar. Ecol. Prog. Ser. 2014, 497, 25–38. [Google Scholar] [CrossRef] [Green Version]

| Region | Station | Latitude | Longitude | Bottom Depth (m) | Feb. | Apr. | Aug. | Oct. |
|---|---|---|---|---|---|---|---|---|
| YS | 307-03 | 36.92 | 126.00 | 37 | o | o | o | o |
| 307-05 | 36.92 | 125.42 | 54 | o | o | o | o | |
| 307-09 | 36.92 | 124.57 | 67 | o | o | o | o | |
| 308-06 | 36.33 | 125.21 | 58 | - | - | o | o | |
| 309-03 | 35.85 | 125.82 | 54 | o | o | o | o | |
| 309-05 | 35.85 | 125.40 | 69 | o | o | o | - | |
| 309-07 | 35.86 | 125.00 | 66 | - | - | - | o | |
| 309-09 | 35.85 | 124.59 | 82 | o | o | o | - | |
| 310-03 | 35.34 | 125.82 | 27 | - | o | - | - | |
| 310-06 | 35.34 | 125.20 | 72 | - | - | - | o | |
| 310-09 | 35.34 | 124.59 | 92 | o | - | - | - | |
| 311-05 | 34.72 | 125.52 | 75 | o | - | - | - | |
| 311-07 | 34.72 | 125.00 | 90 | - | o | o | o | |
| 311-09 | 34.72 | 124.59 | 89 | o | - | o | o | |
| 312-05 | 34.04 | 125.50 | 81 | - | o | - | - | |
| 312-09 | 34.09 | 124.60 | 89 | o | o | o | o | |
| SS | 203-03 | 33.64 | 126.36 | 133 | - | - | - | o |
| 204-04 | 33.90 | 127.25 | 75 | o | o | - | o | |
| 205-03 | 34.08 | 127.94 | 82 | o | o | o | o | |
| 205-05 | 33.62 | 128.15 | 113 | o | o | o | - | |
| 206-03 | 34.37 | 128.82 | 92 | o | o | o | o | |
| 207-03 | 34.89 | 129.25 | 115 | o | o | o | o | |
| 400-14 | 34.21 | 128.40 | 75 | o | - | o | o | |
| 400-25 | 33.55 | 127.56 | 96 | o | - | o | - | |
| 400-27 | 33.51 | 127.08 | 124 | - | o | - | - | |
| EJS | 102-06 | 36.08 | 129.80 | 700 | - | o | - | - |
| 102-07 | 36.08 | 130.00 | 1390 | - | - | - | o | |
| 102-09 | 36.08 | 130.62 | 1880 | - | - | o | - | |
| 103-04 | 36.51 | 129.50 | 110 | - | o | - | - | |
| 103-05 | 36.51 | 129.59 | 205 | - | - | - | o | |
| 103-07 | 36.50 | 130.00 | 850 | o | - | - | - | |
| 103-09 | 36.51 | 130.62 | 2150 | - | - | o | o | |
| 103-10 | 36.51 | 130.93 | 1800 | - | o | - | - | |
| 103-11 | 36.50 | 131.24 | 2100 | o | - | - | - | |
| 104-04 | 37.06 | 129.48 | 110 | - | - | o | - | |
| 104-05 | 37.06 | 129.56 | 220 | - | o | - | - | |
| 104-08 | 37.06 | 130.31 | 720 | - | - | - | o | |
| 104-09 | 37.06 | 130.63 | 2340 | - | o | - | - | |
| 104-11 | 37.06 | 131.26 | 2325 | - | - | o | - | |
| 105-03 | 37.55 | 129.17 | 48 | - | - | - | o | |
| 105-05 | 37.55 | 129.37 | 280 | o | - | - | - | |
| 105-07 | 37.55 | 130.00 | 1480 | o | - | o | - | |
| 105-10 | 37.55 | 130.93 | 1503 | - | o | - | - | |
| 105-11 | 37.55 | 131.24 | 1140 | o | - | o | o | |
| 106-03 | 37.90 | 128.95 | 320 | - | o | - | - | |
| 106-05 | 37.90 | 129.37 | 1120 | - | - | o | - | |
| 106-07 | 37.90 | 130.00 | 1060 | - | - | - | o | |
| 106-10 | 37.90 | 130.94 | 1980 | - | o | - | - | |
| 107-03 | 38.21 | 128.84 | 1120 | - | - | o | o | |
| 107-05 | 38.20 | 129.37 | 1080 | o | - | - | - | |
| 107-07 | 38.20 | 130.00 | 846 | o | o | o | o | |
| 209-04 | 35.79 | 129.55 | 54 | - | - | - | o | |
| 209-05 | 35.75 | 129.64 | 150 | o | - | - | - | |
| 209-07 | 35.61 | 130.01 | 250 | - | o | o | - | |
| 209-08 | 35.60 | 130.00 | 200 | o | - | - | - |
| Region | Month | Light Depth (%) | NH4 | NO2+NO3 | PO4 | SiO2 |
|---|---|---|---|---|---|---|
| µM | ||||||
| YS | Feb. | 100 | 0.9 ± 0.6 | 8.8 ± 2.4 | 0.6 ± 0.1 | 8.9 ± 3.3 |
| 30 | 0.9 ± 0.7 | 8.2 ± 2.1 | 0.5 ± 0.1 | 8.5 ± 3.4 | ||
| 1 | 0.7 ± 0.3 | 9.9 ± 2.8 | 0.6 ± 0.1 | 10.0 ± 3.6 | ||
| Apr. | 100 | 0.6 ± 0.4 | 6.6 ± 3.3 | 0.3 ± 0.1 | 6.8 ± 2.5 | |
| 30 | 0.5 ± 0.5 | 5.8 ± 3.0 | 0.2 ± 0.1 | 6.9 ± 2.9 | ||
| 1 | 0.5 ± 0.5 | 7.1 ± 2.1 | 0.3 ± 0.1 | 7.8 ± 2.7 | ||
| Aug. | 100 | 1.0 ± 1.1 | 1.2 ± 1.6 | 0.1 ± 0.1 | 2.8 ± 1.3 | |
| 30 | 1.2 ± 1.5 | 0.5 ± 0.5 | 0.0 ± 0.0 | 2.4 ± 0.5 | ||
| 1 | 0.7 ± 0.5 | 3.4 ± 1.8 | 0.4 ± 0.2 | 8.7 ± 3.3 | ||
| Oct. | 100 | 0.6 ± 0.1 | 1.7 ± 2.2 | 0.2 ± 0.2 | 4.6 ± 2.4 | |
| 30 | 0.5 ± 0.1 | 1.6 ± 2.1 | 0.2 ± 0.1 | 4.2 ± 1.6 | ||
| 1 | 0.5 ± 0.1 | 5.0 ± 2.7 | 0.4 ± 0.2 | 7.0 ± 3.1 | ||
| SS | Feb. | 100 | 0.2 ± 0.0 | 5.3 ± 1.3 | 0.4 ± 0.0 | 9.2 ± 0.7 |
| 30 | 0.2 ± 0.0 | 5.1 ± 0.8 | 0.4 ± 0.0 | 8.1 ± 1.7 | ||
| 1 | 0.2 ± 0.1 | 5.1 ± 0.8 | 0.4 ± 0.0 | 8.9 ± 0.8 | ||
| Apr. | 100 | 0.1 ± 0.1 | 0.9 ± 0.5 | 0.1 ± 0.0 | 5.6 ± 2.6 | |
| 30 | 0.1 ± 0.1 | 1.0 ± 0.5 | 0.1 ± 0.0 | 6.1 ± 2.4 | ||
| 1 | 0.3 ± 0.2 | 2.1 ± 1.5 | 0.1 ± 0.1 | 6.7 ± 2.2 | ||
| Aug. | 100 | 0.6 ± 0.3 | 1.7 ± 0.2 | 0.1 ± 0.1 | 6.8 ± 1.4 | |
| 30 | 0.5 ± 0.4 | 1.4 ± 0.3 | 0.1 ± 0.1 | 6.2 ± 1.0 | ||
| 1 | 0.1 ± 0.0 | 8.1 ± 2.9 | 0.4 ± 0.2 | 11.3 ± 1.8 | ||
| Oct. | 100 | 0.3 ± 0.2 | 1.6 ± 1.0 | 0.2 ± 0.0 | 5.1 ± 2.9 | |
| 30 | 0.3 ± 0.2 | 1.6 ± 1.0 | 0.2 ± 0.0 | 4.0 ± 2.1 | ||
| 1 | 0.5 ± 0.3 | 3.0 ± 1.1 | 0.2 ± 0.1 | 5.2 ± 2.1 | ||
| EJS | Feb. | 100 | 0.4 ± 0.2 | 6.7 ± 0.9 | 0.4 ± 0.1 | 9.8 ± 1.4 |
| 30 | 0.4 ± 0.2 | 6.4 ± 0.7 | 0.4 ± 0.0 | 8.6 ± 2.4 | ||
| 1 | 0.3 ± 0.2 | 7.5 ± 1.1 | 0.5 ± 0.2 | 10.7 ± 2.8 | ||
| Apr. | 100 | 0.9 ± 0.7 | 1.8 ± 1.0 | 0.1 ± 0.0 | 4.4 ± 1.4 | |
| 30 | 0.9 ± 0.6 | 1.9 ± 1.0 | 0.2 ± 0.1 | 4.2 ± 1.3 | ||
| 1 | 0.9 ± 0.7 | 2.6 ± 1.1 | 0.2 ± 0.0 | 5.0 ± 1.4 | ||
| Aug. | 100 | 0.4 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.0 | 3.7 ± 1.7 | |
| 30 | 0.6 ± 0.6 | 0.4 ± 0.6 | 0.1 ± 0.0 | 3.8 ± 1.6 | ||
| 1 | 0.4 ± 0.2 | 8.7 ± 3.3 | 0.5 ± 0.2 | 11.0 ± 4.1 | ||
| Oct. | 100 | 0.6 ± 0.3 | 1.3 ± 1.1 | 0.1 ± 0.1 | 2.6 ± 1.3 | |
| 30 | 0.6 ± 0.2 | 1.3 ± 1.0 | 0.1 ± 0.1 | 2.4 ± 1.1 | ||
| 1 | 0.5 ± 0.2 | 4.9 ± 4.3 | 0.4 ± 0.3 | 6.9 ± 6.0 | ||
| Resion | Method | Year | Month | Season | PP (mg C m−2 d−1) | Reference |
|---|---|---|---|---|---|---|
| Middle-eastern part | 14C method | 1989 | Aug. | summer | 450 | [41] |
| Oct. | autumn | 130 | ||||
| 1990 | Feb. | winter | 115 | |||
| Aug. | summer | 486 | ||||
| Oct. | autumn | 192 | ||||
| Entire | 14C method | 1992 | Sep. | autumn | 742 | [42] |
| middle-eastern part | 14C method | 1997 | Feb. | winter | 92 | [43] |
| Apr. | spring | 872 | ||||
| Aug. | summer | 899 | ||||
| Oct. | autumn | 667 | ||||
| Dec. | winter | 262 | ||||
| Middle part | Satellite-based | 1998–2003 | May | spring | 947 | [44] |
| Sep. | autumn | 723 | ||||
| Middle-eastern part | Satellite-based | 1998–2003 | May | spring | 734 | |
| Sep. | autumn | 558 | ||||
| Middle part | 14C method | 2008 | Jan. | winter | 56 | [45] |
| Aug. | summer | 649 | ||||
| Middle part | 13C-15N method | 2016 | Aug. | summer | 291 | [24] |
| Middle-eastern part | 13C-15N method | 2018 | Feb. | winter | 26 ± 12 | This study |
| Apr. | spring | 293 ± 394 | ||||
| Aug. | summer | 139 ± 67 | ||||
| Oct. | autumn | 606 ± 178 |
| Region | Method | Year | Month | Season | PP (mg C m−2 d−1) | Reference |
|---|---|---|---|---|---|---|
| Northern part | 14C method | 1989–1990 | Mar. | spring | 310 | [46] |
| Apr. | spring | 1727 | ||||
| Nov. | autumn | 517 | ||||
| Changjjang river mouth to shelf edge (PN-line) | 13C method | 1993–1994 | Feb. | winter | 282 | [47] |
| Aug. | summer | 714 | ||||
| Oct. | autumn | 573 | ||||
| Entire shelf | 14C method | 1997–1998 | Dec. | winter | 235 | [4] |
| Mar. | spring | 213 | ||||
| Jul. | summer | 734 | ||||
| Oct.–Nov. | autumn | 355 | ||||
| Entire shelf | 13C-15N method | 1998 | Mar. | spring | 528 | [48] |
| Oct.–Nov. | autumn | 782 | ||||
| Southern part | satellite-based | 1999 | Oct. | autumn | 543 | [49] |
| Entire | 14C method | 2008–2009 | May | spring | 1375 | [50] |
| Aug. | summer | 414 | ||||
| Nov. | autumn | 245 | ||||
| Feb. | winter | 102 | ||||
| Northern part | 13C-15N method | 2018 | Feb. | winter | 68 ± 35 | This Study |
| Apr. | spring | 426 ± 74 | ||||
| Aug. | summer | 195 ± 125 | ||||
| Oct. | autumn | 487 ± 161 |
| Region | Method | Year | Month | Season | PP (mg C m−2 d−1) | Reference |
|---|---|---|---|---|---|---|
| Southwestern part | 14C method | 1986 | Oct. | autumn | 1505 | [63] |
| Southwestern part | 14C method | 1990 | Oct. | autumn | 1420 | [64] |
| Yamato Basin | 13C method | 1994–1996 | Jan. | winter | 44 | [51] |
| Apr. | spring | 1082 | ||||
| Aug. | summer | 353 | ||||
| Oct. | autumn | 154 | ||||
| West coast of Hokkaido | 1997 | Feb. | winter | 106 | [52] | |
| Mar.–Apr. | spring | 1419 | ||||
| Jul. | summer | 487 | ||||
| Oct. | autumn | 254 | ||||
| Southwestern part | Satellite-based | 1998–2002 | Apr. | spring | 1100 | [65] |
| Nov. | autumn | 650 | ||||
| Monthly | 608 | |||||
| Ulleng Basin | 14C method | 2006 | Apr. | spring | 513 | [66] |
| Ulleng Basin | 13C method | 2010–2011 | May | spring | 1114 | [67] |
| Nov. | autumn | 380 | ||||
| Ulleng Basin | 13C method | 2010 | Jul. | summer | 716 | [68] |
| Northern part | 13C method | 2012 | Nov. | autumn | 181 | [22] |
| 2015 | May | spring | 442 | |||
| Ulleng Basin | 13C method | 2016 | Apr. | spring | 790 | [12] |
| Northwestern part | Apr. | spring | 407 | |||
| Southwestern part | 13C-15N method | 2018 | Feb. | winter | 284 ± 203 | This study |
| Apr. | spring | 491 ± 252 | ||||
| Aug. | summer | 106 ± 77 | ||||
| Oct. | autumn | 428 ± 307 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.-K.; Youn, S.-H.; Joo, H.; Kim, Y.; Kang, J.-J.; Lee, D.; Jo, N.; Kim, K.; Kim, M.-J.; Kim, S.; et al. First Concurrent Measurement of Primary Production in the Yellow Sea, the South Sea of Korea, and the East/Japan Sea, 2018. J. Mar. Sci. Eng. 2021, 9, 1237. https://doi.org/10.3390/jmse9111237
Jang H-K, Youn S-H, Joo H, Kim Y, Kang J-J, Lee D, Jo N, Kim K, Kim M-J, Kim S, et al. First Concurrent Measurement of Primary Production in the Yellow Sea, the South Sea of Korea, and the East/Japan Sea, 2018. Journal of Marine Science and Engineering. 2021; 9(11):1237. https://doi.org/10.3390/jmse9111237
Chicago/Turabian StyleJang, Hyo-Keun, Seok-Hyun Youn, Huitae Joo, Yejin Kim, Jae-Joong Kang, Dabin Lee, Naeun Jo, Kwanwoo Kim, Myung-Joon Kim, Soohyun Kim, and et al. 2021. "First Concurrent Measurement of Primary Production in the Yellow Sea, the South Sea of Korea, and the East/Japan Sea, 2018" Journal of Marine Science and Engineering 9, no. 11: 1237. https://doi.org/10.3390/jmse9111237
APA StyleJang, H.-K., Youn, S.-H., Joo, H., Kim, Y., Kang, J.-J., Lee, D., Jo, N., Kim, K., Kim, M.-J., Kim, S., & Lee, S.-H. (2021). First Concurrent Measurement of Primary Production in the Yellow Sea, the South Sea of Korea, and the East/Japan Sea, 2018. Journal of Marine Science and Engineering, 9(11), 1237. https://doi.org/10.3390/jmse9111237









