Abstract
This study proposes a novel anti-icing model in which silicone rubber with low thermal conductivity is coated at different positions on a material surface to change the continuity of the thermal conductivity of the surface. During the test, the surfaces of aluminum alloy and polymethyl methacrylate (PMMA) are discontinuously coated with silicone rubber. Repeated experiments are conducted to verify the anti-icing effect of the proposed model. Results showed that compared to the conventional surface ice adhesion strength, the rate of reduction of the ice adhesion strength of the aluminum alloy and PMMA could reach 75.07% and 76.70%, respectively, when the novel method is used. Because of the different levels of thermal conductivity at different positions on the material surface, the water attached to the surface locations without the coated silicone rubber had other freezing times. Combined with the heat and phase change of water during the freezing process, changing the stability of the interface between the ice and substrate could act as an active anti-icing power. The ice adhesion strength on the material surface could then be reduced. Compared with the conventional anti-icing methods, the anti-icing method proposed in this study could significantly increase the active anti-icing characteristics of the material and provide a novel anti-icing method for use in engineering applications.
1. Introduction
In a low-temperature environment, water moisture can easily adhere to the surface of components and freeze, becoming ice. Although water adhesion and freezing are common phenomena, they affect the operational performance of the components and can even result in accidents and human casualties. For example, accumulated ice on the rotating parts (such as on aircraft engine blades and fan blades) can alter the dynamic balance and aerodynamic performance of an aircraft [1,2,3]. Ice adhesion can affect the freedom of moving components (such as high-speed rail chassis parts) [4,5] and can increase loads of attached elements (such as transmission lines) [6,7,8]. Accreted ice can also affect the accuracy and efficiency of the telecommunication equipment [9,10] and decrease solar panel transparency, thereby affecting photoelectric conversion efficiency [11,12]. Finally, ice adhesion has been shown to significantly impact offshore platforms, ships, etc. [13,14].
To reduce the hazards of ice adhesion in engineering applications, various anti/de-icing methods have been developed. Based on the anti/de-icing principles, these methods can be divided into three categories: (1) The removal of accreted ice directly using mechanical methods; (2) the melting of accumulated ice or use of thermal de-icing approaches to delay the freezing time of water; and (3) the use of chemical agents that affect the phase temperature of water adhesion on surfaces to delay freezing [7,15,16,17]. However, the existing anti-icing methods have some practical applications. For example, the hot air flow method is a thermal de-icing method that consumes considerable energy, and the use of chemical agents can cause hardening and corrosion of components [18,19]. Therefore, a new type of active anti-icing method that overcomes these disadvantages is urgently required to mitigate the hazards of ice adhesion.
With the development of the material preparation processes, researchers have made material surfaces superhydrophobic by changing the micromorphology of the material surface or coating low-surface-energy materials [20,21,22,23,24,25]. After fabricating the superhydrophobic surfaces with nano/micro-roughness on different substrates, Cohen et al. [26] found that superhydrophobic surfaces could significantly reduce the ice adhesion strength. Spitzner et al. [27] coated pyroelectric polymer polyvinylidene fluoride on a glass surface to prepare a superhydrophobic film on the substrate surface and found the freezing of water into ice was delayed. The superhydrophobic surface is regarded as a promising means of removing accumulated ice owing to the excellent water repellency. However, many studies have reported superhydrophobic surfaces to have low durability, mechanical properties, and corrosion-resistance characteristics [28,29,30,31,32,33,34,35,36,37,38]. Some researchers suggest that the swelling force generated by the freezing process of water filled in the microscopic morphology in a low-temperature environment can destroy the microstructure and result in the loss of surface superhydrophobicity [32,39,40]. Other researchers have considered the reaction between water and substances composed of superhydrophobic surfaces. This reaction can help water molecules adhere to the material surfaces, increasing ice adhesion strength [33]. In summary, many characteristics of superhydrophobic surfaces must be satisfied and improved before they can be applied in actual use. Conventional anti-icing methods should be modified to overcome the current limitations.
After thousands of years of evolution, the surfaces of organisms have developed characteristics and structures that are optimally adapted to the living environments. For example, the body surface of the desert beetle has discontinuous wettability and a non-smooth morphology, which enables the collection of water vapor from the environment and allows the beetle to live in desert areas [41]. Many organisms such as butterflies, lotus, and earthworms have discontinuous characteristics on different scales. As a focus of anti-icing research, superhydrophobic surfaces have discontinuous morphologies on a micro-scale. Compared with the use of complex or high-cost processes to fabricate surfaces with anti/de-icing characteristics, macroscopically altering the properties of materials to endow them with anti-icing function seems feasible.
As a particular phase-change energy-storage material, water has been used in geothermal systems for ice or snow melting on pavements and bridge decks [42,43,44]. However, the expansion phenomenon and swelling force generated from the freezing process are typically neglected in anti/de-icing research. Therefore, inspired by the discontinuous characteristics of organism surfaces and the common phenomenon of water pipes bursting in winter, the continuity of the thermal conductivities of materials is altered in the present study. More specifically, silicone rubber with low thermal conductivity was coated at different positions on a material surface. Various positions in the interior in which water adhered to the material surface had different phase-transition times. As a result, phase-change expansion generated by the freezing of water attached to different positions on the material surface interferes with the water freezing process in adjacent areas. The adhesion stability between the ice and substrate is changed, thus affecting the ice adhesion strength. If the ice adhesion strength can be reduced, the accumulated ice can be removed more efficiently, and the de-icing cost and environmental pollution can be reduced.
2. Materials and Methods
2.1. Anti-Icing Model and Principles
2.1.1. Temperature Characteristics during the Freezing Process
During the freezing process, water not only changes in volume but also releases heat. The change in volume before and after freezing results in the generation of an expansive force. In addition, the released energy can be characterized by a change in the internal temperature of the water droplet. In this study, to show the change in the internal temperature of a single water droplet during the freezing process, an apparatus was designed to observe the freezing process of 5 μL of water on the surface of a 6061 aluminum alloy (5 × 5 × 0.5 mm3). During the test, the Center-309 multi-channel temperature collector (purchased from Center Technology Corporation, Taibei, China) was used to measure and record the internal temperature of the water and substrate temperature during the freezing process. The measured range of the temperature collector is from −200~1370 °C, and the accuracy is ±0.3% rdg +1 °C. The droplet was titrated on the material surface by micropipette. Then, the sample surface temperature was adjusted from the ambient temperature (approximately 20 °C) to the set temperature (−15 °C). The apparatus and procedures are described in detail in a previous study [45]. Figure 1 shows the internal temperature curve of the water droplet during the solidified process. It can be seen that the internal temperature of the water droplet increased from −8.5 °C to −0.4 °C during the phase transition.
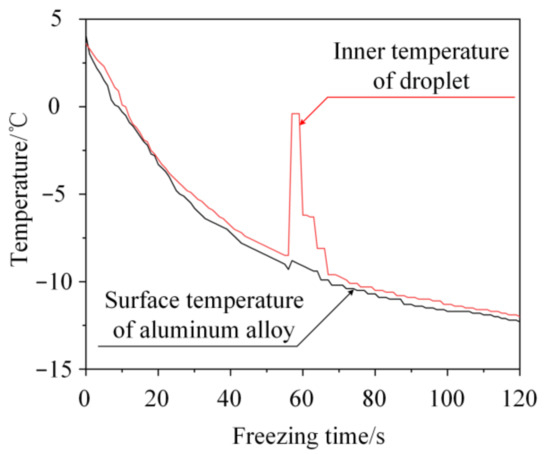
Figure 1.
Inner temperature during the freezing process of a water droplet on an aluminum alloy.
The experimental results showed that the internal temperature of the water droplet changed significantly before and after the phase change. It means that the droplet releases energy in a short duration during the freezing process.
2.1.2. Swelling Force Test
It is well known that the freezing of water in a container in a low-temperature environment will cause the container to burst. Therefore, the apparatus shown in Figure 2 was designed to measure the swelling force. A pit for filling the water with a diameter of 3 mm to a depth of 4 mm was constructed in the center of a cylindrical aluminum rod. The rod had a diameter of 60 mm and a height of 40 mm. An aluminum plate (60 × 60 × 5 mm3 in size) was placed above the aluminum block, and a draft gauge connected with a PC was placed in the center of the plate. Based on the plate and block height, the installation height of the draft gauge was adjusted to ensure it barely touched the aluminum plate. The entire device was placed in a −20 °C environment controlled by a climate chamber with a temperature control accuracy of ±0.01 °C during the test. The accuracy of the draft gauge was ±0.01 N.
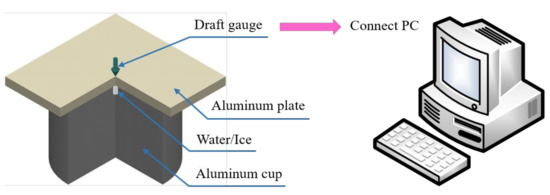
Figure 2.
Swelling force test device.
The swelling force generated by the freezing water as it filled the pit was measured by the designed apparatus, as shown in Figure 3. At −20 °C, when the water in the pit began to phase change and solidify, the generated swelling force increased rapidly and reached the maximum value. After reaching the maximum value of 13.80 N, the swelling force decreased and gradually stabilized.
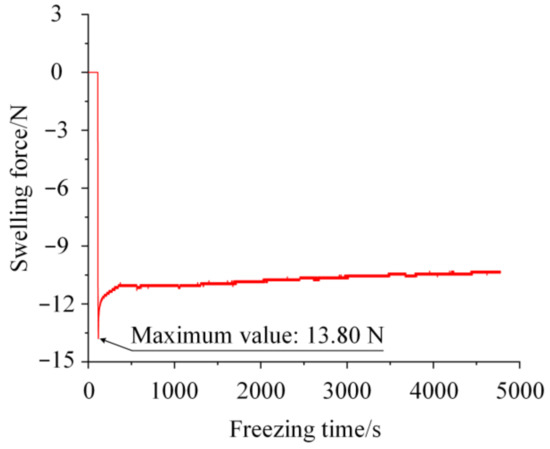
Figure 3.
Swelling force.
2.1.3. Active Anti-Icing Model
This study proposes an active de-icing model, as shown in Figure 4. The de-icing model consisted of silicon rubber with low thermal conductivity and a substrate. The silicone rubber was applied at different positions on the substrate surface to change the thermal continuity of the substrate surface. Due to the different thermal conductivities at different substrate surface positions, the freezing process of the water attached to the surface was affected. A difference in phase transition time was observed with the model. It means that the freezing time of water on the surface of silicone rubber with low thermal conductivity was longer than that on conventional materials without silicone rubber. The proposed anti-icing model utilizes the phase change energy and swelling force generated at different locations to affect the freezing process in adjacent areas, disturb the stability of the contact interface, and actively reduce the ice adhesion strength.
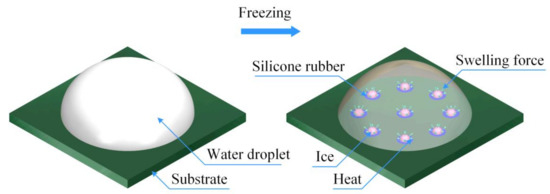
Figure 4.
Schematic of the proposed de-icing prototype.
Water attached to the substrate surface without the silicone rubber freezes at low temperatures, whereas the contact between the substrate and ice gradually stabilizes. In addition, ice formed in the frozen area forms a boundary constraint on the water attached to the surface of the silicone rubber. Then, water attached to the silicone rubber freezes. The energy and phase-change force generated by the freezing process of water that adheres to the silicone rubber will act on the adjacent area. In other words, this study utilizes the expansive force and energy released by the freezing process of water to interfere with the stability of the interface between the ice and substrate. Because the proposed model exhibits active de-icing performance, it can reduce the ice adhesion strength, and the accreted ice can be easily removed.
2.2. Materials
To validate that the anti-icing effect of the proposed model was not affected by the substrate, 6061 aluminum alloy and polymethyl methacrylate (PMMA) with a significant difference in thermal conductivity were used as the substrates in this study. The thermal conductivities of the corresponding substrates were 237 W/m·k and 0.2 W/m·k [46], respectively. During the experiment, all samples were 50 × 50 × 5 mm3 (L × W × H) in size.
One-component room-temperature vulcanized silicone rubber (RTV-1) is silicone rubber with a low thermal conductivity widely used in automotive, electronics, and other engineering fields. It has good adhesion performance, excellent bond strength, and other excellent properties [47]. In our study, RTV-1 was coated on the material surface to change the thermal conductivity of the substrate. The RTV-1 was purchased from Guangdong Hengda New Material Technology Co., Ltd., (Guangdong, China) and its thermal conductivity was 0.13 W/m·k [47].
2.3. Fabrication of a Discontinuous Thermal Conductive Surface
In this study, a discontinuous thermally conductive surface with a pattern was formed using a molding method similar to preparing a superhydrophobic surface. In the test, a round shape that is easy to coat and easy to implement in the engineering field was chosen for coating the silicone rubber on the substrate surface. The preparation process is shown in Figure 5. Firstly, a biaxially oriented polypropylene (BOPP) layer with a thickness of 0.053 mm was pasted on the substrate surface. The pattern was processed on the BOPP surface by a laser engraving machine. BOPP films with different design shapes on the surface were used as the molds in this study. The silicone rubber was then used to fill in the pits of the BOPP. After standing for 1 h, the excess silicone rubber on the BOPP surface was carefully removed by a blade. The BOPP pasted on the substrate surface was slowly removed to obtain a surface with discontinuous thermal conductivity.
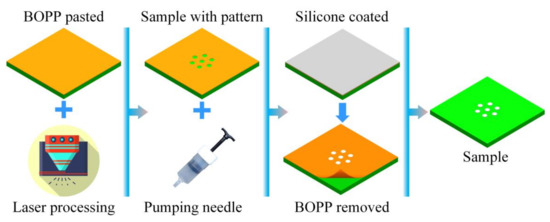
Figure 5.
Surface preparation process with discontinuous thermal conductivity.
During the test, the silicone rubber on the substrate surface was set as the same coating area; the area coated with silicone rubber was half of the ice adhesion area. As Figure 6 shows, the shapes of the silicone rubber specimens covered on the material surface were all circular, and the specimens had diameters of 2, 3, 4, and 5 mm. The thickness of the BOPP film controlled the coated thickness. The coating area of the silicone rubber was calculated. Combined with the characteristics of the test device, the coated silicone rubber was distributed in a circular array on the material surface, and the silicone rubber with different diameters had a similar coating area when the number of circular arrays was adjusted.
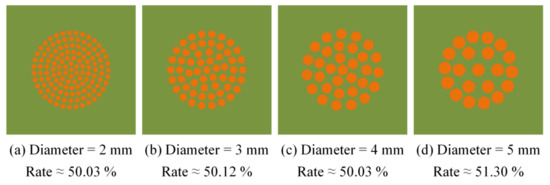
Figure 6.
Anti-icing specimens with different silicone rubber diameters.
2.4. Experiments of Anti-Icing Property
As the ambient temperature decreases, the ice adhesion strength will increase and tend to be stable [45]. Therefore, in our study, the temperature of the test environment was set at −20 °C and controlled by a climate chamber. The refrigeration time was 1 h. The control accuracy of the climate chamber was ±0.01 °C. Water as a special adhesive agent solidified into ice under low-temperature conditions, and adhesion stability was formed between the ice and substrate, making the removal of the accreted ice difficult. During the test, the adhesion force between the ice and surfaces of the different samples was measured using the designed device. The instrument used aluminum cups to prepare the accreted ice on the sample. The inner diameter of the aluminum cup was 32 mm. Before 5 mL of water was added to the aluminum cup, a special tool was used to place the aluminum cup to ensure uniformity of the placement position of the aluminum cup in each test, as shown in Figure 7. Meanwhile, we avoided placing the aluminum cup on the surface of the coated silicone rubber.
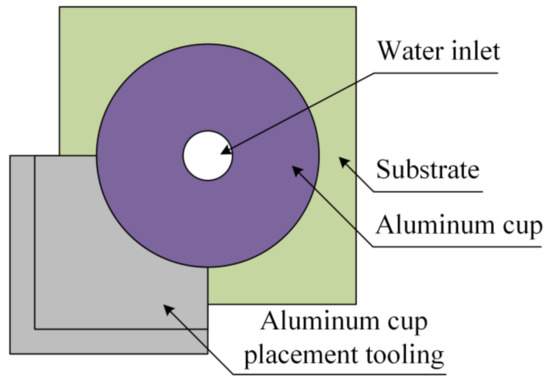
Figure 7.
Placement of aluminum cup.
Figure 8 showw the principle of measuring the ice adhesion force on the substrate surface. A draft gauge with an accuracy of 0.01 N was used to collect the maximum force of the accreted ice during the peeling process. Thus, the ice adhesion strength of the different samples was calculated using the eq. P = F/S, where P is the tangential ice adhesion strength, S is the inner area of the cup, and F is the maximum ice adhesion force during the pull process.
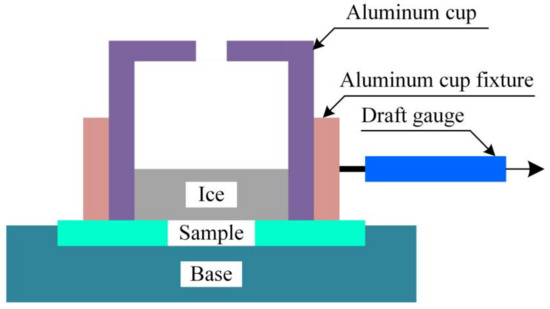
Figure 8.
Schematic diagram of the ice adhesion strength test.
For the test, a sample without silicone rubber on the surface was used as a reference to evaluate the de-icing effect of the proposed de-icing model. The samples were labeled A–E, and the basic characteristics of each sample are shown in Table 1.

Table 1.
Sample descriptions.
3. Results and Discussion
3.1. Ice Adhesion Strength
Five samples were each tested 20 times, and the average value was calculated as the ice adhesion strength on the surfaces of the samples. This value was then used to evaluate the anti-icing effect of the proposed model. During the experiment, the ice adhesion strength on the surface without the coated silicone rubber was used as a reference to calculate the rate of reduction of the ice adhesion strength on the different samples. The experimental results are shown in Figure 9.
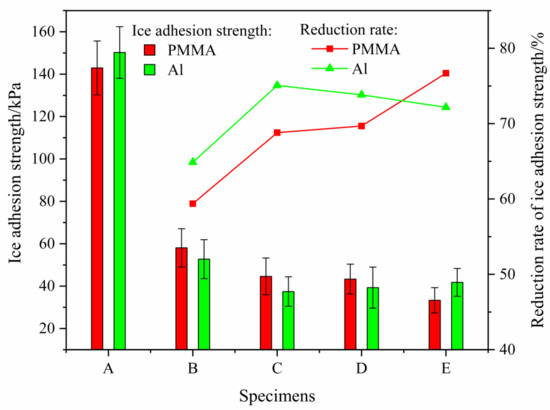
Figure 9.
Shear ice adhesion strength.
Figure 9 showed that the proposed anti-icing model could significantly reduce the ice adhesion strength on the sample surface regardless of whether the substrate was PMMA or aluminum alloy. In addition, the experimental results showed that the proposed model had active anti-icing characteristics. During the study, the average ice adhesion strength on PMMA and Al without silicone rubber was 142.95 kPa and 150.22 kPa, respectively. Of all the samples, only the ice adhesion strength on sample EPMMA was less than that of the sample EAl.
When the substrate was aluminum alloy or PMMA, ice adhesion strength on sample B was greater than the ice adhesion strength on the other sample surface with a discontinuous coating of silicone rubber. The reduction rates of ice adhesion strength on the surfaces of samples CPMMA and DPMMA were similar, and the ice adhesion strength of ice on the surface of sample DPMMA was 43.33 kPa. The reduction rate of the ice adhesion strength on the surface of sample EPMMA was greater than that on other samples, which reached 76.70%. The ice adhesion strength on the surface of EPMMA was 33.31 kPa. It reduced the ice adhesion strength on the surface of PMMA.
When the substrate was aluminum alloy with a discontinuous silicone rubber coating on the surface, CAl had the largest reduction rate of the ice adhesion strength, which was 37.45 kPa. This was close to the ice adhesion strength on the surface of the EPMMA sample. As the diameter of the silicone rubber increased from 3 to 5 mm, the ice adhesion strength of the aluminum alloy surface gradually increased. The reduction rates of ice adhesion strength on the surfaces of samples C, D, and E were 75.07%, 73.83%, and 72.18%, respectively.
3.2. Discussion
The experimental results of ice adhesion strength revealed that the discontinuous coating of silicone rubber with low thermal conductivity on the surface could reduce the ice adhesion strength on the surface. This means that the lower the ice adhesion strength on the surface, the lower the energy consumption and the cost required to remove the ice.
Because of the discontinuous silicone rubber coating on the material surface, the interface between the water and the material surface had different thermal conductivities. At low temperatures, the water attached to the material surface entered a supercooled state at different times. The water on the surface not covered by silicone rubber initially entered a supercooled condition, as shown in Figure 10b. As the heat exchange continued between the water on the material surface and the environment under a low temperature, the water on the material surface without silicone rubber solidified into ice. However, the ice located near the silicone rubber did not freeze because of the silicone rubber with low thermal conductivity. In addition, the area that initially froze formed constraints to the water in the unfrozen interior area.
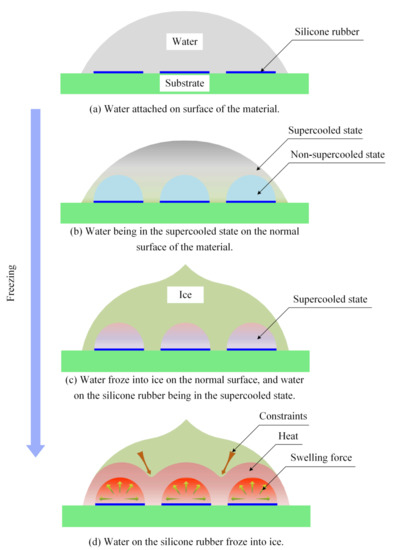
Figure 10.
Schematic of the effects of discontinuous thermal conduction on ice adhesion strength.
Water freezing near the coated region was delayed due to the material surface region with low thermal conductivity. With continuous cooling, the water near the thin layer of silicone rubber on the surface of the substrate transformed into a supercooled state, as shown in Figure 10c. When the water attached to the silicone rubber film began to freeze, heat and swelling forces were generated. For distinction, the areas without and with silicone rubber were named the first and second frozen areas, respectively. The swelling force generated by the second frozen area acted on the constraints formed by the first frozen area on the second frozen area. Because the direction of the expansion force was divergent during the freezing process, the surrounding frozen area tended to move. Therefore, the stability of the adhesion interface between the ice in the first frozen area and the material surface was changed. In addition, the heat generated by the freezing process of the water located on the silicon rubber also influenced the adhesion stability between the ice and material surface, as shown in Figure 10d.
Compared with the thermal conductivity of the silicone rubber, the aluminum alloy substrate exhibited excellent thermal conductivity. When the water adhered to the aluminum alloy surface area that was not coated with silicone rubber, it rapidly froze into ice. A stable contact interface was formed between the ice and substrate. However, the discontinuous coating of the silicone rubber on the surface aluminum alloy delayed the freezing time of water located in the silicone rubber area. This meant that the heat and swelling forces were postponed. Therefore, the rate of reduction of the ice adhesion strength on the aluminum alloy surface was significantly reduced.
In the test, PMMA and the silicone rubber had similar thermal conductivities, lower than that of the aluminum alloy. However, the silicone rubber coated on the PMMA surface further delayed the freezing time of water attached to the coated area. In addition, the time in which a stable contact interface formed between the ice and PMMA surface was longer than that between the ice and aluminum alloy surface. In addition, the difference in freezing times between the areas without and with silicone rubber on the PMMA surface under the same conditions was smaller than that of the aluminum alloy substrate. It caused the rate of reduction of the ice adhesion strength on the PMMA surface to be lower than that of the aluminum alloy surface.
Meanwhile, the adhesion strength of silicone rubber on the substrate surface after 20 freeze–thaw cycles was tested dependent on the [48]. The experimental conditions were the same as those of the ice adhesion strength test. The area of silicone rubber falling off in the central grid area of silicone rubber was calculated by Photoshop 2020. Compared with standard a, it was determined that the adhesion grade of silicone rubber on the substrate surface was 4B after 20 freeze–thaw cycles. As shown by the test results, the silicone rubber had good adhesion on the surface of aluminum alloy and PMMA after several freeze–thaw cycles.
4. Conclusions
In this study, the substrate surface was discontinuously coated with silicone rubber with low thermal conductivity, resulting in the change of single and continuous thermal conductivities of the material surface. The ice adhesion strengths on the aluminum alloy and PMMA were reduced by the swelling force and heat generated during the freezing process of water, thereby endowing the material with an active anti-icing function.
In a low-temperature environment, the water attached to the area without silicone rubber on the surface initially froze into ice, and the interface between the ice and substrate surface gradually stabilized. Then, the water adhesion to the silicone rubber began to freeze. The heat and swelling force generated by the freezing process of the water on the surface of the silicone rubber changed with the stable interface between the accumulated ice and the substrate. This affected the ice adhesion strength on the material surface. Thus, the difficulty and cost of removing ice on the surface of materials were reduced, and the anti-icing ability of the material was improved.
Compared with the conventional anti-icing methods, the proposed anti-icing model could significantly reduce the ice adhesion strength and achieve high efficiency and active de-icing. This model could be widely used in many engineering fields. However, the experimental results showed that the size of the silicone rubber coated on the surface also affected the reduction rate of ice adhesion strength. Therefore, further research is required to determine the size of the silicone rubber coating that most affects the ice adhesion strength on the material surface.
Author Contributions
Conceptualization, T.C., Y.C., L.R., J.J. and Q.C.; methodology, T.C., Y.C. and J.J.; validation, T.C., J.J. and Q.C.; formal analysis, T.C., Y.C., L.R., J.J., Q.C. and K.-L.C.; investigation, T.C., Y.C., J.J. and Q.C.; resources, T.C., L.R., J.J. and Q.C.; data curation, T.C., Y.C. and J.J.; writing—original draft preparation, T.C. and Y.C.; writing—review and editing, T.C., Y.C., J.J., Q.C. and K.-L.C.; supervision, T.C., J.J. and Q.C.; project administration, T.C., J.J. and Q.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Education Department of Jilin Province, China (Grant No. JJKH20211070KJ); the Department of Science and Technology of Jilin Province, China (Grant No. 20200801049GH); and the National Natural Science Foundation of China (Grant No. 51775234).
Data Availability Statement
The data supporting the findings of this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, J.; Dou, R.M.; Cui, D.P.; Zhang, Q.L.; Zhang, Y.F.; Xu, F.J.; Zhou, X.; Wang, J.J.; Song, Y.L.; Jiang, L. Robust prototypical anti-icing coatings with a self-lubricating liquid water layer between ice and substrate. ACS Appl. Mater. Interfaces 2013, 5, 4026–4030. [Google Scholar] [CrossRef] [PubMed]
- Lamraoui, F.; Fortin, G.; Benoit, R.; Perron, J.; Masson, C. Atmospheric icing impact on wind turbine production. Cold Reg. Sci. Technol. 2014, 100, 36–49. [Google Scholar] [CrossRef]
- Cao, Y.H.; Wu, Z.L.; Su, Y.; Xu, Z.D. Aircraft flight characteristics in icing conditions. Prog. Aeosp. Sci. 2015, 74, 62–80. [Google Scholar] [CrossRef]
- Wang, J.B.; Zhang, J.; Zhang, Y.; Xie, F.; Krajnovic, S.; Gao, G.J. Impact of bogie cavity shapes and operational environment on snow accumulating on the bogies of high-speed trains. J. Wind Eng. Ind. Aerodyn. 2018, 176, 211–224. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, J.; Gao, G.; He, K.; Zhang, Y.; Wang, J.; Zhang, Y. Study of snow accumulation on a high-speed train’s bogies based on the discrete phase model. J. Appl. Fluid Mech. 2017, 10, 1729–1745. [Google Scholar] [CrossRef]
- Liu, Y.P.; Cai, W.; Cao, S.; Yao, T.; Huang, D.C.; Chen, Y.; Xu, Z.M. Study of the effect of ice adhesion on electrical power transmission insulator. J. Adhes. Sci. Technol. 2012, 26, 593–602. [Google Scholar] [CrossRef]
- Lv, J.Y.; Song, Y.L.; Jiang, L.; Wang, J.J. Bio-inspired strategies for anti-icing. ACS Nano 2014, 8, 3152–3169. [Google Scholar] [CrossRef]
- Farzaneh, M. Atmospheric Icing of Power Networks, 1st ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 40–78. [Google Scholar]
- Hong, J.W.; Jung, J.H.; Yong, S.M.; Kim, Y.R.; Park, J.; Lee, S.J.; Choi, J.H. Radio-frequency transparent carbon nanotube electrothermal film for radome de-icing application. J. Mater. Res. Technol.-JMRT 2020, 9, 10854–10862. [Google Scholar] [CrossRef]
- Nakamura, K.; Iwasawa, N.; Kawasaki, K.; Yoshida, S.; Takahashi, M. The attenuation characteristics of millimeter-wave by snow accretion. IEICE Commun. Express 2020, 9, 674–678. [Google Scholar] [CrossRef]
- Jelle, B.P. The challenge of removing snow downfall on photovoltaic solar cell roofs in order to maximize solar energy efficiency—Research opportunities for the future. Energy Build. 2013, 67, 334–351. [Google Scholar] [CrossRef] [Green Version]
- Carriveau, R.; Edrisy, A.; Cadieux, P.; Mailoux, R. Ice adhesion issues in renewable energy infrastructure. J. Adhes. Sci. Technol. 2012, 26, 447–461. [Google Scholar] [CrossRef]
- Bhatia, K.; Khan, F. A predictive model to estimate ice accumulation on ship and offshore rig. Ocean Eng. 2019, 173, 68–76. [Google Scholar] [CrossRef]
- Dehghani-Sanij, A.R.; Dehghani, S.R.; Naterer, G.F.; Muzychka, Y.S. Marine icing phenomena on vessels and offshore structures: Prediction and analysis. Ocean Eng. 2017, 143, 1–23. [Google Scholar] [CrossRef]
- Jamil, M.I.; Ali, A.; Haq, F.; Zhang, Q.H.; Zhan, X.L.; Chen, F.Q. Icephobic strategies and materials with superwettability: Design principles and mechanism. Langmuir 2018, 34, 15425–15444. [Google Scholar] [CrossRef]
- Rashid, T.; Khawaja, H.A.; Edvardsen, K. Review of marine icing and anti-/de-icing systems. J. Mar. Eng. Technol. 2016, 15, 79–87. [Google Scholar] [CrossRef]
- Wang, Z.J. Recent progress on ultrasonic de-icing technique used for wind power generation, high-voltage transmission line and aircraft. Energy Build. 2017, 140, 42–49. [Google Scholar] [CrossRef]
- Chen, T.K.; Jin, J.F.; Qi, Y.C.; Tian, W.J.; Cong, Q.; Choy, K.L. Disturbing stability of interface by adopting phase-change temperature gradient to reduce ice adhesion strength. Cold Reg. Sci. Technol. 2019, 158, 69–75. [Google Scholar] [CrossRef]
- Makkonen, L. Ice adhesion-Theory, measurements and countermeasures. J. Adhes. Sci. Technol. 2012, 26, 413–445. [Google Scholar] [CrossRef]
- Buijnsters, J.G.; Zhong, R.; Tsyntsaru, N.; Celis, J.P. Surface wettability of macroporous anodized aluminum oxide. ACS Appl. Mater. Interfaces 2013, 5, 3224–3233. [Google Scholar] [CrossRef]
- Bharathidasan, T.; Kumar, S.V.; Bobji, M.S.; Chakradhar, R.P.S.; Basu, B.J. Effect of wettability and surface roughness on ice-adhesion strength of hydrophilic, hydrophobic and superhydrophobic surfaces. Appl. Surf. Sci. 2014, 314, 241–250. [Google Scholar] [CrossRef]
- Hao, P.F.; Lv, C.J.; Zhang, X.W. Freezing of sessile water droplets on surfaces with various roughness and wettability. Appl. Phys. Lett. 2014, 104, 161609. [Google Scholar] [CrossRef]
- Momen, G.; Jafari, R.; Farzanehnserc, M. Ice repellency behaviour of superhydrophobic surfaces: Effects of atmospheric icing conditions and surface roughness. Appl. Surf. Sci. 2015, 349, 211–218. [Google Scholar] [CrossRef]
- Ramachandran, R.; Kozhukhova, M.; Sobolev, K.; Nosonovsky, M. Anti-icing superhydrophobic surfaces: Controlling entropic molecular interactions to design novel icephobic concrete. Entropy 2016, 18, 132. [Google Scholar] [CrossRef] [Green Version]
- Zou, M.; Beckford, S.; Wei, R.; Ellis, C.; Hatton, G.; Miller, M.A. Effects of surface roughness and energy on ice adhesion strength. Appl. Surf. Sci. 2011, 257, 3786–3792. [Google Scholar] [CrossRef]
- Cohen, N.; Dotan, A.; Dodiuk, H.; Kenig, S. Thermomechanical mechanisms of reducing ice adhesion on superhydrophobic surfaces. Appl. Surf. Sci. 2018, 440, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Spitzner, D.; Bergmann, U.; Apelt, S.; Boucher, R.A.; Wiesmann, H.P. Reversible switching of icing properties on pyroelectric polyvenylidene fluoride thin film coatings. Coatings 2015, 5, 724–736. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, J.; He, M.; Li, K.Y.; Cui, D.P.; Zhang, Q.L.; Zeng, X.P.; Zhang, Y.F.; Wang, J.J.; Song, Y.L. Superhydrophobic surfaces cannot reduce ice adhesion. Appl. Phys. Lett. 2012, 101, 111603. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Jung, S.; Dorrestijn, M.; Raps, D.; Das, A.; Megaridis, C.M.; Poulikakos, D. Are superhydrophobic surfaces best for icephobicity? Langmuir 2011, 27, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Pitchumani, R. Facile fabrication of durable copper–based superhydrophobic surfaces via electrodeposition. Langmuir 2018, 34, 3159–3169. [Google Scholar] [CrossRef] [PubMed]
- Kulinich, S.A.; Farhadi, S.; Nose, K.; Du, X.W. Superhydrophobic surfaces: Are they really ice-repellent? Langmuir 2011, 7, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Mahadik, S.A.; Fernando, P.D.; Hegade, N.D.; Wagh, P.B.; Gupta, S.C. Durability and restoring of superhydrophobic properties in silica–based coatings. J. Colloid Interface Sci. 2013, 405, 262–268. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Hejazi, V. Why superhydrophobic surfaces are not always icephobic. ACS Nano 2012, 6, 8488–8491. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Damle, V.G.; Liu, S.L.Z.; Rykaczewski, K. Bioinspired stimuli-responsive and antifreeze-secreting anti-icing coatings. Adv. Mater. Interfaces 2015, 2, 1400479. [Google Scholar] [CrossRef]
- Villegas, M.; Zhang, Y.X.; Abu Jarad, N.; Soleymani, L.; Didar, T.F. Liquid–infused surfaces: A review of theory, design, and applications. ACS Nano 2019, 13, 8517–8536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lv, F.Y. A review of the recent advances in superhydrophobic surfaces and the emerging energy-related applications. Energy 2015, 82, 1068–1087. [Google Scholar] [CrossRef]
- Zheng, S.L.; Li, C.; Fu, Q.T.; Hu, W.; Xiang, T.F.; Wang, Q.; Du, M.P.; Liu, X.C.; Chen, Z. Development of stable superhydrophobic coatings on aluminum surface for corrosion-resistant, self-cleaning, and anti-icing applications. Mater. Des. 2016, 93, 261–270. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Honda, M.; Zhu, A.L.; Rozhin, A.G.; Du, X.W. The icephobic performance of alkyl-grafted aluminum surfaces. Soft Matter. 2015, 11, 856–861. [Google Scholar] [CrossRef] [Green Version]
- Kulinich, S.A.; Farzaneh, M. On ice-releasing properties of rough hydrophobic coatings. Cold Reg. Sci. Technol. 2011, 65, 60–64. [Google Scholar] [CrossRef]
- Parker, A.R.; Lawrence, R.C. Water capture by a desert beetle. Nature 2011, 414, 33–34. [Google Scholar] [CrossRef]
- Balbay, A.; Esen, M. Experimental investigation of using ground source heat pump system for snow melting on pavements and bridge decks. Sci. Res. Essays. 2010, 5, 3955–3966. [Google Scholar]
- Balbay, A.; Esen, M. Temperature distributions in pavement and bridge slabs heated by using vertical ground-source heat pump systems. Acta Sci.-Technol. 2013, 35, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Esen, M.; Ayhan, T. Development of a model compatible with solar assisted cylindrical energy storage tank and variation of stored energy with time for different phase change materials. Energy Conv. Manag. 1996, 37, 1775–1785. [Google Scholar] [CrossRef]
- Chen, T.K.; Cong, Q.; Sun, C.B.; Jin, J.F.; Choy, K.L. Influence of substrate initial temperature on adhesion strength of ice on aluminum alloy. Cold Reg. Sci. Techol. 2018, 148, 142–147. [Google Scholar] [CrossRef]
- Chen, S.G.; Zhu, G.W.; Li, B.Z. Marine Engineering Manual, 1st ed.; China Communications Press: Beijing, China, 1992; pp. 318–319. [Google Scholar]
- Lai, G.Q. Organic Silicon Chemistry and Technology, 1st ed.; Chemical Industry Press Co., Ltd.: Beijing, China, 2011; pp. 215–225. [Google Scholar]
- ASTM D3359-2017. Standard Test Methods for Rating Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).