Alien Species Threat across Marine Protected Areas of Turkey—An Updated Inventory
Abstract
:1. Introduction
2. Materials and Methods
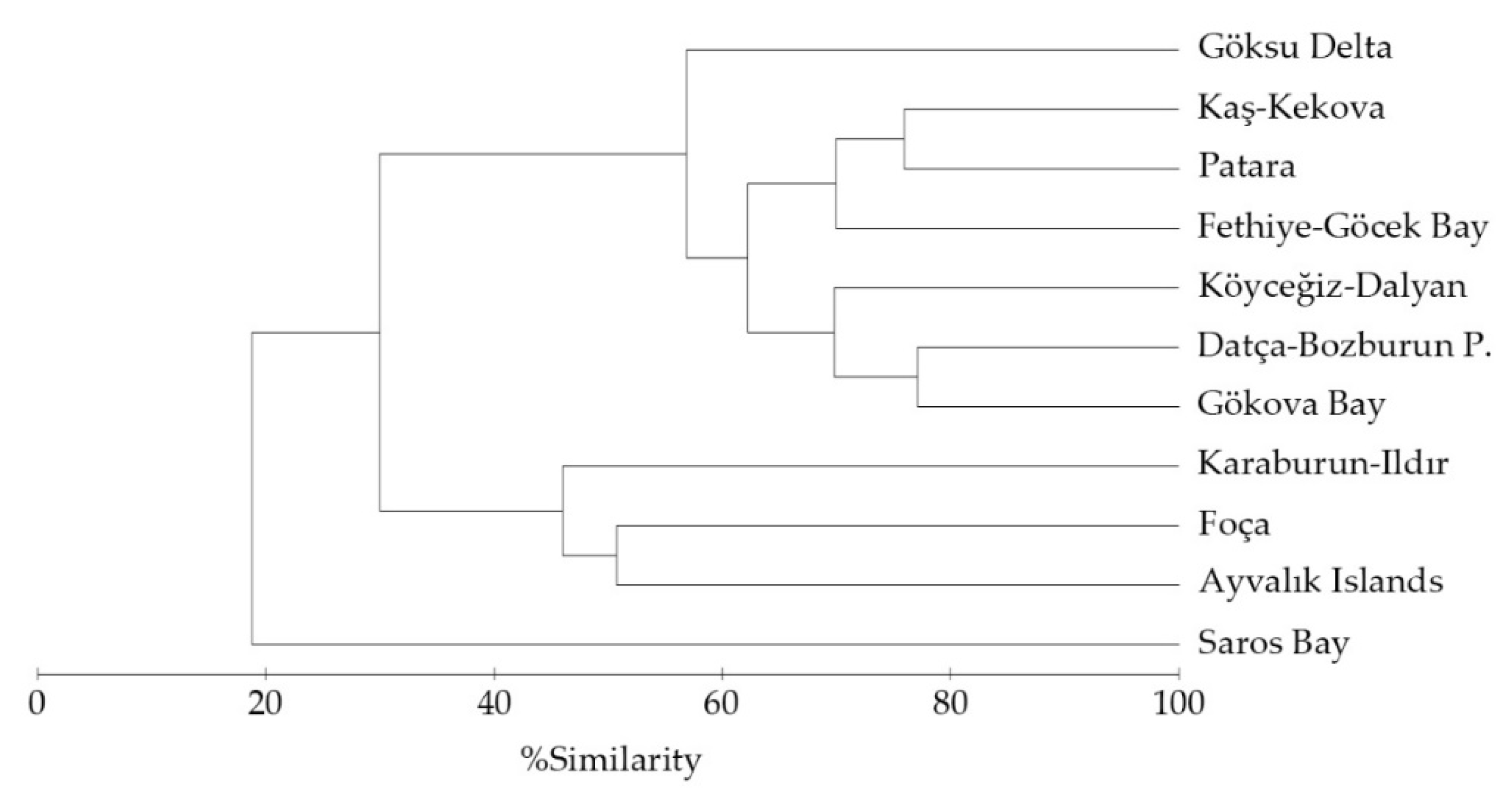
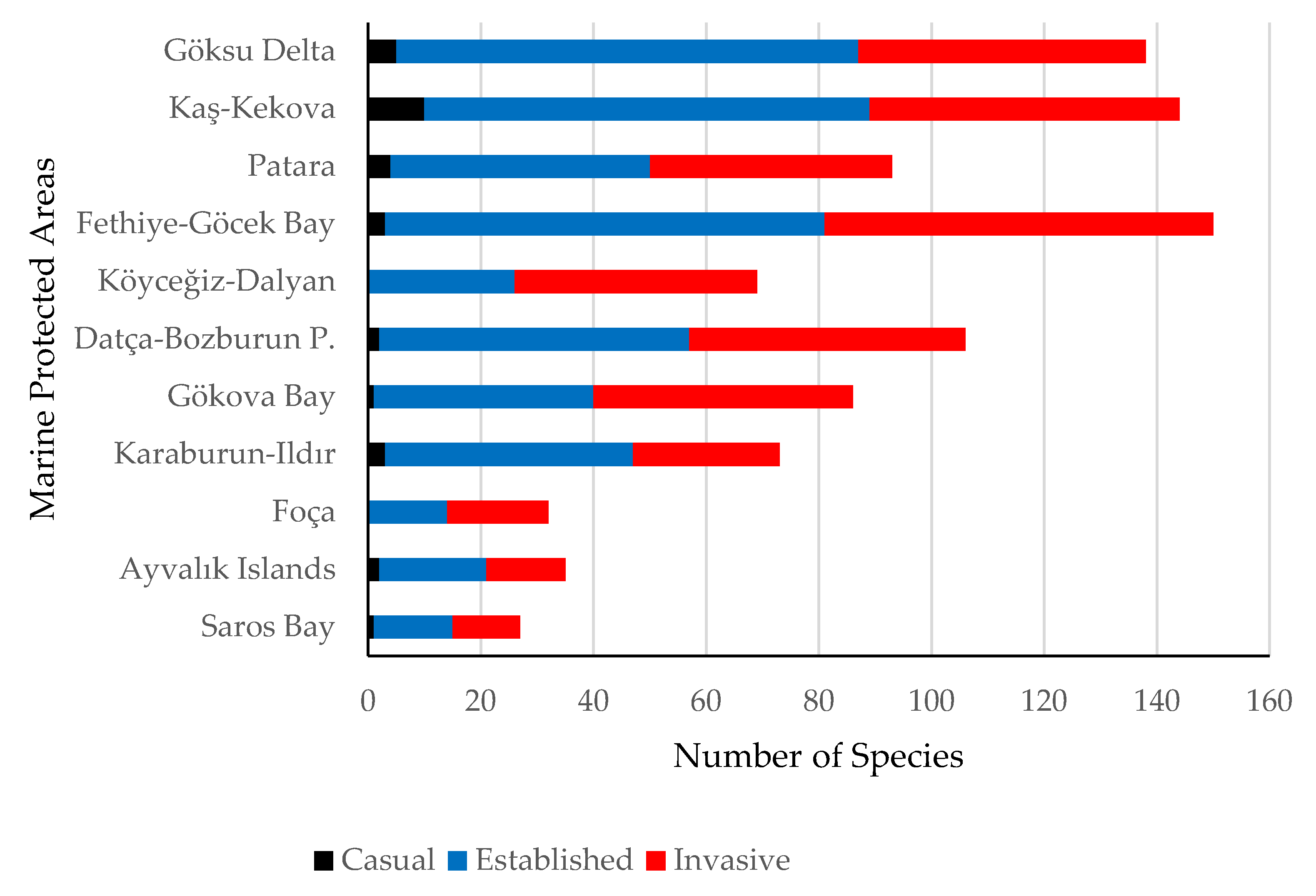
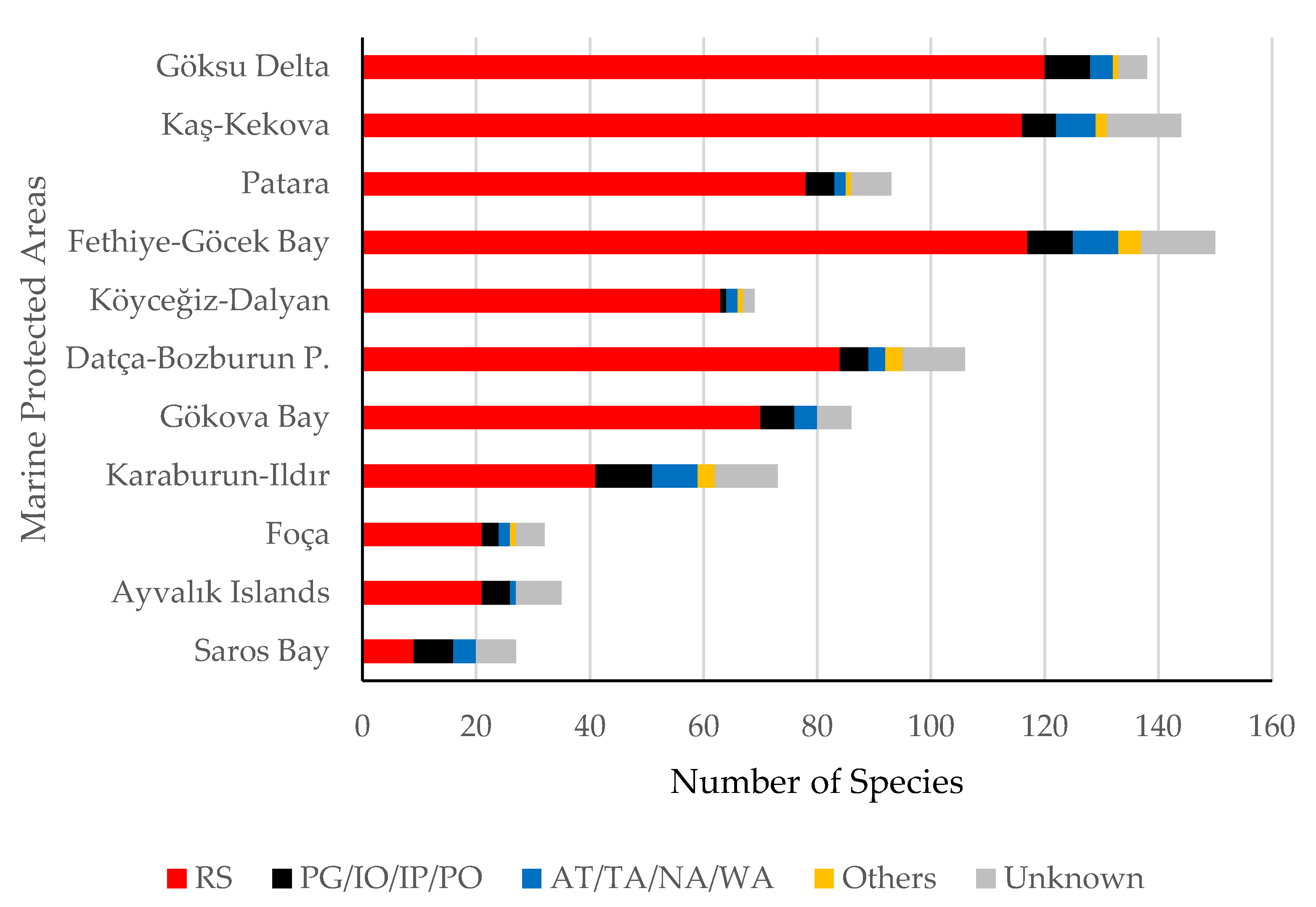
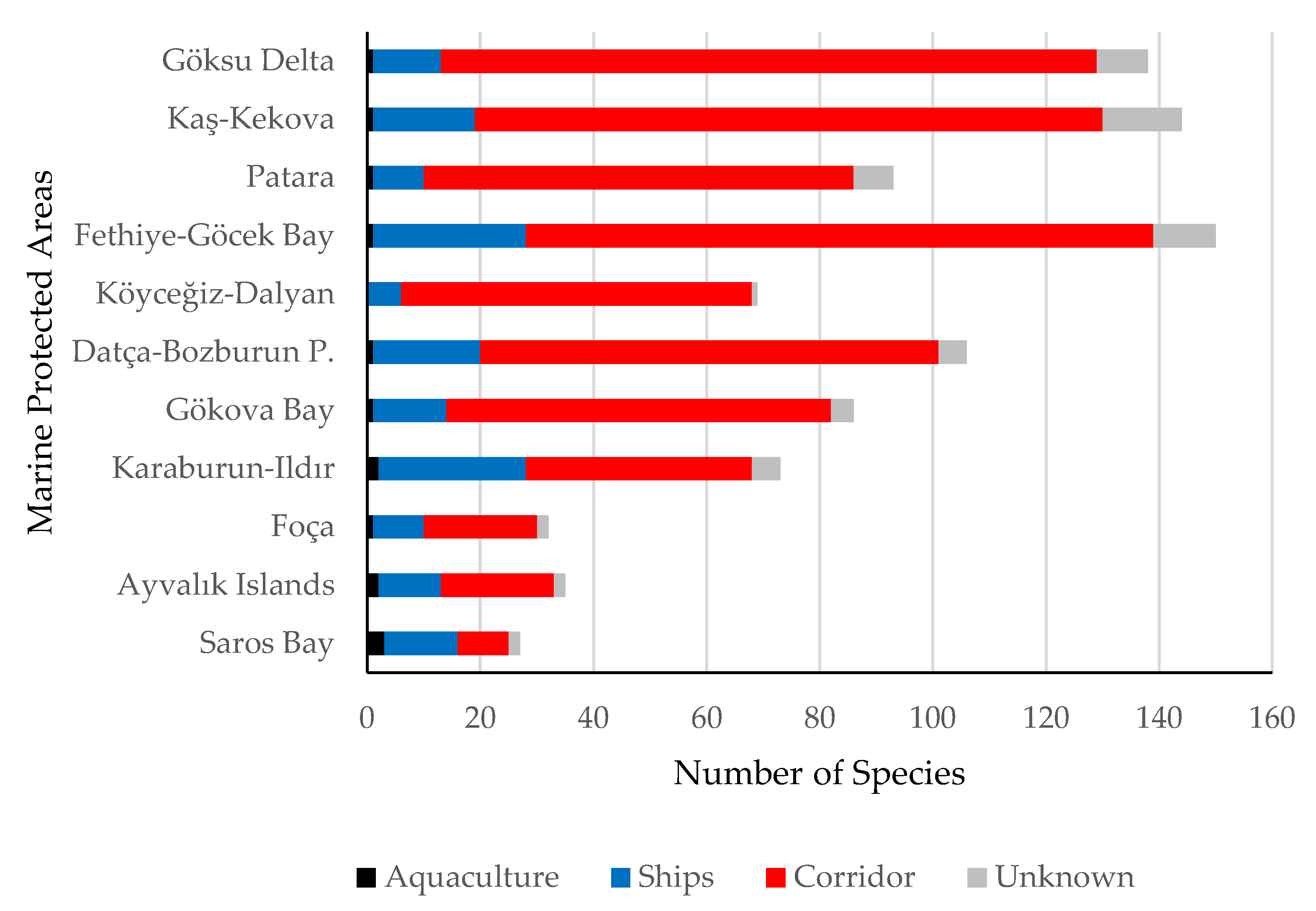
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Species List | Saros Bay | Ayvalık | Foça | Karaburun | Gökova Bay | Datça | Köyceğiz | Fethiye | Patara | Kaş-Kekova | Göksu | ES | O | PW |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ochrophyta | ||||||||||||||
| Botrytella parva (Takamatsu) H.-S.Kim, 1996 | 1 | C | IP | S | ||||||||||
| Cladosiphon zosterae (J.Agardh) Kylin, 1940 | 1 | 1 | 1 | E | AT | S | ||||||||
| Cutleria multifida (Turner) Greville, 1830 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | IP | Aq | ||
| Dictyota cyanoloma Tronholm, De Clerck, Gomez Garreta & Rull Lluch, 2010 | 1 | E | ST | S | ||||||||||
| Halothrix lumbricalis (Kützing) Reinke, 1888 | 1 | 1 | 1 | 1 | 1 | 1 | E | Unk | S | |||||
| Pylaiella littoralis (Linnaeus) Kjellman, 1872 | 1 | 1 | 1 | E | Unk | S | ||||||||
| Sphaerotrichia firma (Gepp) A.D.Zinova, 1940 | 1 | 1 | E | Unk | S | |||||||||
| Stypopodium schimperi (Buchinger ex Kützing) Verlaque & Boudouresque, 1991 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | ?Su | ||
| Chlorophyta | ||||||||||||||
| Caulerpa cylindracea Sonder, 1845 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | |
| Caulerpa racemosa var. lamourouxii f. requienii (Montagne) Weber-van Bosse, 1898 | 1 | 1 | 1 | 1 | E | RS | Su | |||||||
| Caulerpa scalpelliformis (R.Brown ex Turner) C. Agardh, 1817 | 1 | 1 | 1 | E | RS | Su | ||||||||
| Caulerpa taxifolia var. distichophylla (Sonder) Verlaque, Huisman&Procacin, 2013 | 1 | Inv | PO | S | ||||||||||
| Codium fragile subsp. fragile (Suringar) Hariot, 1889 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | Unk | S | |||||
| Codium parvulum (Bory ex Audouin) P.C.Silva, 2003 | 1 | E | RS | Su | ||||||||||
| Codium taylorii P.C. Silva, 1960 | 1 | E | IP | S | ||||||||||
| Pseudocodium okinawense E.J.Faye, M.Uchimura & S.Smimada, 2008 | 1 | C | PO | S | ||||||||||
| Rhodophyta | ||||||||||||||
| Acanthophora nayadiformis (Delile) Papenfuss, 1968 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | |||||
| Asparagopsis armata Harvey, 1855 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | Unk | S | |
| Asparagopsis taxiformis (Delile) Trevisan de Saint-Léon, 1845 | 1 | Inv | RS | Su | ||||||||||
| Bonnemaisonia hamifera Hariot, 1891 | 1 | 1 | 1 | 1 | 1 | Inv | IP | ?S | ||||||
| Botryocladia madagascariensis G. Feldmann, 1945 | 1 | 1 | 1 | 1 | E | Unk | S | |||||||
| Colaconema codicola (Børgesen) H. Stegenga, J.J. Bolton, & R.J. Anderson, 1997 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | Unk | S | ||||
| Ganonema farinosum (Lamouroux) Fan & Wang, 1974 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | |||
| Hypnea spinella (C. Agardh) Kützing, 1847 | 1 | 1 | 1 | 1 | 1 | E | CT | S | ||||||
| Lophocladia lallemandii (Montagne) Schmitz, 1893 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||
| Polysiphonia morrowii Harvey, 1857 | 1 | Inv | PO | S | ||||||||||
| Polysiphonia paniculata Montagne, 1842 | 1 | E | Unk | S | ||||||||||
| Vertebrata fucoides (Hudson) Kuntze 1891 | 1 | 1 | 1 | E | Unk | S | ||||||||
| Tracheophyta | ||||||||||||||
| Halophila stipulacea (Forsskål) Ascherson, 1867 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||
| Foraminifera | ||||||||||||||
| Adelosina longirostra (d’Orbigny, 1826) | 1 | C | Unk | S | ||||||||||
| Amphisorus hemprichii Ehrenberg, 1840 | 1 | 1 | 1 | Inv | Unk | ? | ||||||||
| Amphistegina lobifera Larsen, 1976 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | |||||
| Articulina alticostata Cushman, 1944 | 1 | E | PO | S | ||||||||||
| Astacolus insolitus (Schwager, 1866) | 1 | E | PO | S | ||||||||||
| Bolivina striatula Cushman, 1922 | 1 | E | Unk | ? | ||||||||||
| Clavulina cf. multicamerata Chapman, 1907 | 1 | 1 | E | RS | Su | |||||||||
| Cornuspiroides striolata (Brady) | 1 | E | Unk | S | ||||||||||
| Cyclorbiculina compressa (d’Orbigny, 1839) | 1 | C | Unk | ? | ||||||||||
| Cymbaloporetta plana (Cushman, 1915) | 1 | 1 | 1 | E | RS | Su | ||||||||
| Cymbaloporetta squammosa (d’Orbigny, 1839) | 1 | 1 | 1 | E | Unk | ? | ||||||||
| Entosigmomorphina sp. | 1 | C | PO | S | ||||||||||
| Euthymonacha polita (Chapman, 1904) | 1 | E | Unk | S | ||||||||||
| Haddonia sp. | 1 | 1 | E | RS | Su | |||||||||
| Hauerina diversa Cushman, 1946 | 1 | 1 | E | RS | Su | |||||||||
| Heterostegina depressa d’Orbigny, 1826 | 1 | 1 | E | RS | Su | |||||||||
| Iridia diaphana Heron-Allen and Earland, 1914 | 1 | 1 | E | PO | S | |||||||||
| Miliolinella cf. hybrida (Terquem, 1878) | 1 | C | RS | Su | ||||||||||
| Nodophthalmidium antillarum (Cushman, 1922) | 1 | E | RS | Su | ||||||||||
| Peneroplis arietinus (Batsch, 1791) | 1 | 1 | 1 | E | RS | Su | ||||||||
| Peneroplis pertusus (Forsskål in Niebuhr, 1775) | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||||
| Peneroplis planatus (Fichtel & Moll, 1798) | 1 | 1 | 1 | 1 | 1 | C | RS | Su | ||||||
| Planogypsina acervalis (Brady, 1884) | 1 | E | RS | Su | ||||||||||
| Planogypsina squamiformis (Chapman, 1901) | 1 | 1 | 1 | E | RS | Su | ||||||||
| Pseudomassilina reticulata (Heron-Allen and Earland, 1915) | 1 | C | RS | Su | ||||||||||
| Pseudonodosaria brevis (d’Orbigny, 1846) | 1 | C | PO | S | ||||||||||
| Pulleniatina obliquiloculata (Parker & Jones, 1862) | 1 | C | PO | S | ||||||||||
| Pyrgo denticulata (Brady, 1917) | 1 | E | Unk | ? | ||||||||||
| Quinqueloculina cf. mosharrafai Said, 1949 | 1 | C | RS | Su | ||||||||||
| Schlumbergerina alveoliniformis (Brady, 1879) | 1 | 1 | 1 | E | RS | Su | ||||||||
| Sorites orbiculus Ehrenberg, 1839 | 1 | 1 | 1 | 1 | 1 | E | Unk | ? | ||||||
| Sorites variabilis Lacroix, 1941 | 1 | 1 | 1 | E | RS | Su | ||||||||
| Spiroloculina angulata Cushman, 1917 | 1 | 1 | 1 | E | RS | Su | ||||||||
| Triloculina cf. fichteliana d’Orbigny, 1839 | 1 | 1 | 1 | E | RS | Su | ||||||||
| Vaginulinopsis sublegumen Parr, 1950 | 1 | 1 | E | PO | S | |||||||||
| Hydrozoa | ||||||||||||||
| Clytia linearis (Thorneley, 1900) | 1 | E | RS | Su | ||||||||||
| Filellum serratum (Clarke, 1879) | 1 | E | CT | S | ||||||||||
| Macrorhynchia philippina Kirchenpauer, 1872 | 1 | Inv | RS | Su | ||||||||||
| Sertularia marginata (Kirchenpauer, 1864) | 1 | E | CT | S | ||||||||||
| Scyphozoa | ||||||||||||||
| Cassiopea andromeda (Forsskål, 1775) | 1 | 1 | 1 | 1 | Inv | RS | Su | |||||||
| Phyllorhiza punctata von Lendenfeld, 1884 | 1 | E | RS | Su | ||||||||||
| Rhopilema nomadica Galil, Spanier & Ferguson, 1990 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | |||||
| Ctenophora | ||||||||||||||
| Mnemiopsis leidyi (Agassiz, 1865) | 1 | 1 | 1 | 1 | Inv | NA | S | |||||||
| Sipuncula | ||||||||||||||
| Aspidosiphon (A.) elegans (Chamisso & Eysenhardt, 1821) | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||||
| Annelida | ||||||||||||||
| Aricidea bulbosa Hartley, 1984 | 1 | 1 | E | RS | Su | |||||||||
| Branchiomma bairdi (McIntosh, 1885) | 1 | 1 | Inv | Unk | ?S | |||||||||
| Branchiomma luctuosum Grube, 1869 | 1 | Inv | RS | Su | ||||||||||
| Ceratonereis mirabilis Kinberg, 1866 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||
| Chaetozone corona Berkeley & Berkeley, 1941 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | ?PO | S | ||
| Dorvillea similis (Crossland, 1924) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | |||
| Eurythoe complanata (Pallas, 1766) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | ?RS | ?Su | ||||
| Eusyllis kupfferi Langerhans, 1879 | 1 | 1 | 1 | E | ?AT | S | ||||||||
| Exogone africana (Hartmann-Schröder, 1974) | 1 | E | RS | Su | ||||||||||
| Exogone breviantennata Hartmann-Schröder, 1959 | 1 | 1 | E | RS | Su | |||||||||
| Ficopomatus enigmaticus (Fauvel, 1923) | 1 | Inv | ST | S | ||||||||||
| Glycinde bonhourei Gravier, 1904 | 1 | E | RS | Su | ||||||||||
| Hydroides dirampha Mörch, 1863 | 1 | Inv | CT | S | ||||||||||
| Hydroides elegans (Haswell, 1883) | 1 | 1 | 1 | 1 | 1 | Inv | CT | S | ||||||
| Laonice norgensis Sikorski, 2003 | 1 | C | AT | S | ||||||||||
| Leodice antennata (Savigny, 1820) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | |
| Leonnates indicus Kinberg, 1866 | 1 | 1 | Inv | RS | Su | |||||||||
| Leonnates persicus Wesenberg-Lund, 1949 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | |||
| Linopherus canariensis Langerhans, 1881 | 1 | 1 | 1 | E | AT | S | ||||||||
| Loimia medusa (Savigny, 1818) | 1 | E | RS | ?Su | ||||||||||
| Lumbrineris perkinsi Carrera-Parra, 2001 | 1 | 1 | 1 | 1 | E | RS | ?Su | |||||||
| Lysidice collaris Grube, 1870 | 1 | 1 | 1 | 1 | E | RS | Su | |||||||
| Metasychis gotoi (Izuka, 1902) | 1 | E | RS | Su | ||||||||||
| Notomastus aberans Day, 1957 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||
| Notomastus mossambicus (Thomassin, 1970) | 1 | Inv | RS | Su | ||||||||||
| Palola valida (Gravier, 1900) | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||||
| Phyllodoce longifrons Ben-Eliahu, 1972 | 1 | E | RS | Su | ||||||||||
| Pista unibranchia Day, 1963 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||
| Polycirrus twisti Potts, 1928 | 1 | 1 | 1 | E | RS | Su | ||||||||
| Polydora cornuta Bosc, 1802 | 1 | Inv | WA | S | ||||||||||
| Prionospio (Minuspio) pulchra Imajima 1990 | 1 | 1 | Inv | IP | S | |||||||||
| Prionospio (Prionospio) depauperata Imajima, 1990 | 1 | 1 | Inv | PO | S | |||||||||
| Prionospio (Prionospio) paucipinnulata Blake & Kudenov, 1978 | 1 | E | PO | S | ||||||||||
| Prionospio (Prionospio) saccifera Mackie & Hartley, 1990 | 1 | 1 | 1 | E | RS | Su | ||||||||
| Pseudonereis anomala Gravier, 1900 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | |||
| Pseudopolydora paucibranchiata Okuda, 1937 | 1 | Inv | IP | S | ||||||||||
| Spirorbis marioni Caullery & Mesnil, 1897 | 1 | E | PO | S | ||||||||||
| Streblospio gynobranchiata Rice & Levin, 1998 | 1 | 1 | Inv | WA | S | |||||||||
| Syllis ergeni Çinar, 2005 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Cladocera | ||||||||||||||
| Pleopis schmackeri (Poppe, 1889) | 1 | 1 | 1 | E | IP | Su/S | ||||||||
| Copepoda | ||||||||||||||
| Oithona davisae Ferrari and Orsi, 1984 | 1 | Inv | PO | S | ||||||||||
| Paracartia grani Sars G.O., 1904 | 1 | E | AT | S | ||||||||||
| Stomatopoda | ||||||||||||||
| Clorida albolitura Ahyong & Naiyanetr, 2000 | 1 | E | RS | Su | ||||||||||
| Erugosquilla massavensis (Kossmann, 1880) | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | |||||
| Amphipoda | ||||||||||||||
| Ampithoe bizseli Özaydınlı and Coleman, 2012 | 1 | E | IP | S | ||||||||||
| Latigammaropsis togoensis (Schellenberg, 1925) | 1 | E | Unk | ?S | ||||||||||
| Isopoda | ||||||||||||||
| Paracerceis sculpta Holmes,1904 | 1 | C | IP | S | ||||||||||
| Paradella dianae Menzies,1962 | 1 | E | Unk | ?S | ||||||||||
| Sphaeroma walkeri (Stebbing, 1905) | 1 | 1 | E | RS | Su | |||||||||
| Tanaidacea | ||||||||||||||
| Paradoxapseudes intermedius (Hansen, 1895) | 1 | E | AT | ?S | ||||||||||
| Cumacea | ||||||||||||||
| Eocuma sarsii (Kossmann, 1880) | 1 | E | RS | Su | ||||||||||
| Decapoda | ||||||||||||||
| Alpheus rapacida de Man, 1908 | 1 | 1 | E | RS | Su | |||||||||
| Atergatis roseus (Rüppell, 1830) | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | |||||
| Callinectes sapidus Rathbun, 1896 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | WA | S | |||
| Carupa tenuipes Dana, 1851 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | |||||
| Charybdis hellerii (Milne Edwards, 1867) | 1 | 1 | 1 | 1 | Inv | RS | Su | |||||||
| Charybdis longicollis Leene, 1938 | 1 | 1 | Inv | RS | Su | |||||||||
| Coleusia signata (Paulson, 1875) | 1 | 1 | 1 | 1 | E | RS | Su | |||||||
| Eucrate crenata de Haan, 1835 | 1 | E | RS | Su | ||||||||||
| Gonioinfradens giardi (Nobili, 1905) | 1 | C | IP | S | ||||||||||
| Ixa monodi Holthuis & Gottlieb, 1956 | 1 | 1 | E | RS | Su | |||||||||
| Leptochela pugnax de Man, 1916 | 1 | 1 | E | RS | Su | |||||||||
| Macrophthalmus indicus Davie, 2012 | 1 | E | RS | Su | ||||||||||
| Matuta victor (Fabricius, 1781) | 1 | E | RS | Su | ||||||||||
| Metapenaeopsis aegyptia Galil & Golani, 1990 | 1 | 1 | E | RS | Su | |||||||||
| Metapenaeopsis mogiensis consobrina (Nobili, 1904) | 1 | E | RS | Su | ||||||||||
| Metapenaeus affinis (H. Milne Edwards, 1837) | 1 | E | RS | Su | ||||||||||
| Metapenaeus monoceros (Fabricius, 1798) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Metapenaeus stebbingi (Nobili, 1904) | 1 | Inv | RS | Su | ||||||||||
| Micippa thalia (Herbst, 1803) | 1 | 1 | 1 | E | RS | Su | ||||||||
| Myra subgranulata Kossmann, 1877 | 1 | 1 | 1 | 1 | E | RS | Su | |||||||
| Palaemonella rotumana (Borradaile, 1898) | 1 | E | RS | Su | ||||||||||
| Penaeus aztecus Ives, 1891 | 1 | 1 | E | WA | S | |||||||||
| Penaeus hathor (Burkenroad, 1959) | 1 | 1 | 1 | Inv | RS | Su | ||||||||
| Penaeus pulchricaudatus Stebbing, 1914 (=P. japonicus) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | |||
| Penaeus semisulcatus de Haan, 1844 | 1 | 1 | 1 | 1 | Inv | RS | Su | |||||||
| Percnon gibbesi (H. Milne Edwards, 1853) | 1 | 1 | 1 | 1 | 1 | Inv | TA | S | ||||||
| Pilumnus minutus De Haan,1835 | 1 | 1 | E | RS | Su | |||||||||
| Portunus segnis (Forskål, 1775) | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||||
| Processa macrodactyla Holthuis, 1952 | 1 | 1 | E | TA | S | |||||||||
| Thalamita poissonii (Audouin, 1826) | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||||
| Trachysalambria palaestinensis Steinitz, 1932 | 1 | 1 | 1 | 1 | E | RS | Su | |||||||
| Urocaridella pulchella Yokes & Galil, 2006 | 1 | 1 | E | RS | Su | |||||||||
| Gastropoda | ||||||||||||||
| Diodora ruppellii (Sowerby I, G.B., 1835) | 1 | E | RS | Su | ||||||||||
| Pseudominolia nedyma (Melville, 1897) | 1 | E | RS | Su | ||||||||||
| Smaragdia souverbiana (Montrouzier in Souverbie & Montrouzier, 1863) | 1 | 1 | E | RS | Su | |||||||||
| Cerithidium perparvulum (Watson, R.B., 1886) | 1 | E | PO | S | ||||||||||
| Cerithium scabridum Philippi, 1848 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||||
| Rhinoclavis kochi (Philippi, 1848) | 1 | E | RS | Su | ||||||||||
| Varicopeza pauxilla (A. Adams, 1855) | 1 | E | RS | Su | ||||||||||
| Finella pupoides Adams, A., 1860 | 1 | 1 | 1 | 1 | Inv | RS | Su | |||||||
| Metaxia bacillum (Issel, 1869) | 1 | E | RS | Su | ||||||||||
| Viriola bayani Jousseaume, 1884 | 1 | E | RS | Su | ||||||||||
| Cerithiopsis pulvis (Issel, 1869) | 1 | 1 | 1 | E | RS | Su | ||||||||
| Cerithiopsis tenthrenois (Melvill, 1896) | 1 | E | RS | Su | ||||||||||
| Sticteulima lentiginosa (Adams, A., 1861) | 1 | 1 | E | RS | Su | |||||||||
| Rissoina ambigua (Gould, 1849) | 1 | C | RS | Su | ||||||||||
| Rissoina bertholleti Issel, 1869 | 1 | 1 | E | RS | Su | |||||||||
| Conomurex persicus (Swainson, 1821) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | PG | S | ||||
| Purpuradusta gracilis notata (Gill, 1858) | 1 | E | RS | Su | ||||||||||
| Ergalatax junionae Houart, 2008 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | S | |||||
| Zafra savignyi (Moazzo, 1939) | 1 | 1 | E | RS | Su | |||||||||
| Zafra selasphora (Melvill & Standen, 1901) | 1 | 1 | E | RS | Su | |||||||||
| Pyrgulina fischeri Hornung & Mermod, 1925 | 1 | E | RS | Su | ||||||||||
| Pyrgulina pupaeformis (Souverbie, 1865) | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||||
| Pyrgulina nana Hornung & Mermod, 1924 | 1 | C | RS | ?S | ||||||||||
| Pyrgulina pirinthella Melvill, 1910 | 1 | E | RS | Su | ||||||||||
| Cingulina isseli (Tryon, 1886) | 1 | E | RS | Su | ||||||||||
| Monotygma fulva (Adams, A., 1853) | 1 | E | RS | Su | ||||||||||
| Monotygma lauta (Adams, A., 1853) | 1 | E | RS | Su | ||||||||||
| Odostomia lorioli (Hornung & Mermod, 1924) | 1 | E | RS | Su | ||||||||||
| Oscilla galilae Bogi, Karhan & Yokeş, 2012 | 1 | C | IP | ?S | ||||||||||
| Syrnola fasciata Jickeli, 1882 | 1 | 1 | 1 | Inv | RS | Su | ||||||||
| Syrnola lendix (Adams, A., 1853) | 1 | E | IO | Su | ||||||||||
| Turbonilla edgarii (Melvill, 1896) | 1 | E | RS | Su | ||||||||||
| Leucotina natalensis Smith, E.A., 1910 | 1 | E | RS | Su | ||||||||||
| Bulla arabica Malaquias & Reid, 2008 | 1 | 1 | E | RS | ?Su | |||||||||
| Pyrunculus fourierii (Audouin, 1826) | 1 | 1 | 1 | Inv | RS | Su | ||||||||
| Retusa desgenettii (Audouin, 1826) | 1 | E | RS | Su | ||||||||||
| Lamprohaminoea cyanomarginata (Heller & Thompson, T.E., 1983) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||
| Biuve fulvipunctata (Baba, 1938) | 1 | 1 | E | RS | Su | |||||||||
| Acteocina mucronata (Philippi, 1849) | 1 | E | RS | Su | ||||||||||
| Mnestia girardi (Audouin, 1826) | 1 | E | RS | Su | ||||||||||
| Oxynoe viridis (Pease, 1861) | 1 | 1 | 1 | E | IP | S | ||||||||
| Elysia tomentosa Jensen, 1997 | 1 | E | ?IP | S | ||||||||||
| Bursatella leachii Blainville, 1817 | 1 | 1 | E | RS | ?Su | |||||||||
| Syphonota geographica (Adams, A. & Reeve, 1850) | 1 | 1 | E | RS | Su | |||||||||
| Goniobranchus annulatus (Eliot, 1904) | 1 | E | RS | Su | ||||||||||
| Hypselodoris infucata Rueppel & Leuckart, 1828 | 1 | 1 | 1 | E | RS | Su | ||||||||
| Plocamopherus ocellatus Rüppell & Leuckart, 1828 | 1 | E | RS | Su | ||||||||||
| Baeolidia moebii Bergh, 1888 | 1 | 1 | C | RS | Su | |||||||||
| Coryphellina rubrolineata O’Donoghue, 1929 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||||
| Siphonaria crenata Blainville 1827 | 1 | E | RS | Su | ||||||||||
| Bivalvia | ||||||||||||||
| Brachidontes pharaonis (Fischer, P., 1870) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | |||
| Clementia papyracea (Gmelin, 1791) | 1 | E | RS | Su | ||||||||||
| Dendostrea folium (Linnaeus, 1758) | 1 | 1 | 1 | E | IP | ?S | ||||||||
| Ervilia scaliola Issel, 1869 | 1 | C | RS | Su | ||||||||||
| Fulvia fragilis (Forsskål in Niebuhr, 1775) | 1 | 1 | Inv | RS | Su | |||||||||
| Isognomon legumen (Gmelin, 1791) | 1 | E | RS | Su | ||||||||||
| Magallana gigas (Thunberg, 1793) | 1 | E | PO | Aq | ||||||||||
| Malleus regula (Forsskål in Niebuhr, 1775) | 1 | 1 | 1 | E | RS | Su | ||||||||
| Pinctada imbricata radiata (Leach, 1814) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Ruditapes philippinarum (Adams & Reeve, 1850) | 1 | Inv | PO | Aq | ||||||||||
| Saccostrea cuccullata (Born, 1778) | 1 | E | IP | S | ||||||||||
| Septifer cumingii Récluz, 1849 | 1 | 1 | E | RS | S | |||||||||
| Teredothyra dominicensis (Bartsch, 1921) | 1 | E | WA | S | ||||||||||
| Cephalopoda | ||||||||||||||
| Sepioteuthis lessoniana d’Orbignyi, 1826 | 1 | 1 | Inv | RS | Su | |||||||||
| Bryozoa | ||||||||||||||
| Amathia verticillata (delle Chiaje, 1822) | 1 | 1 | Inv | AT | S | |||||||||
| Celleporaria brunnea (Hincks, 1884) | 1 | 1 | Inv | AT | S | |||||||||
| Echinodermata | ||||||||||||||
| Ophiactis savignyi (Müller & Troschel, 1842) | 1 | E | RS | Su | ||||||||||
| Diadema setosum (Leske, 1778) | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||||
| Synaptula reciprocans (Forrskål, 1775) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Tunicata | ||||||||||||||
| Ascidiella aspersa (Müller, 1776) | 1 | E | NA | S | ||||||||||
| Clavelina oblonga Herdman, 1880 | 1 | E | WA | S | ||||||||||
| Diplosoma listerianum (Milne Edwards, 1841) | 1 | 1 | E | ?AT | S | |||||||||
| Microcosmus exasperatus Heller, 1878 | 1 | 1 | E | RS | Su | |||||||||
| Phallusia nigra Savignyi, 1816 | 1 | 1 | 1 | 1 | Inv | WA | ?S | |||||||
| Pyura (=Herdmania) momus (Savigny, 1816) | 1 | 1 | 1 | 1 | E | RS | Su | |||||||
| Rhodosoma turcicum (Savigny, 1816) | 1 | E | CT | S | ||||||||||
| Styela plicata (Lesueur, 1823) | 1 | 1 | 1 | Inv | ?AT | S | ||||||||
| Symplegma brakenhielmi (Michaelsen, 1904) | 1 | Inv | RS | Su | ||||||||||
| Actinopterygii | ||||||||||||||
| Acanthopagrus bifasciatus (Forsskål, 1775) | 1 | C | RS | Su | ||||||||||
| Alepes djedaba (Forsskål, 1775) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Apogonichthyoides pharaonis (Bellotti, 1874) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||
| Atherinomorus forskalii (Rüppell, 1838) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Bregmaceros nectabanus Whitley, 1941 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||
| Callionymus filamentosus Valenciennes, 1837 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||||
| Champsodon nudivittis (Ogilby, 1895) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||
| Cheilodipterus novemstriatus (Rüppell, 1838) | 1 | 1 | E | RS | Su | |||||||||
| Cynoglossus sinusarabici (Chabanaud, 1913) | 1 | 1 | E | RS | Su | |||||||||
| Diplogrammus randalli Fricke, 1983 | 1 | C | RS | Su | ||||||||||
| Dussumieria elopsoides Bleeker, 1849 | 1 | Inv | RS | Su | ||||||||||
| Equulites klunzingeri (Steindachner, 1898) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Equulites popei (Whitley, 1932) | 1 | Inv | RS | Su | ||||||||||
| Etrumeus golanii DiBatistta, Randall and Bowen, 2012 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | |||
| Fistularia commersonii (Rüppell, 1835) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Fistularia petimba Lacepède, 1803 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Hazeus ingressus Engin, Larson, Irmak, 2018 | 1 | C | RS | Su | ||||||||||
| Hemiramphus far (Forsskål, 1775) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||
| Herklotsichthys punctatus (Rüppell, 1837) | 1 | E | RS | Su | ||||||||||
| Jaydia queketti (Gilchrist, 1903) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||
| Jaydia smithi Kotthaus, 1970 | 1 | E | RS | Su | ||||||||||
| Lagocephalus guentheri (Richardson, 1844) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Lagocephalus sceleratus (Gmelin, 1789) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su |
| Lagocephalus suezensis Clark & Gohar, 1953 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Liza carinata (Valenciennes, 1836) | 1 | E | RS | Su | ||||||||||
| Nemipterus randalli Russell, 1986 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Ostorhinchus fasciatus (White, 1790) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||
| Oxyurichthys petersi (Klunzinger, 1871) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||
| Paranthias furcifer (Valenciennes, 1828) | 1 | C | AT | ? | ||||||||||
| Parexocoetus mento (Valenciennes, 1846) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||
| Parupeneus forskalli (Fourmanoir & Guézé, 1976) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Pelates quadrilineatus (Bloch, 1790) | 1 | E | RS | Su | ||||||||||
| Pempheris rhomboidea Kossmann & Räuber, 1877 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||
| Petroscirtes ancylodon Rüppell, 1838 | 1 | E | RS | Su | ||||||||||
| Planiliza haematocheilus (Temminck & Schlegel, 1845) | 1 | 1 | 1 | Inv | PO | Aq | ||||||||
| Pomadasys stridens (Forsskål, 1775) | 1 | E | RS | Su | ||||||||||
| Priacanthus sagittarius Starnes, 1988 | 1 | C | RS | Su | ||||||||||
| Pteragogus trispilus Randall, 2013 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||
| Pterois miles (Bennett, 1828) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | |||
| Sargocentron rubrum (Forsskål, 1775) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||
| Saurida lessepsianus (Russell, Golani and Tikochinski, 2015) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Scarus ghobban Forsskål, 1775 | 1 | C | RS | Su | ||||||||||
| Scomberomorus commerson Lacepède, 1800 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||
| Siganus luridus (Rüppell, 1829) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | |
| Siganus rivulatus Forsskål, 1775 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su |
| Sillago suezensis Golani, Fricke and Tikochinski, 2014 | 1 | 1 | E | RS | Su | |||||||||
| Sphyraena chrysotaenia Klunzinger, 1884 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||
| Sphyraena flavicauda Rüppell, 1838 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||||
| Stephanolepis diaspros Fraser-Brunner, 1940 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su | ||||
| Synchiropus sechellensis Regan, 1908 | 1 | E | RS | Su | ||||||||||
| Torquigener flavimaculosus Hardy & Randall, 1983 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Tylerius spinosissimus (Regan, 1908) | 1 | C | RS | Su | ||||||||||
| Upeneus moluccensis (Bleeker, 1855) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||
| Upeneus pori Ben-Tuvia & Golani, 1989 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Inv | RS | Su | ||||
| Vanderhorstia mertensi Klausewitz, 1974 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | E | RS | Su |
References
- Meola, B.; Webster, C. The 2016 Status of Marine Protected Areas in the Mediterranean; MedPAN & RAC/SPA: Tunis, Tunisia, 2019; p. 222. [Google Scholar]
- Gabrié, C.; Lagabrielle, E.; Bissery, C.; Crochelet, E.; Meola, B.; Webster, C.; Claudet, J.; Chassanite, A.; Marinesque, S.; Robert, P. The Status of Marine Protected Areas in the Mediterranean Sea 2012; MedPAN & RAC/SPA: Marseille, France, 2012; p. 257. [Google Scholar]
- Gownaris, N.J.; Santora, C.M.; Davis, J.B.; Pikitch, E.K. Gaps in protection of important ocean areas: A spatial meta-analysis of ten global mapping initiatives. Front. Mar. Sci. 2019, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kelleher, G. Guidelines for Marine Protected Areas; IUCN: Gland, Switzerland; Cambridge, UK, 1999; p. 107. [Google Scholar]
- Rodríguez-Rodríguez, D.; Merkohasanaj, M.; Lopez, I. Social and economic sustainability of multiple-use marine protected areas in Spain: A mixed methods, multi-scale study. Ocean Coast. Manag. 2019, 171, 47–55. [Google Scholar] [CrossRef]
- Sala, E.; Giakoumi, S. No-take marine reserves are the most effective protected areas in the ocean. Ices J. Mar. Sci. 2018, 75, 1166–1168. [Google Scholar] [CrossRef] [Green Version]
- MedPAN; UNEP/MAP-RAC/SPA. The 2016 Status of Marine Protected Areas in the Mediterranean Main Findings; MedPAN & RAC/SPA: Marseille, France, 2016; p. 16. [Google Scholar]
- Gomei, M.; Abdulla, A.; Schröder, C.; Yadav, S.; Sánchez, A.; Rodríguez, D.; Abdul Malak, D. Towards 2020: How Mediterranean Countries Are Performing to Protect Their Sea; WWF: Malaga, Spain, 2019; p. 38. [Google Scholar]
- Bruno, J.F.; Bates, A.E.; Cacciapaglia, C.; Pike, E.P.; Amstrup, S.C.; van Hooidonk, R.; Henson, S.A.; Aronson, R.B. Climate change threatens the world's marine protected areas. Nat. Clim. Chang. 2018, 8, 499–503. [Google Scholar] [CrossRef]
- Galil, B. Eyes wide shut: Managing bio-invasions in Mediterranean marine protected areas. In Management of Marine Protected Areas: A Network Perspective; Goriup, P.D., Ed.; John Wiley & Sons Ltd: Oxford, UK, 2017; pp. 187–206. [Google Scholar]
- Katsanevakis, S.; Coll, M.; Piroddi, C.; Steenbeek, J.; Lasram, F.B.; Zenetos, A.; Cardoso, A.C. Invading the Mediterranean Sea: Biodiversity patterns shaped by human activities. Front. Mar. Sci. 2014, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Çinar, M.E.; Bilecenoglu, M.; Yokes, M.B.; Ozturk, B.; Taskin, E.; Bakir, K.; Dogan, A.; Acik, S. Current status (as of end of 2020) of marine alien species in Turkey. PLoS ONE 2021, 16, e0251086. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Lasram, F.B.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [Green Version]
- Mannino, A.M.; Parasporo, M.; Crocetta, F.; Balistreri, P. An updated overview of the marine alien and cryptogenic species from the Egadi Islands Marine Protected Area (Italy). Mar. Biodivers. 2017, 47, 469–480. [Google Scholar] [CrossRef]
- Monaco, A.; Genovesi, P. European Guidelines on Protected Areas and Invasive Alien Species; Council of Europe and Regional Parks Agency: Rome, Italy, 2014; p. 60. [Google Scholar]
- Burfeind, D.D.; Pitt, K.A.; Connolly, R.M.; Byers, J.E. Performance of non-native species within marine reserves. Biol. Invasions 2013, 15, 17–28. [Google Scholar] [CrossRef]
- D'Amen, M.; Azzurro, E. Lessepsian fish invasion in Mediterranean marine protected areas: A risk assessment under climate change scenarios. Ices J. Mar. Sci. 2020, 77, 388–397. [Google Scholar] [CrossRef]
- Mazaris, A.D.; Katsanevakis, S. The threat of biological invasions is under-represented in the marine protected areas of the European Natura 2000 network. Biol. Conserv. 2018, 225, 208–212. [Google Scholar] [CrossRef]
- Belle, E.; Kingston, N.; Burgess, N.; Sandwith, T.; Ali, N.; MacKinnon, K. Protected Planet Report 2018, Tracking Progress towards Global Targets for Protected Areas; UNEP-WCMC, IUCN & NGS: Cambridge, UK; Gland, Switzerland; Washington, DC, USA, 2018; p. 70. [Google Scholar]
- Okus, E.; Sur, H.; Yuksek, A.; Yilmaz, I.; Aslan-Yilmaz, A.; Karhan, S.; Oz, M.; Demirel, N.; Tas, S.; Altiok, A. Datça-Bozburun özel Çevre Koruma Bölgesinin Denizsel ve Kıyısal Alanlarının Biyolojik Çeşitliliğinin Tespiti Final Raporu; T.C. Çevre ve Orman Bakanlığı Özel Çevre Koruma Kurumu Başkanlığı: Ankara, Turkey, 2004; p. 291. (In Turkish) [Google Scholar]
- Okus, E.; Yüksek, A.; Yokes, M.B.; Yilmaz, I.N.; Aslan-Yilmaz, A.; Karhan, S.U.; Demirel, N.; Demir, V.; Zeki, S.; Tas, S.; et al. Gökova Özel Çevre Koruma Bölgesinin kıyı ve deniz Alanlarının Biyolojik Çeşitliliginin Tespiti Projesi Final Raporu; T.C. Çevre ve Orman Bakanlığı Özel Çevre Koruma Kurulu Başkanlığı: Ankara, Turkey, 2006; p. 352. [Google Scholar]
- Çinar, M.E.; Kocatas, A.; Katagan, T.; Yilmaz, A.; Onen, M.; Tarkan, A.N.; Yalciner, A.C.; Oztürk, B.; Bilecenoglu, M.; Düzgün, Ş.; et al. Fethiye Göcek özel Çevre Koruma Bölgesi Kiyi ve Deniz Alanlarinin Biyolojik Çeşitlilik Tespiti Projesi Final Raporu; T.C. Çevre ve Orman Bakanligi Özel Çevre Koruma Kurumu Başkanliği: Ankara, Turkey, 2010; p. 299. [Google Scholar]
- UNEP-MAP. Action Plan Concerning Species Introductions and Invasive Species in the Mediterranean Sea; UN Environment/MAP: Athens, Greece, 2017; p. 14. [Google Scholar]
- MoAF. National Biodiversity Action Plan 2018-2028; Republic of Turkey, Ministry of Agriculture and Forestry (MoAF), General Directorate of Nature Conservation and National Parks: Ankara, Turkey, 2019; p. 118.
- Marchini, A.; Galil, B.S.; Occhipinti-Ambrogi, A. Recommendations on standardizing lists of marine alien species: Lessons from the Mediterranean Sea. Mar. Pollut. Bull. 2015, 101, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Latombe, G.; Pysek, P.; Jeschke, J.M.; Blackburn, T.M.; Bacher, S.; Capinha, C.; Costello, M.J.; Fernandez, M.; Gregory, R.D.; Hobern, D.; et al. A vision for global monitoring of biological invasions. Biol. Conserv. 2017, 213, 295–308. [Google Scholar] [CrossRef]
- Vanderhoeven, S.; Adriaens, T.; Desmet, P.; Strubbe, D.; Backeljau, T.; Barbier, Y.; Brosens, D.; Cigar, J.; Coupremanne, M.; De Troch, R.; et al. Tracking Invasive Alien Species (TrIAS): Building a data-driven framework to inform policy. Res. Ideas Outcomes 2017, 3, e13414. [Google Scholar] [CrossRef] [Green Version]
- Zenetos, A.; Galanidi, M. Mediterranean non indigenous species at the start of the 2020s: Recent changes. Mar. Biodivers. Rec. 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bilecenoglu, M. Preface-special issue on: Marine animal diversity of Turkey. Turk. J. Zool 2014, 38, i–ii. [Google Scholar]
- Bizsel, K.C.; Kozludere, S.; Besiktepe, S.; Bizsel, N.; Sayin, E.; Yüksek, A.; Kaboglu, G.; Akçali, B.; Yilmaz, E.C.; Kavcioglu, R.; et al. Köyceğiz-Dalyan özel Çevre Koruma Bölgesi Deniz ve kıyı Alanlarında Biyolojik Çeşitliliğin Tespiti Projesi Sonuç Raporu; T.C. Çevre ve Orman Bakanlığı Özel Çevre Koruma Kurumu Başkanlığı: Ankara, Turkey, 2010; p. 206. [Google Scholar]
- SualtiArastirmalariDernegi. Foça özel Çevre Koruma Bölgesi kıyı Alanları Taşıma Kapasitesinin Belirlenmesi Projesi; T.C. Çevre ve Orman Bakanlığı Özel Çevre Koruma Kurumu Başkanlığı: Ankara, Turkey, 2008; p. 528. [Google Scholar]
- Tural, U. MedPAN South Turkey Pilot Project, Developing a Management Plan for Kaş-Kekova Specially Protected Area (Spa); WWF MedPO: İstanbul, Turkey, 2012; p. 20. [Google Scholar]
- Yigit, N.; Albay, M.; Altinagaç, U.; Okudan Aslan, E.S.; Cevik, C.; Muftuoglu, E.; Balkis, N.; Topçu, N.E.; Kuyucuoglu, B.; Dalyan, C.; et al. Saros Körfezi Özel Çevre Koruma Bölgesi Karasal ve Denizel Biyolojik Çeşitliliğin Tespiti Projesi; T.C. Çevre ve Şehircilik Bakanlığı Tabiat Varlıklarını Koruma Genel Müdürlüğü: Ankara, Turkey, 2014; p. 427. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Natural Environment Research Council: Swindon, UK, 1994.
- Çinar, M.E.; Bilecenoglu, M.; Ozturk, B.; Katagan, T.; Yokes, M.B.; Aysel, V.; Dagli, E.; Acik, S.; Ozcan, T.; Erdogan, H. An updated review of alien species on the coasts of Turkey. Mediterr. Mar. Sci. 2011, 12, 257–315. [Google Scholar] [CrossRef]
- Olden, J.D.; Comte, L.; Giam, X. Biotic homogenisation. In eLS; John Wiley & Sons, Ltd: Chichester, UK, 2016; pp. 1–8. [Google Scholar]
- Galil, B.S.; Mienis, H.K.; Hoffman, R.; Goren, M. Non-indigenous species along the Israeli Mediterranean coast: Tally, policy, outlook. Hydrobiologia 2021, 848, 2011–2029. [Google Scholar] [CrossRef]
- Servello, G.; Andaloro, F.; Azzurro, E.; Castriota, L.; Catra, M.; Chiarore, A.; Crocetta, F.; D’alessandro, M.; Denitto, F.; Froglia, C.; et al. Marine alien species in Italy: A contribution to the implementation of descriptor D2 of the marine strategy framework directive. Mediterr. Mar. Sci. 2019, 20, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Zenetos, A.; Corsini-Foka, M.; Crocetta, F.; Gerovasileiou, V.; Karachle, P.K.; Simboura, N.; Tsiamis, K.; Pancucci-Papadopoulou, M.-A. Deep cleaning of alien and cryptogenic species records in the Greek Seas (2018 update). Manag. Biol. Invasions 2018, 9, 209–226. [Google Scholar] [CrossRef] [Green Version]
- Ounifi-Ben Amor, K.; Rifi, Μ.; Ghanem, R.; Draeif, I.; Zaouali, J.; Ben Souissi, J. Update of alien fauna and new records from Tunisian marine waters. Mediterr. Mar. Sci. 2016, 17, 124–143. [Google Scholar] [CrossRef]
- Shakman, E.; Eteayb, K.; Taboni, I.; Ben Abdalla, A. Status of marine alien species along the Libyan coast. J. Black Sea/Mediterr. Environ. 2019, 25, 188–209. [Google Scholar]
- Otero, M.; Cebrian, E.; Francour, P.; Galil, B.; Savini, D. Monitoring Marine Invasive Species in Mediterranean Marine Protected Areas (Mpas), A Strategy and Practical Guide for Managers; IUCN: Malaga, Spain, 2013; p. 136. [Google Scholar]
- Giakoumi, S.; Pey, A. Assessing the Effects of Marine Protected Areas on Biological Invasions: A Global Review. Front. Mar. Sci. 2017, 4, 1–6. [Google Scholar] [CrossRef]
- Reise, K.; Olenin, S.; Thieltges, D.W. Are aliens threatening European aquatic coastal ecosystems? Helgol. Mar. Res. 2006, 60, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Huseyinoglu, M.F.; Demir, V.; Arda, Y.; Draman, M.; Yokes, M.B. Spatio-temporal distribution of lionfish, Pterois miles (Bennett, 1828) in Kas-Kekova Special Environmental Protected Area, Turkey. Estuar. Coast. Shelf Sci. 2021, 254, 107331. [Google Scholar] [CrossRef]
- Giakoumi, S.; Guilhaumon, F.; Kark, S.; Terlizzi, A.; Claudet, J.; Felline, S.; Cerrano, C.; Coll, M.; Danovaro, R.; Fraschetti, S.; et al. Space invaders; biological invasions in marine conservation planning. Divers. Distrib. 2016, 22, 1220–1231. [Google Scholar] [CrossRef] [Green Version]
- Giakoumi, S.; Katsanevakis, S.; Albano, P.G.; Azzurro, E.; Cardoso, A.C.; Cebrian, E.; Deiduni, A.; Edelist, D.; Francour, P.; Jimenez, C.; et al. Management priorities for marine invasive species. Sci. Total Environ. 2019, 688, 976–982. [Google Scholar] [CrossRef]
- Guidetti, P.; Baiata, P.; Ballesteros, E.; Di Franco, A.; Hereu, B.; Macpherson, E.; Micheli, F.; Pais, A.; Panzalis, P.; Rosenberg, A.A. Large-scale assessment of Mediterranean marine protected areas effects on fish assemblages. PLoS ONE 2014, 9, e91841. [Google Scholar] [CrossRef] [Green Version]
- Mannino, A.M.; Balistreri, P. Invasive alien species in Mediterranean Marine Protected Areas: The Egadi Islands (Italy) case study. Biodiversity 2021, 22, 13–23. [Google Scholar] [CrossRef]
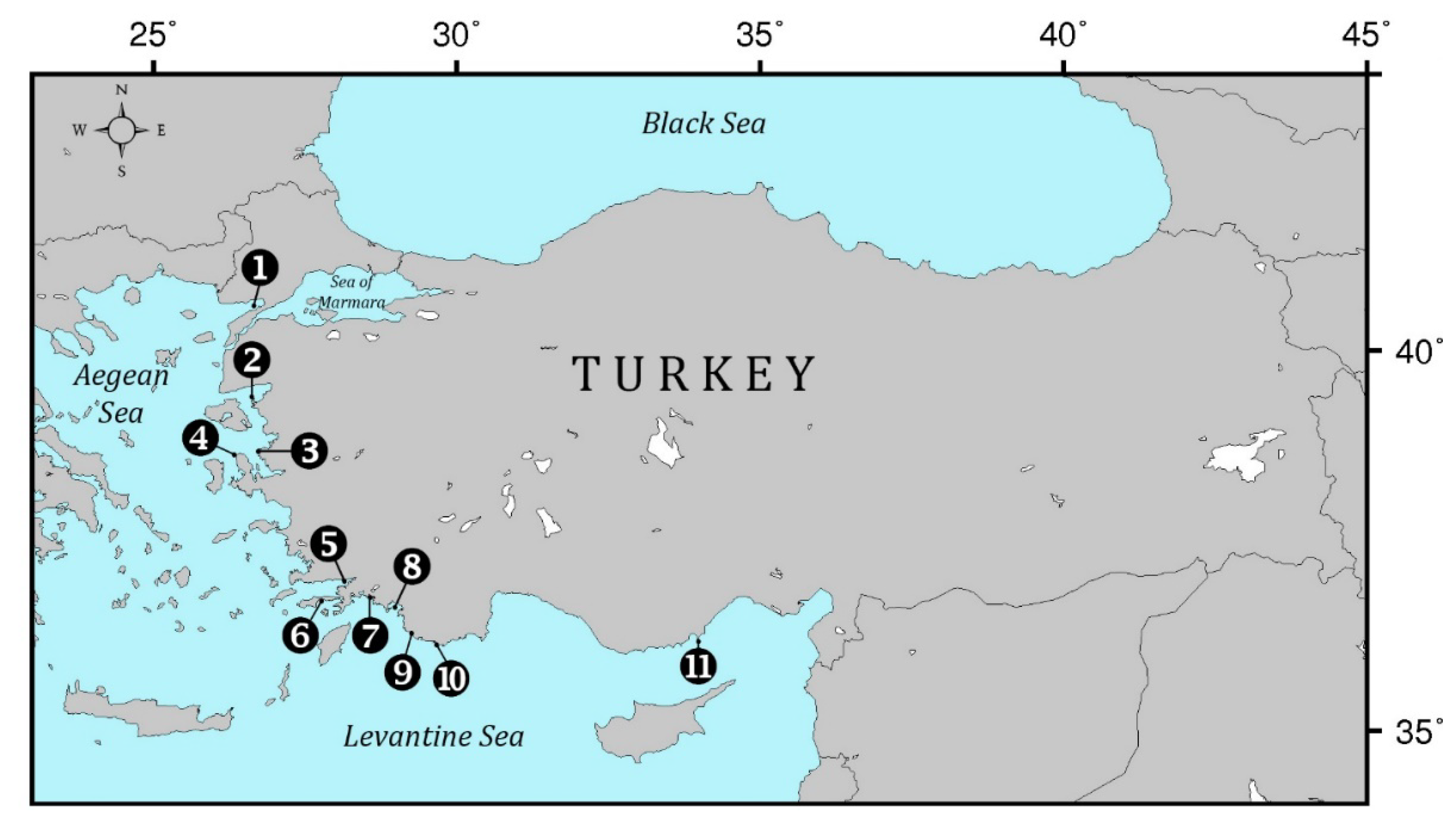
| MPA Name | Protection Status | Year Founded | Surface Area (km2) | Marine Coverage (km2) | Coastal Length (km) |
|---|---|---|---|---|---|
| Aegean Sea | |||||
| 1. Saros Bay | SEPA | 2010 | 730 | 538 | 62 |
| 2. Ayvalik Islands | NP | 1995 | 180 | 142 | 110 |
| 3. Foça | SEPA | 1990 | 71 | 52 | 28 |
| 4. Karaburun-Ildır Bay | SEPA | 2019 | 947 | 502 | 127 |
| 5. Gökova Bay | SEPA | 1988 | 1093 | 820 | 193 |
| 6. Datça-Bozburun Peninsula | SEPA | 1990 | 1444 | 737 | 417 |
| 7. Köyceğiz-Dalyan | SEPA | 1988 | 461 | 41 | 26 |
| Levantine Sea | |||||
| 8. Fethiye-Göcek Bay | SEPA | 1988 | 805 | 339 | 196 |
| 9. Patara | SEPA | 1990 | 197 | 45 | 23 |
| 10. Kaş-Kekova | SEPA | 1990 | 258 | 158 | 81 |
| 11. Göksu Delta | SEPA | 1990 | 229 | 98 | 35 |
| Phyla | Saros | Ayvalık | Foça | Karaburun | Gökova | Datça | Köyceğiz | Fethiye | Patara | Kaş | Göksu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ochrophyta | 11.1 | 20.0 | 3.1 | 8.2 | 2.3 | 2.8 | 2.0 | 3.2 | 2.1 | 2.2 | |
| Chlorophyta | 7.4 | 8.6 | 6.3 | 4.1 | 2.3 | 1.9 | 1.4 | 2.0 | 4.3 | 2.1 | 1.4 |
| Rhodophyta | 14.8 | 11.4 | 6.3 | 12.3 | 5.8 | 4.7 | 5.3 | 8.6 | 5.6 | 5.1 | |
| Tracheophyta | 2.9 | 3.1 | 1.4 | 1.2 | 0.9 | 1.4 | 0.7 | 1.1 | 0.7 | ||
| Foraminifera | 18.5 | 17.1 | 19.2 | 1.2 | 12.3 | 1.3 | 14.0 | 15.3 | |||
| Cnidaria | 2.3 | 4.7 | 4.3 | 2.0 | 0.7 | 0.7 | |||||
| Ctenophora | 3.7 | 1.2 | 0.7 | 0.7 | |||||||
| Sipuncula | 3.1 | 1.4 | 1.2 | 0.9 | 0.7 | ||||||
| Annelida | 7.4 | 20.0 | 34.4 | 19.2 | 14.0 | 12.3 | 14.5 | 18.0 | 8.6 | 13.2 | 12.3 |
| Arthropoda | 18.5 | 5.7 | 3.1 | 12.3 | 17.4 | 10.4 | 10.1 | 16.7 | 10.8 | 13.9 | 12.3 |
| Mollusca | 7.4 | 3.1 | 6.8 | 9.3 | 12.3 | 13.0 | 16.7 | 8.6 | 16.0 | 30.4 | |
| Bryozoa | 2.7 | 1.3 | |||||||||
| Echinodermata | 2.3 | 1.9 | 2.9 | 2.0 | 1.1 | 1.4 | 0.7 | ||||
| Tunicata | 6.3 | 2.8 | 1.4 | 5.3 | 2.2 | 1.4 | 0.7 | ||||
| Chordata | 11.1 | 14.3 | 31.3 | 12.3 | 39.5 | 32.1 | 50.7 | 25.3 | 37.6 | 27.1 | 34.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilecenoğlu, M.; Çınar, M.E. Alien Species Threat across Marine Protected Areas of Turkey—An Updated Inventory. J. Mar. Sci. Eng. 2021, 9, 1077. https://doi.org/10.3390/jmse9101077
Bilecenoğlu M, Çınar ME. Alien Species Threat across Marine Protected Areas of Turkey—An Updated Inventory. Journal of Marine Science and Engineering. 2021; 9(10):1077. https://doi.org/10.3390/jmse9101077
Chicago/Turabian StyleBilecenoğlu, Murat, and Melih Ertan Çınar. 2021. "Alien Species Threat across Marine Protected Areas of Turkey—An Updated Inventory" Journal of Marine Science and Engineering 9, no. 10: 1077. https://doi.org/10.3390/jmse9101077
APA StyleBilecenoğlu, M., & Çınar, M. E. (2021). Alien Species Threat across Marine Protected Areas of Turkey—An Updated Inventory. Journal of Marine Science and Engineering, 9(10), 1077. https://doi.org/10.3390/jmse9101077







