Assessment of Marine Litter in the Coralligenous Habitat of a Marine Protected Area along the Ionian Coast of Sicily (Central Mediterranean)
Abstract
1. Introduction
2. Materials and Methods
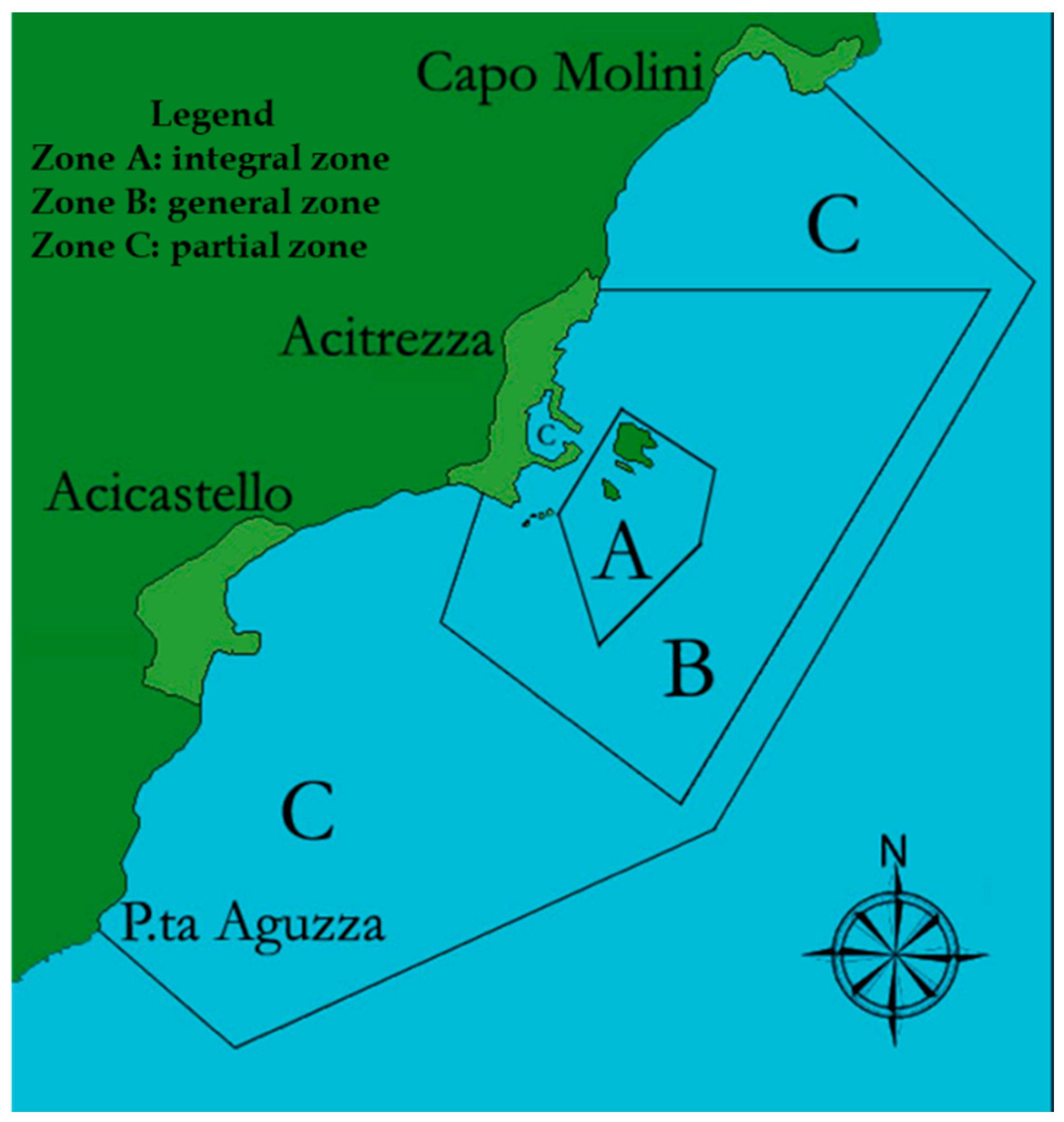
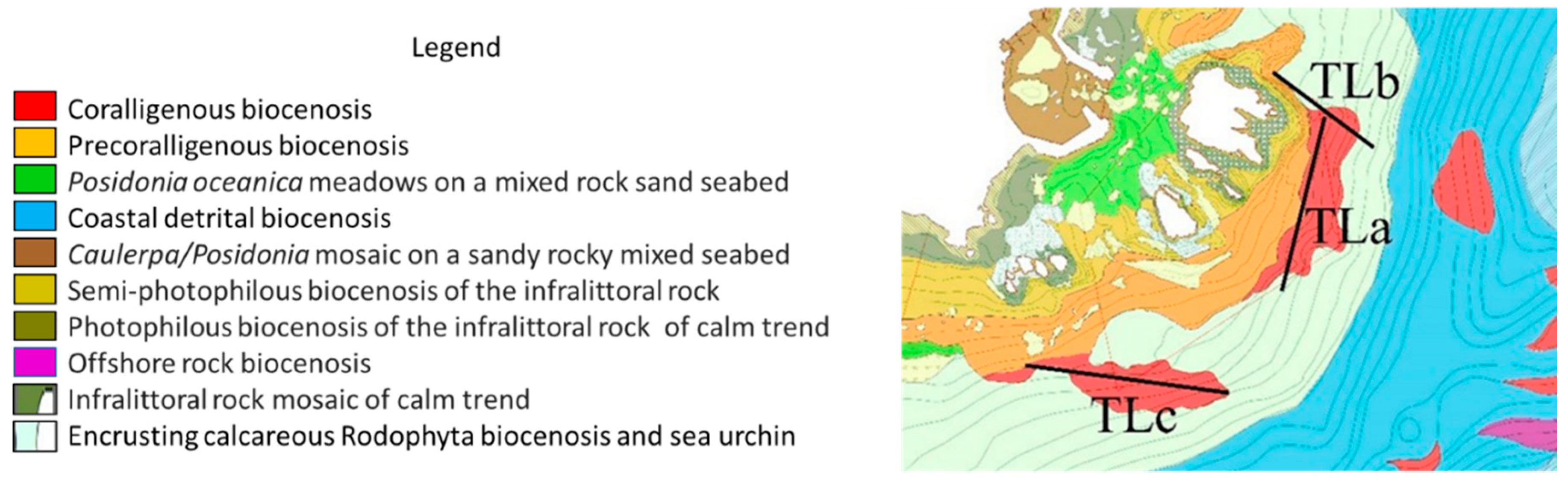
2.1. Study Area
2.2. Experimental Design
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biol. Ann. Rev. 2006, 44, 123–195. [Google Scholar]
- Ballesteros, E. Els Vegetals i la Zonació Litoral: Espècies, Comunitats i Factors Que Influeixen la Seva Distribució; Arxius de la Seccó de Ciències CI, Institut d’Estudis Catalans: Barcelona, Spain, 1992; pp. 1–613. [Google Scholar]
- Boudouresque, C.F.; Blanfuné, A.; Harmelin-Vivien, M.; Personnic, S.; Ruitton, S.; Thibaut, T.; Verlaque, M. Where seaweed forests meet animal forests: The examples of macroalgae in coral reefs and the Mediterranean coralligenous ecosystem. In Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Rossi, S., Bramanti, L., Gori, A., Orejas, C., Eds.; Springer International Publishing: New York, NY, USA, 2016; pp. 1–28. [Google Scholar]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean Bioconstructions along the Italian Coast. Adv. Mar. Biol. 2018, 79, 61–136. [Google Scholar] [PubMed]
- Ballesteros, E. The Coralligenous in the Mediterranean Sea: Definition of the Coralligenous Assemblage in the Mediterranean, Its Main Builders, Its Richness and Key Role in Benthic Ecology as Well as Its Threats: Project for the Preparation of a Strategic Action Plan for the Conservation of the Biodiversity in the Mediterranean Region (SAP BIO); RAC/SPA: Tunis City, Tunis, 2003; pp. 1–83. [Google Scholar]
- Piazzi, L.; Gennaro, P.; Balata, D. Threats to macroalgal coralligenous assemblages in the Mediterranean Sea. Mar. Pollut. Bull. 2012, 64, 2623–2629. [Google Scholar] [CrossRef]
- Collie, J.S.; Hall, S.J.; Kaiser, M.J.; Poiner, I.R. A quantitative analysis of fishing impacts on shelf-sea benthos. J. Anim. Ecol. 2000, 69, 785–798. [Google Scholar] [CrossRef]
- Carr, A. Impact of non degradable marine debris on the ecology and survival outlook of sea turtles. Mar. Pollut. Bull. 1987, 18, 352–356. [Google Scholar] [CrossRef]
- Matsuoka, T.; Nakashima, T.; Nagasawa, N. A review of ghost fishing: Scientific approaches to evaluation and solutions. Fish. Sci. 2005, 71, 691–702. [Google Scholar] [CrossRef]
- Brown, J.; Macfadyen, G. Ghost fishing in European waters: Impacts and management responses. Mar. Policy 2007, 31, 488–504. [Google Scholar] [CrossRef]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef]
- Vieira, R.P.; Raposo, I.P.; Sobral, P.; Gonçalves, J.M.; Bell, K.L.; Cunha, M.R. Lost fishing gear and litter at Gorringe Bank (NE ATLantic). J. Sea Res. 2015, 100, 91–98. [Google Scholar] [CrossRef]
- Bavestrello, G.; Cerrano, C.; Zanzi, D.; Cattaneo-Vietti, R. Damage by fishing activities to the gorgonian coral Paramuricea clavata in the Ligurian Sea. Aquat. Conserv. 1997, 7, 253–262. [Google Scholar] [CrossRef]
- Bo, M.; Bava, S.; Canese, S.; Angiolillo, M.; Cattaneo-Vietti, R.; Bavestrello, G. Fishing impact on deep Mediterranean rocky habitats as revealed by ROV investigation. Biol. Conserv. 2014, 171, 167–176. [Google Scholar] [CrossRef]
- Bo, M.; Bavestrello, G.; Angiolillo, M.; Calcagnile, L.; Canese, S.; Cannas, R.; Cau, A.L.; D’Elia, M.; D’Oriano, F.; Follesa, M.C.; et al. Persistence of pristine deep-sea coral gardens in the Mediterranean Sea (SW Sardinia). PLoS ONE 2015, 10, e0119393. [Google Scholar] [CrossRef] [PubMed]
- Cau, A.; Follesa, M.C.; Moccia, D.; Alvito, A.; Bo, M.; Angiolillo, M.; Canese, S.; Paliaga, E.M.; Orrù, P.E.; Sacco, F.; et al. Deep-water corals biodiversity along roche du large ecosystems with different habitat complexity along the south Sardinia continental margin (CW Mediterranean Sea). Mar. Biol. 2015, 162, 1865–1878. [Google Scholar] [CrossRef]
- Cau, A.; Alvito, A.; Moccia, D.; Canese, S.; Pusceddu, A.; Rita, C.; Angiolillo, M.; Follesa, M.C. Submarine canyons along the upper Sardinian slope (Central Western Mediterranean) as repositories for derelict fishing gears. Mar. Pollut. Bull. 2017, 123, 357–364. [Google Scholar] [CrossRef]
- Taviani, M.; Angeletti, L.; Canese, S.; Cannas, R.; Cardone, F.; Cau, A.; Cau, A.B.; Follesa, M.C.; Marchese, F.; Tessarolo, C. The “Sardinian Cold-Water Coral Province” in the context of the Mediterranean coral ecosystems. Deep Sea Res. PT. II. 2017, 145, 61–78. [Google Scholar] [CrossRef]
- Consoli, P.; Andaloro, F.; Altobelli, C.; Battaglia, P.; Campagnuolo, S.; Canese, S.; Castriota, L.; Cillari, T.; Falautano, M.; Peda, C.; et al. Marine litter in an EBSA (Ecologically or Biologically Significant Area) of the central Mediterranean Sea: Abundance, composition, impact on benthic species and basis for monitoring entanglement. Environ. Pollut. 2018, 236, 405–415. [Google Scholar] [CrossRef]
- Galgani, F.; Hanke, G.; Werner, S.D.V.L.; De Vrees, L. Marine litter within the European marine strategy framework directive. ICES J. Mar. Sci. 2013, 70, 1055–1064. [Google Scholar] [CrossRef]
- Cristofolini, R. La massa subvulcanica di Aci Trezza (Etna). Rend Soc. Ital. Miner. Petrol. 1975, 30, 741–770. [Google Scholar]
- Corsaro, R.A.; Cristofolini, R. Geology, geochemistry and mineral chemistry of tholeitic to transitional Etnean magmas. Acta Vulcanol. 1997, 9, 55–66. [Google Scholar]
- Sciuto, F.; Rosso, A.; Sanfilippo, R.; Di Martino, E. Ostracods from mid-outer shelf bottoms of the Ciclopi Islands Marine Protected Area (Ionian Sea, Eastern Sicily). Boll. Soc. Paleontol. Ital. 2015, 54, 131–145. [Google Scholar]
- Toscano, F.; Alongi, G.; Conti, E.; Turnaturi, R.; Mulder, C. Capitalizing the blue world: What can we learn from an eastern mediterranean case study? Ecol. Indic. 2020, 115, 106420. [Google Scholar] [CrossRef]
- Aguilar, R.; Pastor, X.; Torriente, A.; Garcia, S. Deep-sea coralligenous beds observed with ROV on four seamounts in the western Mediterranean. In Proceedings of the 1st Mediterranean Symposium on the Conservation of the Coralligenous and Others Calcareous Bio-Concretions, Tabarka, Tunis, 15–16 January 2009; pp. 147–149. [Google Scholar]
- Ferrigno, F.; Russo, G.F.; Sandulli, R. Coralligenous Bioconstructions Quality Index (CBQI): A synthetic indicator to assess the status of different types of coralligenous habitats. Ecol. Indic. 2017, 82, 271–279. [Google Scholar] [CrossRef]
- Ferrigno, F.; Appolloni, L.; Russo, G.F.; Sandulli, R. Impact of fishing activities on different coralligenous assemblages of Gulf of Naples (Italy). J. Mar. Biol. Assoc. UK 2018, 98, 41–50. [Google Scholar] [CrossRef]
- Bergmann, M.; Klages, M. Increase of litter at the Arctic deep-sea observatory, HAUSGARTEN. Mar. Pollut. Bull. 2012, 64, 2734–2741. [Google Scholar] [CrossRef]
- Angiolillo, M.; Di Lorenzo, B.; Farcomeni, A.; Bo, M.; Bavestrello, G.; Santangelo, G.; Cau, A.; Mastascusa, V.; Cau, A.; Sacco, F.; et al. Distribution and assessment of marine debris in the deep Tyrrhenian Sea (NW Mediterranean Sea, Italy). Mar. Pollut. Bull. 2015, 92, 149–159. [Google Scholar] [CrossRef]
- Melli, V.; Angiolillo, M.; Ronchi, F.; Canese, S.; Giovanardi, O.; Querin, S.; Fortibuoni, T. The first assessment of marine debris in a site of community importance in the North-western Adriatic Sea (Mediterranean Sea). Mar. Pollut. Bull. 2017, 114, 821–830. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bava, S.; Bavestrello, G.; Betti, F.; Lanteri, L.; Bo, M. Artisanal fishing impact on deep coralligenous animal forests: A Mediterranean case study of marine vulnerability. Ocean Coast Manag. 2019, 177, 112–126. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Grundfragen und Aufgaben der Pflanzensoziologie. In Vistas in Botany; Pergamon Press: London, UK, 1959; pp. 145–171. [Google Scholar]
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11. [Google Scholar] [CrossRef]
- Villarrubia-Gómez, P.; Cornell, S.E.; Fabres, J. Marine plastic pollution as a planetary boundary threat—The drifting piece in the sustainability puzzle. Mar. Policy 2018, 96, 213–220. [Google Scholar] [CrossRef]
- Pham, C.K.; Gomes-Pereira, J.N.; Isidro, E.J.; Santos, R.S.; Morato, T. Abundance of litter on Condor seamount (Azores, Portugal, Northeast ATLantic). Deep Sea Res. 2013, 98, 204–208. [Google Scholar] [CrossRef]
- Galgani, F.; Souplet, A.; Cadiou, Y. Accumulation of debris on the deep-sea floor off the French Mediterranean coast. Mar. Ecol. Prog. Ser. 1996, 142, 225–234. [Google Scholar] [CrossRef]
- Galgani, F.; Leaute, J.P.; Moguedet, P.; Souplet, A.; Verin, Y.; Carpentier, A.; Goraguer, H.; Latrouite, D.; Andral, B.; Cadiou, Y.; et al. Litter on the sea floor along European coasts. Mar. Pollut. Bull. 2000, 40, 516–527. [Google Scholar] [CrossRef]
- Pham, C.K.; Ramirez-Llodra, E.; Alt, C.H.; Amaro, T.; Bergmann, M.; Canals, M.; Company, J.B.; Davies, J.; Duineveld, G.; Galgani, F.; et al. Marine litter distribution and density in European seas, from the shelves to deep basins. PLoS ONE 2014, 9, e9583. [Google Scholar] [CrossRef]
- Schlining, K.; Von Thun, S.; Kuhnz, L.; Schlining, B.; Lundsten, L.; Stout, N.J.; Chaney, L.; Connor, J. Debris in the deep: Using a 22-year video annotation database to survey marine litter in Monterey Canyon, central California, USA. Deep Sea Res. 2013, 79, 96–105. [Google Scholar] [CrossRef]
- Bauer, L.J.; Kendall, M.S.; Jeffrey, C.F. Incidence of marine debris and its relationships with benthic features in Gray’s Reef National Marine Sanctuary, Southeast USA. Mar. Pollut. Bull. 2008, 56, 402–413. [Google Scholar] [CrossRef]
- Chiappone, M.; Dienes, H.; Swanson, D.W.; Miller, S.L. Impacts of lost fishing gear on coral reef sessile invertebrates in the Florida Keys National Marine Sanctuary. Biol. Conserv. 2005, 121, 221–230. [Google Scholar] [CrossRef]
- Watters, D.L.; Yoklavich, M.M.; Love, M.S.; Schroeder, D.M. Assessing marine debris in deep seafloor habitats off California. Mar. Pollut. Bull. 2010, 60, 131–138. [Google Scholar] [CrossRef]
- Miyake, H.; Shibata, H.; Furushima, Y. Deep-Sea Litter Study Using Deep-Sea Observation Tools. In Interdisciplinary Studies on Environmental Chemistry—Marine Environmental Modeling and Analysis; Omori, K., Guo, X., Yoshie, N., Fujii, N., Handoh, I.C., Isobe, A., Tanabe, S., Eds.; TERRAPUB: Tokyo, Japan, 2011; pp. 261–269. [Google Scholar]
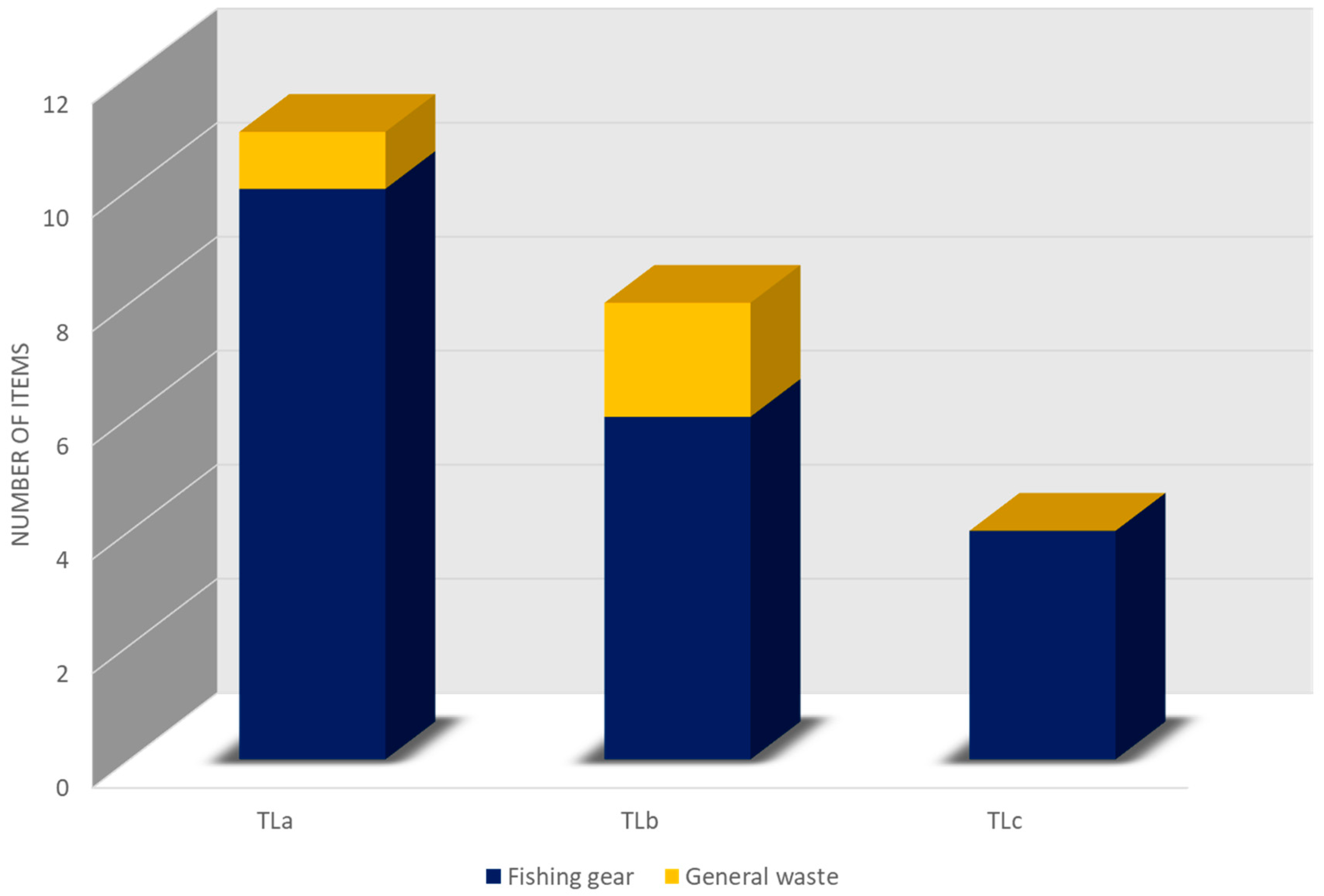

| Transect | Recording Minutes | Number of Frames | Number of Items (Frequency) | Density | Depth Range | Lenght | Exposure | Latitude and Longitude | |
|---|---|---|---|---|---|---|---|---|---|
| Initial Point of the Transect | Final Point of the Transect | ||||||||
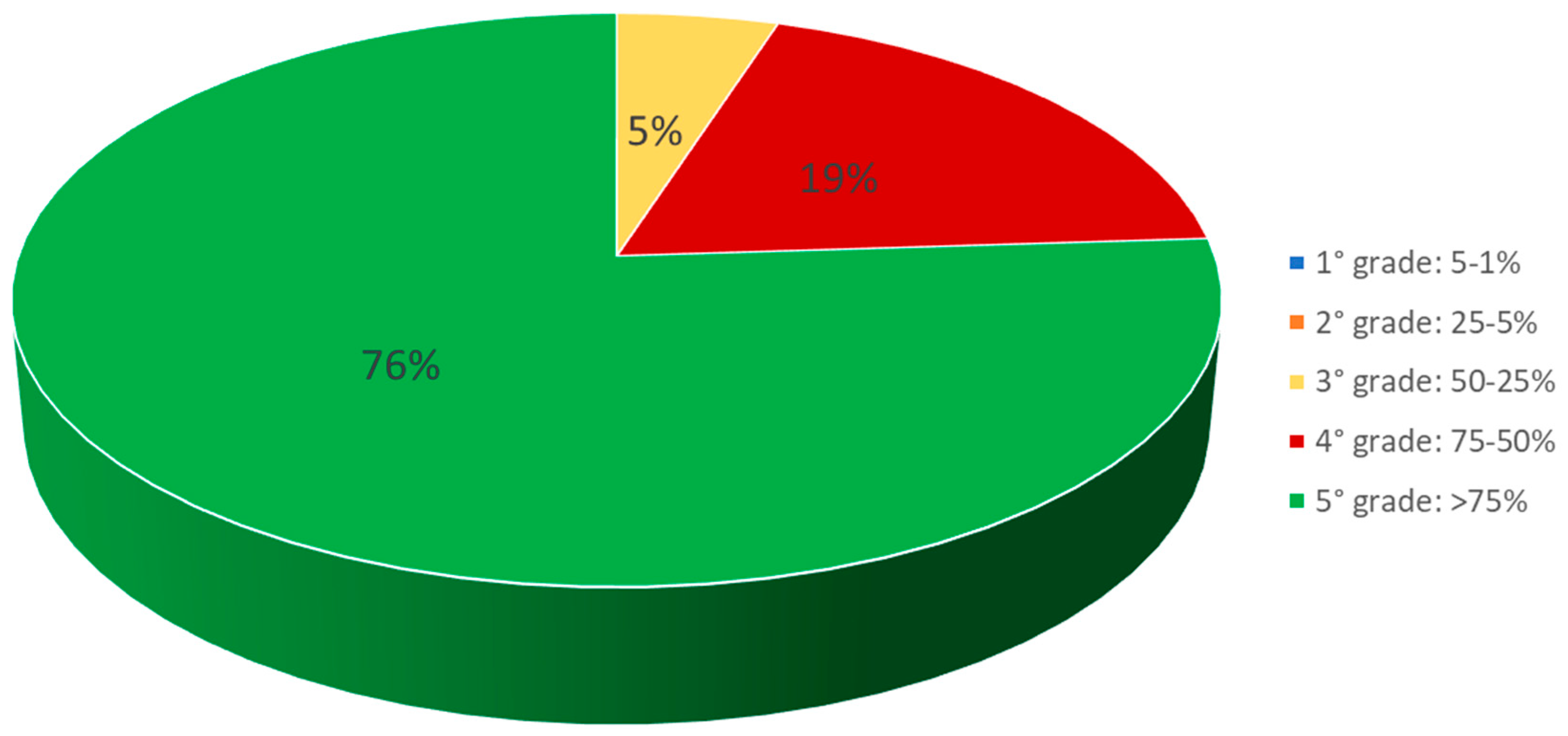
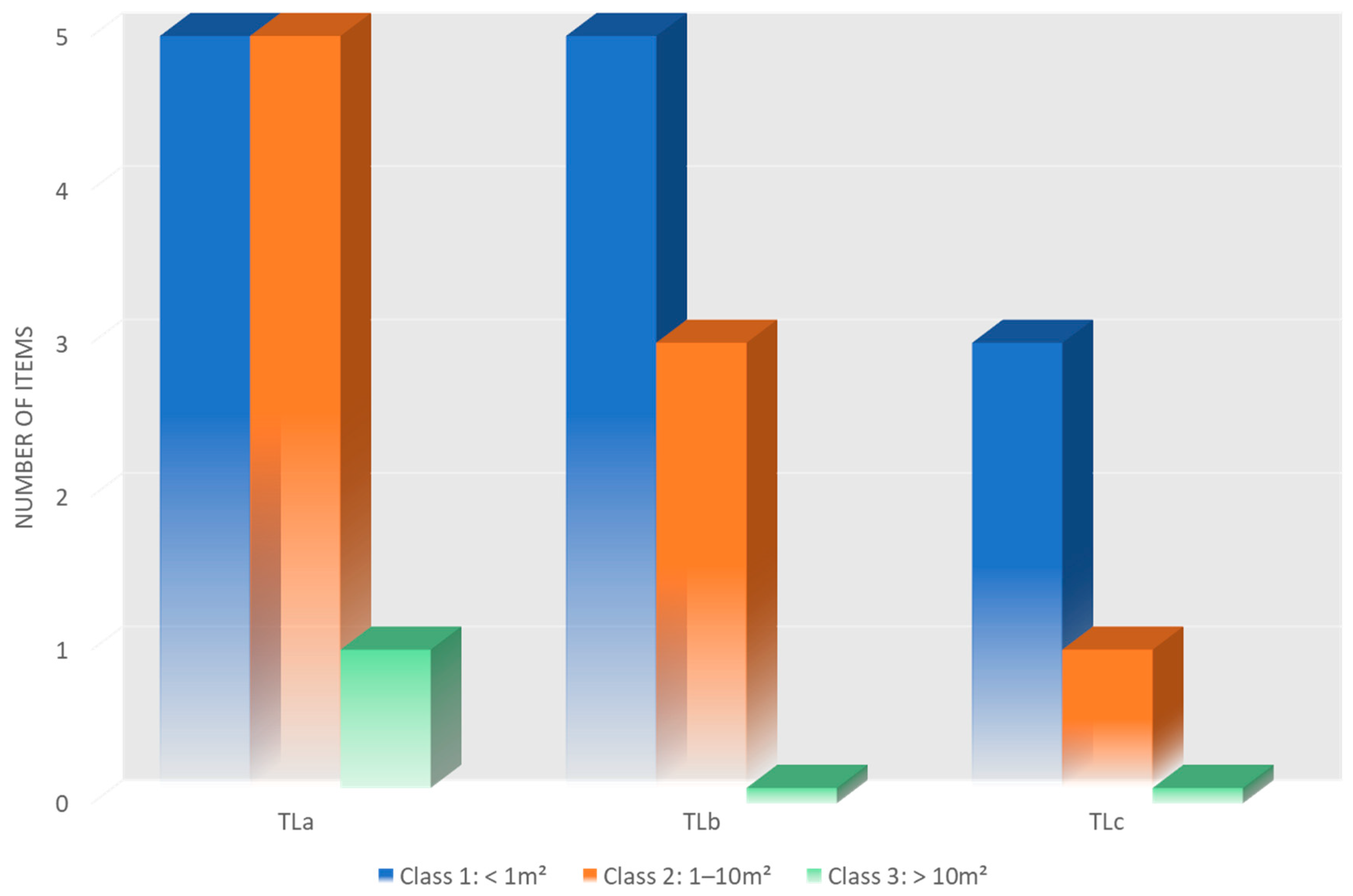
| TLa | 132 | 10 | 11 (47.8%) | 5.5 items/ 100 m2 | 35 m | ~200 m | East–South/East | 37°33′41.50″ N 15°10′6.26″ E | 37°33′34.82″ N 15°10′4.98″ E |
| TLb | 68 | 7 | 8 (34.8%) | 4 items/ 100 m2 | 33 m | ~200 m | North/East–North | 37°33′44.02″ N 15°10′1.85″ E | 37°33′40.56″ N 15°10′8.73″ E |
| TLc | 87 | 4 | 4 (17.4%) | 2 items/ 100 m2 | 35 m | ~200 m | South–South/West | 37°33′25.09″ N 15°09′47.47″ E | 37°33′24.79″ N 15°09′56.47″ E |
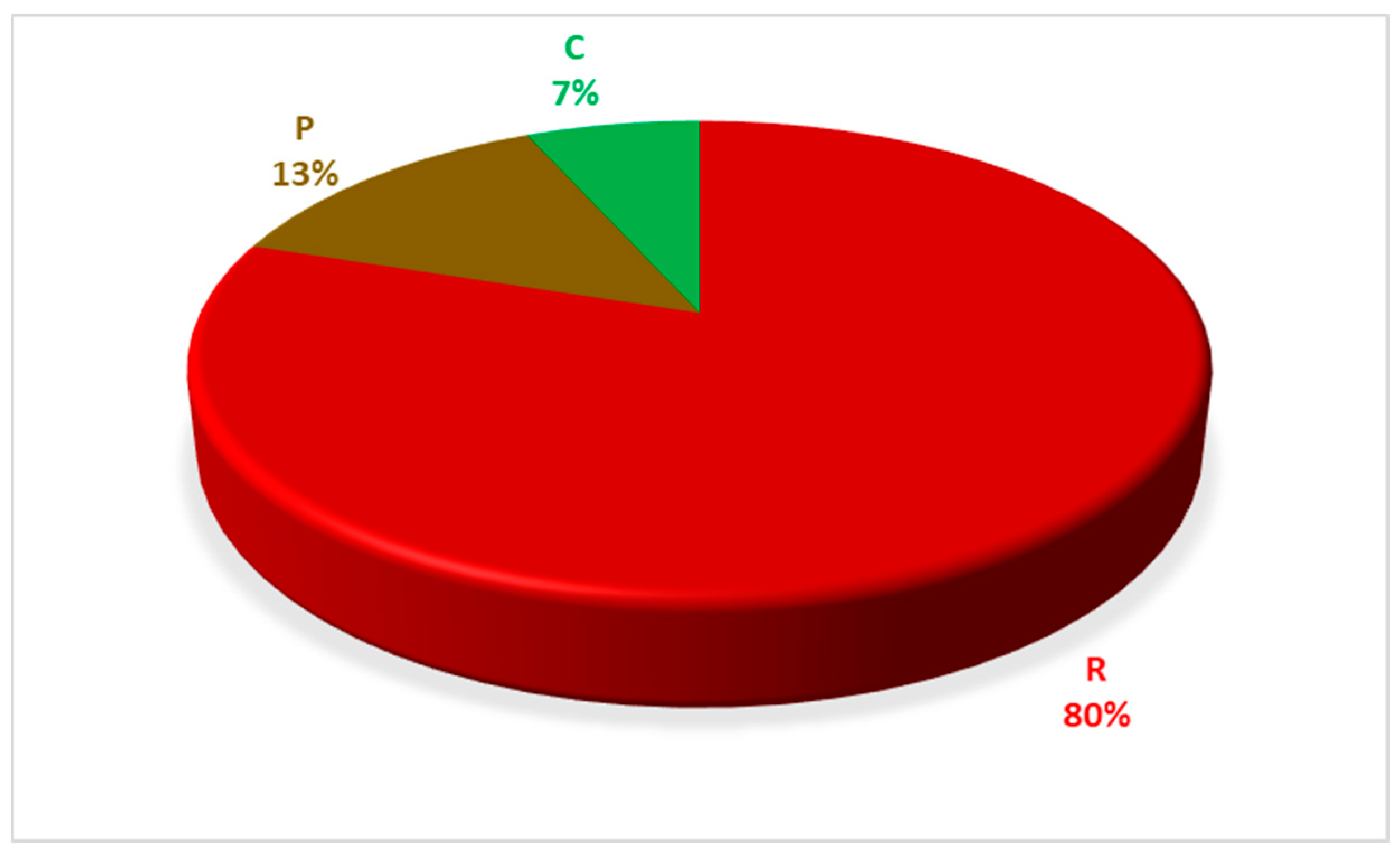
| Items | Group of Marine Litter | Number of Findings | Frequencies of the Groups |
|---|---|---|---|
| Rubber | General waste | 1 | 3 (13.04%) |
| Block of iron and concrete | General waste | 1 | |
| Glass bottle | General waste | 1 | |
| Rope | Fishing gear | 8 | 20 (86.96%) |
| Fishing line | Fishing gear | 7 | |
| Set net | Fishing gear | 2 | |
| Floating rope | Fishing gear | 2 | |
| Buoy chain | Fishing gear | 1 |
| Genera. |
|---|
| Antithamnion Nägeli |
| Apoglossum (J.Agardh) J.Agardh |
| CaulacanthusKützing |
| Ceramium Roth |
| Champia Desvaux |
| Contarinia Zanardini |
| Erythrotrichia Areschoug |
| Gelidiella Feldmann & G.Hamel |
| Gloiocladia J.Agardh |
| HeterosiphoniaMontagne |
| Hydrolithon (Foslie) Foslie |
| Irvinea Guiry |
| Jania J.V.Lamouroux |
| Kallymenia J.Agardh |
| Lophosiphonia Falkenberg |
| Mesophyllum Me.Lemoine |
| Peyssonnelia Decaisne |
| Phyllophora Greville |
| PneophyllumKützing |
| Pterothamnion Nägeli |
| Rhodophyllis Kützing |
| Rhodymenia Greville |
| Seirospora Harvey |
| Spermothamnion Areschoug |
| Dictyota J.V.Lamouroux |
| Halopteris Kützing |
| Lobophora J.Agardh |
| Sphacelaria Lyngbye |
| Derbesia Solier |
| Palmophyllum Kützing |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanzo, L.G.; Marletta, G.; Alongi, G. Assessment of Marine Litter in the Coralligenous Habitat of a Marine Protected Area along the Ionian Coast of Sicily (Central Mediterranean). J. Mar. Sci. Eng. 2020, 8, 656. https://doi.org/10.3390/jmse8090656
Costanzo LG, Marletta G, Alongi G. Assessment of Marine Litter in the Coralligenous Habitat of a Marine Protected Area along the Ionian Coast of Sicily (Central Mediterranean). Journal of Marine Science and Engineering. 2020; 8(9):656. https://doi.org/10.3390/jmse8090656
Chicago/Turabian StyleCostanzo, Luca Giuseppe, Giuliana Marletta, and Giuseppina Alongi. 2020. "Assessment of Marine Litter in the Coralligenous Habitat of a Marine Protected Area along the Ionian Coast of Sicily (Central Mediterranean)" Journal of Marine Science and Engineering 8, no. 9: 656. https://doi.org/10.3390/jmse8090656
APA StyleCostanzo, L. G., Marletta, G., & Alongi, G. (2020). Assessment of Marine Litter in the Coralligenous Habitat of a Marine Protected Area along the Ionian Coast of Sicily (Central Mediterranean). Journal of Marine Science and Engineering, 8(9), 656. https://doi.org/10.3390/jmse8090656





