Relative Influence of Environmental Factors on Biodiversity and Behavioural Traits of a Rare Mesopelagic Fish, Trachipterus trachypterus (Gmelin, 1789), in a Continental Shelf Front of the Mediterranean Sea
Abstract
:1. Introduction
2. Materials and Methods
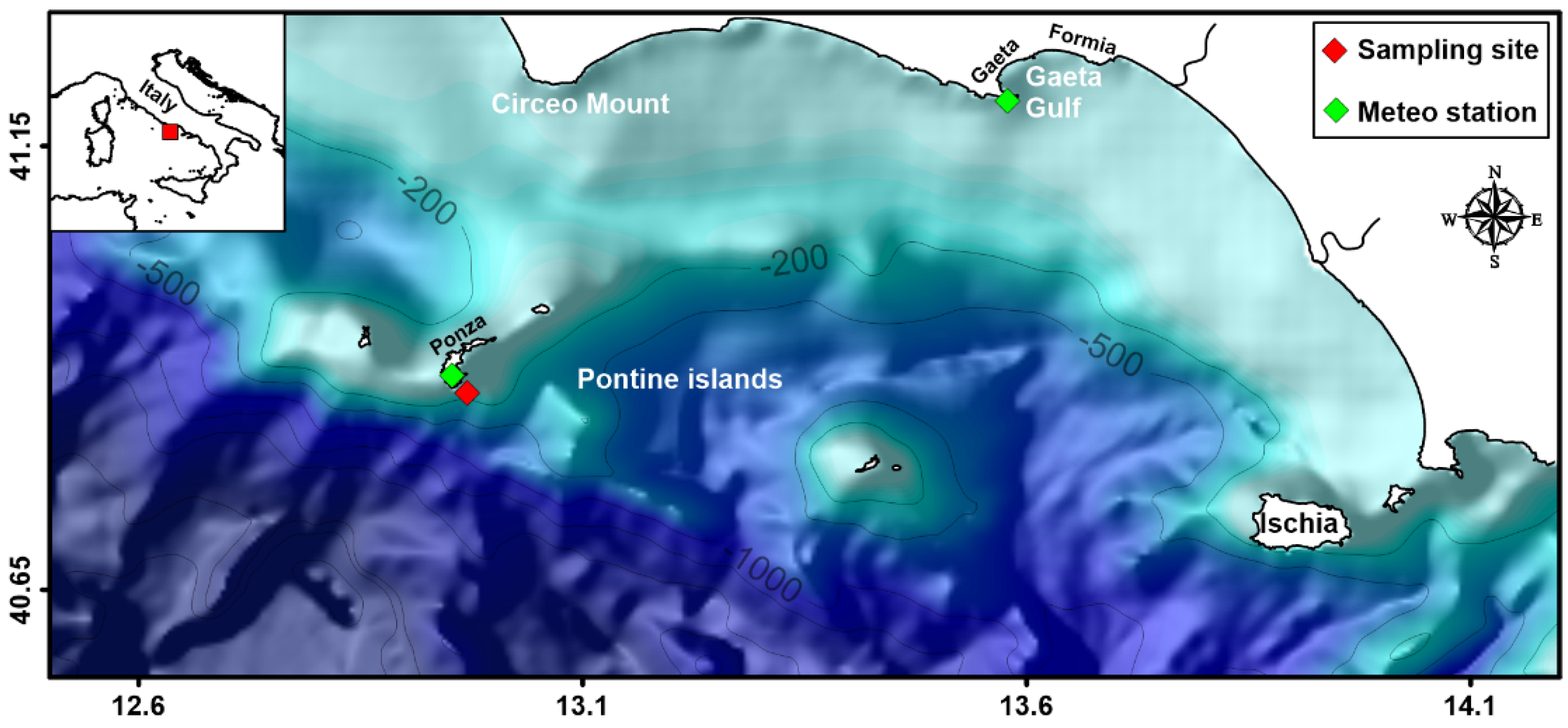
2.1. Study Area and Sampling Design
2.2. Environmental Data Collection
2.3. Data Analysis
3. Results
3.1. Oceanographic Landscape
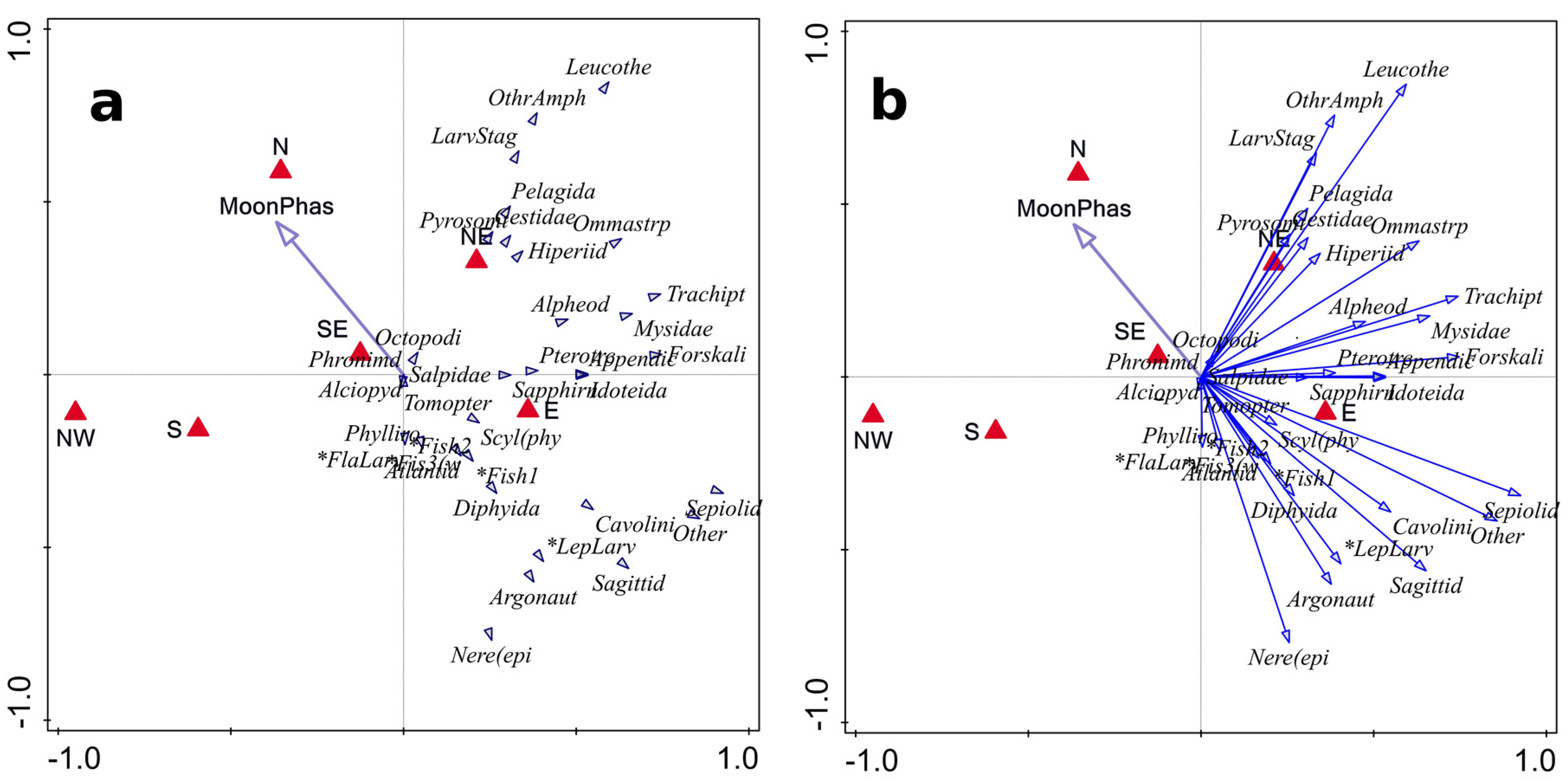
3.2. Assemblages
3.3. Role of Environmental Factors
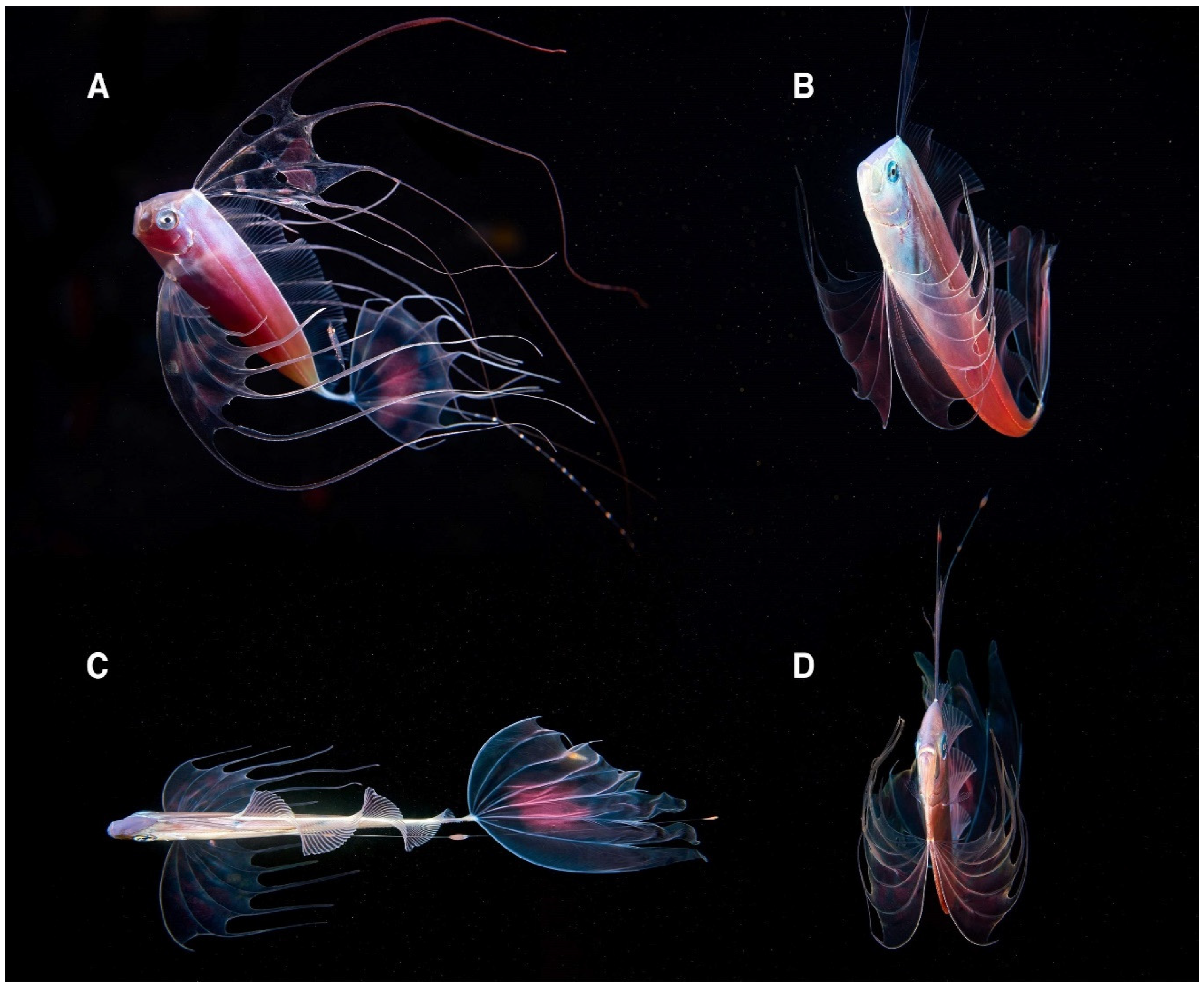
3.4. Behavioral Traits of T. trachypterus
4. Discussion
4.1. Ponza Island Coastal front and Pelagic Biodiversity
4.2. Ecological Insight on the Uncommon Mesopelagic Fish Trachipterus trachypterus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lévy, M.; Jahn, O.; Dutkiewicz, S.; Follows, M.J.; D’Ovidio, F. The dynamical landscape of marine phytoplankton diversity. J. R. Soc. Interface 2015, 12, 20150481. [Google Scholar] [CrossRef]
- Ferrari, R.; Wunsch, C. Ocean Circulation Kinetic Energy: Reservoirs, Sources, and Sinks. Annu. Rev. Fluid Mech. 2009, 41, 253–282. [Google Scholar] [CrossRef] [Green Version]
- McGillicuddy, D.J. Mechanisms of Physical-Biological-Biogeochemical Interaction at the Oceanic Mesoscale. Ann. Rev. Mar. Sci. 2016, 8, 125–159. [Google Scholar] [CrossRef] [Green Version]
- Mahadevan, A.; Campbell, J.W. Biogeochemical patchiness at the sea surface. Geophys. Res. Lett. 2002, 29, 31–32. [Google Scholar] [CrossRef]
- Lehahn, Y.; D’Ovidio, F.; Koren, I. A Satellite-Based Lagrangian View on Phytoplankton Dynamics. Ann. Rev. Mar. Sci. 2018, 10, 99–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McManus, M.A.; Woodson, C.B. Plankton distribution and ocean dispersal. J. Exp. Biol. 2012, 215, 1008–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, L.L. Sampling rare populations. In Sampling Rare or Elusive Species; Thompson, W., Ed.; Island Press: Washington, DC, USA, 2004; pp. 11–42. [Google Scholar]
- Kelly, R.P.; Port, J.A.; Yamahara, K.M.; Martone, R.G.; Lowell, N.; Thomsen, P.F.; Mach, M.E.; Bennett, M.; Prahler, E.; Caldwell, M.R.; et al. Harnessing DNA to improve environmental management. Science 2014, 344, 1455–1456. [Google Scholar] [CrossRef] [PubMed]
- Wuertz, M. Mediterranean Pelagic Habitat: Oceanographic and Biological Processes, an Overview; IUCN: Gland, Switzerland, 2010; ISBN 978-2-8317-1242-0. [Google Scholar]
- Bonanno, A.; Zgozi, S.; Basilone, G.; Hamza, M.; Barra, M.; Genovese, S.; Rumolo, P.; Nfate, A.; Elsger, M.; Goncharov, S.; et al. Acoustically detected pelagic fish community in relation to environmental conditions observed in the Central Mediterranean sea: A comparison of Libyan and Sicilian–Maltese coastal areas. Hydrobiologia 2015, 755, 209–224. [Google Scholar] [CrossRef]
- Giannoulaki, M.; Iglesias, M.; Tugores, M.P.; Bonanno, A.; Patti, B.; De Felice, A.; Leonori, I.; Bigot, J.L.; Tičina, V.; Pyrounaki, M.M.; et al. Characterizing the potential habitat of European anchovy Engraulis encrasicolus in the Mediterranean Sea, at different life stages. Fish. Oceanogr. 2013, 22, 69–89. [Google Scholar] [CrossRef]
- Tugores, P.; Giannoulaki, M.; Iglesias, M.; Bonanno, A.; Tičina, V.; Leonori, I.; MacHias, A.; Tsagarakis, K.; Díaz, N.; Giráldez, A.; et al. Habitat suitability modelling for sardine Sardina pilchardus in a highly diverse ecosystem: The Mediterranean Sea. Mar. Ecol. Prog. Ser. 2011, 443, 181–205. [Google Scholar] [CrossRef] [Green Version]
- Damgaard, C.; Weiner, J. It’s About Time: A Critique of Macroecological Inferences Concerning Plant Competition. Trends Ecol. Evol. 2017, 32, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.L.; Harvey, E.S.; Fitzpatrick, B.M.; Langlois, T.J.; Shedrawi, G. Assessing reef fish assemblage structure: How do different stereo-video techniques compare? Mar. Biol. 2010, 157, 1237–1250. [Google Scholar] [CrossRef]
- Olney, J.E.; McBride, R.S. Intraspecific variation in batch fecundity of American shad: Revisiting the paradigm of reciprocal latitudinal trends in reproductive traits. Am. Fish. Soc. Symp. 2003, 2003, 185–192. [Google Scholar]
- Walters, V.; Fitch, J. The families and genera of the lampridiform (Allotriognath) suborder Trachipteroidei. Calif. Fish Game 1960, 46, 441–451. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. Available online: www.fishbase.org (accessed on 1 May 2018).
- Dulčić, J. First record of ribbon fish larva, Trachipterus trachypterus, from the Eastern Adriatic. Cybium 1996, 20, 101–102. [Google Scholar]
- Dulčić, J. First record of scalloped ribbon fish, Zu cristatus (Pisces: Trachipteridae), eggs in the Adriatic Sea. J. Plankton Res. 2002, 24, 1245–1246. [Google Scholar] [CrossRef]
- Borme, D.; Voltolina, F. On the occurrence of ribbon fish Trachipterus trachypterus (Gmelin, 1789) in the Gulf of Trieste (northern Adriatic Sea). Ann. Ser. Historia Nat. 2006, 16, 181. [Google Scholar]
- Psomadakis, P.N.; Scacco, U.; Vacchi, M. Recent findings of some uncommon fishes from the central Tyrrhenian Sea. Cybium 2006, 30, 297–304. [Google Scholar]
- Psomadakis, P.N.; Bottaro, M.; Vacchi, M. On two large specimens of Zu cristatus (Trachipteridae) from the Gulf of Genoa (NW Mediterranean). Cybium 2007, 31, 480–482. [Google Scholar]
- Bradai, M.N.; El Ouaer, A. New record of the scalloped ribbon fish, Zu cristatus (Osteichthyes: Trachipteridae) in Tunisian waters (central Mediterranean). Mar. Biodivers. Rec. 2014, 5, e59. [Google Scholar] [CrossRef]
- Mytilineou, C.; Anastasopoulou, A.; Christides, G.; Bekas, P.; Smith, C.J.; Papadopoulou, K.N.; Lefkaditou, E.; Kavadas, S. New records of rare deep-water fish species in the Eastern Ionian Sea (Mediterranean Sea). J. Nat. Hist. 2013, 47, 1645–1662. [Google Scholar] [CrossRef]
- Bono, G.; Gancitano, S.; Bosch-Belmar, M.; Giusto, G.B.; Okpala, C.O.R.; Scannella, D.; Geraci, M.L.; Falsone, F. Occurrence of two rare species from order Lampriformes: Crestfish Lophotus lacepede (Giorna, 1809) and scalloped ribbonfish Zu cristatus(Bonelli, 1819) in the northern coast of Sicily, ItalyPojava dviju rijetkih vrsta iz reda Lampriformes: B. Acta Adriat. 2017, 58, 137–146. [Google Scholar] [CrossRef]
- Bianco, P.G.; Zupo, V.; Ketmaier, V. Occurrence of the scalloped ribbonfish Zu cristatus (Lampridiformes) in coastal waters of the central Tyrrhenian Sea, Italy. J. Fish Biol. 2006, 68, 150–155. [Google Scholar] [CrossRef]
- Crocetta, F.; Agius, D.; Balistreri, P.; Bariche, M.; Bayhan, Y.K.; Çakir, M.; Ciriaco, S.; Corsini-Foka, M.; Deidun, A.; El Zrelli, R.; et al. New mediterranean biodiversity records (October 2015). Mediterr. Mar. Sci. 2015, 16, 682–702. [Google Scholar] [CrossRef] [Green Version]
- Tiralongo, F.; Lillo, A.O.; Tibullo, D.; Tondo, E.; Martire, C.L.; D’Agnese, R.; Macali, A.; Mancini, E.; Giovos, I.; Coco, S.; et al. Monitoring uncommon and non-indigenous fishes in Italian waters: One year of results for the AlienFish project. Reg. Stud. Mar. Sci. 2019, 28, 100606. [Google Scholar] [CrossRef]
- Paladini de Mendoza, F.; Bonamano, S.; Martellucci, R.; Melchiorri, C.; Consalvi, N.; Piermattei, V.; Marcelli, M. Circulation during storms and dynamics of suspended matter in a sheltered coastal area. Remote Sens. 2018, 10, 602. [Google Scholar] [CrossRef] [Green Version]
- Martellucci, R.; Pierattini, A.; Paladini de Mendoza, F.; Melchiorri, C.; Piermattei, V.; Marcelli, M. Physical and biological water column observations during summer sea/land breeze winds in the coastal northern Tyrrhenian Sea. Water (Switzerland) 2018, 10, 1673. [Google Scholar] [CrossRef] [Green Version]
- Kronfeld-Schor, N.; Dominoni, D.; de la Iglesia, H.; Levy, O.; Herzog, E.D.; Dayan, T.; Helfrich-Forster, C. Chronobiology by moonlight. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123088. [Google Scholar] [CrossRef] [Green Version]
- Last, K.S.; Hobbs, L.; Berge, J.; Brierley, A.S.; Cottier, F. Moonlight Drives Ocean-Scale Mass Vertical Migration of Zooplankton during the Arctic Winter. Curr. Biol. 2016, 26, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Benoit-Bird, K.J.; Au, W.W.L.; Wisdom, D.W. Nocturnal light and lunar cycle effects on diel migration of micronekton. Limnol. Oceanogr. 2009, 54, 1789–1800. [Google Scholar] [CrossRef] [Green Version]
- Drazen, J.C.; De Forest, L.G.; Domokos, R. Micronekton abundance and biomass in Hawaiian waters as influenced by seamounts, eddies, and the moon. Deep Sea Res. Part I Oceanogr. Res. Pap. 2011, 58, 557–566. [Google Scholar] [CrossRef]
- Rubolini, D.; Maggini, I.; Ambrosini, R.; Imperio, S.; Paiva, V.H.; Gaibani, G.; Saino, N.; Cecere, J.G. The Effect of Moonlight on Scopoli’s Shearwater Calonectris diomedea Colony Attendance Patterns and Nocturnal Foraging: A Test of the Foraging Efficiency Hypothesis. Ethology 2015, 121, 284–299. [Google Scholar] [CrossRef]
- Aksnes, D.L.; Røstad, A.; Kaartvedt, S.; Martinez, U.; Duarte, C.M.; Irigoien, X. Light penetration structures the deep acoustic scattering layers in the global ocean. Sci. Adv. 2017, 3, e1602468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochoa, J.; Maske, H.; Sheinbaum, J.; Candela, J. Diel and lunar cycles of vertical migration extending to below 1000 m in the ocean and the vertical connectivity of depth-tiered populations. Limnol. Oceanogr. 2013, 58, 1207–1214. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 2001, 58, 626–639. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008; pp. 1–214. [Google Scholar]
- Villegas-Zurita, F.; Castillejos-Moguel, F.; Benítez-Villalobos, F.; Urbán-Ramírez, J. Alpha diversity of marine mammals of the Mexican South Pacific. Rev. Mex. Biodivers. 2018, 89, 898–909. [Google Scholar] [CrossRef]
- Morris, C. Multivariate Analysis of Ecological Data Using Canoco 5. Afr. J. Range Forage Sci. 2015, 32, 289–290. [Google Scholar] [CrossRef]
- Bakun, A.; Agostini, V.N. Seasonal patterns of wind-induced upwelling/downwelling in the Mediterranean Sea. Sci. Mar. 2001, 65, 243–257. [Google Scholar] [CrossRef]
- Haury, L.R.; McGowan, J.A.; Wiebe, P.H. Patterns and Processes in the Time-Space Scales of Plankton Distributions. In Spatial Pattern in Plankton Communities; Springer: New York, NY, USA, 1978; pp. 277–327. [Google Scholar]
- Le Fèvre, J. Aspects of the Biology of Frontal Systems. Adv. Mar. Biol. 1987, 23, 163–299. [Google Scholar] [CrossRef]
- Joyce, T.M. Varieties of ocean fronts. In Baroclinic Instability and Ocean Fronts; Technical Report No. 83-41; Woods Hole Oceanographic Institution: Falmouth, MA, USA, 1983; Volume 1, p. 59. [Google Scholar]
- Belkin, I.M.; Cornillon, P.C.; Sherman, K. Fronts in Large Marine Ecosystems. Prog. Oceanogr. 2009, 81, 223–236. [Google Scholar] [CrossRef]
- Franks, P.J.S. Phytoplankton blooms at fronts: Patterns, scales, and physical forcing mechanisms. Rev. Aquat. Sci. 1992, 6, 121–137. [Google Scholar]
- Genin, A.; Jaffe, J.S.; Reef, R.; Richter, C.; Franks, P.J.S. Swimming against the flow: A mechanism of zooplankton aggregation. Science 2005, 308, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Bost, C.A.; Cotté, C.; Bailleul, F.; Cherel, Y.; Charrassin, J.B.; Guinet, C.; Ainley, D.G.; Weimerskirch, H. The importance of oceanographic fronts to marine birds and mammals of the southern oceans. J. Mar. Syst. 2009, 78, 363–376. [Google Scholar] [CrossRef]
- Bignami, F.; Böhm, E.; D’Acunzo, E.; D’Archino, R.; Salusti, E. On the dynamics of surface cold filaments in the Mediterranean Sea. J. Mar. Syst. 2008, 74, 429–442. [Google Scholar] [CrossRef]
- Wang, D.-P.; Vieira, M.E.C.; Salat, J.; Tintoré, J.; La Violette, P.E. A shelf/slope frontal filament off the northeast Spanish Coast. J. Mar. Res. 2008, 46, 321–332. [Google Scholar] [CrossRef]
- Siokou-Frangou, I.; Christaki, U.; Mazzocchi, M.G.; Montresor, M.; Ribera D’Alcala, M.; Vaque, D.; Zingone, A. Plankton in the open mediterranean Sea: A review. Biogeosciences 2010, 7, 1543–1586. [Google Scholar] [CrossRef] [Green Version]
- Di Carlo, B.S.; Ianora, A.; Fresi, E.; Hure, J. Vertical zonation patterns for mediterranean copepods from the surface to 3000 m at a fixed station in the Tyrrhenian Sea. J. Plankton Res. 1984, 6, 1031–1056. [Google Scholar] [CrossRef]
- de Puelles, M.L.F.; Pinot, J.-M.; Valencia, J. Variabilite saisonniere et interannuelle du zooplancton au large de Majorque (mer des Baleares, Mediterranee occidentale). Oceanol. Acta 2003, 5, 673–686. [Google Scholar]
- Chakraborty, U. Effects of different phases of the lunar month on living organisms. Biol. Rhythm Res. 2020, 51, 254–282. [Google Scholar] [CrossRef]
- Farias, I.; Moura, T.; Figueiredo, I.; Vieira, A.R.; Serra-Pereira, B.; Serrano Gordo, L. Northernmost occurrence of the ribbonfish Trachipterus trachypterus (Gmelin, 1789) in the NE Atlantic: The Portuguese continental shelf: Short communication. J. Appl. Ichthyol. 2010, 26, 143–144. [Google Scholar] [CrossRef]
- Consoli, P.; Esposito, V.; Battaglia, P.; Altobelli, C.; Perzia, P.; Romeo, T.; Canese, S.; Andaloro, F. Fish distribution and habitat complexity on banks of the strait of sicily (central mediterranean sea) from Remotely-Operated Vehicle (ROV) explorations. PLoS ONE 2016, 11, e0167809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houde, E.D. Fish Early Life Dynamics and Recruitment Variability. In Proceedings of the American Fisheries Society Symposium, 10 Annual Larval Fish Conference, Miami, FL, USA, 18–23 May 1987. [Google Scholar]
- Anderson, J.T. A Review of Size Dependant Survival during Pre-recruit Stages of Fishes in Relation to Recruitment. J. Northwest Atl. Fish. Sci. 1988, 8, 55–66. [Google Scholar] [CrossRef]
- Hare, J.A.; Cowen, R.K. Size, growth, development, and survival of the planktonic larvae of Pomatomus saltatrix (Pisces: Pomatomidae). Ecology 1997, 78, 2415–2431. [Google Scholar] [CrossRef]
- Wells, K.D. The Origin and Evolution of Larval Forms. Copeia 2000. [Google Scholar] [CrossRef]
- Miller, B.S.; Kendall, A.W. Early Life History of Marine Fishes; University of California Press: Berkeley, CA, USA, 2009; ISBN 9780520249721. [Google Scholar]
- Greer, A.T.; Woodson, C.B.; Guigand, C.M.; Cowen, R.K. Larval fishes utilize Batesian mimicry as a survival strategy in the plankton. Mar. Ecol. Prog. Ser. 2016, 551, 1–12. [Google Scholar] [CrossRef]
- Huheey, J.E. Mathematical Models of Mimicry. Am. Nat. 1988, 131, S22–S41. [Google Scholar] [CrossRef]
- Caley, M.J.; Schluter, D. Predators favour mimicry in a tropical reef fish. Proc. R. Soc. B Biol. Sci. 2003, 270, 667–672. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, S.; Hirosaki, Y. Observations on the swimming behavior of some taeniosomous fishes in aquaria and in nature. Publ. Seto Mar. Biol. Lab. 1964, 12, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Moritz, T.; Stümer, D.; Jakobsen, K.; Jakobsen, J. Observations on two live specimens of Trachipterus arcticus (Lampriformes: Trachipteridae) from the Azores. Cybium 2015, 39, 78–80. [Google Scholar]
- Sazima, I. Juvenile grunt (Haemulidae) mimicking a venomous leatherjacket (Carangidae), with a summary of Batesian mimicry in marine fishes. J. Ichthyol. Aquat. Biol. 2002, 6, 61–68. [Google Scholar]
- Reininger, M. Mimicry in juvenile wrasses: Ecological and behavioural aspects of a coris-amphiprion relationship in the northern red sea. Zool. Middle East 2011, 54, 23–34. [Google Scholar] [CrossRef]
- Tan, H.H. Apparent mimicry of jellyfish by juvenile pomfret, Pampus chinensis (Teleostei: Stromateidae). Nat. Singap. 2008, 1, 139–142. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macali, A.; Semenov, A.; Paladini de Mendoza, F.; Dinoi, A.; Bergami, E.; Tiralongo, F. Relative Influence of Environmental Factors on Biodiversity and Behavioural Traits of a Rare Mesopelagic Fish, Trachipterus trachypterus (Gmelin, 1789), in a Continental Shelf Front of the Mediterranean Sea. J. Mar. Sci. Eng. 2020, 8, 581. https://doi.org/10.3390/jmse8080581
Macali A, Semenov A, Paladini de Mendoza F, Dinoi A, Bergami E, Tiralongo F. Relative Influence of Environmental Factors on Biodiversity and Behavioural Traits of a Rare Mesopelagic Fish, Trachipterus trachypterus (Gmelin, 1789), in a Continental Shelf Front of the Mediterranean Sea. Journal of Marine Science and Engineering. 2020; 8(8):581. https://doi.org/10.3390/jmse8080581
Chicago/Turabian StyleMacali, Armando, Alexander Semenov, Francesco Paladini de Mendoza, Alessia Dinoi, Elisa Bergami, and Francesco Tiralongo. 2020. "Relative Influence of Environmental Factors on Biodiversity and Behavioural Traits of a Rare Mesopelagic Fish, Trachipterus trachypterus (Gmelin, 1789), in a Continental Shelf Front of the Mediterranean Sea" Journal of Marine Science and Engineering 8, no. 8: 581. https://doi.org/10.3390/jmse8080581
APA StyleMacali, A., Semenov, A., Paladini de Mendoza, F., Dinoi, A., Bergami, E., & Tiralongo, F. (2020). Relative Influence of Environmental Factors on Biodiversity and Behavioural Traits of a Rare Mesopelagic Fish, Trachipterus trachypterus (Gmelin, 1789), in a Continental Shelf Front of the Mediterranean Sea. Journal of Marine Science and Engineering, 8(8), 581. https://doi.org/10.3390/jmse8080581








