Marine Biofouling: A European Database for the Marine Renewable Energy Sector
Abstract
1. Introduction
2. Review of Biofouling Aspects
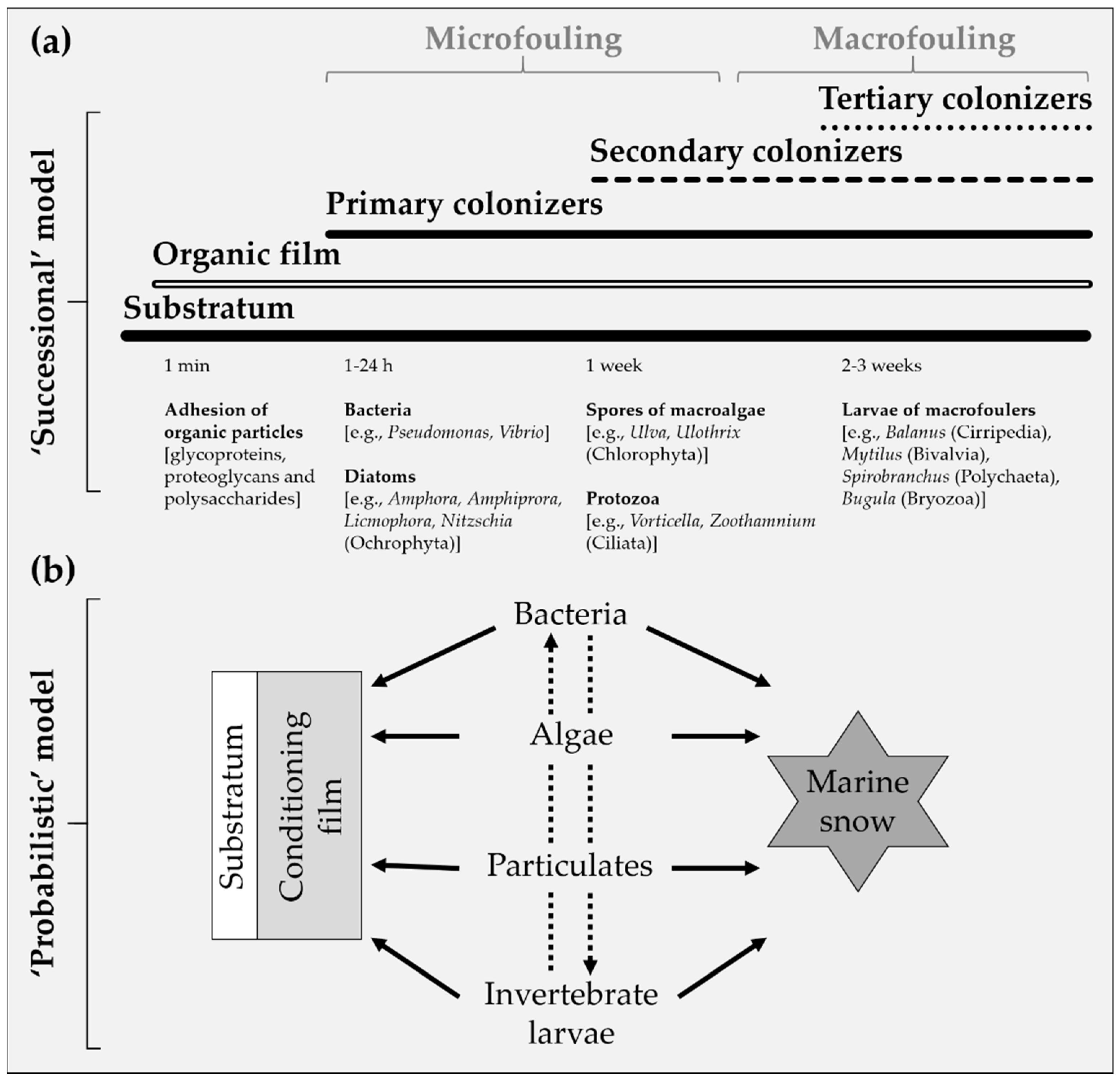
2.1. Colonization of Artificial Substrata
- Within minutes to hours of submersion, the substrata adsorb a biochemical conditioning biofilm, consisting of organic material such as glycoproteins, proteoglycans and polysaccharides naturally dissolved in the seawater.
- Within hours, primary colonizers, assemblages of unicellular organisms that secrete extracellular polymeric substances (EPS) adhere to the substrata. Together, the microorganisms and EPS facilitate the settlement of macrofoulers.
- Within days to weeks, secondary colonizers consisting of sessile macrofoulers, including soft- and hard-foulers, develop and overgrow the microfouling. As they grow and age, macrofoulers provide “micro-habitats” that attract further settlements.
- Within weeks to months, the substrata are fouled by tertiary colonizers which typically reside within the sessile biofouling. The biofouling communities reach maturity within a few years, accompanied by an increase in species diversity and richness. The communities are characterized by a variety of sessile and mobile benthic and epibenthic organisms.
2.2. Factors Influencing Marine Biofouling
2.2.1. Seawater Temperature
2.2.2. Depth and Light Availability
2.2.3. Currents and Distance to Shore
2.2.4. Material of Substrata
2.2.5. Topography and Wettability of Substrata
2.2.6. Color of Substrata
2.3. Key Macrofouling Groups to the MRE Sector
2.3.1. Kelp (Phylum Ochrophyta, Class Phaeophyceae, Order Laminariales)
Overview
Life Cycle
2.3.2. Bryozoans (Phylum Bryozoa)
Overview
Life Cycle
2.3.3. Mussels (Phylum Mollusca, Class Bivalvia, Family Mytilidae)
Overview
Life Cycle
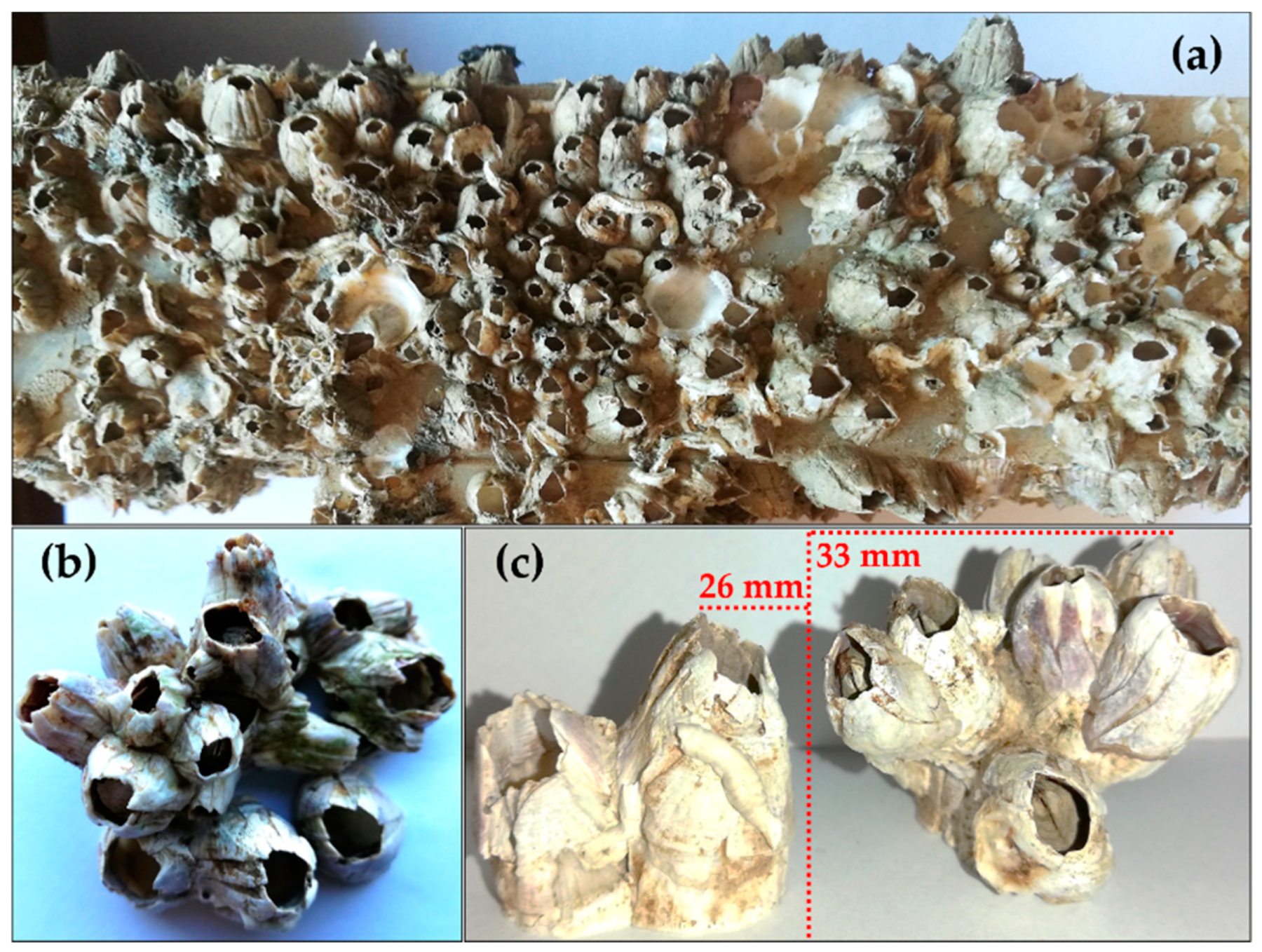
2.3.4. Acorn Barnacles (Phylum Arthropoda, Subphylum Crustacea, Infraclass Cirripedia)
Overview
Life Cycle
2.3.5. Calcareous Tubeworms (Phylum Annelida, Class Polychaeta, Family Serpulidae)
Overview
Life Cycle
2.4. Impact of Key Biofouling Groups to MRE Equipment
2.5. Biofouling Control
2.6. Artificial Reef Effect: Non-Native Species and Regulatory Framework
- 2004: The United Nations International Maritime Organization (IMO; http://www.imo.org) hosts the International Convention for the Control and Management of Ships’ Ballast Water and Sediments (BWM) which provides standards and guidelines to prevent, minimize and furtherly eliminate the transfer of harmful organisms and pathogens in ballast waters and sediments.
- 2008: The EU Marine Strategy Framework Directive (MSFD, Directive 2008/56/EC; https://ec.europa.eu) enters into force aiming at a more effective protection of the marine environment and biodiversity. The MSFD intends for the Members States to achieve “Good Environmental Status” with assessment of 11 Descriptors including the D2—Non-Indigenous Species, through an adaptive management approach which must be kept up-to-date and reviewed every six years.
- 2011: The IMO Marine Environment Protection Committee (MEPC) adopts the IMO guidelines for the control and management of ships’ biofouling to minimize the transfer of invasive aquatic species (resolution MEPC.207(62)), which was further supplemented by the 2012 guidance for minimizing the transfer of invasive aquatic species as biofouling (hull fouling) for recreational craft (MEPC.1/Circ.792).
- 2015: The EU Regulation on invasive alien species (IAS Regulation 1143/2014; https://ec.europa.eu) enters into force setting out rules to prevent, minimize and mitigate the adverse impacts caused by invasive species. The Regulation requires the Member States to study the introduction routes and spread of invasive species and to set up surveillance systems and action plans to ascertain the adequate preventive measures, among others.
3. European Biofouling Database
3.1. Database Description
- Realm, Province and Ecoregion (after [127]): Allows an overview of the countries encompassed in the mapping and provides insights of possible biofouling community patterns.
- Country and Site: Defines the country and location of the sampled. Non-European sites in the Mediterranean were included.
- Distance to shore: Defines the distance between the sampled site and the closest shore.
- Type of equipment/structure: Describes the surfaces surveyed, including equipment/structures from the MRE and oil and gas sectors; deliberate artificial reefs and test panels were included.
- Period of submersion: Defines the period (months/years) during which the biofouling could grow (i.e., from the equipment deployment until data was gathered).
- Depth: Defines the depth at which data was retrieved.
- Temperature and wave height: Presents temperature and wave height data in the area sampled (or for the closest area). Data were retrieved from the cited work or from WindGuru (http://www.windguru.cz) for the area and fouling period.
- Biofouling data: Includes qualitative and quantitative information on samples collected in the field and on biofouling organisms found in the samples: kelp, bryozoans, mussels, acorn barnacles and calcareous tubeworms and other relevant sessile biofoulers including NNS. Taxonomy was standardized according to WoRMS (World Record of Marine Species) (http://www.marinespecies.org). Quantitative data on samples include biofouling weight and thickness and the biofoulers weight and size (length or height, depending on the species). Weight was standardized mainly to kg fresh weight m−2.
- Reference: Identifies the source of the biofouling data presented.
3.2. Database Indicators
- The number of species increases with depth within the euphotic region down to about 40 m. Upper sections present greater biofouling weight (0–10 m) and thickness (0–20 m).
- Upper intertidal sections (0–6 m) are dominated by ephemeral green, red and brown algae and barnacles (e.g., Semibalanus balanoides). Below, kelp (e.g., Laminaria sp.) may develop down to mid water column sections depending on light availability and on seawater temperature, especially in the North Sea.
- From the lower intertidal to the infralittoral (down to about 30 m), bands of barnacles (e.g., S. balanoides, Balanus crenatus) and mussels (e.g., M. edulis, M. galloprovincialis) occur. The biomass of these organisms decreases with increasing depth down to 90 m. They are accompanied by a plethora of additional sessile organisms including serpulids (e.g., Spirobranchus sp., Hydroides sp.), anemones (e.g., Metridium senile, Sagartia sp.), hydrozoans (e.g., Tubularia/Ectopleura sp.), soft corals (e.g., Alcyonium digitatum) and sea-squirts (e.g., Ascidiella aspersa). In addition, a great variety of mobile organisms such as crustaceans (e.g., decapods: Pachygrapsus marmoratus, Pilumnus hirtellus; amphipods: Jassa sp.) and echinoderms (e.g., Asterias rubens, Paracentrotus lividus) is found.
- The seabed is occupied by mobile organisms such as decapods and starfishes which predate on organisms such as mussels and barnacles and restrict their lower (deeper) limit.
4. Discussion
- Only sessile organisms were included. Mobile organisms such as decapods, amphipods or starfish may still be a dominant constituent of the communities at certain depths (regarding density or coverage, since the bulk of weight and thickness is generally associated to sessile organisms). Sessile organisms are unable to freely move along artificial structures and allow for an easier understanding of biofouling zonation across depths. Furthermore, the stationary fouling organisms represent the main challenge associated with cleaning and maintenance of marine structures.
- The weight and thickness of the biofouling communities were used throughout the study and extracted from the literature as the biofouling parameters most responsible for the impacts to the MRE as opposed to density (number of organisms) and/or coverage percentage. This is because weight and thickness better reflect the magnitude of the biofouling impact such as loading, potential increased drag or altered hydrodynamic properties. Furthermore, several of the attached organisms are challenging to quantify by other means and large coverage or density does not necessarily translate into a great weight or thickness.
- The month/season during which data were obtained and the bathymetry where the sampled structured is placed were not included due to scarcity of data. Time of sampling remains an important factor, particularly for short periods of biofouling growth where sampling after the spring–summer period (which is the period when growth and reproduction rates are enhanced) could yield different results compared to sampling after the autumn–winter period (when severe environmental conditions may not only cause reduced growth and reproduction rates, but also may cause the displacement of organisms by larger waves). Nonetheless, the database includes data from an extensive range of submersion periods (up to 17 years) which is perceived as extensive enough to cover for seasonality bias. The depth (bathymetry) influences the structure of biofouling communities, especially in shallow waters which are generally more turbulent and have greater variation in physical-chemical parameters such as temperature and turbidity that influence the biofouling composition and abundance (biomass, density and coverage), compared to deeper waters. The bathymetry parameter may be added to the database in the future (including for the present sites).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Titah-Benbouzid, H.; Benbouzid, M. Biofouling issue on marine renewable energy converters: A state of the art review on impacts and prevention. Int. J. Energy Convers. 2017, 5, 67–78. [Google Scholar] [CrossRef]
- Loxton, J.; Macleod, A.K.; Nall, C.R.; McCollin, T.; Machado, I.; Simas, T.; Vance, T.; Kenny, C.; Want, A.; Miller, R.G. Setting an agenda for biofouling research for the marine renewable energy industry. Int. J. Mar. Energy 2017, 19, 292–303. [Google Scholar] [CrossRef]
- Ayers, J.; Turner, H. The principal fouling organisms. In Marine Fouling and Its Prevention; Redfield, A., Ketchum, B., Eds.; United States Naval Institute: Annapolis, MD, USA, 1952; pp. 118–164. [Google Scholar]
- Richmond, M.; Seed, R. A review of marine macrofouling communities with special reference to animal fouling. Biofouling 1991, 3, 151–168. [Google Scholar] [CrossRef]
- Lejars, M.N.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef]
- Videla, H.A.; Herrera, L.K. Microbiologically influenced corrosion: Looking to the future. Int. Microbiol. 2005, 8, 169–180. [Google Scholar]
- Jia, R.; Unsal, T.; Xu, D.; Lekbach, Y.; Gu, T. Microbiologically influenced corrosion and current mitigation strategies: A state of the art review. Int. Biodeterior. Biodegrad. 2019, 137, 42–58. [Google Scholar] [CrossRef]
- Kleemann, K. Biocorrosion by Bivalves. Mar. Ecol. 1996, 17, 145–158. [Google Scholar] [CrossRef]
- Blackwood, D.J.; Lim, C.S.; Teo, S.L.; Hu, X.; Pang, J. Macrofouling induced localized corrosion of stainless steel in Singapore seawater. Corros. Sci. 2017, 129, 152–160. [Google Scholar] [CrossRef]
- Pinori, E.; Berglin, M.; Brive, L.M.; Hulander, M.; Dahlström, M.; Elwing, H. Multi-seasonal barnacle (Balanus improvisus) protection achieved by trace amounts of a macrocyclic lactone (ivermectin) included in rosin-based coatings. Biofouling 2011, 27, 941–953. [Google Scholar] [CrossRef]
- Pinori, E.; Elwing, H.; Berglin, M. The impact of coating hardness on the anti-barnacle efficacy of an embedded antifouling biocide. Biofouling 2013, 29, 763–773. [Google Scholar] [CrossRef]
- Keller, R.P.; Geist, J.; Jeschke, J.M.; Kühn, I. Invasive species in Europe: Ecology, status, and policy. Environ. Sci. Eur. 2011, 23, 1–17. [Google Scholar] [CrossRef]
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: Some basic aspects. Mar. Ecol. Prog. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef]
- Abarzua, S.; Jakubowski, S. Biotechnological investigation for the prevention of biofouling. I. Biological and biochemical principles for the prevention of biofouling. Mar. Ecol. Prog. Ser. 1995, 123, 301–312. [Google Scholar] [CrossRef]
- Railkin, A.I. Marine Biofouling: Colonization Processes and Defenses; CRC press: Boca Raton, FL, USA, 2003; p. 303. [Google Scholar]
- Clare, A.; Rittschof, D.; Gerhart, D.; Maki, J. Molecular approaches to nontoxic antifouling. Invertebr. Reprod. Dev. 1992, 22, 67–76. [Google Scholar] [CrossRef]
- Maki, J.; Mitchell, R. Biofouling in the marine environment. In Encyclopedia of Environmental Microbiology; Bitton, G., Ed.; Wiley & Sons: New York, NY, USA, 2002; pp. 610–619. [Google Scholar]
- Roberts, D.; Rittschof, D.; Holm, E.; Schmidt, A. Factors influencing initial larval settlement: Temporal, spatial and surface molecular components. J. Exp. Mar. Biol. Ecol. 1991, 150, 203–221. [Google Scholar] [CrossRef]
- Terlizzi, A.; Faimali, M. Fouling on artificial substrata. In Biofouling; Dürr, S., Thomason, J.C., Eds.; Blackwell Publishing Ltd.: Chichester, UK, 2010; pp. 170–184. [Google Scholar]
- Rittschof, D. Research on practical environmentally benign antifouling coatings. In Biofouling; Dürr, S., Thomason, J.C., Eds.; Blackwell Publishing Ltd.: Chichester, UK, 2010; pp. 396–409. [Google Scholar]
- Lehaitre, M.; Delauney, L.; Compère, C. Biofouling and underwater measurements. In Real-Time Observation Systems for Ecosystem Dynamics Harmful Algal Blooms: Theory, Instrumentation Modelling; Babin, M., Roesler, C.S., Cullen, J.J., Eds.; UNESCO Publishing: Paris, France, 2008; pp. 463–493. [Google Scholar]
- Cao, S.; Wang, J.; Chen, H.; Chen, D. Progress of marine biofouling and antifouling technologies. Chin. Sci. Bull. 2011, 56, 598–612. [Google Scholar] [CrossRef]
- Van Der Stap, T.; Coolen, J.W.P.; Lindeboom, H.J. Marine Fouling Assemblages on Offshore Gas Platforms in the Southern North Sea: Effects of Depth and Distance from Shore on Biodiversity. PLoS ONE 2016, 11, e0146324. [Google Scholar] [CrossRef] [PubMed]
- Dayton, P.K. Competition, disturbance, and community organization: The provision and subsequent utilization of space in a rocky intertidal community. Ecol. Monogr. 1971, 41, 351–389. [Google Scholar] [CrossRef]
- Raffaelli, D.; Hawkins, S.J. Intertidal Ecology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; p. 365. [Google Scholar]
- Johnson, L.E.; Strathmann, R.R. Settling barnacle larvae avoid substrata previously occupied by a mobile predator. J. Exp. Mar. Biol. Ecol. 1989, 128, 87–103. [Google Scholar] [CrossRef]
- Newell, R.; Branch, G. The influence of temperature on the maintenance of metabolic energy balance in marine invertebrates. In Advances in Marine Biology; Blaxter, J.H.S., Russell, F.S., Yonge, M., Eds.; Elsevier: London, UK, 1980; Volume 17, pp. 329–396. [Google Scholar]
- Hellio, C.; Yebra, D. Advances in Marine Antifouling Coatings and Technologies; CRC Press: Boca Raton, FL, USA, 2009; p. 785. [Google Scholar]
- Almeida, L.P.; Coolen, J.W.P. Modelling thickness variations of macrofouling communities on offshore platforms in the Dutch North Sea. J. Sea Res. 2020, 156, 1–8. [Google Scholar] [CrossRef]
- Bartsch, I.; Wiencke, C.; Bischof, K.; Buchholz, C.M.; Buck, B.H.; Eggert, A.; Feuerpfeil, P.; Hanelt, D.; Jacobsen, S.; Karez, R. The genus Laminaria sensu lato: Recent insights and developments. Eur. J. Phycol. 2008, 43, 1–86. [Google Scholar] [CrossRef]
- Foster, B. Barnacle ecology and adaptation. In Barnacle Biology; Southward, A.J., Ed.; A.A. Balkema: Rotterdam, The Netherlands, 1987; pp. 113–133. [Google Scholar]
- Koehl, M. Mini review: Hydrodynamics of larval settlement into fouling communities. Biofouling 2007, 23, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Swain, G.; Anil, A.; Baier, R.E.; Chia, F.S.; Conte, E.; Cook, A.; Hadfield, M.; Haslbeck, E.; Holm, E.; Kavanagh, C. Biofouling and barnacle adhesion data for fouling-release coatings subjected to static immersion at seven marine sites. Biofouling 2000, 16, 331–344. [Google Scholar] [CrossRef]
- Kamino, K. Mini-review: Barnacle adhesives and adhesion. Biofouling 2013, 29, 735–749. [Google Scholar] [CrossRef]
- Rittschof, D.; Orihuela, B.; Stafslien, S.; Daniels, J.; Christianson, D.; Chisholm, B.; Holm, E. Barnacle reattachment: A tool for studying barnacle adhesion. Biofouling 2008, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.; Verran, J. The effect of substratum properties on the survival of attached microorganisms on inert surfaces. In Marine and Industrial Biofouling; Flemming, H.C., Murthy, P.S., Venkatesan, R., Cooksey, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 13–33. [Google Scholar]
- Pomerat, C.; Weiss, C. The influence of texture and composition of surface on the attachment of sedentary marine organisms. Biol. Bull. 1946, 91, 57–65. [Google Scholar] [CrossRef]
- Aldred, N.; Scardino, A.; Cavaco, A.; De Nys, R.; Clare, A.S. Attachment strength is a key factor in the selection of surfaces by barnacle cyprids (Balanus amphitrite) during settlement. Biofouling 2010, 26, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Scardino, A.; Guenther, J.; De Nys, R. Attachment point theory revisited: The fouling response to a microtextured matrix. Biofouling 2008, 24, 45–53. [Google Scholar] [CrossRef]
- Scardino, A.J.; De Nys, R. Mini review: Biomimetic models and bioinspired surfaces for fouling control. Biofouling 2011, 27, 73–86. [Google Scholar] [CrossRef]
- Clare, A.; Aldred, N. Surface colonisation by marine organisms and its impact on antifouling research. In Advances in Marine Antifouling Coatings and Technologies; Hellio, C., Yebra, D., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2009; pp. 46–79. [Google Scholar]
- Dahlem, C.; Moran, P.; Grant, T. Larval settlement of marine sessile invertebrates on surfaces of different colour and position. Ocean Sci. Eng. 1984, 9, 225–236. [Google Scholar]
- Swain, G.; Herpe, S.; Ralston, E.; Tribou, M. Short-term testing of antifouling surfaces: The importance of colour. Biofouling 2006, 22, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Dahms, H.U.; Qian, P.Y. Inhibition of biofouling by marine microorganisms and their metabolites. Biofouling 2006, 22, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Abed, R.M.; Voolstra, C.R. The effect of surface colour on the formation of marine micro and macrofouling communities. Biofouling 2013, 29, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Gallardo, T. Marine Algae: General Aspect (Biology, Systematics, Fields and Laboratory Techniques). In Marine Algae: Biodiversity, Taxonomy, Environmental Asessment, Biotechnology; Pereira, L., Neto, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–67. [Google Scholar]
- Streftaris, N.; Zenetos, A.; Papathanassiou, E. Globalisation in marine ecosystems: The story of non-indigenous marine species across European seas. In Oceanography and Marine Biology; Gibson, R.N., Atkinson, R.J.A., Gordon, J.D.M., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 419–453. [Google Scholar]
- Guiry, M.; Guiry, G. AlgaeBase: World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2019; Available online: https://www.algaebase.org/ (accessed on 23 March 2020).
- Ryland, J. Behaviour, Settlement and Metamorphosis of Bryzoan Larvae: A Review. Thalass. Jugosl. 1974, 10, 239–262. [Google Scholar]
- Soule, J.D.; Soule, D.F. Fouling and bioadhesion: Life strategies of bryozoans. In Biology of Bryozoans; Woollacott, R.M., Zimmer, R.L., Eds.; Academic Press: New York, NY, USA, 1977; pp. 437–457. [Google Scholar]
- Ostrovsky, A.N. Evolution of Sexual Reproduction in Marine Invertebrates: Example of Gymnolaemate Bryozoans; Springer: Dordrecht, The Netherlands, 2013; p. 356. [Google Scholar]
- Mihm, J.W.; Banta, W.C.; Loeb, G.I. Effects of adsorbed organic and primary fouling films on bryozoan settlement. J. Exp. Mar. Biol. Ecol. 1981, 54, 167–179. [Google Scholar] [CrossRef]
- Prendergast, G.S. Settlement and behaviour of marine fouling organisms. In Biofouling; Dürr, S., Thomason, J.C., Eds.; Blackwell Publishing Ltd.: Chichester, UK, 2010; pp. 30–59. [Google Scholar]
- Westerbom, M.; Kilpi, M.; Mustonen, O. Blue mussels, Mytilus edulis, at the edge of the range: Population structure, growth and biomass along a salinity gradient in the north-eastern Baltic Sea. Mar. Biol. Res. 2002, 140, 991–999. [Google Scholar]
- Bayne, B. Marine Mussels: Their Ecology and Physiology; Cambridge University Press: Cambridge, UK, 1976; Volume 10, p. 506. [Google Scholar]
- Widdows, J. Physiological ecology of mussel larvae. Aquaculture 1991, 94, 147–163. [Google Scholar] [CrossRef]
- Dinesen, G.E.; Morton, B. Review of the functional morphology, biology and perturbation impacts on the boreal, habitat-forming horse mussel Modiolus modiolus (Bivalvia: Mytilidae: Modiolinae). Mar. Biol. Res. 2014, 10, 845–870. [Google Scholar] [CrossRef]
- Southgate, T.; Myers, A. Mussel fouling on the Celtic Sea Kinsale field gas platforms. Estuar. Coast. Shelf Sci. 1985, 20, 651–659. [Google Scholar] [CrossRef]
- Bao, W.Y.; Satuito, C.G.; Yang, J.L.; Kitamura, H. Larval settlement and metamorphosis of the mussel Mytilus galloprovincialis in response to biofilms. Mar. Biol. Res. 2007, 150, 565–574. [Google Scholar] [CrossRef]
- Holm, E.R. Barnacles and Biofouling. Integr. Comp. Biol. 2012, 52, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Wendt, D.; Kowalke, G.; Kim, J.; Singer, I. Factors that influence elastomeric coating performance: The effect of coating thickness on basal plate morphology, growth and critical removal stress of the barnacle Balanus amphitrite. Biofouling 2006, 22, 1–9. [Google Scholar] [CrossRef]
- Larsson, A.I.; Mattsson-Thorngren, L.; Granhag, L.M.; Berglin, M. Fouling-release of barnacles from a boat hull with comparison to laboratory data of attachment strength. J. Exp. Mar. Biol. Ecol. 2010, 392, 107–114. [Google Scholar] [CrossRef]
- Southward, A. Barnacles: Keys and Notes for the Identification of British Species; Field Studies Council: Shrewsbury, UK, 2008; Volume 57, p. 140. [Google Scholar]
- Khandeparker, L.; Anil, A.C. Underwater adhesion: The barnacle way. Int. J. Adhes. Adhes. 2007, 27, 165–172. [Google Scholar] [CrossRef]
- Essock-Burns, T.; Gohad, N.V.; Orihuela, B.; Mount, A.S.; Spillmann, C.M.; Wahl, K.J.; Rittschof, D. Barnacle biology before, during and after settlement and metamorphosis: A study of the interface. J. Exp. Biol. 2017, 220, 194–207. [Google Scholar] [CrossRef]
- Anil, A.C.; Desai, D.; Khandeparker, L. Larval development and metamorphosis in Balanus amphitrite Darwin (Cirripedia; Thoracica): Significance of food concentration, temperature and nucleic acids. J. Exp. Mar. Biol. Ecol. 2001, 263, 125–141. [Google Scholar] [CrossRef]
- Crisp, D.J.; Spencer, C. The control of the hatching process in barnacles. Proc. R. Soc. B Biol. Sci. 1958, 149, 278–299. [Google Scholar]
- Clare, A.S.; Matsumura, K. Nature and perception of barnacle settlement pheromones. Biofouling 2000, 15, 57–71. [Google Scholar] [CrossRef]
- Abramova, A.; Lind, U.; Blomberg, A.; Rosenblad, M.A. The complex barnacle perfume: Identification of waterborne pheromone homologues in Balanus improvisus and their differential expression during settlement. Biofouling 2019, 35, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, M.G. Biofilms and Marine Invertebrate Larvae: What Bacteria Produce That Larvae Use to Choose Settlement Sites. Annu. Rev. Mar. Sci. 2011, 3, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Holmström, C.; Rittschof, D.; Kjelleberg, S. Inhibition of Settlement by Larvae of Balanus amphitrite and Ciona intestinalis by a Surface-Colonizing Marine Bacterium. Appl. Environ. Microbiol. 1992, 58, 2111–2115. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, G.H.; Vega, I.E.; Wahl, K.J.; Orihuela, B.; Beyley, V.; Rodriguez, E.N.; Everett, R.K.; Bonaventura, J.; Rittschof, D. Barnacle cement: A polymerization model based on evolutionary concepts. J. Exp. Biol. 2009, 212, 3499–3510. [Google Scholar] [CrossRef]
- Ten Hove, H.; Kupriyanova, E. Taxonomy of Serpulidae (Annelida, Polychaeta): The state of affairs. Zootaxa 2009, 2036, 1–126. [Google Scholar] [CrossRef]
- Kupriyanova, E.; Nishi, E.; Hove, H.; Rzhavsky, A. Life-history patterns in serpulimorph polychaetes: Ecological and Evolutionary perspectives. Oceanogr. Mar. Biol. 2001, 39, 1–101. [Google Scholar]
- Toonen, R.J.; Pawlik, J.R. Settlement of the tube worm Hydroides dianthus (Polychaeta: Serpulidae): Cues for gregarious settlement. Mar. Biol. 1996, 126, 725–733. [Google Scholar] [CrossRef]
- Bruno, P. Escape Hatches for the Clonal Offspring of Serpulid Polychaetes. Biol. Bull. 2001, 200, 107–117. [Google Scholar]
- Jusoh, I.; Wolfram, J. Effects of marine growth and hydrodynamic loading on offshore structures. J. Mek. 1996, 1, 77–96. [Google Scholar]
- Decurey, B.; Schoefs, F.; Barillé, A.L.; Soulard, T. Model of Bio-Colonisation on Mooring Lines: Updating Strategy Based on a Static Qualifying Sea State for Floating Wind Turbines. J. Mar. Sci. Eng. 2020, 8, 108. [Google Scholar] [CrossRef]
- Relini, G.; Tixi, F.; Relini, M.; Torchia, G. The macrofouling on offshore platforms at Ravenna. Int. Biodeterior. Biodegrad. 1998, 41, 41–55. [Google Scholar] [CrossRef]
- Miller, R.; Macleod, A. Marine Growth Mapping and Monitoring: Feasibility of Predictive Mapping of Marine Growth; Shree Rajasthan Syntex Ltd.: Glasgow, UK, 2016; p. 51. [Google Scholar]
- Oliveira, D.; Granhag, L. Matching Forces Applied in Underwater Hull Cleaning with Adhesion Strength of Marine Organisms. J. Mar. Sci. Eng. 2016, 4, 66. [Google Scholar] [CrossRef]
- Schultz, M.P.; Kavanagh, C.J.; Swain, G.W. Hydrodynamic forces on barnacles: Implications on detachment from fouling-release surfaces. Biofouling 1999, 13, 323–335. [Google Scholar] [CrossRef]
- Holm, E.R.; Kavanagh, C.J.; Meyer, A.E.; Wiebe, D.; Nedved, B.T.; Wendt, D.; Smith, C.M.; Hadfield, M.G.; Swain, G.; Wood, C.D.; et al. Interspecific variation in patterns of adhesion of marine fouling to silicone surfaces. Biofouling 2006, 22, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, J.; Boud, R. Future Marine Energy. Results of the Marine Energy Challenge: Cost Competitiveness and Growth of Wave and Tidal Stream Energy; Carbon Trust: London, UK, 2006; pp. 1–36. [Google Scholar]
- Mérigaud, A.; Ringwood, J.V. Condition-based maintenance methods for marine renewable energy. Renew. Sustain. Energy Rev. 2016, 66, 53–78. [Google Scholar] [CrossRef]
- Borthwick, A.G.L. Marine Renewable Energy Seascape. Engineering 2016, 2, 69–78. [Google Scholar] [CrossRef]
- Dobretsov, S.; Coutinho, R.; Rittschof, D.; Salta, M.; Ragazzola, F.; Hellio, C. The oceans are changing: Impact of ocean warming and acidification on biofouling communities. Biofouling 2019, 35, 585–595. [Google Scholar] [CrossRef]
- Redfield, A.; Ketchum, B. Marine Fouling and Its Prevention; United States Naval Institute: Annapolis, MD, USA, 1952; p. 388. [Google Scholar]
- Fischer, E.; Castelli, V.; Rodgers, S.; Bleile, H. Technology for Control of Marine Biofouling—A Review. In Marine Biodeterioration: An Interdisciplinary Study; Costlaw, J.D., Tipper, R.C., Eds.; Naval Institute Press: Annapolis, MD, USA, 1984; pp. 261–299. [Google Scholar]
- Swain, G. Redefining antifouling coatings. J. Prot. Coat. Linings 1999, 16, 26–35. [Google Scholar]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J. Modern Approaches to Marine Antifouling Coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Fusetani, N.; Clare, A. Antifouling Compounds; Springer: Berlin/Heidelberg, Germany, 2006; p. 226. [Google Scholar]
- Almeida, E.; Diamantino, T.C.; De Sousa, O. Marine paints: The particular case of antifouling paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Magin, C.M.; Cooper, S.P.; Brennan, A.B. Non-toxic antifouling strategies. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Salta, M.; Wharton, J.A.; Stoodley, P.; Dennington, S.P.; Goodes, L.R.; Werwinski, S.; Mart, U.; Wood, R.J.K.; Stokes, K.R. Designing biomimetic antifouling surfaces. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4729–4754. [Google Scholar] [CrossRef] [PubMed]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Poll. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef]
- Buskens, P.; Wouters, M.; Rentrop, C.; Vroon, Z. A brief review of environmentally benign antifouling and foul-release coatings for marine applications. J. Coat. Technol. Res. 2013, 10, 29–36. [Google Scholar] [CrossRef]
- Gittens, J.E.; Smith, T.J.; Suleiman, R.; Akid, R. Current and emerging environmentally-friendly systems for fouling control in the marine environment. Biotechnol. Adv. 2013, 31, 1738–1753. [Google Scholar] [CrossRef]
- Legg, M.; Yücel, M.K.; Garcia de Carellan, I.; Kappatos, V.; Selcuk, C.; Gan, T.H. Acoustic methods for biofouling control: A review. Ocean Eng. 2015, 103, 237–247. [Google Scholar] [CrossRef]
- Ciriminna, R.; Bright, F.V.; Pagliaro, M. Ecofriendly Antifouling Marine Coatings. ACS Sustain. Chem. Eng. 2015, 3, 559–565. [Google Scholar] [CrossRef]
- Amara, I.; Miled, W.; Slama, R.B.; Ladhari, N. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Environ. Toxicol. Pharmacol. 2018, 57, 115–130. [Google Scholar] [CrossRef]
- Xie, Q.; Pan, J.; Ma, C.; Zhang, G. Dynamic surface antifouling: Mechanism and systems. Soft Matter 2019, 15, 1087–1107. [Google Scholar] [CrossRef]
- Verma, S.; Mohanty, S.; Nayak, S. A review on protective polymeric coatings for marine applications. J. Coat. Technol. Res. 2019, 16, 307–338. [Google Scholar] [CrossRef]
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Hunsucker, K.Z.; Braga, C.; Gardner, H.; Jongerius, M.; Hietbrink, R.; Salters, B.; Swain, G. Using ultraviolet light for improved antifouling performance on ship hull coatings. Biofouling 2019, 35, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Wake, H.; Takahashi, H.; Takimoto, T.; Takayanagi, H.; Ozawa, K.; Kadoi, H.; Okochi, M.; Matsunaga, T. Development of an electrochemical antifouling system for seawater cooling pipelines of power plants using titanium. Biotechnol. Bioeng. 2006, 95, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Dickenson, N.C.; Krumholz, J.S.; Hunsucker, K.Z.; Radicone, M. Iodine-infused aeration for hull fouling prevention: A vessel-scale study. Biofouling 2017, 33, 955–969. [Google Scholar] [CrossRef]
- Gunari, N.; Brewer, L.H.; Bennett, S.M.; Sokolova, A.; Kraut, N.D.; Finlay, J.A.; Meyer, A.E.; Walker, G.C.; Wendt, D.E.; Callow, M.E. The control of marine biofouling on xerogel surfaces with nanometer-scale topography. Biofouling 2011, 27, 137–149. [Google Scholar] [CrossRef]
- Kirschner, C.M.; Brennan, A.B. Bio-inspired antifouling strategies. Annu. Rev. Mater. Res. 2012, 42, 211–229. [Google Scholar] [CrossRef]
- Hanssen, K.Ø.; Cervin, G.; Trepos, R.; Petitbois, J.; Haug, T.; Hansen, E.; Andersen, J.H.; Pavia, H.; Hellio, C.; Svenson, J. The bromotyrosine derivative ianthelline isolated from the arctic marine sponge Stryphnus fortis inhibits marine micro-and macrobiofouling. Mar. Biotechnol. 2014, 16, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Moodie, L.W.; Trepos, R.; Cervin, G.; Brathen, K.A.; Lindgard, B.; Reiersen, R.; Cahill, P.; Pavia, H.; Hellio, C.; Svenson, J. Prevention of marine biofouling using the natural allelopathic compound batatasin-III and synthetic analogues. J. Nat. Prod. 2017, 80, 2001–2011. [Google Scholar] [CrossRef]
- Moodie, L.W.; Trepos, R.; Cervin, G.; Larsen, L.; Larsen, D.S.; Pavia, H.; Hellio, C.; Cahill, P.; Svenson, J. Probing the structure-activity relationship of the natural antifouling agent polygodial against both micro-and macrofoulers by semisynthetic modification. J. Nat. Prod. 2017, 80, 515–525. [Google Scholar] [CrossRef]
- Moodie, L.W.; Cervin, G.; Trepos, R.; Labriere, C.; Hellio, C.; Pavia, H.; Svenson, J. Design and biological evaluation of antifouling dihydrostilbene oxime hybrids. Mar. Biotechnol. 2018, 20, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Bixler, G.D.; Theiss, A.; Bhushan, B.; Lee, S.C. Anti-fouling properties of microstructured surfaces bio-inspired by rice leaves and butterfly wings. J. Coll. Interface Sci. 2014, 419, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Dundar Arisoy, F.; Kolewe, K.W.; Homyak, B.; Kurtz, I.S.; Schiffman, J.D.; Watkins, J.J. Bioinspired photocatalytic shark-skin surfaces with antibacterial and antifouling activity via nanoimprint lithography. ACS Appl. Mater. Interfaces 2018, 10, 20055–20063. [Google Scholar] [CrossRef]
- Trepos, R.; Cervin, G.; Hellio, C.; Pavia, H.; Stensen, W.; Stensvag, K.; Svendsen, J.S.; Haug, T.; Svenson, J. Antifouling compounds from the sub-arctic ascidian Synoicum pulmonaria: Synoxazolidinones A and C, pulmonarins A and B, and synthetic analogues. J. Nat. Prod. 2014, 77, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Trepos, R.; Cervin, G.; Pile, C.; Pavia, H.; Hellio, C.; Svenson, J. Evaluation of cationic micropeptides derived from the innate immune system as inhibitors of marine biofouling. Biofouling 2015, 31, 393–403. [Google Scholar] [CrossRef]
- Trepos, R.; Pinori, E.; Jonsson, P.; Berglin, M.; Svenson, J.; Coutinho, R.; Lausmaa, J.; Hellio, C. Innovative approaches for the development of new copper-free marine antifouling paints. J. Ship Ocean Technol. 2014, 9, 7–18. [Google Scholar]
- Inger, R.; Attrill, M.J.; Bearhop, S.; Broderick, A.C.; James Grecian, W.; Hodgson, D.J.; Mills, C.; Sheehan, E.; Votier, S.C.; Witt, M.J.; et al. Marine renewable energy: Potential benefits to biodiversity? An urgent call for research. J. Appl. Ecol. 2009, 46, 1145–1153. [Google Scholar] [CrossRef]
- Wilhelmsson, D.; Malm, T. Fouling assemblages on offshore wind power plants and adjacent substrata. Estuar. Coast. Shelf Sci. 2008, 79, 459–466. [Google Scholar] [CrossRef]
- Langhamer, O.; Wilhelmsson, D.; Engström, J. Artificial reef effect and fouling impacts on offshore wave power foundations and buoys—A pilot study. Estuar. Coast. Shelf Sci. 2009, 82, 426–432. [Google Scholar] [CrossRef]
- Lewis, J.; Coutts, A. Biofouling invasions. In Biofouling; Dürr, S., Thomason, J., Eds.; Wiley-Blackwell: Singapore, 2009; pp. 348–365. [Google Scholar]
- Mineur, F.; Cook, E.J.; Minchin, D.; Bohn, K.; MacLeod, A.; Maggs, C.A. Changing coasts: Marine aliens and artificial structures. In Oceanography and Marine Biology—An Annual Review; Gibson, R.N., Atkinson, R.J.A., Gordon, J.D.M., Hughes, R.N., Eds.; CRC Press: Abingdon, UK, 2012; pp. 189–234. [Google Scholar]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Want, A.; Porter, J. BioFREE: An International Study of Biofouling Impacts on the Marine Renewable Energy Industry. In Proceedings of the 2018 OCEANS MTS/IEEE Kobe Techno-Oceans, Kobe, Japan, 28–31 May 2018; pp. 1874–1880. [Google Scholar]




| Author | Title |
|---|---|
| Redfield and Ketchum, 1952 [90] | Marine fouling and its prevention (Part III: Prevention of fouling) |
| Fisher et al., 1984 [91] | Technology for control of marine biofouling—A review |
| Wahl, 1989 [13] | Marine Epibiosis. I. Fouling and antifouling: Some basic aspects |
| Swain, 1999 [92] | Redefining antifouling coatings |
| Yebra et al., 2004 [46] | Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings |
| Chambers et al., 2006 [93] | Modern approaches to marine antifouling coatings |
| Fusetani and Clare, 2006 [94] | Antifouling compounds |
| Almeida et al., 2007 [95] | Marine paints: The particular case of antifouling paints |
| Hellio and Yebra, 2009 [28] | Advances in marine antifouling coatings and technologies |
| Magin et al., 2010 [96] | Non-toxic antifouling strategies |
| Salta et al., 2010 [97] | Designing biomimetic antifouling surfaces |
| Callow and Callow, 2011 [98] | Trends in the development of environmentally friendly fouling-resistant marine coatings |
| Cao et al., 2011 [22] | Progress of marine biofouling and antifouling technologies |
| Dafforn et al., 2011 [99] | Antifouling strategies: History and regulation, ecological impacts and mitigation |
| Lejars et al., 2012 [5] | Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings |
| Buskens et al., 2013 [100] | A brief review of environmentally benign antifouling and foul-release coatings for marine applications |
| Gittens et al., 2013 [101] | Current and emerging environmentally-friendly systems for fouling control in the marine environment |
| Legg et al., 2015 [102] | Acoustic methods for biofouling control: A review |
| Ciriminna et al., 2015 [103] | Ecofriendly antifouling—Marine coatings |
| Amara et al., 2018 [104] | Antifouling processes and toxicity effects of antifouling paints on marine environment: A review |
| Xie et al., 2019 [105] | Dynamic surface antifouling: Mechanism and systems |
| Verma et al., 2019 [106] | A review on protective polymeric coatings for marine applications |
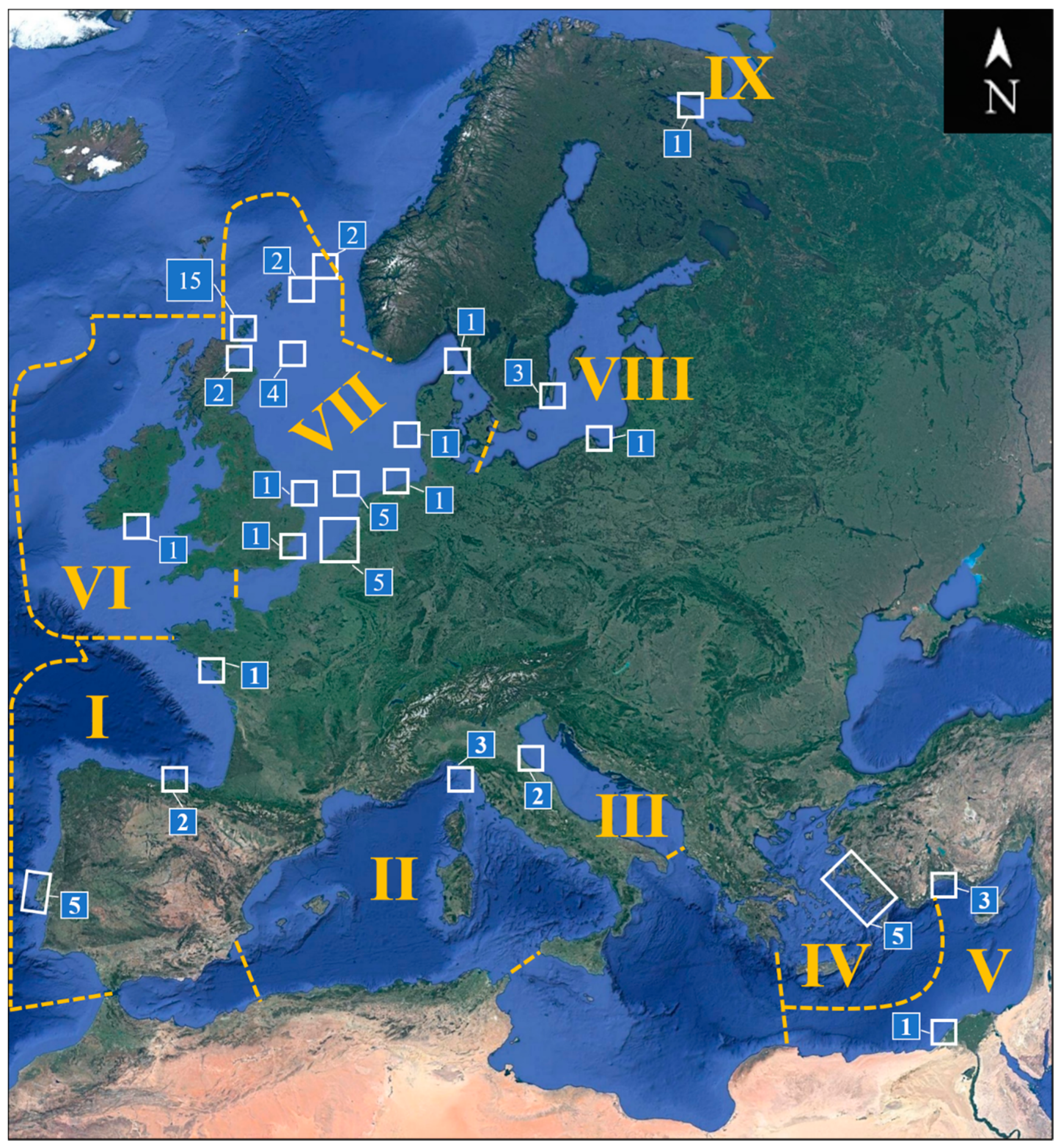
| Region | Ecoregion | Countries |
|---|---|---|
| I | South European Atlantic Shelf | Portugal, Spain (north coast), France (west coast) |
| II | Western Mediterranean Sea | Italy (west coast) |
| III | Adriatic Sea | Italy (east coast) |
| IV | Aegean Sea | Turkey (west coast) |
| V | Levantine Sea | Turkey (south coast), Egypt |
| VI | Celtic Seas | Ireland |
| VII | North Sea | England, Scotland, Belgium, Netherlands, Germany, Denmark, Sweden (west coast), “North Sea” |
| VIII | Baltic Sea | Sweden (east coast), Poland |
| IX | White Sea | Russia (west coast) |
| Region | Country/Location | Records a | Structures | Period (months) | Depth (m) | Distance (km) |
|---|---|---|---|---|---|---|
| I | France | 1 | Moorings | 17–19 | 0–25 | 20 |
| I | Portugal | 5 | Metallic and plastic panels, Rubber seals | 7.5–17 | 0–10 | Onshore–0.4 |
| I | Spain | 2 | Metallic and plastic panels | 5 and 9 | 25 | 1.7 |
| II | Italy | 3 | Buoys, panels | 3–70 | 0–39 | 55 and 68.5 |
| III | Italy | 2 | Gas platform piles | 204–209 | 0–12 | 10.5 |
| IV | Turkey | 5 | Asbestos-cement panels | 3–12 | 3 | 0.1 |
| V | Egypt | 1 | Polystyrene panels | 1 | 0–5.5 | 0.5 |
| V | Turkey | 3 | Wood panels | 13 | 0–1 | 0.1 |
| VI | Ireland | 1 | Gas platform piles | 15–51 | 3–90 | 50 |
| VII | Belgium | 4 | Monopiles, concrete foundations | 2–45 | 0–25 | 30 and 49 |
| VII | Denmark | 1 | Monopiles | 12–42 | 0–10 | 14 |
| VII | England | 2 | Monopiles | 31 | 0–10 | 10–50 |
| VII | Germany | 1 | Jacket foundation | 21–51 | 0–28 | 45 |
| VII | Netherlands | 6 | Monopiles, jacket foundations | 18–468 | 0–43 | 18–177 |
| VII | North Sea | 4 | Oil and gas platforms, clamps | 36–180 | 0–67 | 100–115 |
| VII | Scotland | 21 | Jacket foundations, piles, buoys, chains, ADCP, WEC, pontoons, harbor walls, metallic panels | 11–60 | 0–90 | 0.1–195 |
| VII | Sweden | 1 | Concrete foundation | 2–26 | 0–25 | 2 |
| VIII | Poland | 1 | PVC panels | 0.3–2 | 3–7 | 0.35 |
| VIII | Sweden | 3 | Bridge pillars, monopiles, boulders | 24–300 | 1.5–5 | Onshore–12 |
| IX | Russia | 1 | Ceramic panels | 2–13 | 1.5 | 0.1 |
| Sampling Location Information | |||||
| Realm a | Temperate Northern Atlantic | ||||
| Province a | Lusitanean | ||||
| Ecoregion a | South European Atlantic Shelf | ||||
| Country-city | Portugal-Peniche | ||||
| Site | AW-Energy WaveRoller test site | ||||
| Coordinates | 39°22′56.64″ N, 9°18′58.68″ W | ||||
| Distance to land | 0.4 km | ||||
| Equipment | Metallic and plastic test panels | ||||
| Fouling period | 12 months | ||||
| Depth | 5–10 m | ||||
| Temperature | 12.9 °C (January)–20.2 °C (August) | ||||
| Wave height | 1.6 m (September)–3.1 m (February) | ||||
| Biofouling Sample | |||||
| Sample maximum thickness | 83.7 mm (at 5 m) | ||||
| Sample maximum weight | 33.5 kg fresh weight m−2 (at 5 m) | ||||
| Biofouling Organisms | |||||
| Group b | Sub-group b | Species b | Common Name b | Max. Size c | Max Weight d |
| Polychaeta | Serpulidae | Hydroides sp. | Tubeworm | - | - |
| Spirobranchus sp. | Christmas tree worms | - | - | ||
| Bryozoa | Gymnolaemata | Bugula sp. | Bryozoan | - | 3.7 e |
| Crustacea | Cirripedia | Perforatus perforatus | Acorn barnacle | 22.1 e | 5.1 e |
| Mollusca | Bivalvia | Anomia ephippium | Saddle oyster | 5.9 f | 0.001 f |
| Hiatella arctica | Wrinkled rock borer | 12.1 e | 0.05 e | ||
| Musculus costulatus | Flat striped shell | - | - | ||
| Mytilus galloprovincialis | Mediterranean mussel | 89.6 e | 23.8 e | ||
| Biofouling | Group a | Species | Common Name | Country b | |
|---|---|---|---|---|---|
| Algae | Soft-fouling | Cl. Phaeophyceae | Colpomenia peregrina | Oyster thief | Sco |
| Ph. Chlorophyta | Codium fragile fragile | Sponge seaweed | Sco | ||
| Ph. Rhodophyta | Dasysiphonia japonica | Siphoned Japan weed | Sco | ||
| Invertebrates | Or. Diptera | Telmatogeton japonicus | Marine splash midge | Bel, Den, Net, Swe | |
| S.Ph. Crustacea | Jassa marmorata | Amphipod | Bel, Den, Eng, Net | ||
| Caprella mutica | Japanese skeleton shrimp | Den, Net, Sco | |||
| Hemigrapsus sanguineus | Pacific crab, Asian shore crab | Bel | |||
| Ph. Chordata | Corella eumyota | Orange-tipped sea squirt | Sco | ||
| Hard-fouling | Ph. Bryozoa | Schizoporella japonica | Orange ripple bryozoan | Sco | |
| S.Ph. Crustacea | Amphibalanus amphitrite | Striped barnacle | Bel | ||
| Austrominius modestus | Australasian barnacle | Bel, Sco, Eng, Net, Pt | |||
| Balanus glandula | North American barnacle | Bel | |||
| Megabalanus coccopoma | Titan acorn barnacle | Bel, Net | |||
| Megabalanus tintinnabulum | Sea tulip | Bel | |||
| Perforatus perforatus | Acorn barnacle | Bel, Net | |||
| Ph. Mollusca | Magallana gigas | Pacific oyster | Bel, Ger, Ita, Net | ||
| Crepidula fornicata | American slipper limpet | Bel | |||
| Patella vulgata | Common limpet | Bel | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinagre, P.A.; Simas, T.; Cruz, E.; Pinori, E.; Svenson, J. Marine Biofouling: A European Database for the Marine Renewable Energy Sector. J. Mar. Sci. Eng. 2020, 8, 495. https://doi.org/10.3390/jmse8070495
Vinagre PA, Simas T, Cruz E, Pinori E, Svenson J. Marine Biofouling: A European Database for the Marine Renewable Energy Sector. Journal of Marine Science and Engineering. 2020; 8(7):495. https://doi.org/10.3390/jmse8070495
Chicago/Turabian StyleVinagre, Pedro Almeida, Teresa Simas, Erica Cruz, Emiliano Pinori, and Johan Svenson. 2020. "Marine Biofouling: A European Database for the Marine Renewable Energy Sector" Journal of Marine Science and Engineering 8, no. 7: 495. https://doi.org/10.3390/jmse8070495
APA StyleVinagre, P. A., Simas, T., Cruz, E., Pinori, E., & Svenson, J. (2020). Marine Biofouling: A European Database for the Marine Renewable Energy Sector. Journal of Marine Science and Engineering, 8(7), 495. https://doi.org/10.3390/jmse8070495






