The More You Search, the More You Find: A New Mediterranean Endemism of the Genus Ocenebra Gray, 1847 (Mollusca: Gastropoda: Muricidae) from a Submarine Cave of the Messina Strait Area (Italy)
Abstract
:1. Introduction
2. Material and Methods
2.1. Field Work
2.2. Laboratory Work
2.3. Nomenclature, Abbreviations, and Acronyms
3. Results
3.1. Systematics
3.2. Material Examined
3.3. Type Locality
3.4. Description
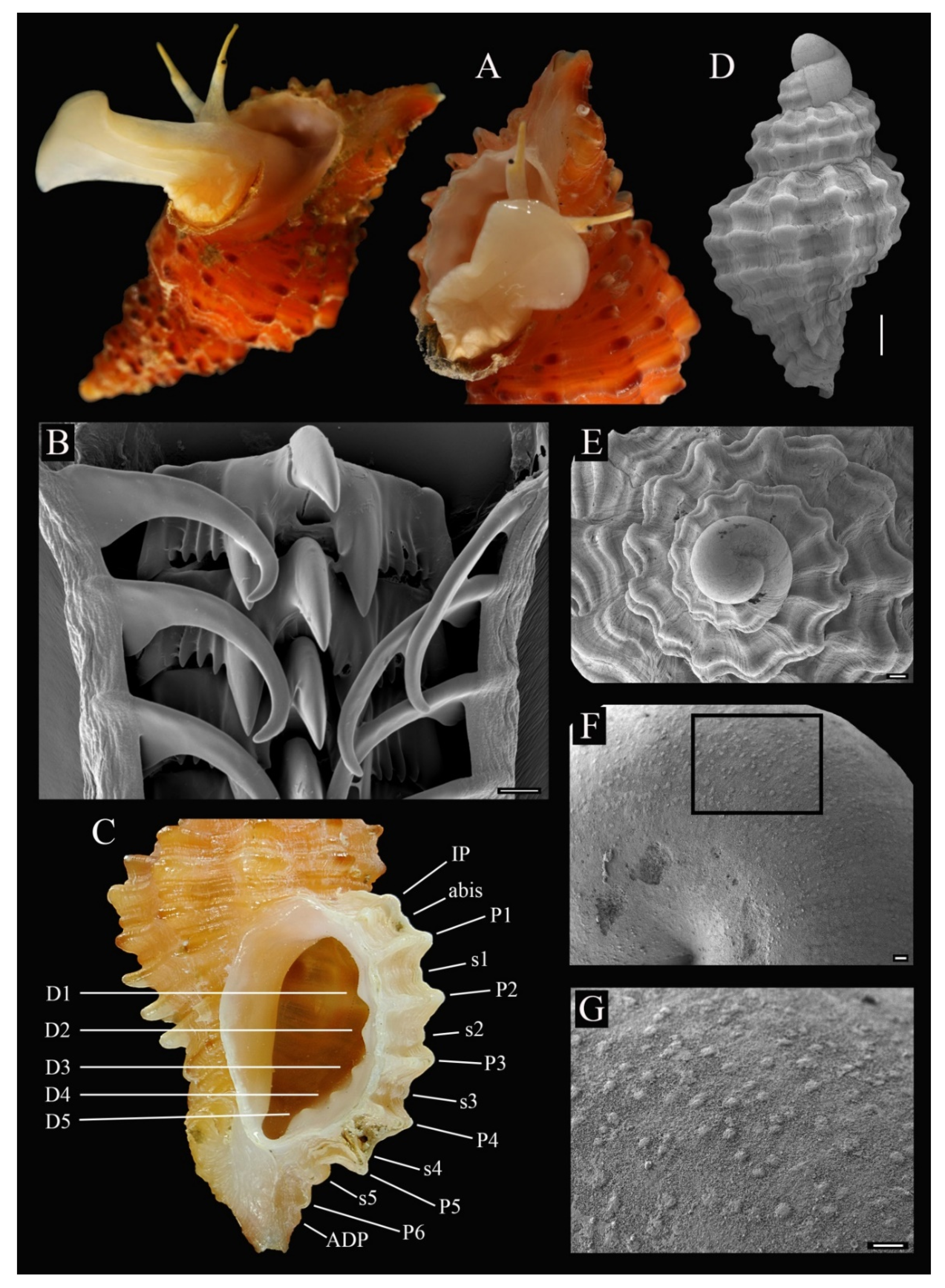
3.5. Etymology
3.6. Distribution
3.7. Taxonomic Remarks
| O. [erinaceus] erinaceus (Linnaeus, 1758) | O. [erinaceus] brevirobusta Houart, 2000 | O. chavesi Houart, 1996 | O. edwardsii (Payraudeau, 1826) complex | |
| Figures | 2A–E | |||
| Shell TH (in mm) | up to 65 | up to 42 | up to 21.4 | up to 26.82 |
| Protoconch w: whorls m: microsculpture | w: 1.25–1.5 m: smooth | w: 1.5 m: smooth | w: 1.5 m: smooth | w: 1.25–1.5 m: smooth or with small granules |
| Teleoconch ga: general aspect w: whorls cp: colour pattern | ga: fusiform, rounded whorls w: up to 7 cp: whitish, light tan, pale brown, occasionally with darker spiral bands | ga: rounded w: up to 6 cp: pale or dark brown | ga: rounded, slightly scalariform w: up to 6 cp: light tan or pale brown | ga: rounded, scalariform w: up to 6.5 cp: various (from whitish to dark brown, usually light tan with brown blotches or whitish spiral bands) |
| Teleoconch sculpture of the convex part of the last whorl a: axial s: spiral t: threads | a: 3–11 low or high, rounded, occasionally strong varices s: 6 primary cords alternated by smaller secondary cords t: often present | a: 4–6 broad, large, rounded varices, occasionally very low s: 6 primary cords (with obsolete P2) alternated by smaller secondary cords t: occasionally present | a: 6–7 broad, high, squamous ribs s: 6 primary cords alternated by smaller secondary cords t: often present | a: from 7–9 low, rounded, nodose or occasionally spinose ribs, usually with 1–2 erratically placed varices s: 6 narrow and strong primary cords alternated by smaller secondary cords t: present, occasionally absent |
| Aperture ga: general aspect cl: columellar lip ol: outer lip d: denticles ID: infrasutural apertural denticle lt: labral tooth | ga: moderately large, elongate-ovate, whitish internal colour cl: narrow, smooth, adherent adapically ol: weakly crenulate d: from absent to 5 weak or strong, occasionally some could appear double ID: occasionally present lt: absent | ga: moderately large, broad, roundy-ovate, whitish internal colour cl: narrow, smooth, adherent adapically ol: crenulate d: 5 strong, occasionally some could appear double ID: occasionally present lt: absent | ga: moderately large, ovate, whitish internal colour cl: smooth, adherent adapically ol: crenulate d: 5 weak, sometimes one could appear double ID: occasionally present lt: absent | ga: large, ovate, white or pale brown internal colour, with occasionally whitish spiral bands cl: narrow, smooth, adherent ol: crenulate, erect. d: 5 weak, sometimes one could appear double ID: absent lt: absent |
| Radula rachidian cusps c: central l: lateral | c: elongate but quite thick l: elongate but quite thick | unknown | c: elongate but quite thick l: elongate but quite thick | c: elongate l: elongate |
| Animal general colour pattern | creamish | unknown | unknown | creamish |
| Depth range (in m) | 0–130 | littoral | 10–22 | 0–70 |
| Distribution | Atlantic-Mediterranean | Atlantic | Atlantic | Atlantic-Mediterranean |
| Notes | 1 | 1 | 2 | |
| O. helleri (Brusina, 1865) | O. hybrida (Aradas and Benoît, 1876) - O. piantonii (Cecalupo, Buzzurro, and Mariani, 2008) | O. inordinata Houart and Abreu, 1994 | O. miscowichae (Pallary, 1920) | |
| Figures | 2F | 3A, possibly 3B, and 3C (see Notes) | 3D | 3E |
| Shell TH (in mm) | up to 23 | up to 18.5 | up to 21 | up to 22 |
| Protoconch w: whorls m: microsculpture | w: 1.5 m: smooth with small and dense granules, often with well visible dense growth lines on the last half whorl | w: 1.25–1.5 m: uncertain, presumably with small granules | w: 1.25–1.5 m: unknown | w: 1.25 m: unknown |
| Teleoconch ga: general aspect w: whorls cp: colour pattern | ga: slender, scalariform w: up to 6.5 cp: from pale orange to light tan, often with a whitish spiral band in median zone (sometimes two) | ga: rounded, scalariform w: up to 5 cp: various (uniformly light or dark brown, blackish or whitish, occasionally with one or two spiral bands in median zone) | ga: shouldered, strongly nodose w: up to 6 cp: light brown | ga: rounded, scalariform w: up to 5 cp: uniformly light tan or light tan with darker spiral bands |
| Teleoconch sculpture of the convex part of the last whorl a: axial s: spiral t: threads | a: 6–13 broad, rounded, nodose ribs, occasionally with an erratically placed varix s: 6 high and rounded primary cords alternated by smaller secondary cords t: absent | a: from 3–5 to 6–7 narrow, high, spinose varices, occasionally with weak nodose ribs s: 6 narrow and strong rounded primary cord alternated by smaller secondary cords t: present | a: 4–5 varices alternated by 1–2 high strong nodes s: 6 low shallow primary cords alternated by smaller secondary cords t: occasionally present | a: 7–9 low, weakly nodose ribs s: 6 strong and narrow primary cords alternated by smaller secondary cords t: occasionally present |
| Aperture ga: general aspect cl: columellar lip ol: outer lip d: denticles ID: infrasutural apertural denticle lt: labral tooth | ga: moderately large, ovate, brown internal colour cl: narrow, smooth, weakly erect abapically, adherent adapically ol: crenulate, erect d: 5 strong and well-marked, often one (or two) appear/s double ID: occasionally present lt: absent | ga: large, ovate, white or black internal colour cl: smooth, adherent, weakly erect abapically ol: crenulate d: 5 strong, sometimes one could appear double ID: absent lt: absent | ga: moderately large, ovate, white internal colour cl: smooth, margin partially weakly erect, adherent adapically ol: crenulate, erect d: 5 strong, sometimes one could appear double ID: absent lt: absent | ga: moderately large, ovate, white internal colour with brown spiral bands cl: narrow, smooth, adherent ol: crenulate, erect d: 5 weak, sometimes one could appear double ID: absent lt: absent |
| Radula rachidian cusps c: central l: lateral | unknown | c: elongate l: elongate | unknown | unknown |
| Animal general colour pattern | creamish | creamish | unknown | unknown |
| Depth range (in m) | 20–80 | 0–8 | 14–86 | infralittoral |
| Distribution | Mediterranean | Mediterranean | Atlantic | Atlantic |
| Notes | 3 | |||
| O. nicolai (Monterosato, 1884) | O. paddeui (Bonomolo and Buzzurro, 2006) | O. purpuroidea (Pallary, 1920) | O. vazzanai sp. nov. | |
| Figures | 3F | 4A | 4B | 1A–G and 4C |
| Shell TH (in mm) | up to 19.74 | up to 15.03 | up to 16 | up to 18.4 |
| Protoconch w: whorls m: microsculpture | w: unknown m: unknown | w: 1.15 m: smooth with growth lines | w: 1.25–1.5 m: unknown | w: 1.25–1.5 m: with small granules |
| Teleoconch ga: general aspect w: whorls cp: colour pattern | ga: rounded, slightly scalariform w: up to 6.5 cp: from uniformly whitish to light tan or pale brown with a whitish spiral band in median zone (sometimes two) | ga: slender, not scalariform w: up to 5.5 cp: pale brown with whitish spiral bands in median zone (always two but even more) | ga: broad, not scalariform w: up to 5 cp: light tan with brown blotches | ga: slender, slightly scalariform w: up to 6 cp: uniformly pale brown/reddish/orangish, often with a whitish spiral band in median zone (sometimes two), dark spots on ribs |
| Teleoconch sculpture of the convex part of the last whorl a: axial s: spiral t: threads | a: 7–9 rounded and nodose ribs, sometimes with 1–2 erratically placed varices s: 6 low and strong primary cords alternated by smaller secondary cords t: occasionally present | a: 6–7 low ribs, occasionally with an erratically placed varix s: 6 low and weak primary cords alternated by smaller secondary cords t: occasionally present | a: obsolete, with rarely broad, very low ribs s: 6 high and narrow primary cords and approximately similarly sized secondary cords t: occasionally present | a: 8–9 rounded and nodose/spinose ribs, sometimes with 1–2 erratically placed varices s: 6 nodose and rounded primary cords alternated by smaller secondary cords t: often present |
| Aperture ga: general aspect cl: columellar lip ol: outer lip d: denticles ID: infrasutural apertural denticle lt: labral tooth | ga: moderately large, ovate, pale brown internal colour cl: narrow, smooth, adherent ol: crenulate, erect d: 5 weak ID: absent lt: absent | ga: narrow and elongate, ovate, shiny white internal colour with brown spiral bands cl: smooth, weakly erect abapically, adherent adapically ol: crenulate, erect d: 5 weak ID: absent lt: absent | ga: large and broad, roundly-ovate, white internal colour with brown spiral bands cl: narrow, smooth, adherent ol: crenulate d: 5 weak pairs ID: absent lt: absent | ga: slightly narrow, elongate-ovate, pale brown internal colour cl: smooth, slightly expanded ventrally, erect abapically and adherent adapically ol: crenulate, erect d: 5 strong, rarely one could appear double ID: absent lt: absent |
| Radula rachidian cusps c: central l: lateral | unknown | unknown | unknown | c: short and thick l: short and thick |
| Animal general colour pattern | unknown | unknown | unknown | creamish |
| Depth range (in m) | circalittoral | 80–120 | infralittoral | 50–52 |
| Distribution | Mediterranean | Mediterranean | Atlantic | Mediterranean |
| Notes |
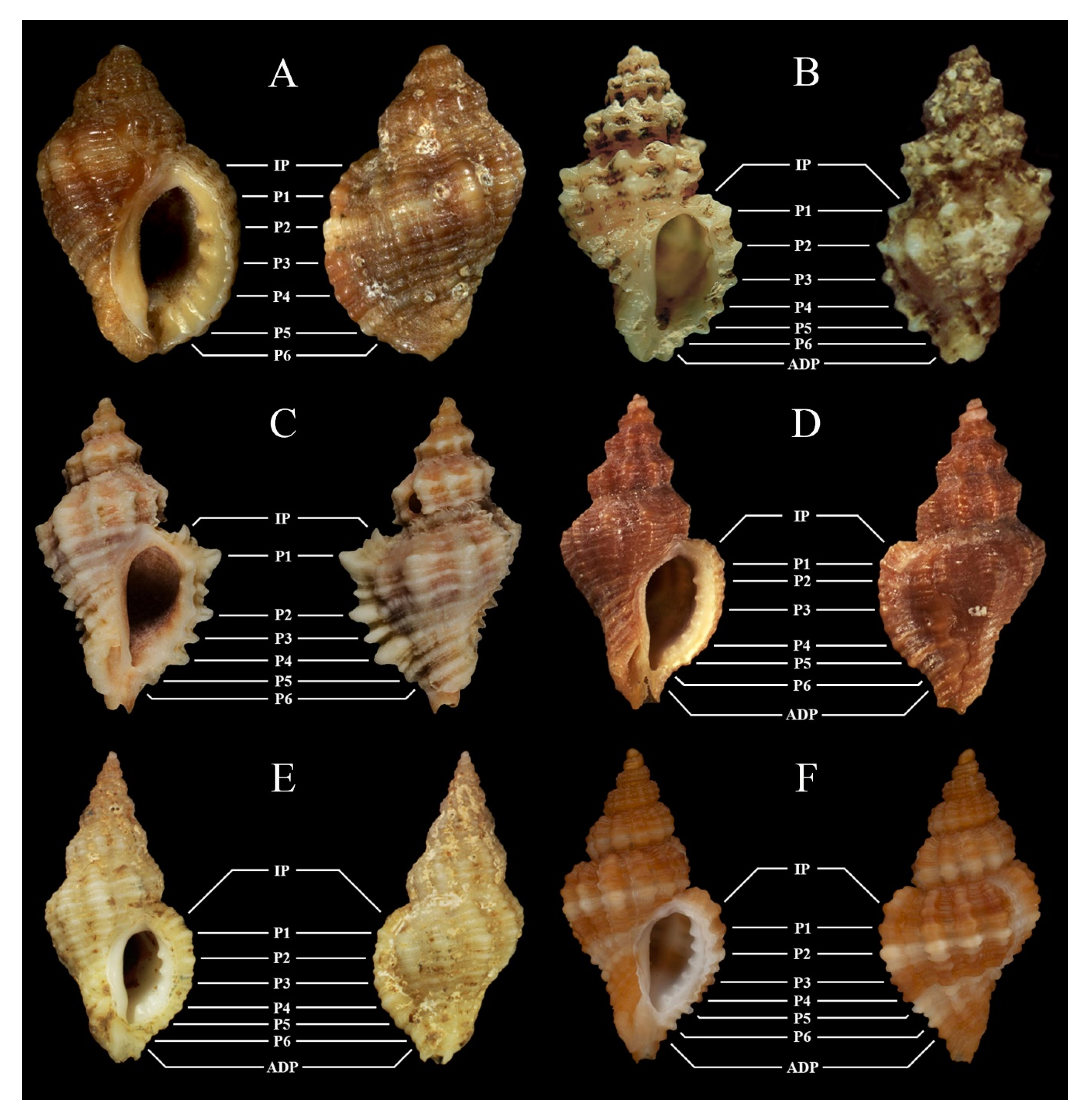
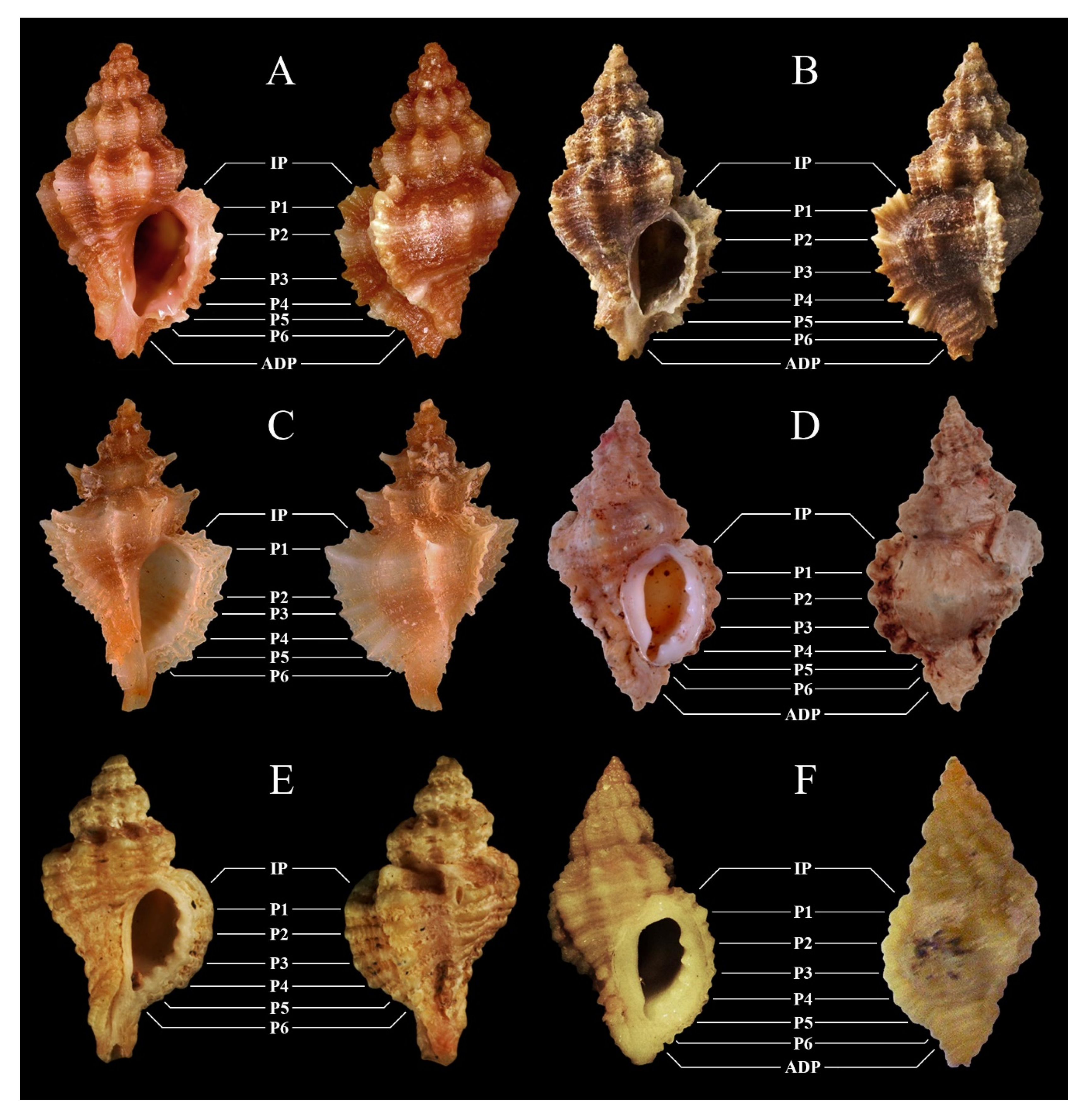
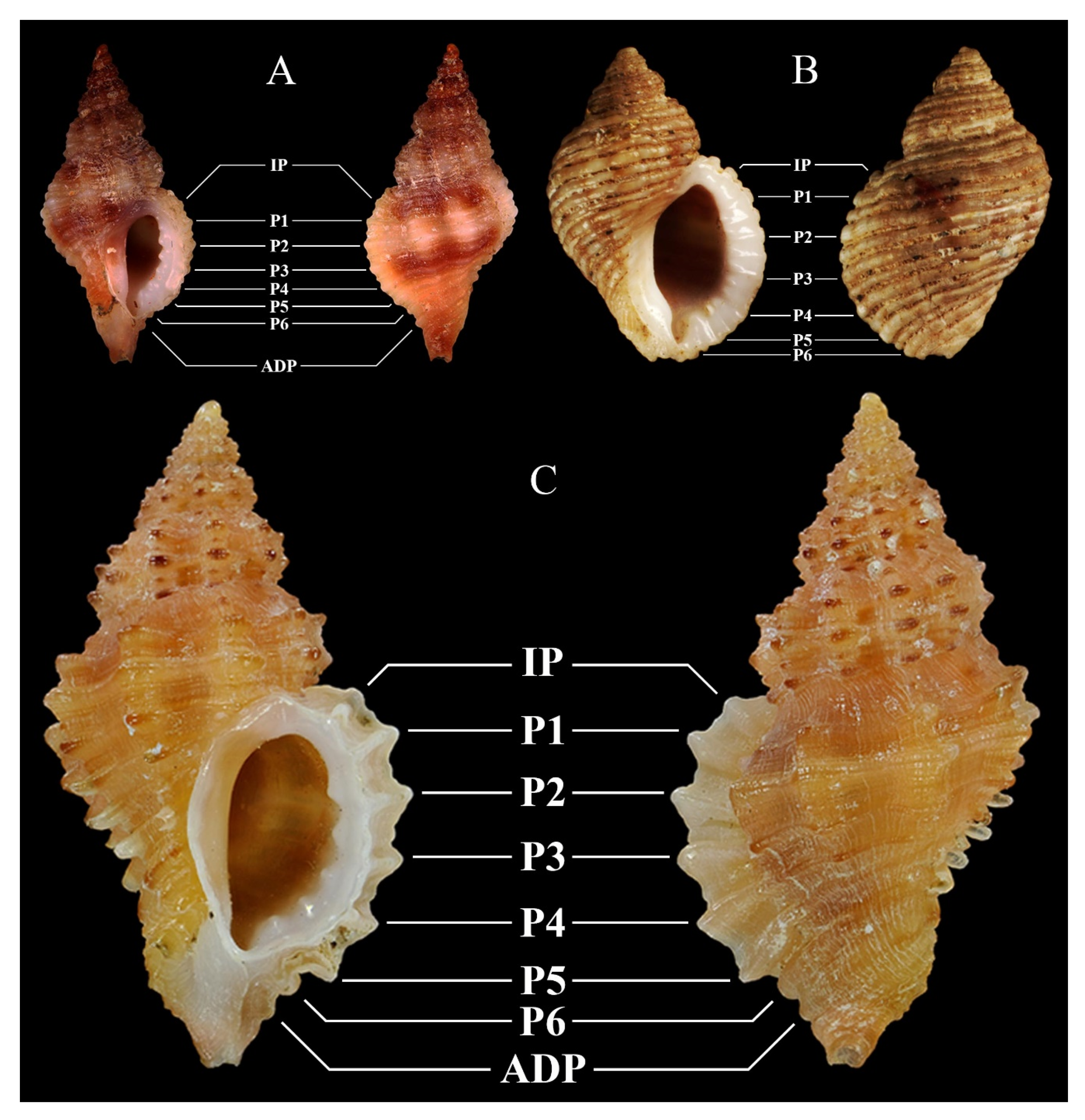
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Lasram, F.B.R.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, C.N.; Morri, C.; Chiantore, M.; Montefalcone, M.; Parravicini, V.; Rovere, A. Mediterranean Sea biodiversity between the legacy from the past and a future of change. In Life in the Mediterranean Sea: A Look at Habitat Changes; Stambler, N., Ed.; Nova Science Publishers: New York, NY, USA, 2012; pp. 1–55. [Google Scholar]
- Gosliner, T.M.; Cervera, J.L.; Ghiselin, M.T. Scientific exploration in the Mediterranean Region. Biodiversity of the Mediterranean Opisthobranch gastropod fauna: Historical and phylogenetic perspective. Proc. Calif. Acad. Sci. 2008, 59, 117–137. [Google Scholar]
- Sabelli, B.; Taviani, M. The making of the Mediterranean molluscan biodiversity. In The Mediterranean Sea: Its History and Present Challenges; Goffredo, S., Dubinsky, Z., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 285–396. [Google Scholar]
- Agnarsson, I.; Kuntner, M. Taxonomy in a Changing World: Seeking Solutions for a Science in Crisis. Syst. Biol. 2007, 56, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Wägele, H.; Klussmann-Kolb, A.; Kuhlmann, M.; Haszprunar, G.; Lindberg, D.; Koch, A.; Wägele, J.W. The taxonomist—An endangered race. A practical proposal for its survival. Front. Zool. 2011, 8, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scacchi, A. Catalogus Conchyliorum Regni Neapolitani quae usque adhuc reperit A. Scacchi; Typis Filiatre-Sebetii: Napoli, Italy, 1836. [Google Scholar]
- Crosse, H. Description d’espèces nouvelles. J. Conchyliol. 1865, 13, 213–215. [Google Scholar]
- Crosse, H. Description d’un Murex nouveau de l’Adriatique. J. Conchyliol. 1866, 14, 274–276. [Google Scholar]
- Jousseaume, F.P. Division méthodique de la famille des Purpuridae. Naturaliste 1880, 2, 335–336. [Google Scholar]
- Di Monterosato, T.A. Molluschi viventi e quaternari raccolti lungo le coste della Tripolitania dall’ing Camillo Crema. Boll. Soc. Zool. Ital. 1917, 3, 1–28. [Google Scholar]
- De Gregorio, A. Studi su Talune Conchiglie Mediterranee Viventi e Fossili; Tipografia all’insegna dell’ancora: Siena, Italy, 1885. [Google Scholar]
- Pallary, P. Diagnoses de quelques coquilles nouvelles provenant du Maroc. J. Conchyliol. 1902, 49, 226–228. [Google Scholar]
- Pallary, P. Addition à la faune malacologique du Golfe de Gabès. J. Conchyliol. 1904, 52, 212–248. [Google Scholar]
- Pallary, P. Sur la faune de l’ancienne lagune de Tunis. Bull. Soc. Hist. Nat. Afrique N. 1912, 3, 215–228. [Google Scholar]
- Coen, G.S. Contributo allo studio della Fauna malacologica Adriatica. R. Com. Tal. Ital. 1914, Mem. 46, 1–34. [Google Scholar]
- Coen, G.S. Saggio di una Sylloge Molluscorum Adriaticorum. R. Com. Tal. Ital. 1933, Mem. 192, 1–186. [Google Scholar]
- Franc, A. Recherches sur le développement d’Ocinebra aciculata, Lamarck (Mollusque Gastéropode). Bull. Biol. Fr. Belg. 1940, 74, 327–345. [Google Scholar]
- Nordsieck, F. Die Europäischen Meeres-Gehäuseschnecken (Prosobranchia); G. Fischer: Stuttgart, Germany, 1968. [Google Scholar]
- Franchini, D. Guide to Mediterranean conchology. MURICACEA (III). La Conchiglia 1972, 4, 10–11. [Google Scholar]
- Settepassi, F. Atlante Malacologico. I Molluschi Marini Viventi nel Mediterraneo; Museo di Zoologia: Roma, Italy, 1977; Volume 2. [Google Scholar]
- Houart, R.; Abreu, A.D. The Muricidae (Gastropoda) from Madeira with the description of a new species of Ocenebrinae. Apex 1994, 9, 119–130. [Google Scholar]
- Houart, R. The west African Muricidae. II. Ocenebrinae, Ergalataxinae, Tripterotyphinae, Typhinae, Trophoninae & Rapaninae. Apex 1997, 12, 49–91. [Google Scholar]
- Houart, R. New species of Muricidae (Gastropoda) from the northeastern Atlantic and the Mediterranean Sea. Zoosystema 2000, 22, 459–469. [Google Scholar]
- Houart, R. A Review of the Recent Mediterranean and Northeastern Atlantic Species of Muricidae; Edizioni Evolver: Rome, Italy, 2001. [Google Scholar]
- Bonomolo, G.; Buzzurro, G. Description of a new Muricid for the Mediterranean sea: Ocinebrina paddeui (Mollusca, Gastropoda, Muricidae, Ocenebrinae). Triton 2006, 13, 1–4. [Google Scholar]
- Cecalupo, A.; Buzzurro, G.; Mariani, M. Contributo alla conoscenza della malacofauna del Golfo di Gabès (Tunisia). Quad. Civ. Staz. Idrobiol. Milano 2008, 31, 1–267. [Google Scholar]
- Crocetta, F.; Bonomolo, G.; Albano, P.G.; Barco, A.; Houart, R.; Oliverio, M. The status of the northeastern Atlantic and Mediterranean small mussel drills of the Ocinebrina aciculata complex (Mollusca: Gastropoda: Muricidae), with the description of a new species. Sci. Mar. 2012, 76, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Barco, A.; Corso, A.; Oliverio, M. Endemicity in the Gulf of Gabés: The small mussel drill Ocinebrina hispidula is a distinct species in the Ocinebrina edwardsii complex (Muricidae: Ocenebrinae). J. Moll. Stud. 2013, 79, 273–276. [Google Scholar] [CrossRef] [Green Version]
- Barco, A.; Houart, R.; Bonomolo, G.; Crocetta, F.; Oliverio, M. Molecular data reveal cryptic lineages within the northeastern Atlantic and Mediterranean small mussel drills of the Ocinebrina edwardsii complex (Mollusca: Gastropoda: Muricidae). Zool. J. Linn. Soc. 2013, 169, 389–407. [Google Scholar] [CrossRef] [Green Version]
- Barco, A.; Herbert, G.; Houart, R.; Fassio, G.; Oliverio, M. A molecular phylogenetic framework for the subfamily Ocenebrinae (Gastropoda, Muricidae). Zool. Scripta 2017, 46, 322–335. [Google Scholar] [CrossRef] [Green Version]
- Barco, A.; Aissaoui, C.; Houart, R.; Bonomolo, G.; Crocetta, F.; Oliverio, M. Revision of the Ocinebrina aciculata species complex (Mollusca: Gastropoda: Muricidae) in the northeastern Atlantic Ocean and Mediterranean Sea. J. Moll. Stud. 2018, 84, 19–29. [Google Scholar] [CrossRef]
- Merle, D.; Garrigues, B.; Pointier, J.-P. Fossil & Recent Muricidae of the World—Part Muricinae; ConchBooks: Hackenheim, Germany, 2011. [Google Scholar]
- Marzouk, Z.; Aurelle, D.; Said, K.; Chenuil, A. Cryptic lineages and high population genetic structure in the exploited marine snail Hexaplex trunculus (Gastropoda: Muricidae). Biol. J. Linn. Soc. 2017, 122, 411–428. [Google Scholar] [CrossRef] [Green Version]
- Berrou, V.; Merle, D.; Dommergues, J.-L.; Crônier, C.; Néraudeau, D. Comparative morphology of Pliocene, Quaternary and Recent shells of Ocenebra erinaceus (Linnaeus, 1758) and O. brevirobusta Houart, 2000 (Mollusca, Muricidae, Ocenebrinae): Reflections on the intra- and interspecific variations. Geodiversitas 2004, 26, 263–295. [Google Scholar]
- Crocetta, F.; Spanu, M. Molluscs associated with a Sardinian deep water population of Corallium rubrum (Linné, 1758). Med. Mar. Sci. 2008, 9, 63–85. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, B.; Buzzurro, G.; Benli, H.A. Marine molluscs from Cyprus: New data and checklist. Boll. Malacol. 2004, 39, 49–78. [Google Scholar]
- Öztürk, B.; Dogan, A.; Bitlis-Bakir, B.; Salman, A. Marine molluscs of the Turkish coasts: An updated checklist. Turk. J. Zool. 2014, 38, 832–879. [Google Scholar] [CrossRef]
- Crocetta, F.; Bitar, G.; Zibrowius, H.; Oliverio, M. Increase in knowledge of the marine gastropod fauna of Lebanon since the 19th century. Bull. Mar. Sci. 2020, 96, 1–22. [Google Scholar] [CrossRef]
- Vazzana, A. La malacofauna del Circalitorale di Scilla (Stretto di Messina). Boll. Malacol. 2010, 46, 65–74. [Google Scholar]
- Vazzana, A. Biodiversità Marina Lungo le Coste della Provincia di Reggio Calabria; Laruffa: Reggio Calabria, Italy, 2011. [Google Scholar]
- Mistri, M. Ecological observations on a population of the Mediterranean gorgonian Paramuricea clavata. Boll. Zool. 1994, 61, 163–166. [Google Scholar] [CrossRef]
- Mistri, M.; Ceccherelli, V.U. Growth and secondary production of the Mediterranean gorgonian Paramuricea clavata. Mar. Ecol. Prog. Ser. 1994, 103, 291–296. [Google Scholar] [CrossRef]
- Merle, D. The spiral cords and the internal denticles of the outer lip in the Muricidae: Terminology and methodological comments. Novapex 2001, 2, 69–91. [Google Scholar]
- Merle, D. The spiral cords of the Muricidae (Gastropoda, Neogastropoda): Importance of ontogenetic and topological correspondences for delineating structural homologies. Lethaia 2005, 38, 367–379. [Google Scholar] [CrossRef]
- MolluscaBase. Muricidae Rafinesque, 1815. Accessed through: World Register of Marine Species. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails&id=148 (accessed on 31 March 2020).
- Chirli, C. Malacofauna Pliocenica Toscana. Superfamiglia Muricoidea; Stamperia e Legatoria Pisana: Pisa, Italy, 2000; Volume 2. [Google Scholar]
- Landau, B.M.; Houart, R.; da Silva, C.M. The Early Pliocene Gastropoda (Mollusca) of Estepona, southern Spain. Part 7: Muricidae. Palaeontos 2007, 11, 1–87. [Google Scholar]
- Goret, B.; Ledon, D.; Pons, J. Les Muricidae (Gastropoda, Muricoidea) du Pliocène inférieur de Catalogne (France, Espagne). Palaeontos 2013, 23, 1–43. [Google Scholar]
- Goret, B.; Pons, J. Les Muricidae (Gastropoda, Muricoidea) du Miocène de Montpeyroux (Languedoc, France). Palaeontos 2013, 23, 53–70. [Google Scholar]
- Landau, B.M.; Harzhauser, M.; Islamoğlu, Y.; Marques da Silva, C. Systematics and palaeobiogeography of the gastropods of the middle Miocene (Serravallian) Karaman Basin, Turkey. Cainoz. Res. 2013, 11–13, 3–584. [Google Scholar]
- Landau, B.M.; Merle, D.; Ceulemans, L.; Van Dingenen, F. The upper Miocene gastropods of northwestern France, 3. Muricidae. Cainoz. Res. 2019, 19, 3–44. [Google Scholar]
- Brunetti, M.M. Il giacimento di Cava Lustrelle e la sua fauna malacologica. Soc. Reggiana Sci. Nat.—Notiziario 2011, 21–34. [Google Scholar]
- Ardovini, R.; Cossignani, T. West African Seashells; L’Informatore Piceno: Ancona, Italy, 2004. [Google Scholar]
- Cossignani, T.; Ardovini, R. Malacologia Mediterranea. Atlante delle Conchiglie del Mediterraneo—7.500 Foto a Colori; L’Informatore Piceno: Ancona, Italy, 2011. [Google Scholar]
- Renda, W. La Rubrica dei Record di dimensioni delle Conchiglie marine. Notiziario S.I.M. 2012, 30, 1–2. [Google Scholar]
- Appolloni, M.; Smriglio, C.; Amati, B.; Lugliè, L.; Nofroni, I.; Tringali, L.P.; Mariottini, P.; Oliverio, M. Catalogue of the primary types of marine molluscan taxa described by Tommaso Allery Di Maria, Marquis of Monterosato, deposited in the Museo Civico di Zoologia, Roma. Zootaxa 2018, 4477, 1–138. [Google Scholar] [CrossRef]
- Jablonski, D.; Lutz, R.A. Larval ecology of marine benthic invertebrates: Paleobiological implications. Biol. Rev. 1983, 58, 21–89. [Google Scholar] [CrossRef]
- Aissaoui, C.; Puillandre, N.; Bouchet, P.; Fassio, G.; Modica, M.V.; Oliverio, M. Cryptic diversity in Mediterranean gastropods of the genus Aplus (Neogastropoda: Buccinidae). Sci. Mar. 2016, 80, 521–533. [Google Scholar] [CrossRef] [Green Version]
- Calvo, M.; Templado, J.; Oliverio, M.; Machordom, A. Hidden Mediterranean biodiversity: Molecular evidence for a cryptic species complex within the reef building vermetid gastropod Dendropoma petraeum (Mollusca: Caenogastropoda). Biol. J. Linn. Soc. 2009, 96, 898–912. [Google Scholar] [CrossRef] [Green Version]
- Amati, B.; Smriglio, C.; Oliverio, M. Revision of the Recent Mediterranean species of Mitromorpha, with seven new species. Zootaxa 2015, 3931, 151–195. [Google Scholar] [CrossRef]
- Aissaoui, C.; Puillandre, N.; Bouchet, P. New insights in the taxonomy of Mediterranean Diodora (Mollusca, Gastropoda, Fissurellidae). J. Mar. Biol. Assoc. UK 2017, 97, 1527–1536. [Google Scholar] [CrossRef]
- Aissaoui, C.; Galindo, L.A.; Puillandre, N.; Bouchet, P. The nassariids from the Gulf of Gabès revisited (Neogastropoda, Nassariidae). Mar. Biol. Res. 2017, 13, 370–389. [Google Scholar] [CrossRef]
- Pola, M.; Paz-Sedano, S.; Macali, A.; Minchin, D.; Marchini, A.; Vitale, F.; Licchelli, C.; Crocetta, F. What is really out there? Review of the genus Okenia Menke, 1830 (Nudibranchia: Goniodorididae) in the Mediterranean Sea with description of two new species. PLoS ONE 2019, 14, e0215037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Hervás, M.R.; Carmona, L.; Jensen, K.; Licchelli, C.; Vitale, F.; Cervera, J.L. Description of a new pseudocryptic species of Elysia Risso, 1818 (Heterobranchia, Sacoglossa) in the Mediterranean Sea. Bull. Mar. Sci. 2020, 96, 127–143. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Voultsiadou, E. Mediterranean marine caves as biodiversity reservoirs: A preliminary overview. In Proceedings of the 1st Mediterranean Symposium on the Conservation of Dark Habitats, Portorož, Slovenia, 31 October 2014. [Google Scholar]
- Warén, A.; Carrozza, F.; Rocchini, R. Description of two new species of Hyalogyrinidae (Gastropoda, Heterobranchia) from the Mediterranean. Boll. Malacol. 1997, 32, 57–66. [Google Scholar]
- La Perna, R. A new Mediterranean Skeneoides (Gastropoda: Skeneidae) from a shallow-water cave. J. Conchol. 1999, 36, 21–27. [Google Scholar]
- Palazzi, S.; Villari, A. Molluschi e Brachiopodi delle grotte sottomarine del Taorminense. La Conchiglia 2001, 297 (Suppl. 56), 1–56. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crocetta, F.; Houart, R.; Bonomolo, G. The More You Search, the More You Find: A New Mediterranean Endemism of the Genus Ocenebra Gray, 1847 (Mollusca: Gastropoda: Muricidae) from a Submarine Cave of the Messina Strait Area (Italy). J. Mar. Sci. Eng. 2020, 8, 443. https://doi.org/10.3390/jmse8060443
Crocetta F, Houart R, Bonomolo G. The More You Search, the More You Find: A New Mediterranean Endemism of the Genus Ocenebra Gray, 1847 (Mollusca: Gastropoda: Muricidae) from a Submarine Cave of the Messina Strait Area (Italy). Journal of Marine Science and Engineering. 2020; 8(6):443. https://doi.org/10.3390/jmse8060443
Chicago/Turabian StyleCrocetta, Fabio, Roland Houart, and Giuseppe Bonomolo. 2020. "The More You Search, the More You Find: A New Mediterranean Endemism of the Genus Ocenebra Gray, 1847 (Mollusca: Gastropoda: Muricidae) from a Submarine Cave of the Messina Strait Area (Italy)" Journal of Marine Science and Engineering 8, no. 6: 443. https://doi.org/10.3390/jmse8060443
APA StyleCrocetta, F., Houart, R., & Bonomolo, G. (2020). The More You Search, the More You Find: A New Mediterranean Endemism of the Genus Ocenebra Gray, 1847 (Mollusca: Gastropoda: Muricidae) from a Submarine Cave of the Messina Strait Area (Italy). Journal of Marine Science and Engineering, 8(6), 443. https://doi.org/10.3390/jmse8060443






