Trajectories of Changes in Phytoplankton Biomass, Phaeocystis globosa and Diatom (incl. Pseudo-nitzschia sp.) Abundances Related to Nutrient Pressures in the Eastern English Channel, Southern North Sea
Abstract
:1. Introduction
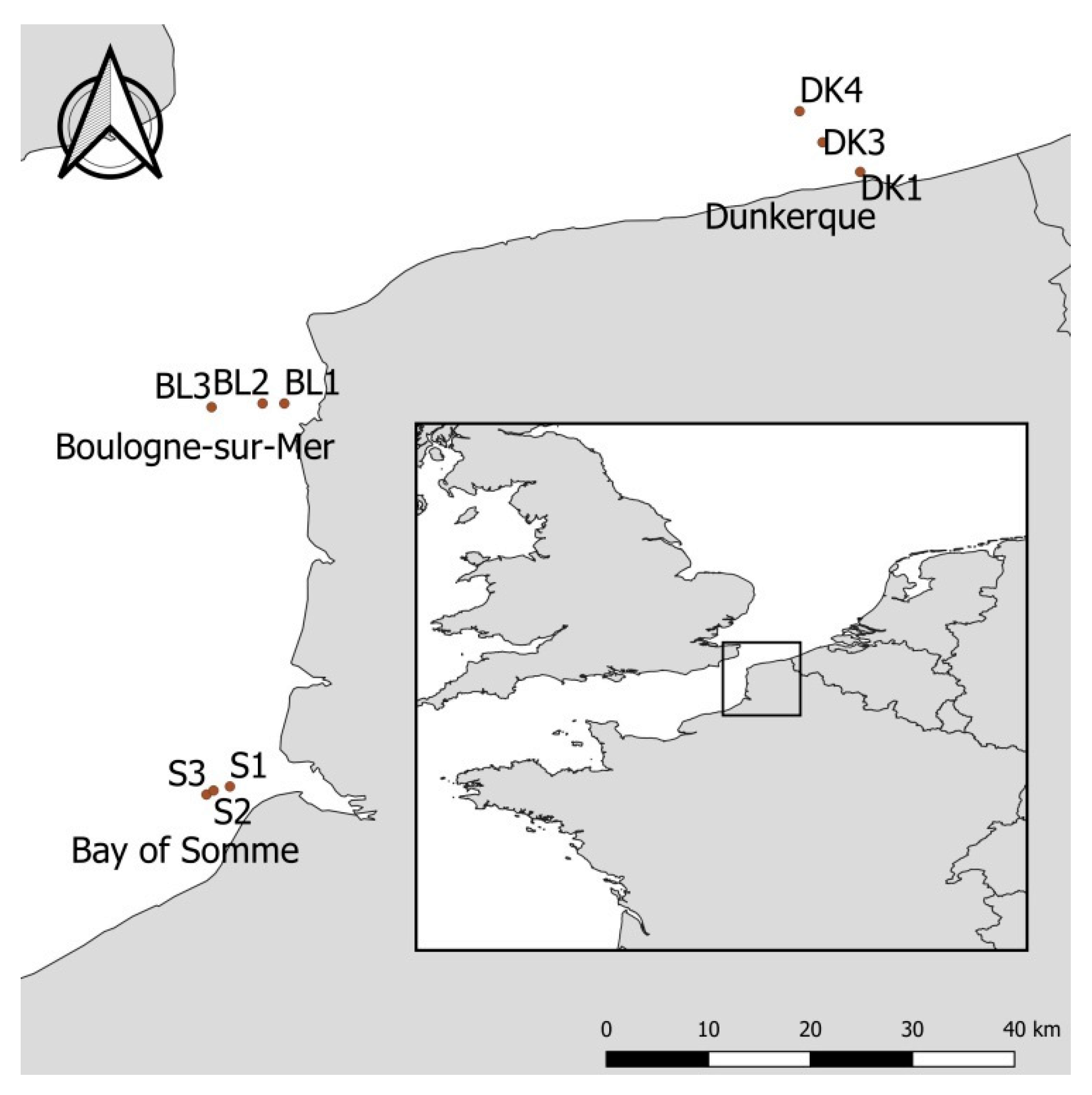
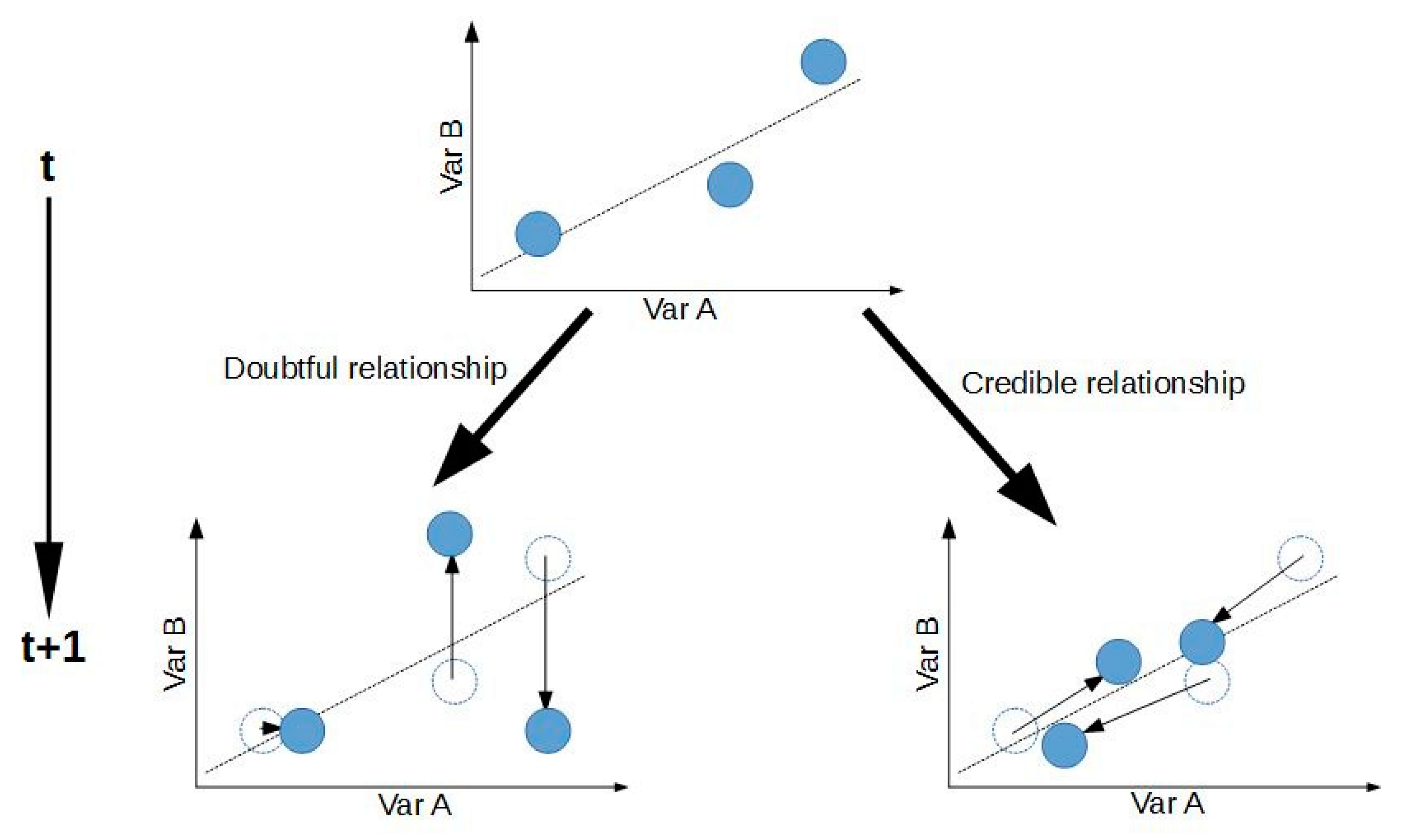
2. Materials and Methods
3. Results
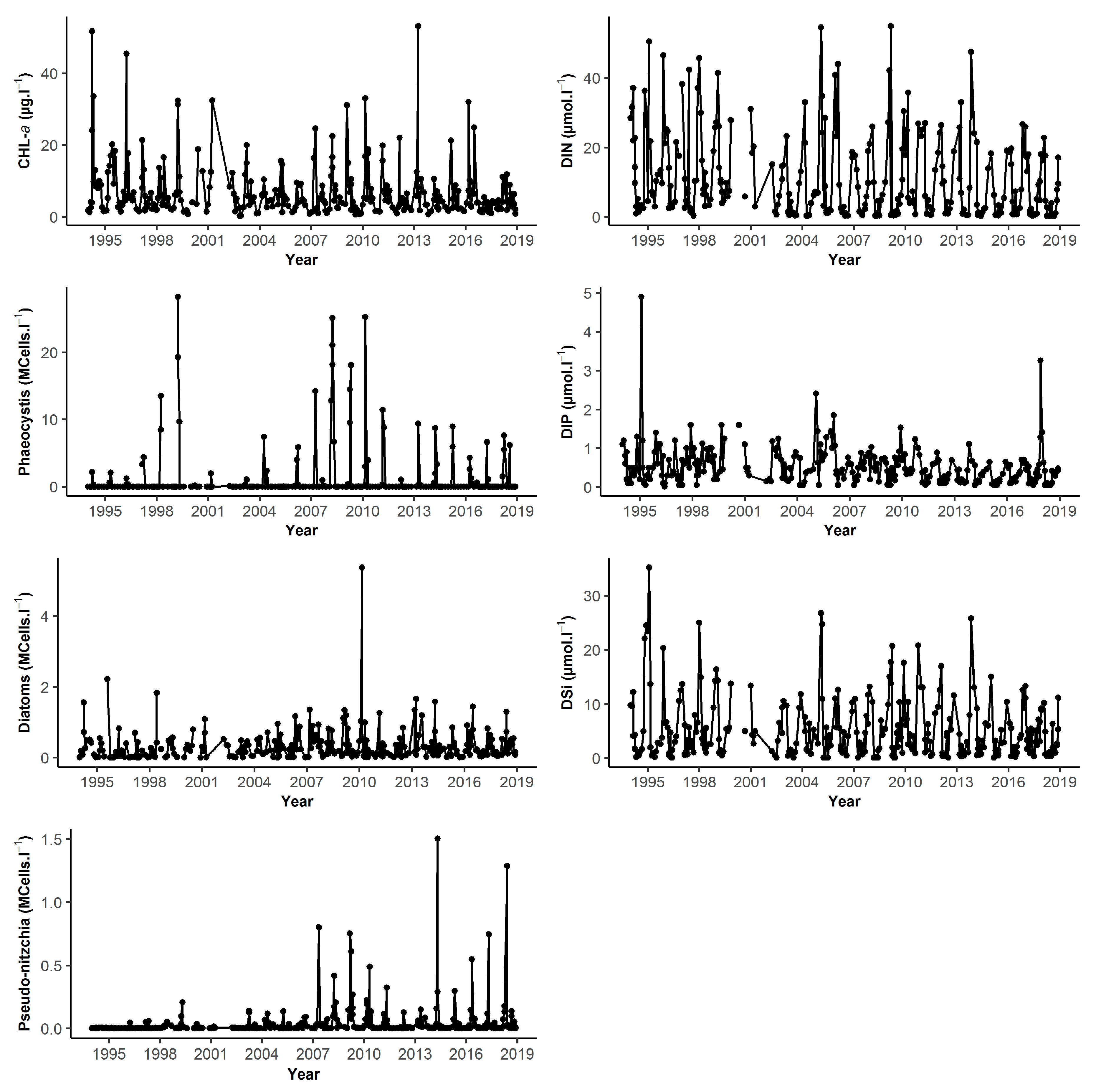
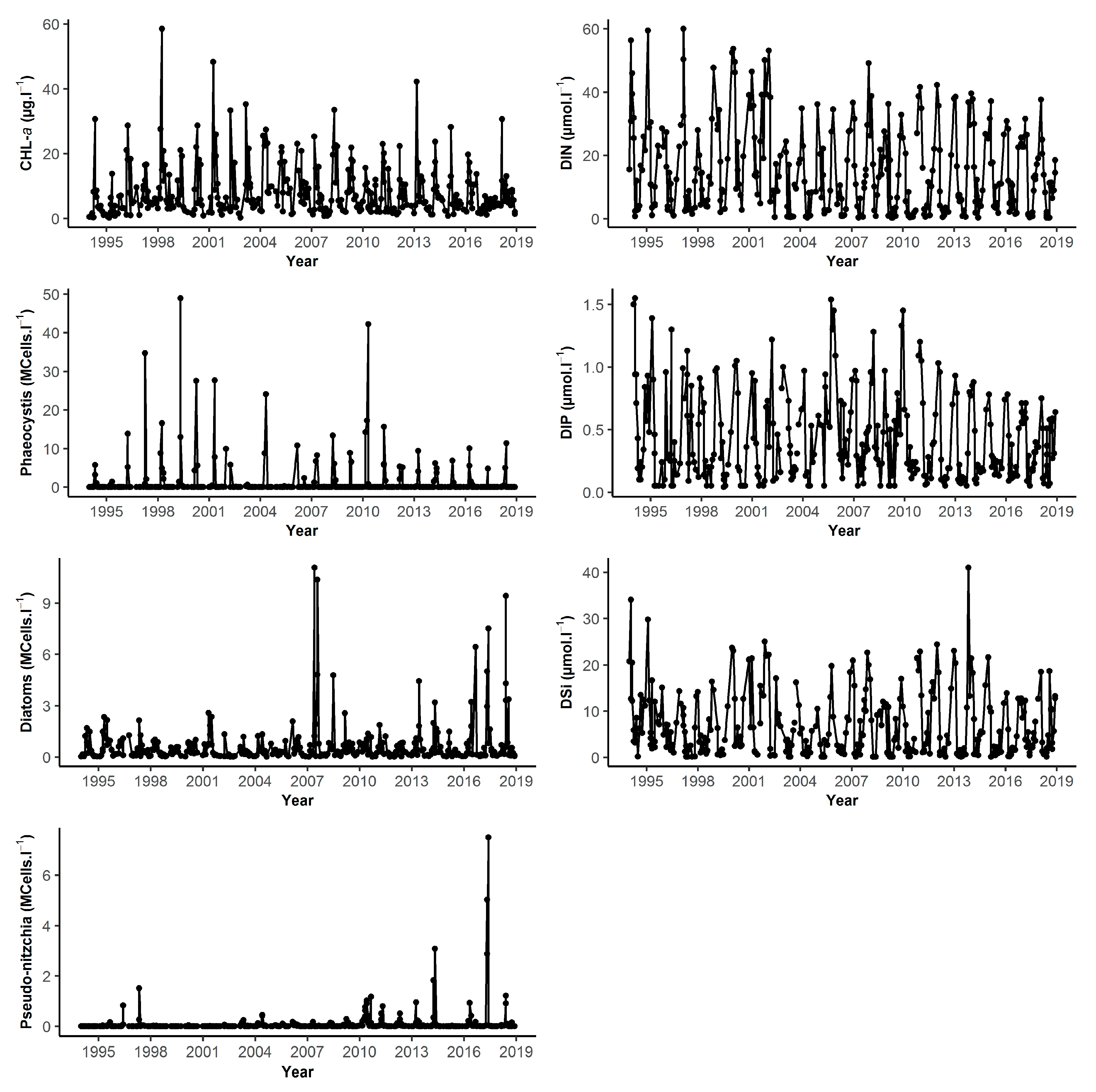
3.1. Environmental Characteristics, Seasonnality and Trends
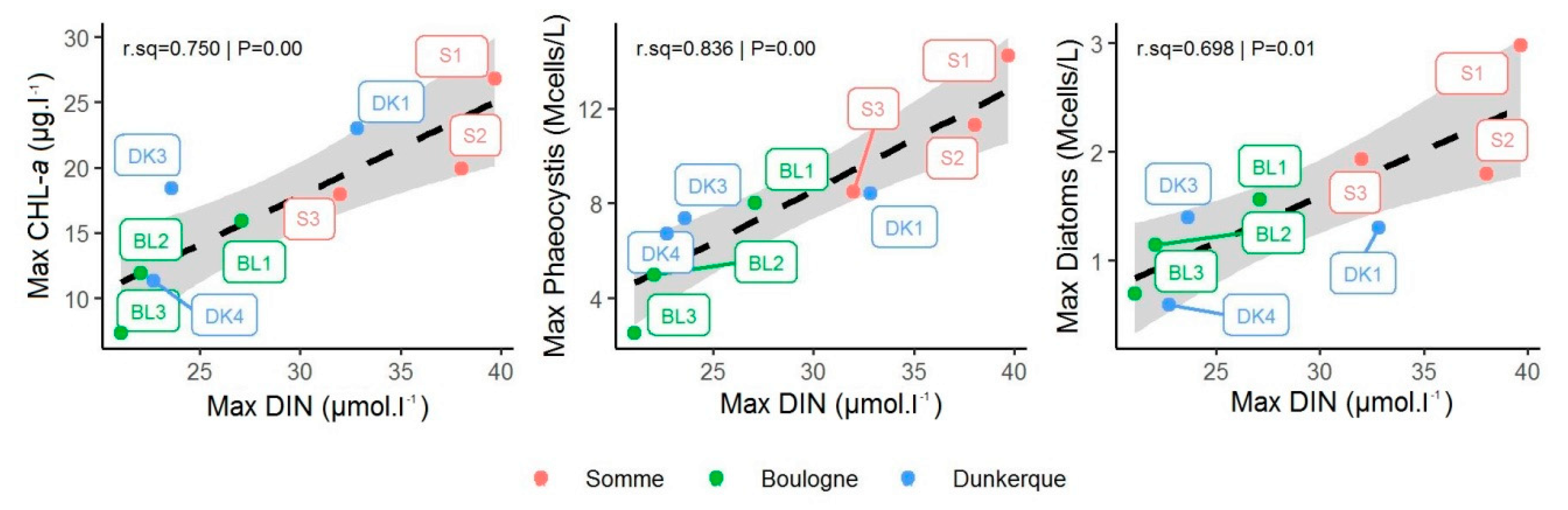
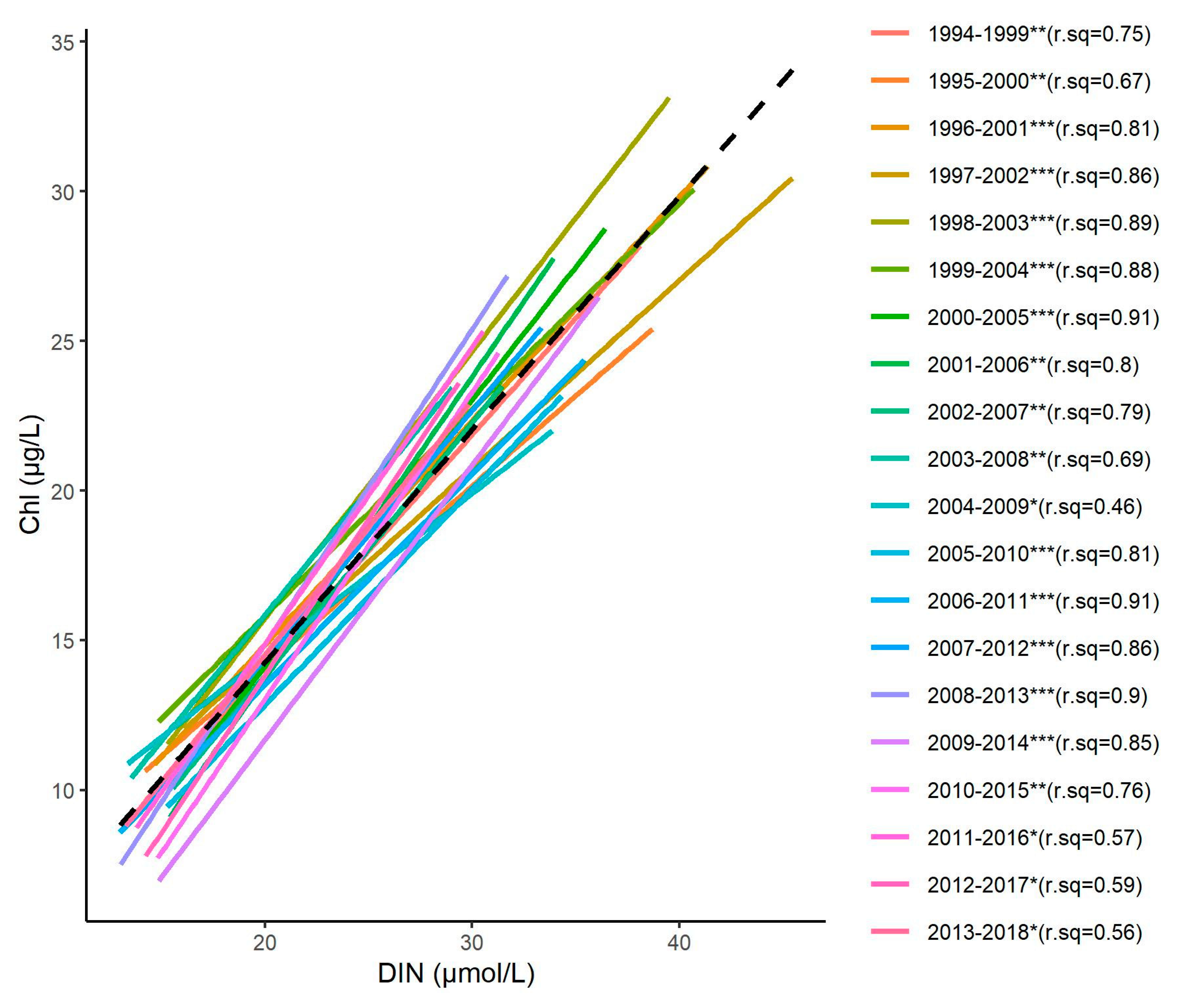
3.2. Relationship between Phytoplankton Biomass, Abundance and Nutrients
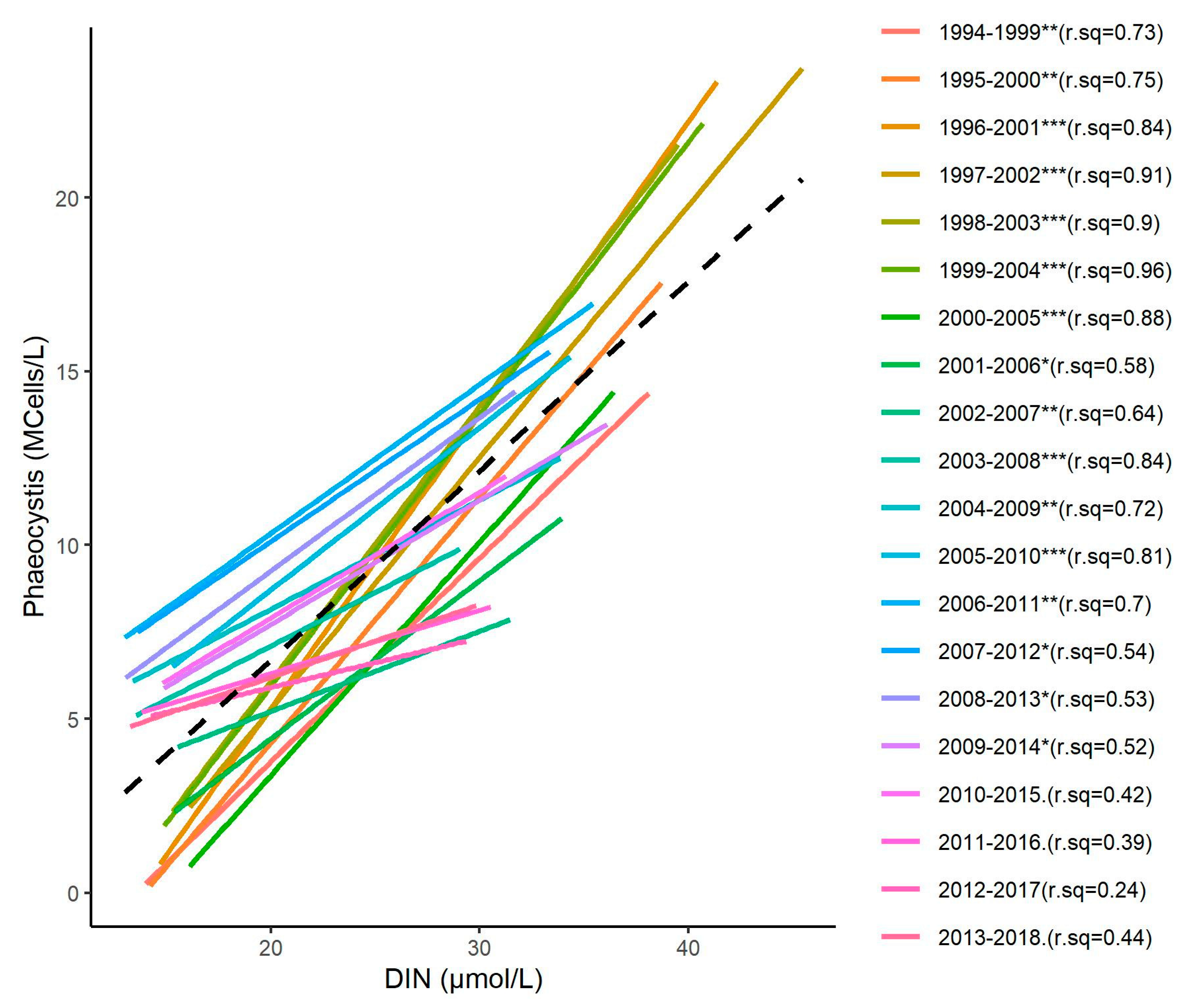
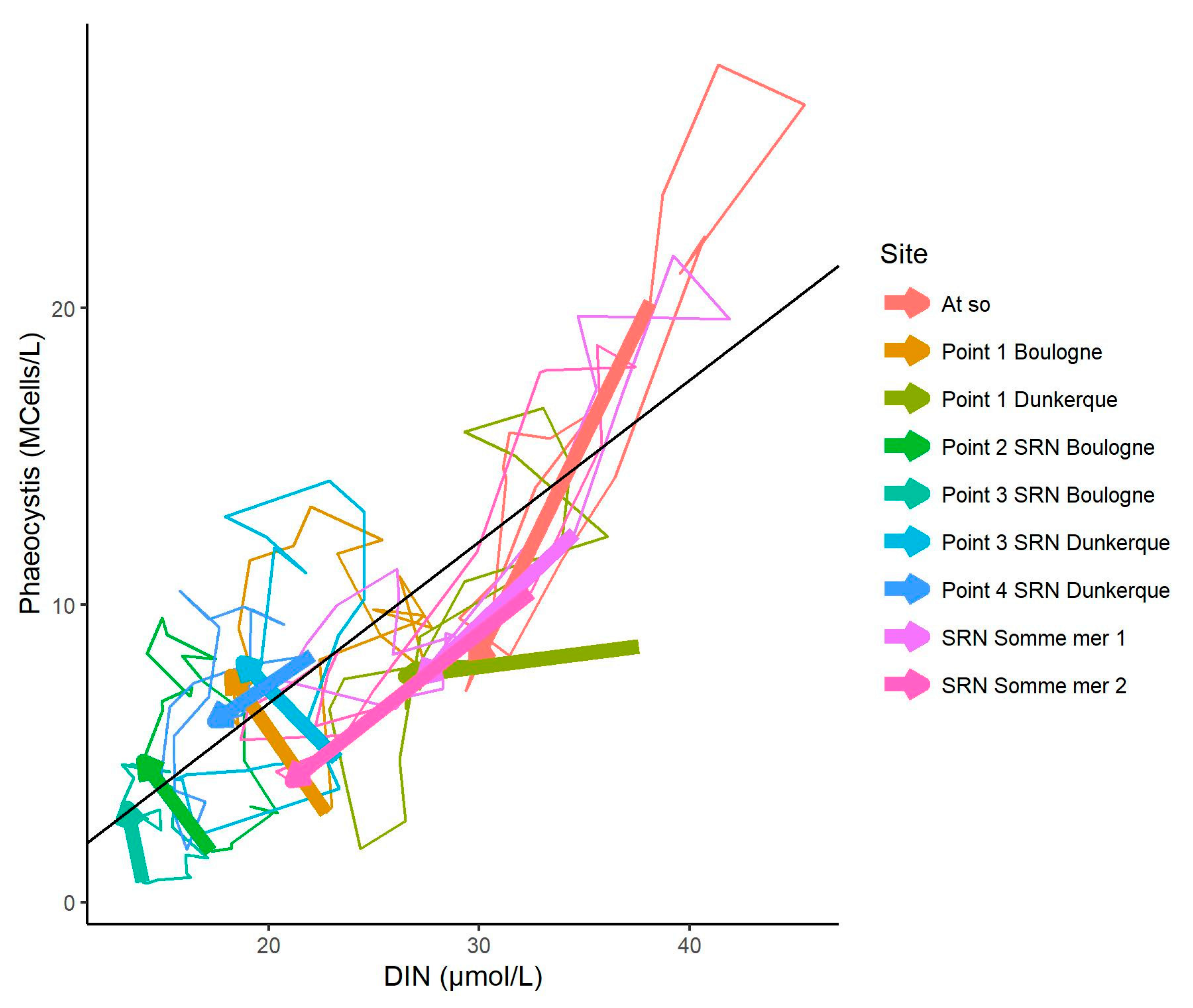
3.3. Relationship between Phaeocystis and DIN Concentrations
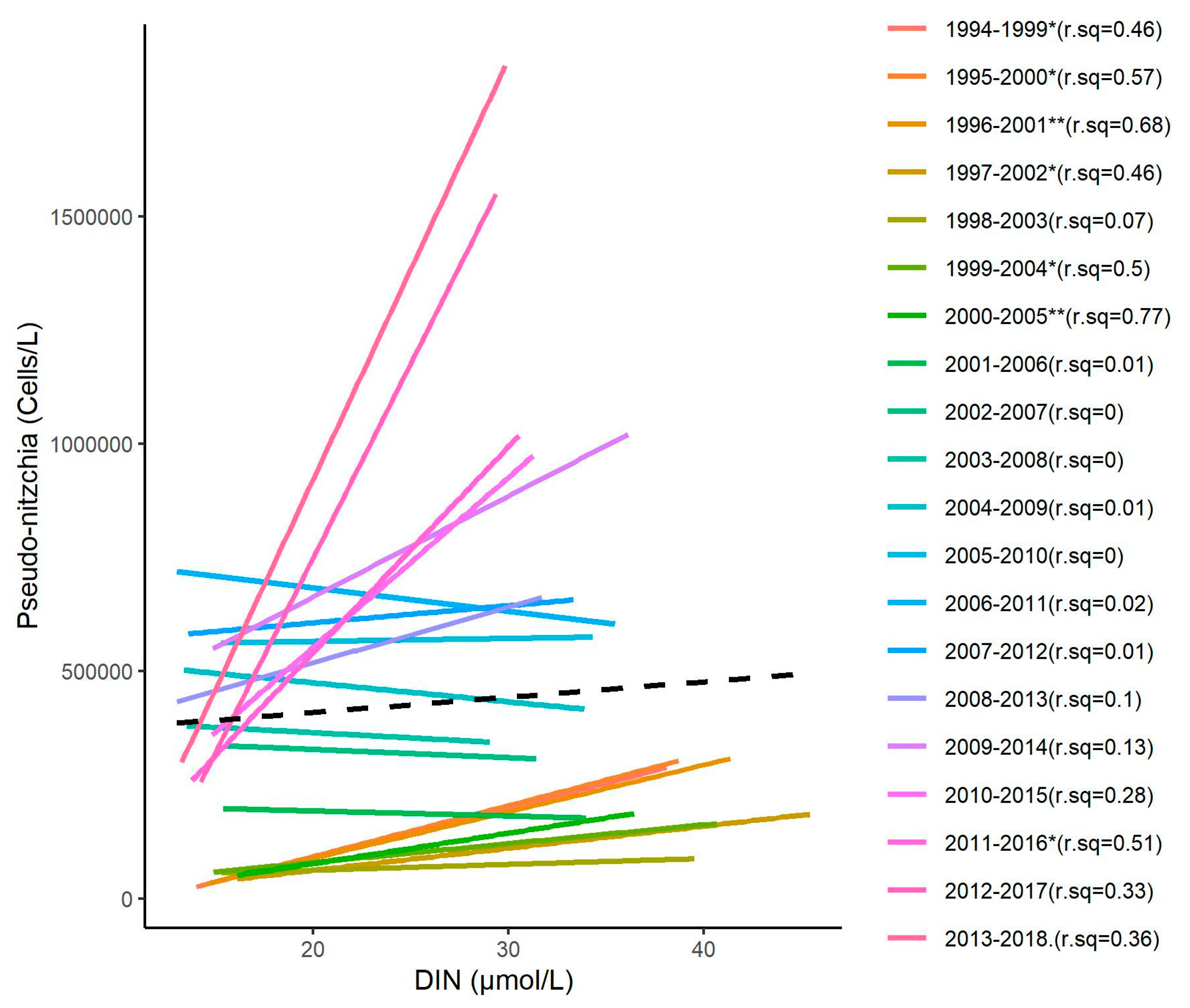
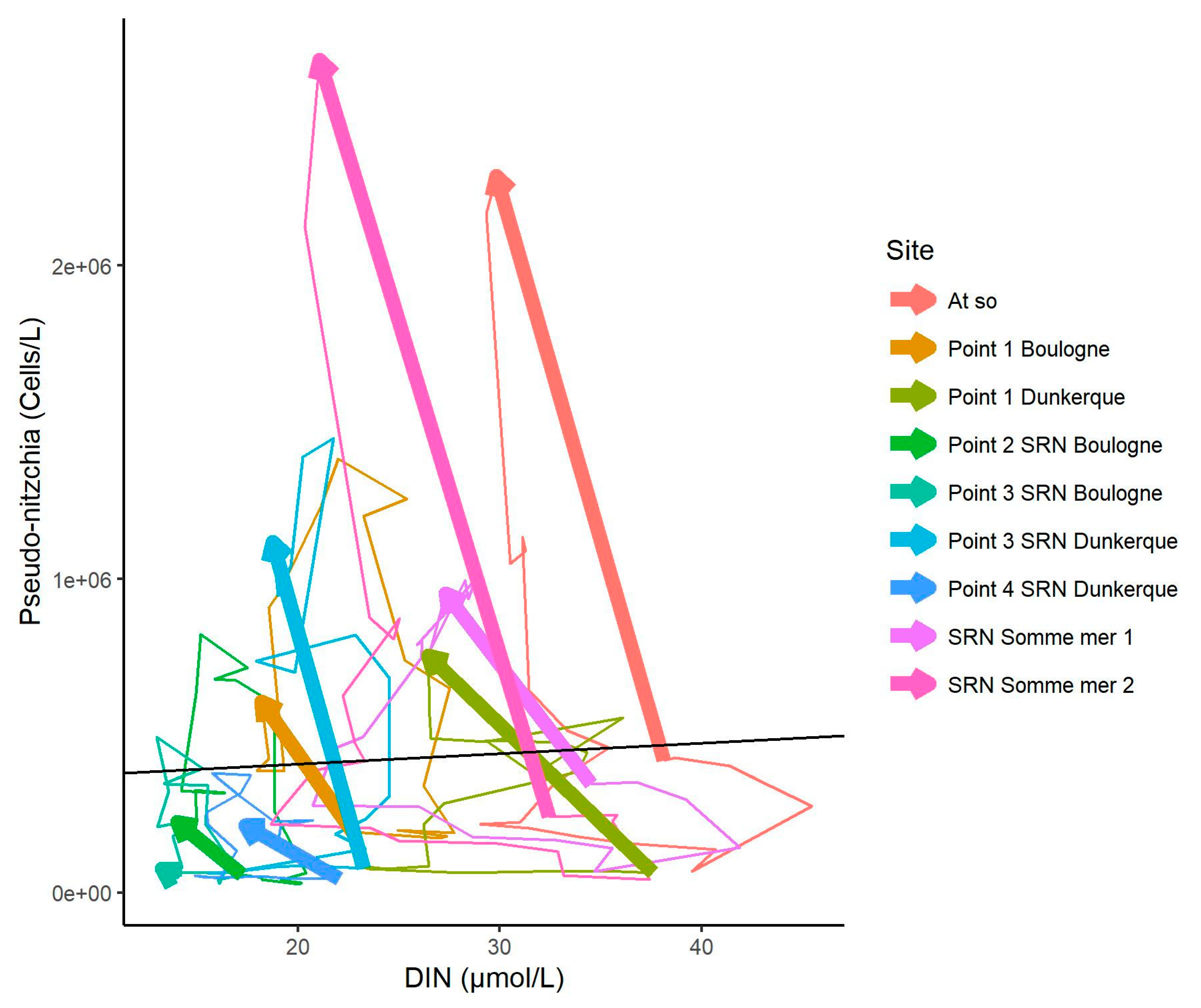
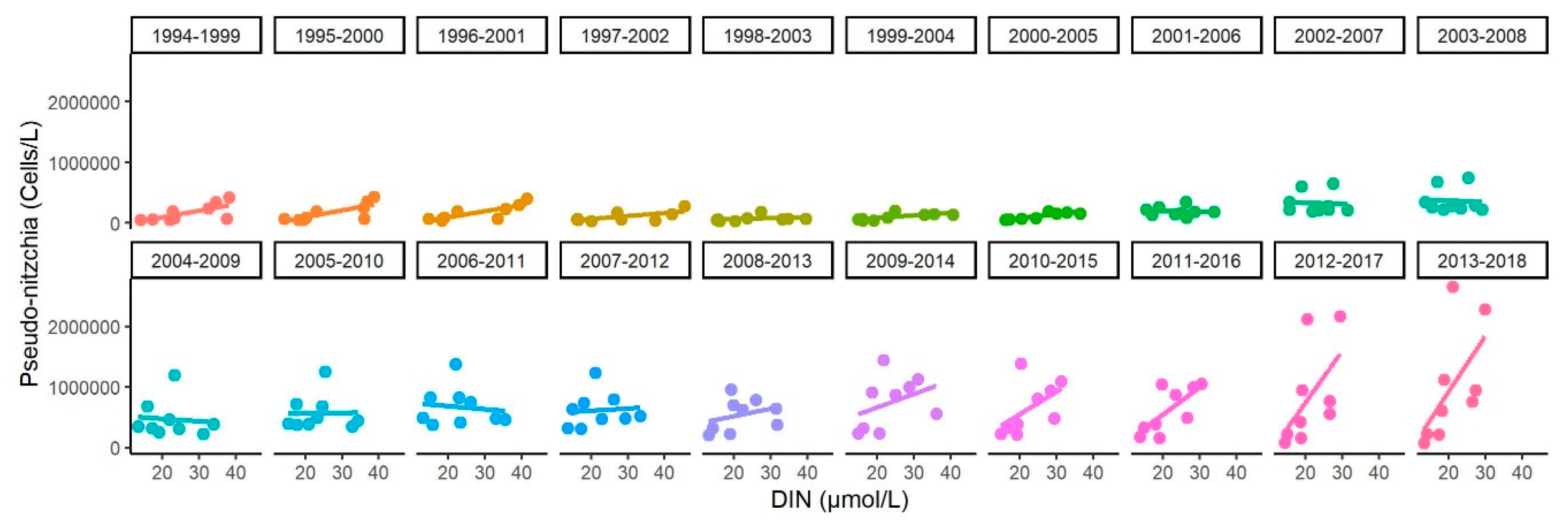
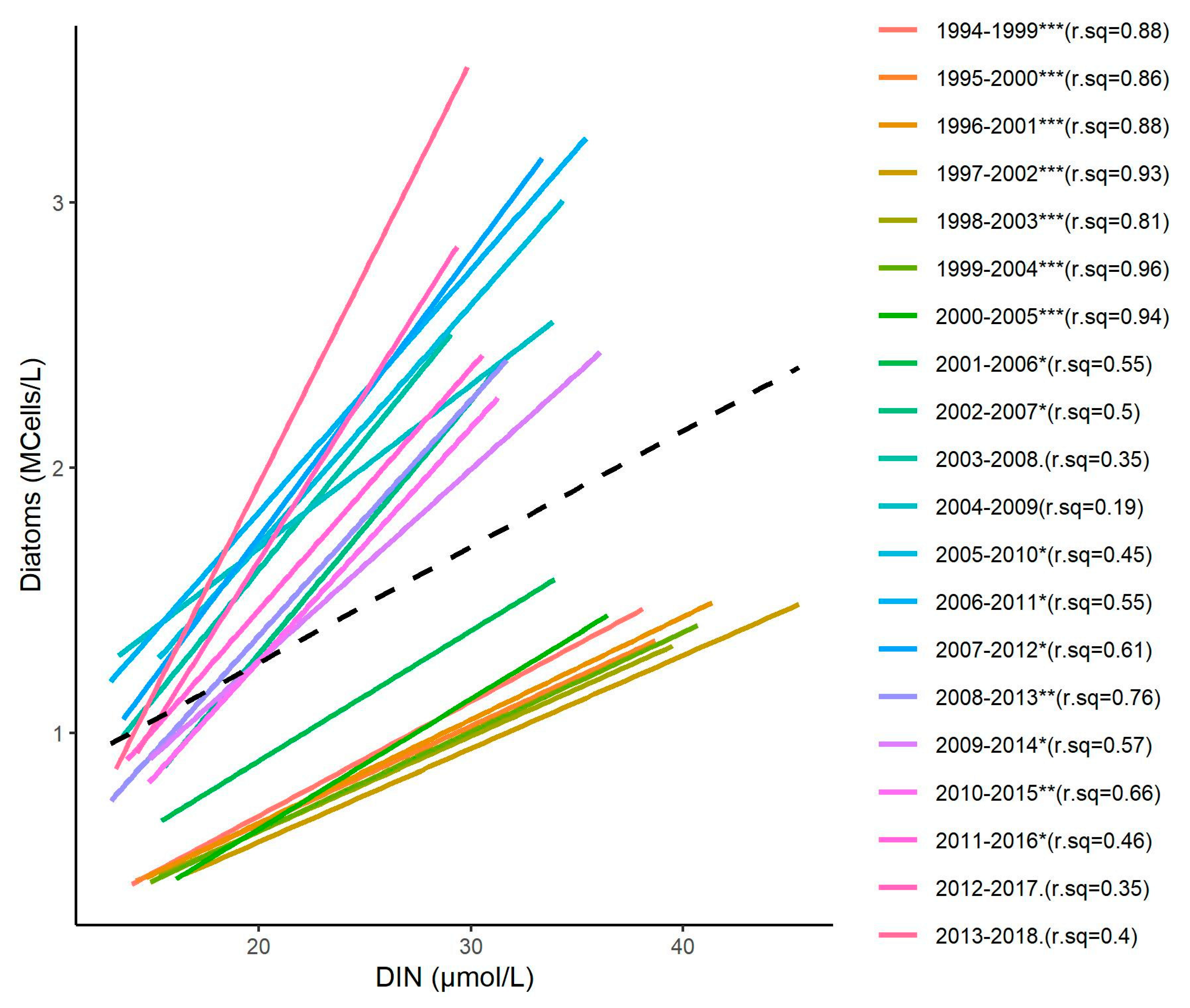
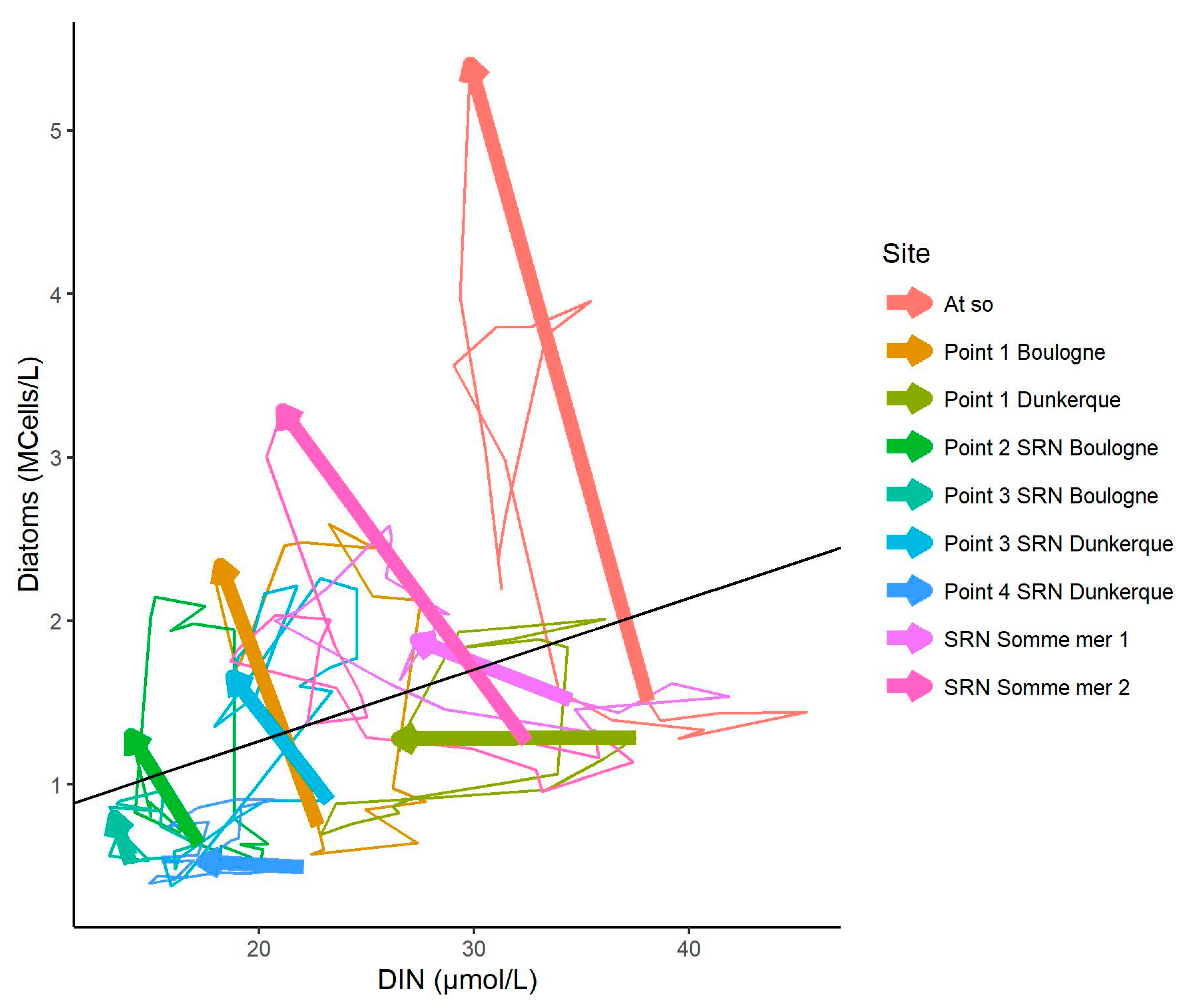
3.4. Relationship between DIN and Diatom Concentrations
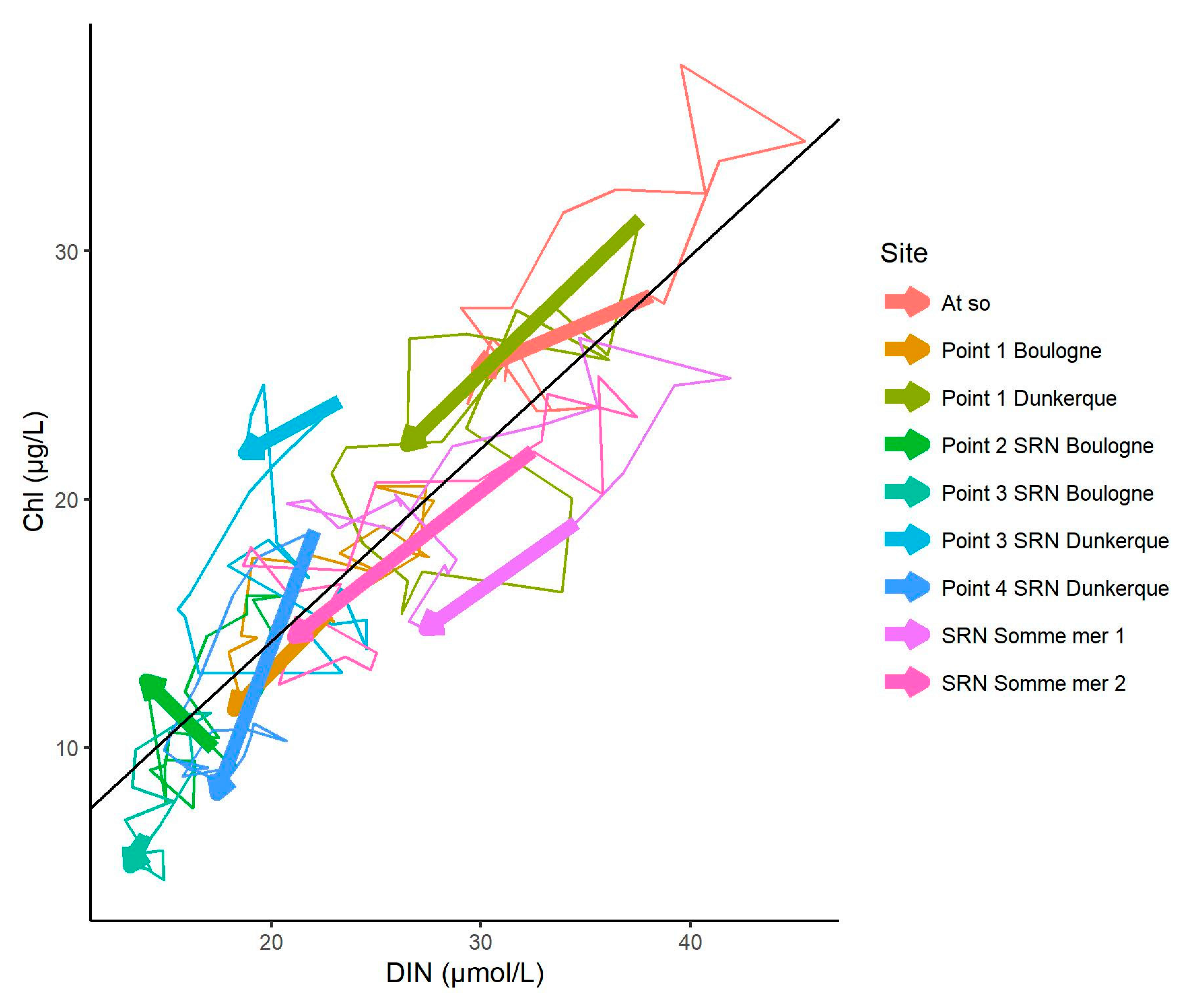
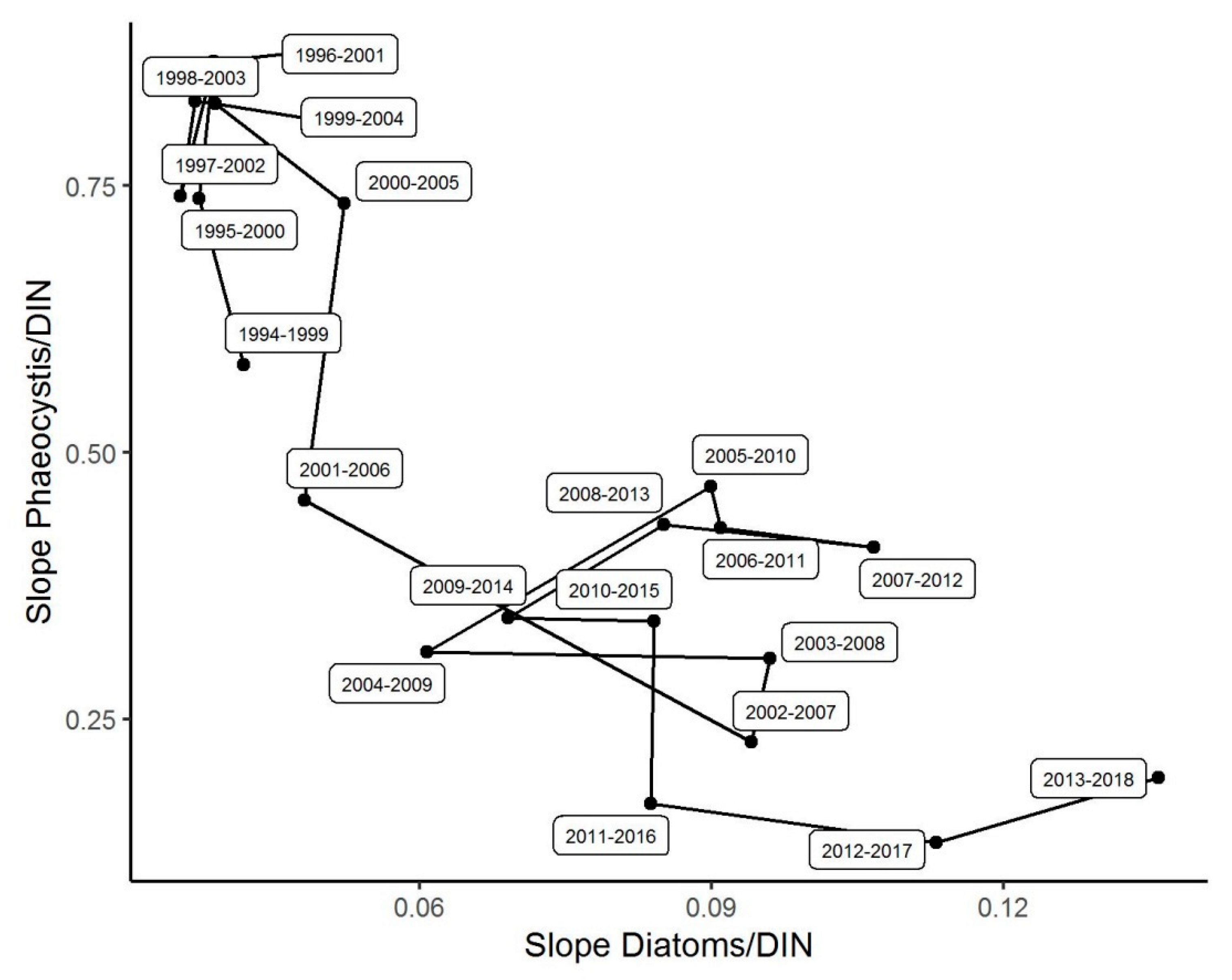
3.5. Changes in the Competitive Advantage of Diatoms Versus Phaeocystis
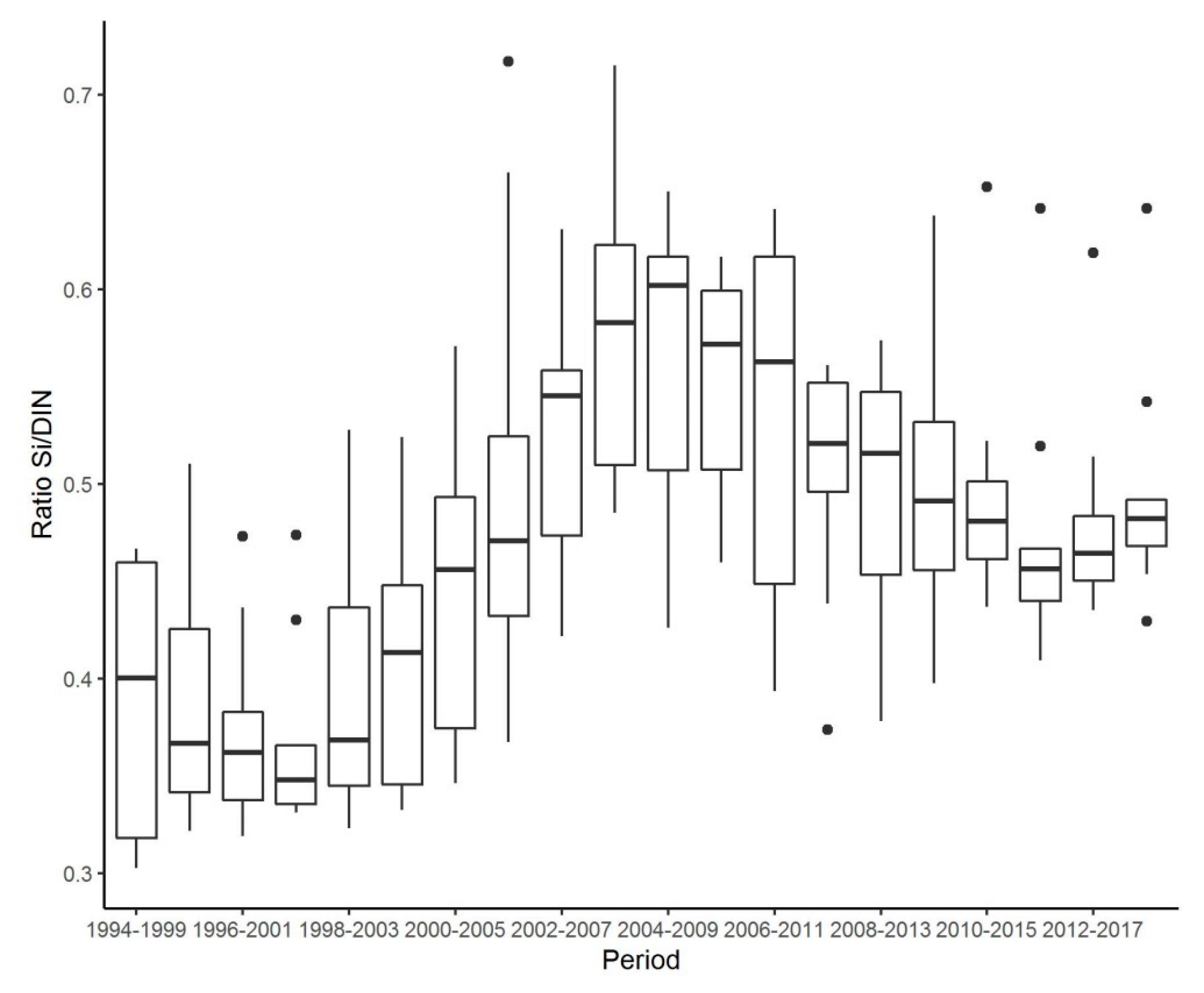
3.6. Changes in DSi/DIN Ratio
4. Discussion
4.1. Long-Term Data Series, Research and Scientific Expertise and Advice
4.2. Relationships between Nutrient Concentrations and The Indicator of Phytoplankton Biomass
4.3. Relationships between Nutrients Concentration and The Indicator Phytoplankton Species
4.4. Change Towards an Unstable Status
4.5. Limitations of Our Approach—Advice for Monitoring and Indicator Development
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
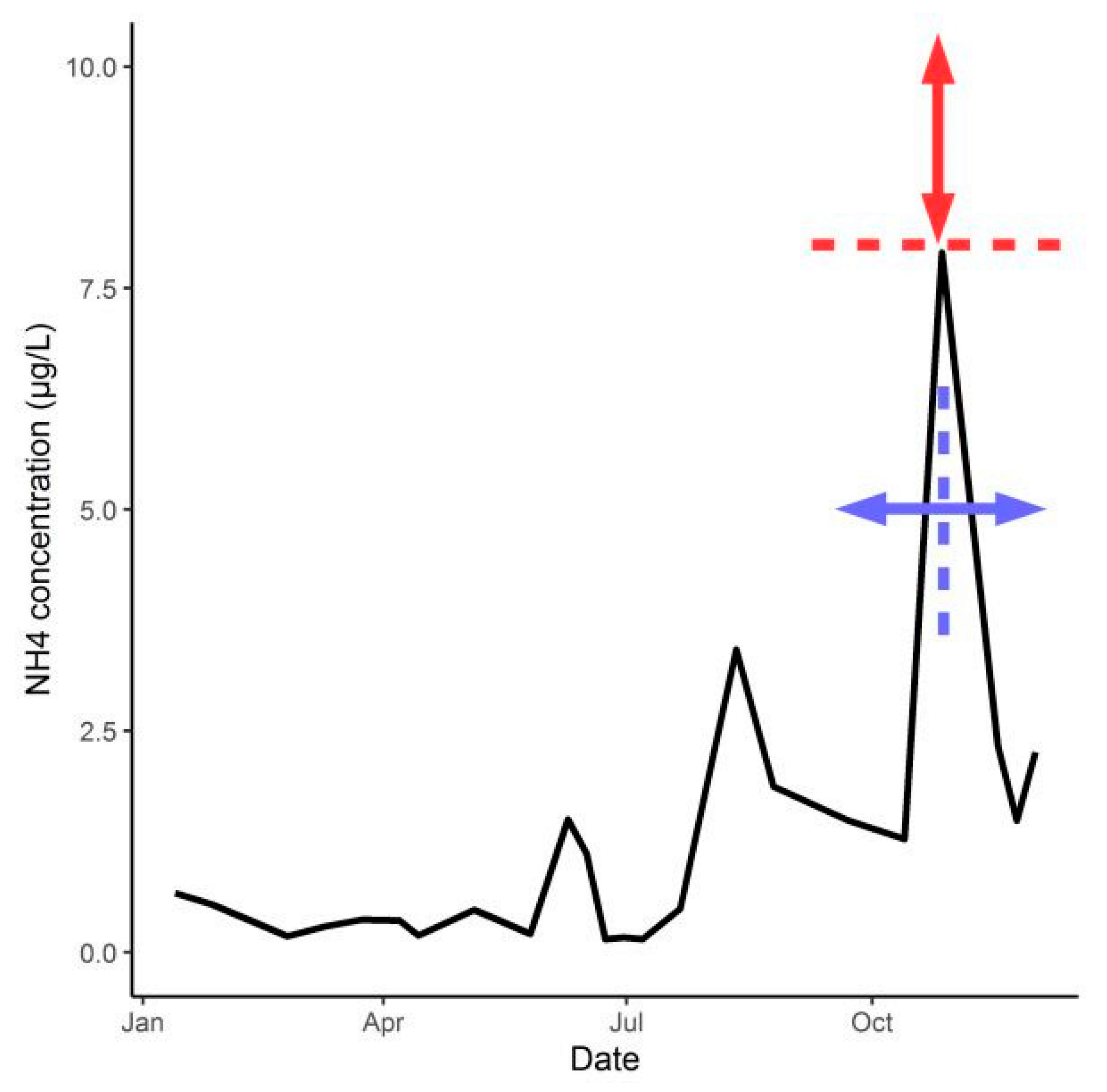
Appendix B
| Dependent Variable | Parameter | Mean [sd] | R² |
|---|---|---|---|
| Chl-a | Intercept | −3.05 [2.82] | 0.87 |
| Slope | 0.85 [0.11] *** | ||
| Phaeocystis | Intercept | −2.63 [1.72] | 0.84 |
| Slope | 0.45 [0.07] *** | ||
| Diatoms | Intercept | −0.51 [0.54] | 0.63 |
| Slope | 0.08 [0.02] ** |
Appendix C
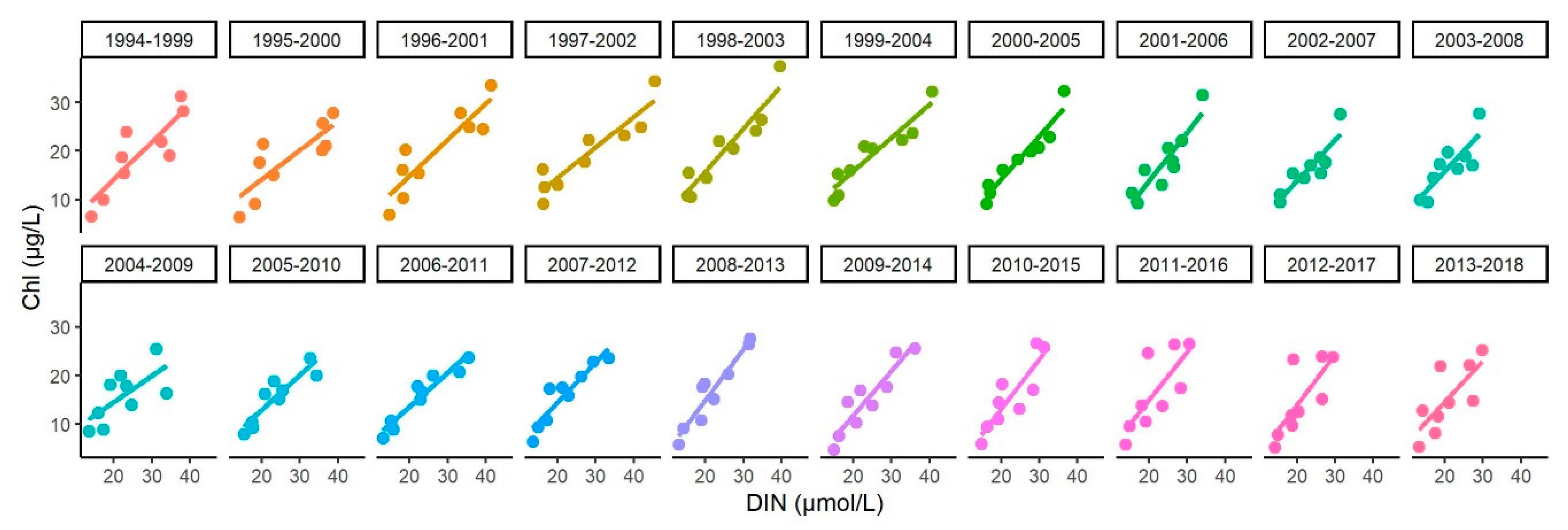
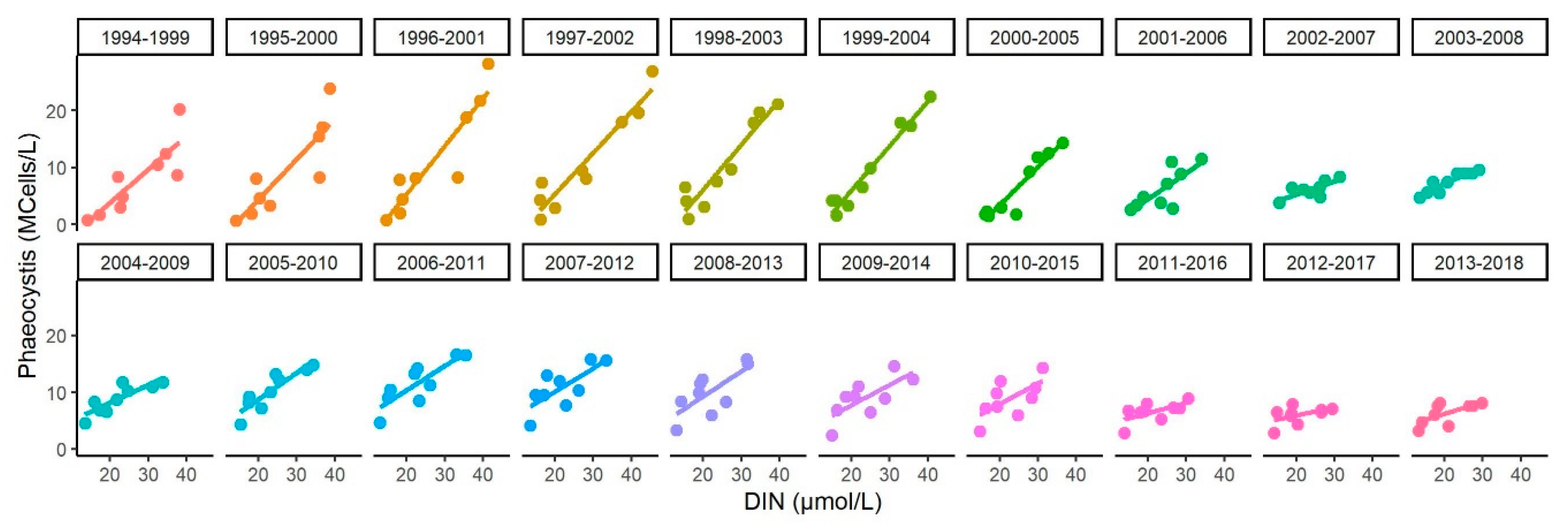
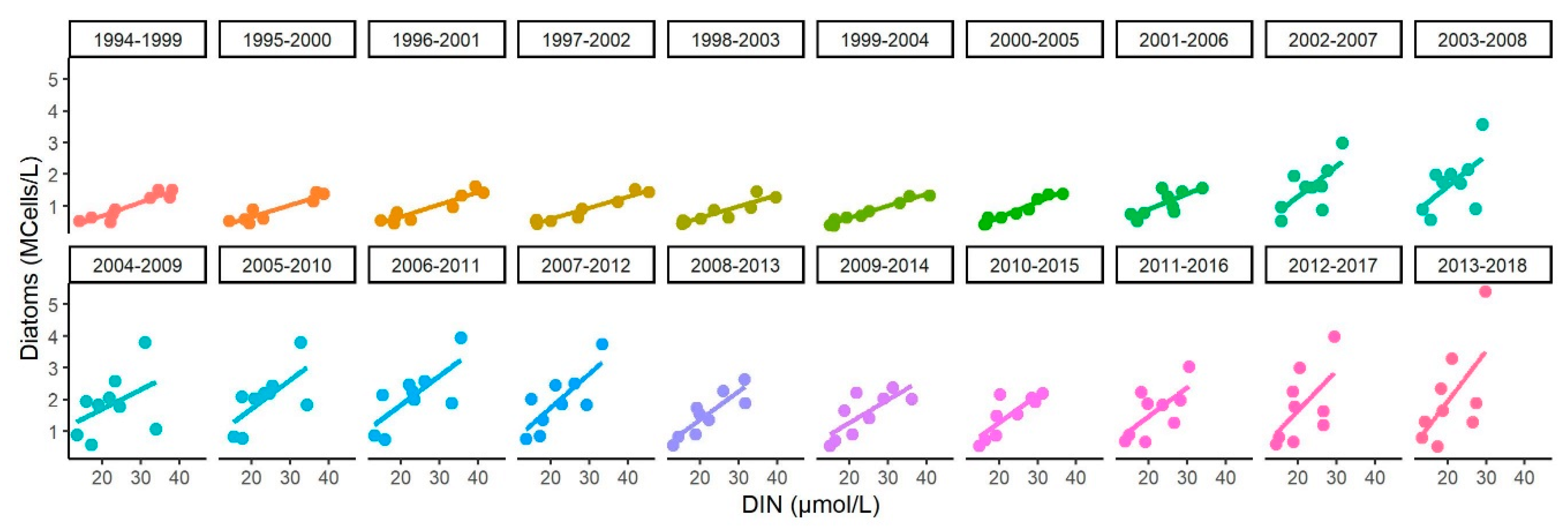
Appendix D
References
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Gobler, C.J. Climate Change and Harmful Algal Blooms: Insights and perspective. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef] [PubMed]
- Belin, C.; Soudant, D.; Amzil, Z. Three decades of data on phytoplankton and phycotoxins on the French coast: Lessons from REPHY and REPHYTOX. Harmful Algae 2020. [Google Scholar] [CrossRef]
- Lancelot, C. The mucilage phenomenon in the continental coastal waters of the North Sea. Sci. Total Environ. 1995, 165, 83–102. [Google Scholar] [CrossRef]
- Verity, P.G.; Brussaard, C.P.; Nejstgaard, J.C.; van Leeuwe, M.A.; Lancelot, C.; Medlin, L.K. Current understanding of Phaeocystis ecology and biogeochemistry, and perspectives for future research. Phaeocystis Major Link Biogeochem. Cycl. Clim. Elem. 2007, 83, 311–330. [Google Scholar]
- Gieskes, W.W.C.; Leterme, S.C.; Peletier, H.; Edwards, M.; Reid, P.C. Phaeocystis colony distribution in the North Atlantic Ocean since 1948, and interpretation of long-term changes in the Phaeocystis hotspot in the North Sea. Biogeochemistry 2007, 83, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Prins, T.C.; Baretta-Bekker, J.G. WFD phytoplankton metric Phaeocystis—Application in the Netherlands; Marine Ecologie: Deltares, The Netherlands, 2010; p. 39. [Google Scholar]
- Rousseau, V.; Mathot, S.; Lancelot, C. Calculating carbon biomass of Phaeocystis sp. from microscopic observations. Mar. Biol. 1990, 107, 305–314. [Google Scholar] [CrossRef]
- Peperzak, L.; Poelman, M. Mass mussel mortality in The Netherlands after a bloom of Phaeocystis globosa (prymnesiophyceae). J. Sea Res. 2008, 60, 220–222. [Google Scholar] [CrossRef]
- Grattepanche, J.D.; Breton, E.; Brylinski, J.M.; Lecuyer, E.; Christaki, U. Succession of primary producers and micrograzers in a coastal ecosystem dominated by Phaeocystis globosa blooms. J. Plankton Res. 2011, 33, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Fariñas, T.; Soudant, D.; Barillé, L.; Belin, C.; Lefebvre, A.; Bacher, C. Temporal changes in the phytoplankton community along the French coast of the eastern English Channel and the southern Bight of the North Sea. ICES J. Mar. Sci. 2014, 71, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Sazhin, A.F.; Artigas, L.F.; Nejstgaard, J.C.; Frischer, M.E. The colonization of two Phaeocystis species (Prymnesiophyceae) by pennate diatoms and other protists: A significant contribution to colony biomass. Biogeochemistry 2007, 83, 137–145. [Google Scholar] [CrossRef]
- Rousseau, V.; Lantoine, F.; Rodriguez, F.; LeGall, F.; Chrétiennot-Dinet, M.J.; Lancelot, C. Characterization of Phaeocystis globosa (Prymnesiophyceae), the blooming species in the Southern North Sea. J. Sea Res. 2013, 76, 105–113. [Google Scholar] [CrossRef]
- Delegrange, A.; Lefebvre, A.; Gohin, F.; Courcot, L.; Vincent, D. Pseudo-nitzschia sp. diversity and seasonality in the southern North Sea, domoic acid levels and associated phytoplankton communities. Estuar. Coast. Shelf Sci. 2018, 214, 194–206. [Google Scholar] [CrossRef] [Green Version]
- Jahan, R.; Choi, J.K. Climate Regime Shift and Phytoplankton Phenology in a Macrotidal Estuary: Long-Term Surveys in Gyeonggi Bay, Korea. Estuaries Coasts 2014, 37, 1169–1187. [Google Scholar] [CrossRef]
- Gregg, W.W.; Casey, N.W.; McClain, C.R. Recent trends in global ocean chlorophyll. Geophys. Res. Lett. 2005, 32, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Bedford, J.; Johns, D.; McQuatters-Gollop, A. A century of change in North Sea plankton communities explored through integrating historical datasets. ICES J. Mar. Sci. 2019, 76, 104–112. [Google Scholar] [CrossRef]
- Rombouts, I.; Simon, N.; Aubert, A.; Cariou, T.; Feunteun, E.; Guérin, L.; Hoebeke, M.; McQuatters-Gollop, A.; Rigaut-Jalabert, F.; Artigas, L.F. Changes in marine phytoplankton diversity: Assessment under the Marine Strategy Framework Directive. Ecol. Indic. 2019, 102, 265–277. [Google Scholar] [CrossRef] [Green Version]
- Tett, P.; Gowen, R.; Mills, D.; Fernandes, T.; Gilpin, L.; Huxham, M.; Kennington, K.; Read, P.; Service, M.; Wilkinson, M.; et al. Defining and detecting undesirable disturbance in the context of marine eutrophication. Mar. Pollut. Bull. 2007, 55, 282–297. [Google Scholar] [CrossRef]
- Prins, T. Phaeocystis assessments as indicator for eutrophication. In Proceedings of the Intersessional Correspondence Group on Eutrophication (ICG EUT), Secretariat, London, UK, 15–16 May 2018; Volume 2, pp. 22–24. [Google Scholar]
- Lancelot, C.; Passy, P.; Gypens, N. Model assessment of present-day Phaeocystis colony blooms in the Southern Bight of the North Sea (SBNS) by comparison with a reconstructed pristine situation. Harmful Algae 2014, 37, 172–182. [Google Scholar] [CrossRef]
- Lefebvre, A.; Guiselin, N.; Barbet, F.; Artigas, F.L. Long-term hydrological and phytoplankton monitoring (19922007) of three potentially eutrophic systems in the eastern English Channel and the Southern Bight of the North Sea. ICES J. Mar. Sci. 2011, 68, 2029–2043. [Google Scholar] [CrossRef] [Green Version]
- Desmit, X.; Nohe, A.; Borges, A.V.; Prins, T.; De Cauwer, K.; Lagring, R.; Van der Zande, D.; Sabbe, K. Changes in chlorophyll concentration and phenology in the North Sea in relation to de-eutrophication and sea surface warming. Limnol. Oceanogr. 2019. [Google Scholar] [CrossRef] [Green Version]
- Schramm, W. Factors influencing seaweed responses to eutrophication: Some results from EU-project EUMAC. J. Appl. Phycol. 1999, 11, 69–78. [Google Scholar] [CrossRef]
- Viaroli, P.; Bartoli, M.; Giordani, G.; Naldi, M.; Orfanidis, S.; Zaldivar, J.M. Community shifts, alternative stable states, biogeochemical controls and feedbacks in eutrophic coastal lagoons: A brief overview. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, S105–S117. [Google Scholar] [CrossRef]
- Duarte, C.M.; Conley, D.J.; Carstensen, J.; Sánchez-Camacho, M. Return to Neverland: Shifting baselines affect eutrophication restoration targets. Estuaries Coasts 2009, 32, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Borja, Á.; Dauer, D.M.; Elliott, M.; Simenstad, C.A. Medium-and Long-term Recovery of Estuarine and Coastal Ecosystems: Patterns, Rates and Restoration Effectiveness. Estuaries Coasts 2010, 33, 1249–1260. [Google Scholar] [CrossRef] [Green Version]
- McCrackin, M.L.; Jones, H.P.; Jones, P.C.; Moreno-Mateos, D. Recovery of lakes and coastal marine ecosystems from eutrophication: A global meta-analysis. Limnol. Oceanogr. 2017, 62, 507–518. [Google Scholar] [CrossRef]
- Derolez, V.; Bec, B.; Munaron, D.; Fiandrino, A.; Pete, R.; Simier, M.; Souchu, P.; Laugier, T.; Aliaume, C.; Malet, N. Recovery trajectories following the reduction of urban nutrient inputs along the eutrophication gradient in French Mediterranean lagoons. Ocean Coast. Manag. 2019, 171, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, A.; Poisson-Caillault, E. High resolution overview of phytoplankton spectral groups and hydrological conditions in the eastern English Channel using unsupervised clustering. Mar. Ecol. Prog. Ser. 2019, 608, 73–92. [Google Scholar] [CrossRef]
- Cloern, J.E. Phytoplankton bloom dynamics in coastal ecosystems: A review with some general lessons from sustained investigation of San Francisco Bay, California. Rev. Geophys. 1996, 34, 127–168. [Google Scholar] [CrossRef]
- SRN. SRN Dataset—Regional Observation and Monitoring Program for Phytoplankton and Hydrology in the Eastern English Channel; SEANOE: Issy-les-Moulineaux, France, Semptember 2017. [Google Scholar] [CrossRef]
- REPHY. REPHY—French Observation and Monitoring Program for Phytoplankton and Hydrology in Coastal Waters. 1987–2016. Metropolitan Dat; SEANOE: Issy-les-Moulineaux, France, Semptember 2017. [Google Scholar] [CrossRef]
- Aminot, A.; Kérouel, R. Hydrologie des Écosystèmes Marins: Paramètres et Analyses; Ifremer: Moulineaux, France, 2004; p. 340. [Google Scholar]
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- WFD. Directive 2000/60/Ec Of The European Parliament And Of The Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Communities 2000, L 327, 1–72. [Google Scholar]
- R Development Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Vienna, Austria, 2016. [Google Scholar]
- Grolemund, G.; Wickham, H. Dates and Times Made Easy with lubridate. J. Stat. Softw. 2011, 40, 1–25. [Google Scholar] [CrossRef]
- Devreker, D.; Lefebvre, A. TTAinterfaceTrendAnalysis: An R GUI for routine Temporal Trend Analysis and diagnostics. J. Oceanogr. Res. Data 2014, 6, 1–18. [Google Scholar]
- European Commission. European Commission MSFD Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental policy (Marine Strategy Framework Directive). (Text with EEA Relevance); European Commission: Brussels, Belgium, 2008. [Google Scholar]
- Gohin, F.; van der Zande, D.; Tilstone, G.; Eleveld, M.A.; Lefebvre, A.; Andrieux-Loyer, F.; Blauw, A.N.; Bryère, P.; Devreker, D.; Garnesson, P.; et al. Twenty years of satellite and in situ observations of surface chlorophyll-a from the northern Bay of Biscay to the eastern English Channel. Is the water quality improving? Remote Sens. Environ. 2019, 233, 111343. [Google Scholar] [CrossRef]
- Karasiewicz, S.; Breton, E.; Lefebvre, A.; Hernández Fariñas, T.; Lefebvre, S. Realized niche analysis of phytoplankton communities involving HAB: Phaeocystis spp. as a case study. Harmful Algae 2018, 72, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nohe, A.; Goffin, A.; Tyberghein, L.; Lagring, R.; De Cauwer, K.; Vyverman, W.; Sabbe, K. Marked changes in diatom and dinoflagellate biomass, composition and seasonality in the Belgian Part of the North Sea between the 1970s and 2000s. Sci. Total Environ. 2020, 716, 136316. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.A.; O’Brien, T.D.; Paerl, H.W.; Peierls, B.L.; Harrison, P.J.; Robb, M. Precipitation as a driver of phytoplankton ecology in coastal waters: A climatic perspective. Estuar. Coast. Shelf Sci. 2015, 162, 1–11. [Google Scholar] [CrossRef]
- Breton, E.; Rousseau, V.; Parent, J.-Y.; Ozer, J.; Lancelot, C. Hydroclimatic modulation of diatom/Phaeocystis blooms in nutrient-enriched Belgian coastal waters (North Sea). Limnol. Oceanogr. 2006, 51, 1401–1409. [Google Scholar] [CrossRef]
- Elliott, M.; Burdon, D.; Hemingway, K.L.; Apitz, S.E. Estuarine, coastal and marine ecosystem restoration: Confusing management and science—A revision of concepts. Estuar. Coast. Shelf Sci. 2007, 74, 349–366. [Google Scholar] [CrossRef]
- EDL DCE. Escaut, Somme et côtiers Manche Mer du Nord, Meuse (Partie Sambre); Agence l’Eau Artois Picardie: ONEMA DREAL Nord Pas Calais, France, 2013; p. 144. [Google Scholar]
- Prins, T.C.; Desmit, X.; Baretta-Bekker, J.G. Phytoplankton composition in Dutch coastal waters responds to changes in riverine nutrient loads. J. Sea Res. 2012, 73, 49–62. [Google Scholar] [CrossRef]
- Cadée, G.C.; Hegeman, J. Seasonal and annual variation in phaeocystis pouchetii (haptophyceae) in the westernmost inlet of the Wadden Sea during the 1973 to 1985 period. Netherlands J. Sea Res. 1986, 20, 29–36. [Google Scholar] [CrossRef]
- Alvarez-Fernandez, S.; Riegman, R. Chlorophyll in North Sea coastal and offshore waters does not reflect long term trends of phytoplankton biomass. J. Sea Res. 2014, 91, 35–44. [Google Scholar] [CrossRef]
- Gobler, C.J.; Baumann, H. Hypoxia and acidification in ocean ecosystems: Coupled dynamics and effects on marine life. Biol. Lett. 2016, 12, 20150976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes-Banda, P.; García-Mendoza, E.; Ponce-Rivas, E.; Blanco, J.; Almazán-Becerril, A.; Galindo-Sánchez, C.; Cembella, A. Association of the toxigenic dinoflagellate Alexandrium ostenfeldii with spirolide accumulation in cultured mussels (Mytilus galloprovincialis) from Northwest Mexico. Front. Mar. Sci. 2018, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gómez, F.; Souissi, S. The impact of the 2003 summer heat wave and the 2005 late cold wave on the phytoplankton in the north-eastern English Channel. Comptes Rendus-Biol. 2008, 331, 678–685. [Google Scholar] [CrossRef]
- Kopp, D.; Lefebvre, S.; Cachera, M.; Villanueva, M.C.; Ernande, B. Reorganization of a marine trophic network along an inshore-offshore gradient due to stronger pelagic-benthic coupling in coastal areas. Prog. Oceanogr. 2015, 130, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Giraldo, C.; Ernande, B.; Cresson, P.; Kopp, D.; Cachera, M.; Travers-Trolet, M.; Lefebvre, S. Depth gradient in the resource use of a fish community from a semi-enclosed sea. Limnol. Oceanogr. 2017, 62, 2213–2226. [Google Scholar] [CrossRef] [Green Version]
- OSPAR. OSPAR CEMP Guideline Common Indicator: PH1/FW5 Plankton Lifeforms; OSPAR Commission: London, UK, 2018; pp. 1–17. [Google Scholar]
- Glibert, P.M.; Louchart, A.; Lizon, F.; Lefebvre, A.; Didry, M.; Schmitt, F.G.; Artigas, L.F.; Giering, S.L.C.; Cavan, E.L.; Basedow, S.L.; et al. Coastal ocean and nearshore observation: A French case study. Front. Mar. Sci. 2019, 6, 390–402. [Google Scholar]
- Cocquempot, L.; Delacourt, C.; Paillet, J.; Riou, P.; Aucan, J.; Castelle, B.; Charria, G.; Claudet, J.; Conan, P.; Coppola, L.; et al. Coastal ocean and nearshore observation: A French case study. Front. Mar. Sci. 2019, 6, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Garnier, J.; Riou, P.; Le Gendre, R.; Ramarson, A.; Billen, G.; Cugier, P.; Schapira, M.; Théry, S.; Thieu, V.; Ménesguen, A. Managing the agri-food system of watersheds to combat coastal eutrophication: A land-to-sea modelling approach to the french coastal English channel. Geosciemce 2019, 9, 441. [Google Scholar] [CrossRef] [Green Version]
- Grassi, K.; Poisson Caillault, E.; Lefebvre, A. Multilevel Spectral Clustering for extreme event characterization. In Proceedings of the OCEANS 2019—Marseille, Marseille, France, 17–20 June 2019; pp. 1–7. [Google Scholar]
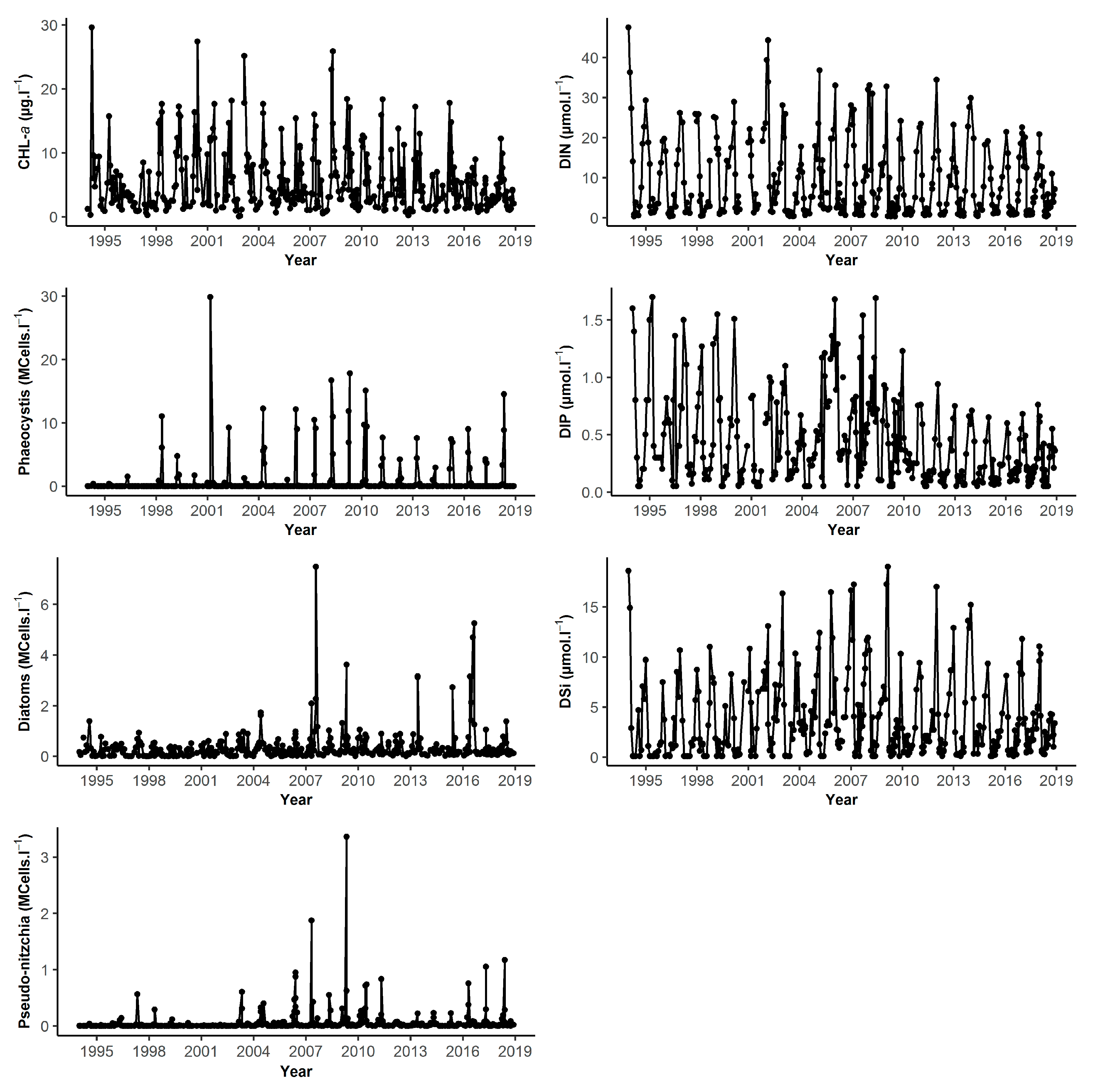
| DK1 | Min | Q1 | Median | Mean | Q3 | Max | NA | N |
| Chl-a (µg.L−1) | 0.24 | 2.55 | 4.55 | 6.95 | 8.60 | 53.18 | 6 | 321 |
| DIN (µmol.L−1) | 0.30 | 1.51 | 5.35 | 10.47 | 17.65 | 54.92 | 17 | 310 |
| DIP (µmol.L−1) | 0.01 | 0.17 | 0.40 | 0.51 | 0.69 | 4.90 | 15 | 312 |
| DSi (µmol.L−1) | 0.1 | 1.05 | 3.13 | 5.05 | 6.57 | 35.20 | 14 | 313 |
| Diatoms (cell.L−1) | 900 | 79,470 | 202,600 | 335,000 | 426,400 | 5,365,000 | 19 | 308 |
| Dinoflagellates (cell.L−1) | 0 | 675 | 4685 | 11890 | 13,520 | 202,200 | 19 | 308 |
| Phaeocystis (cell.L−1) | 0 | 0 | 465,800 | 4,061,000 | 6,473,000 | 28,230,000 | 2 | 78 |
| Pseudo-nitzchia (cell.L−1) | 0 | 400 | 7016 | 51,600 | 34,650 | 1,505,000 | 19 | 308 |
| BL1 | Min | Q1 | Median | Mean | Q3 | Max | NA | N |
| Chl-a (µg.L−1) | 0.04 | 2.01 | 3.67 | 5.52 | 7.44 | 29.60 | 5 | 399 |
| DIN (µmol.L−1) | 0.30 | 1.39 | 4.25 | 8.40 | 12.90 | 47.51 | 25 | 379 |
| DIP (µmol.L−1) | 0.05 | 0.13 | 0.28 | 0.40 | 0.60 | 1.70 | 18 | 386 |
| DSi (µmol.L−1) | 0.10 | 0.47 | 1.64 | 3.25 | 4.32 | 19.01 | 18 | 386 |
| Diatoms (cell.L−1) | 400 | 88,740 | 210,800 | 374,300 | 417,000 | 7,492,000 | 5 | 399 |
| Dinoflagellates (cell.L−1) | 0 | 1020 | 5362 | 10,450 | 12,600 | 237,500 | 5 | 399 |
| Phaeocystis (cell.L−1) | 0 | 36,270 | 1,330,000 | 3,499,000 | 6,043,000 | 17,800,000 | 1 | 89 |
| Pseudo-nitzchia (cell.L−1) | 0 | 400 | 8536 | 73,310 | 39,880 | 3,361,000 | 5 | 399 |
| S1 | Min | Q1 | Median | Mean | Q3 | Max | NA | N |
| Chl-a (µg.L−1) | 0.21 | 3.10 | 5.77 | 8.41 | 10.81 | 58.53 | 7 | 362 |
| DIN (µmol.L−1) | 0.30 | 3.01 | 10.37 | 14.44 | 22.78 | 60.04 | 15 | 354 |
| DIP (µmol.L−1) | 0.04 | 0.13 | 0.27 | 0.40 | 0.59 | 1.55 | 14 | 355 |
| DSi (µmol.L−1) | 0.10 | 1.13 | 3.67 | 6.30 | 10.18 | 41.00 | 15 | 354 |
| Diatoms (cell.L−1) | 2900 | 122,100 | 295,700 | 679,800 | 746,000 | 11,070,000 | 4 | 365 |
| Dinoflagellates (cell.L−1) | 0 | 400 | 2900 | 12,460 | 10,920 | 815,100 | 4 | 365 |
| Phaeocystis (cell.L−1) | 0 | 18,210 | 1,913,000 | 5,777,000 | 6,360,000 | 48,930,000 | 0 | 83 |
| Pseudo-nitzchia (cell.L−1) | 0 | 0 | 3750 | 123,400 | 33,070 | 7,503,000 | 4 | 365 |
| Parameter | Trend Statistics | DK1 | BL1 | S1 |
|---|---|---|---|---|
| Chl-a (µg.L−1) | sen.slope | −0.055 | −0.0152 | 0.0235 |
| sen.slope.pct | −0.0138 | −0.0063 | 0.0052 | |
| p-value | 0.005 | 0.2953 | 0.3774 | |
| DIN (µmol.L−1) | sen.slope | −0.173 | −0.0595 | −0.1272 |
| sen.slope.pct | −0.028 | −0.0152 | −0.0157 | |
| p-value | 0 | 1.00 × 10−4 | 0.0021 | |
| DIP (µmol.L−1) | sen.slope | −0.01 | −0.0087 | −0.004 |
| sen.slope.pct | −0.0242 | −0.0247 | −0.0128 | |
| p-value | 0 | 0 | 0.0149 | |
| DSi (µmol.L−1) | sen.slope | −0.0162 | 0.0278 | −0.0554 |
| sen.slope.pct | −0.0063 | 0.024 | −0.0144 | |
| p-value | 0.3093 | 0.0017 | 0.0158 | |
| Diatoms (cell.L−1) | sen.slope | 2313 | 3411 | 2008 |
| sen.slope.pct | 0.032 | 0.0376 | 0.0117 | |
| p-value | 0.0126 | 0.0023 | 0.1931 | |
| Dinoflagellates (cell.L−1) | sen.slope | 178 | 186 | 183 |
| sen.slope.pct | 0.1329 | 0.1264 | 0.2407 | |
| p-value | 0 | 0 | 0 | |
| Phaeocystis (cell.L−1) | sen.slope | 25,767 | 247,220 | −7345 |
| sen.slope.pct | 0.0854 | 0.3777 | −0.008 | |
| p-value | 0.4026 | 0.0109 | 0.922 | |
| Pseudo-nitzschia (cell.L−1) | sen.slope | 400 | 460 | 311 |
| sen.slope.pct | 0.3385 | 0.4951 | 1.2563 | |
| p-value | 0 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefebvre, A.; Dezécache, C. Trajectories of Changes in Phytoplankton Biomass, Phaeocystis globosa and Diatom (incl. Pseudo-nitzschia sp.) Abundances Related to Nutrient Pressures in the Eastern English Channel, Southern North Sea. J. Mar. Sci. Eng. 2020, 8, 401. https://doi.org/10.3390/jmse8060401
Lefebvre A, Dezécache C. Trajectories of Changes in Phytoplankton Biomass, Phaeocystis globosa and Diatom (incl. Pseudo-nitzschia sp.) Abundances Related to Nutrient Pressures in the Eastern English Channel, Southern North Sea. Journal of Marine Science and Engineering. 2020; 8(6):401. https://doi.org/10.3390/jmse8060401
Chicago/Turabian StyleLefebvre, Alain, and Camille Dezécache. 2020. "Trajectories of Changes in Phytoplankton Biomass, Phaeocystis globosa and Diatom (incl. Pseudo-nitzschia sp.) Abundances Related to Nutrient Pressures in the Eastern English Channel, Southern North Sea" Journal of Marine Science and Engineering 8, no. 6: 401. https://doi.org/10.3390/jmse8060401
APA StyleLefebvre, A., & Dezécache, C. (2020). Trajectories of Changes in Phytoplankton Biomass, Phaeocystis globosa and Diatom (incl. Pseudo-nitzschia sp.) Abundances Related to Nutrient Pressures in the Eastern English Channel, Southern North Sea. Journal of Marine Science and Engineering, 8(6), 401. https://doi.org/10.3390/jmse8060401






