Abstract
Bivalve aquaculture is an important component of the economy in eastern Canada. Because of current social, environmental, economic, and resource constraints, offshore mussel cultivation seems to be a promising strategy. With the objective of optimizing farming strategies that support the sustainability and development of the mussel industry at a microgeographic scale, we evaluated, after a traditional two year production cycle, the commercial performance of spat from several mussel (Mytilus edulis) stocks originating from sites separated by less than 65 km and cultivated at two different grow-out sites (shallow lagoon and offshore waters). The spatiotemporal variation in spat performance was studied through a multiyear in situ “stock-site” spat transfer design. The spat supply originating from the Bassin du Havre-Aubert lagoon systematically exhibited a larger size at sleeving time when compared to other stocks, and a better productivity level when harvested. Nevertheless, an alternative strategy would be to collect spat from the Havre-aux-Maisons lagoon, mostly because of the important commercial volumes of spat that can be collected there. Commercial performance (net income) was three times higher in the deep offshore grow-out site than in the shallow lagoon site. This better productivity in the open sea confirms the highly valuable strategy of offshore mussel farming in this area, where it was hypothesized that the less stressful environmental conditions positively influence reproduction, survival, and growth trends.
1. Introduction
The aquaculture of marine bivalves has increased sharply over the past few decades and will keep growing given the rising global demand [1]. In eastern Canada, mussel culture dominates the shellfish market, with an annual production of more than 30,000 tons and a total value of 22 million Canadian dollars in 2015 [2]. Worldwide, mussel culture development has occurred almost exclusively in protected near-shore waters [3,4] or in estuarine habitats [5,6], with off-bottom culture techniques using rafts, pole racks, and longline systems [7]. However, nearshore bivalve culture is severely constrained by space limitation and user conflicts [8,9], climate change and fluctuating environmental conditions [6,10], carrying capacity limits or other environmental concerns [4,11], and the need for improving mussel performance and productivity [8,12]. On the basis of several pilot projects conducted in different countries over more than a decade, the establishment of offshore suspended long-line mussel farms has been shown to be promising [4,8,9,10,13] in areas like the Îles-de-la-Madeleine Archipelago (Gulf of St. Lawrence, Québec, Canada) [14,15].
In Canada, blue mussel (Mytilus edulis, L. 1758) farming is exclusively supplied by wild spat using ropes as artificial collectors. For more than 25 years, mussel culture in the Îles-de-la-Madeleine has relied on the collection of wild juveniles in a small lagoon, the Bassin du Havre-Aubert (BHA). This area is used to supply spat because this local stock has better performance in terms of survival and growth compared to neighboring sites (<65 km between the most distant mussel populations) [16]. Indeed, several studies have demonstrated that this high performance is related to specific metabolic and genetic features that provide better resistance to stressful conditions and lower vulnerability to summer mortality episodes [17,18,19,20,21,22]. Despite this advantage, BHA is small (3 km2) and shallow (2–3 m), and has a large interannual variability in spat supply; all these restrict the development of local mussel farming [23]. Because of this, alternative supply scenarios need to be identified by reassessing the commercial potential of all stocks of mussel spat in the Îles-de-la-Madeleine, particularly for offshore culture.
Stock performance depends on its genetic heterogeneity, the intrinsic adaptive flexibility of individuals and the environmental variability of grow-out sites [24,25]. A “stock-site” spat transfer design is a reliable way to assess the differential performance (survival and growth) of stocks farmed in specific conditions [26] and represents a method of identifying the best stock to farm [16,26,27,28,29]. This design is also used to determine if the adaptive genetic variability remains stable through multiple combinations of stocks and/or sites, and if the adaptive flexibility differently integrates environmental variations [30,31]. Considering the evolution of oceanographic conditions in the Gulf of St. Lawrence following global change trends [32] and the annual spat transfers of the high performing BHA stock for 25 years to all grow-out sites of the Îles-de-la-Madeleine, we hypothesize that the differential performance of mussels stocks previously described [16] may have been remodeled by phenotypic plasticity and genotypic selection. Thus, the aim of this study was to determine if the pattern of spat performance persists over years at a microgeographic scale. By applying a stock-site design, we first compared the quality of different mussel stocks in 2014 and 2015 and then assessed their related commercial productivity after a traditional two- year production cycle [14] in lagoon and offshore grow-out sites. Commercial productivity was estimated by an original calculation of the net incomes produced by each stock-site scenario, considering the costs of local industrial processes.
2. Materials and Methods
2.1. Experimental Design
Our study compares the productivity and commercial performance of different stocks of mussel spat collected in the Îles-de-la-Madeleine archipelago in the southern Gulf of St. Lawrence, Canada (Figure 1). Spat from four sites were sampled, three in the lagoons of Bassin du Havre-Aubert (BHA), Havre-aux-Maisons (HAM), and Grande-Entrée (GE), and one in the offshore site of Baie de Plaisance (BP). In June–July, when the first competent larvae were observed in the water column [33,34], ten artificial collectors (1 m long propylene ropes) were submerged 3 m below the surface on long-line structures in lagoons and 10 m below the surface at the offshore site. Five collectors were harvested in November (4–5-month-old spat) and five in May (10–11-month-old spat) respectively for fall and spring season stock recoveries (Table 1). Thus, experimental stocks were characterized by (i) the origin and (ii) the season; and experiments (two year production cycle) began in 2014 (hereafter Year 1) and 2015 (Year 2). A brining operation was conducted on all collectors in early August to reduce the predation pressure of benthic species like sea stars (Asterias sp.) and spatial competition with fouling filamentous algae (Cladophora sp. and Enteromorpha sp.), as described by Bourque and Myrand [35,36]. A total of 16 mussel stocks were collected between 2014 and 2016, including two BHA stocks harvested in fall, which are commonly used by local mussel farmers and served as our control stocks.
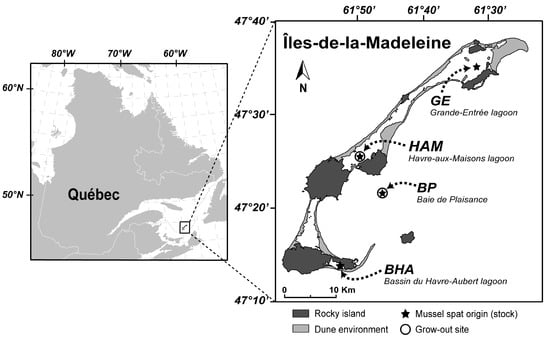
Figure 1.
Location of mussel stocks and production sites in the Îles-de-la-Madeleine archipelago, southern Gulf of St. Lawrence, Canada.

Table 1.
Summary of the stock-site design conducted into the Îles-de-la-Madeleine.
2.2. Spat Supply Performance
The cumulative settlement rates and size frequency distributions were immediately determined after each stock sampling to determine if the industrial criteria required for grow-out processing (sock sleeving) were met. These criteria were (i) a minimum of 35% of spat (size >6 mm) exceeding 15 mm in length on each collector, and (ii) production of at least 2 m of mussel socks (40 mm opening) from a single 1 m collector (“sock:collector ratio” > 2:1). These criteria lead to the usual commercial density of around 680 to 830 mussels m−1 [37,38,39]. Five socks (3 m in length) were sleeved per stock and suspended on long-lines in the lagoon (HAM) and offshore (BP) grow-out sites. Socks were suspended 3 m below the surface in HAM and 10 m below the surface in BP.
2.3. Commercial Harvest Performance
To compare production performance among the stock-site scenarios, all socks were harvested after a traditional two year production cycle (22–24-month-old mussels), corresponding to 12 and 18 months of growth in sites for stocks sleeved in spring and fall, respectively (Table 1). Immediately after sampling, total density, size frequency, and mussel fresh mass were assessed on a randomly selected 30 cm section of each sock. The productivity of each stock-site scenario was evaluated by determining the proportion (%) and fresh mass (kg m−1 of sock) of commercial mussels (≥ 50 mm) in order to estimate wholesale volumes of raw, non-transformed mussels at the market price of $1.47 kg−1, as applied by national processing plants during the 2014–2017 period (here $ refers to the Canadian dollar). The commercial performance (net incomes) was evaluated through a standardized method integrating spat supply and productivity performance at the scale of a standard long-line (122 m long). In addition, variable expenses including working hours along the production cycle, i.e., from supply to harvest, were estimated. Net incomes were calculated following the technical-economical method described in Laplante and Bourque [40]:
- -
- The length of mussel socks produced with one long-line containing 488 m of spat collectors, by applying the sock:collector ratio calculated previously;
- -
- The required quantity of grow-out long-lines, by considering a mean length of 549 m of mussel socks per unit;
- -
- The associated variable costs (human and material resources), including seven distinct steps: (i) spat collector installations, (ii) brining [35,36], (iii) harvesting, (iv) spat stripping, de-clumping, and size-ranging, (v) sock sleeving, (vi) installations to grow-out, (vii) harvesting and preparation of raw mussels;
- -
- The raw income of the production purchase at $1.47 kg−1.
2.4. Environmental and Trophic Parameters
Environmental and trophic parameters were recorded from mid-May to mid-November 2015 (Year 2) and 2016 (Year 3) in each grow-out site to document conditions experienced by Year 1 and Year 2 mussel stocks, respectively. The seawater temperature was recorded hourly with multi-parameter YSI 6600 probes (Yellow Spring Inc., Yellow Springs, OH, USA) deployed on long-lines, close to the mussel socks. The trophic conditions were monitored every 7–10 days by estimating particulate organic matter (POM), chlorophyll-a, and planktonic group composition. The surrounding seawater was sampled with a Niskin bottle (from a depth of 2 m), immediately sieved on a 20 µm square mesh and stored in opaque bottles. Because the oligotrophic seawater of the archipelago is characterized by small-sized particles [41,42,43], we pre-filtered all samples in accordance with the range of size detected for future flow-cytometer analyses. Triplicate samples of 2 L were filtered on GF/C glass fiber filters (1.2 µm; Whatman Ltd., Maidstone, UK), and POM concentration was quantified by gravimetry according to Aminot and Chaussepied [44]. Triplicate samples of 0.5 L were filtered in the dark on GF/F glass fiber filters (0.7 µm; Whatman Ltd., Maidstone, UK), and chlorophyll-a concentration was measured after an overnight extraction in 90% acetone using a Trilogy fluorimeter (Turner Designs Inc., San Jose, CA, USA). For planktonic composition, duplicate samples of 4.5 mL were preserved in 0.1% glutaraldehyde, stored at −80 °C, and analyzed with an Epics Altra flow cytometer (Beckman Coulter Inc., Fullerton, CA, USA) as described in Belzile et al. [45] and Tremblay et al. [46] for the enumeration of pico (0.2–2 µm) and nano (2–20 µm) eukaryotes and cyanobacteria as well as heterotrophic bacteria.
2.5. Statistical Analyses
For each season (fall and spring), a series of two-way analyses of variance (ANOVA) was used to study the effects of year (two fixed levels) and spat origin (four fixed levels) on the dependent variables describing spat supply performance, i.e., spat density, proportion exceeding 15 mm (%), and the sock:collector ratio. ANOVAs were conducted separately for fall and spring to eliminate a potential seasonal effect on spat performance. For a given year, fall and spring spat were not independent since they both came from the same spawning events. A second series of two-way ANOVAs was performed to test the effects of spat stock (nine fixed levels) and grow-out site (two fixed levels) on the commercial performance variables, i.e., proportion of commercial mussels (%), mass of commercial mussels, and net incomes. ANOVAs were conducted after testing assumptions of normality and homoscedasticity with Kolmogorov–Smirnov and Levene tests, respectively, using R version 3.5.2 [47]. When assumptions were not met, data were log transformed, except for percentage or proportion data, which were arcsine-square-root transformed [48]. When significance occurred, Tukey HSD post-hoc tests were conducted to determine pairwise differences.
For each experimental year, the differences between environmental conditions (temperature and the seven trophic variables) of the two grow-out sites (HAM and BP) were tested through a one-way permutational multivariate analysis of variance (PERMANOVA with 9999 permutations) based on Euclidean dissimilarities using PRIMER 7 version 7.0.12 (PRIMER-e, Quest Research Ltd., Auckland, New Zealand) [49]. Assumptions of homoscedasticity had previously been verified with a PERMDISP test [50] and data were log transformed when necessary. In addition, a similar percent analysis routine (SIMPER) was performed on untransformed data for each experimental year to assess the contribution of environmental variables to dissimilarities between grow-out sites. Finally, two-way ANOVAs were performed to test the effects of grow-out site (two fixed levels) and time (from 14 to 18 levels, according to the year and site) on environmental variables during each experimental year. A significant threshold of α = 0.05 was adopted for all statistical analyses.
3. Results
3.1. Spat Supply Performance
In both seasons of spat recovery (fall and spring), an interaction effect between year and origin was observed on mussel spat density (Figure 2a). For both years, the control stock (BHA-Fall) had the lowest density (<5000 ind m−1 of collector) compared to the other stocks (HAM, GE, and BP), which could reach up to 9000 ind m−1. In spring, GE stocks density were drastically lower than in the fall, and the BHA and GE stocks had the lowest densities (<3000 ind m−1). Values for HAM and BP stocks were more than twice as high, but unfortunately their spring stocks were lost in Year 1 due to harsh winter conditions (Figure 2a). The overall interseasonal mean density decreased by 44% between fall and spring. Among the 14 recovered spat stocks, 10 showed a proportion of large spat (>15 mm) exceeding the industrial threshold of 35% for sleeving (Figure 2b). We observed interaction between the year and origin effects in the fall and an origin effect in the spring. On Figure 2b,c, all pairwise comparisons (post-hoc test) have been represented to highlight the interseasonal, interannual, and intersite variability existing among the stocks assessed. For both years and seasons, BHA stocks showed the highest proportions (>75%) of mussels > 15 mm compared to other stocks (10% to 65%). Overall, this proportion was 27% lower in the fall than in the spring. BHA stocks were similar between years and seasons, and they were the only stocks to systematically show values above the industrial threshold of 35% for sleeving. Because of strong sea star predation on collectors, the volume of spat for the BHA-Year2-Spring stock was too low to produce enough socks for experimental grow-out processing. Thus, only nine stocks that met the second industrial criteria of a sock:collector ratio > 2:1 were used for sleeving (Figure 2c). In both fall and spring, this ratio showed year and origin effects without interaction. Maximal values were observed with HAM-Year1-Fall and HAM-Year2-Spring, with one single collector supplying spat to sleeve more than 9 m of socks.
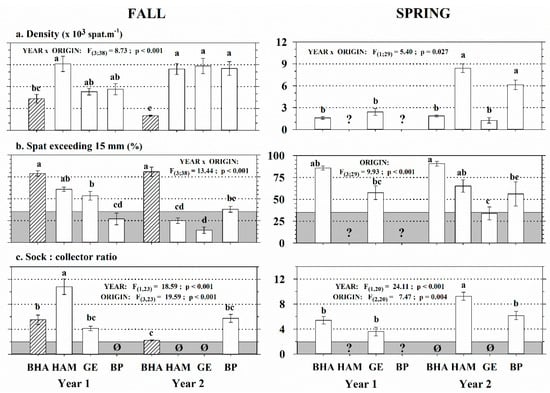
Figure 2.
Mussel spat stock performance (origin × year × season) from the Îles-de-la-Madeleine, with spat density (a), proportion of spat > 15 mm (b) and sock:collector ratio (c). Data are Mean ± SE; n = 6 collectors. BHA, HAM, GE, and BP = spat origin. Striped bars = control stocks. Year 1 and Year 2 = spawning year (2014, 2015). Horizontal gray area (b and c) = industrial criteria to grow-out a mussel stock (threshold of 35% of spat > 15 mm and sock:collector ratio > 2:1). ? = collectors lost during winter. Ø = unsleeved stocks (industrial criteria not met). For each season of spat recovery, significant results of two-way ANOVAs are presented and letters indicate significant differences (p < 0.05) among stocks. BHA, Bassin du Havre-Aubert; HAM, Havre-aux-Maisons; GE, Grande-Entrée; BP, Baie de Plaisance.
3.2. Commercial Performance
The socks produced with the nine spat stocks selected using the industrial criteria were grown in the HAM (lagoon) and BP (offshore) sites, for a total of 18 stock-site scenarios. Unfortunately, the socks produced with the GE-Year1-Fall stock were lost in BP during the winter, so 17 combinations remained at the end of the experiment. Stock × site interactions were observed for every parameter characterizing commercial performance of each combination harvested after a two year production cycle (Figure 3). Whatever the stock, the percentage of commercial mussels was higher in BP (>40%) than in HAM (<35%) (Figure 3a). The overall mean was three times higher offshore than at the lagoon site (55 ± 2% and 19 ± 2%, respectively). The most efficient stocks in BP were BHA-Year1-Fall, BP-Year2-Fall, HAM-Year2-Spring, and BP-Year2-Spring with >55% commercial mussels. In HAM, the best stocks were BHA-Year1-Fall, BHA-Year2-Fall, and HAM-Year1-Fall with 30%–35% commercial mussels. Surprisingly, the HAM-Year2-Spring stock provided the highest rate of commercial mussels in BP (72.4 ± 2.2%) but was one of the least productive stocks in HAM (5.3 ± 2.4%). The proportions of commercial mussels for the stocks sleeved in spring were always drastically lower in HAM (<10%) than in BP (>40%). In addition, the mass of commercial mussels produced per meter of sock was twice as high in BP compared to HAM (Figure 3b), with mean values of 5.2 ± 0.4 and 2.2 ± 0.3 kg m−1 of sock, respectively. The most efficient stocks in BP were BHA-Year1-Fall, BHA-Year2-Fall, and HAM-Year2-Spring, with a production mass of more than 6 kg of commercial mussels per meter of sock. In HAM, the most performant stocks had masses of 3–4 kg m−1 of sock. There was a drastic difference between grow-out sites with the HAM-Year2-Spring and BP-Year2-Spring stocks: HAM-Year2-Spring was 25 times more productive offshore (6.7 ± 0.5 kg m−1) than at the lagoon site (0.27 ± 0.09 kg m−1) while BP-Year2-Spring was six times more productive in BP (3.6 ± 0.2 kg m−1) than in HAM (0.61 ± 0.15 kg m−1). Finally, the mean net income estimated for the 17 stock-site scenarios was $13,111 ± 1574, with great variability related to stock and site (Figure 3c). Overall, mussel culture was three times more lucrative in BP ($20,045 ± 2305) than in HAM ($6573 ± 1475) at the end of the two year production cycle. Stocks BHA-Year1-Fall and HAM-Year2-Spring generated the highest net incomes in BP, with respective mean values of $29,170 and $40,278. In parallel, two stocks cultivated in HAM, HAM-Year2-Spring and BP-Year2-Spring, were unprofitable. Among the 17 stock-site scenarios, five were very profitable (exceeding $20,000), including four stocks grown in BP (BHA-Year1-Fall, HAM-Year1-Fall, HAM-Year2-Spring, BP-Year2-Fall) and just one in HAM (HAM-Year1-Fall).
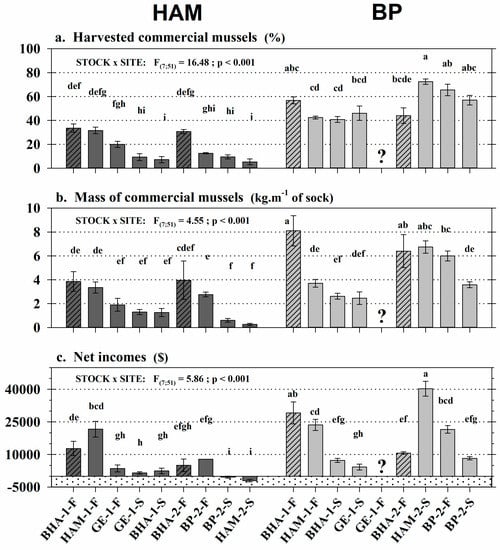
Figure 3.
Commercial performance of the nine spat stocks sleeved and harvested after a two year production cycle at the HAM (dark gray) and BP (light gray) grow-out sites: (a) Proportion and (b) mass of commercial mussels (>50 mm) as well as the (c) net incomes associated. Data are Mean ± SE; n = 2–5 mussel socks. BHA, HAM, GE, and BP = spat origin. Striped bars = control stocks. 1 and 2 = spawning years (2014, 2015). F and S = fall and spring spat collector recovery. Stippled area = negative net incomes. ? = lost stocks. Significant results of two-way ANOVAs are presented and letters indicate significant differences (p < 0.05) among stock-site combinations.
3.3. Environmental and Trophic Conditions in Grow-out Sites
The lagoon (HAM) and offshore (BP) sites showed strong differences in their environmental conditions from May to November for both years (Table 2, Figure 4 and Figure 5). The SIMPER analysis carried out on data from each year showed that all parameters contributed almost equally to the difference between sites (Table 3), with contributions to the dissimilarity ranging between 10% and 14%. However, total heterotrophic bacteria and POM concentrations had slightly higher influences on the differentiation observed each year. According to the average squared Euclidean distances calculated, the dissimilarity between sites was higher in Year 2 than in Year 3, with respective values of δ = 20.44 and δ = 18.71. Two-way ANOVAs performed on each of the eight environmental parameters showed site × date interactions, but values were generally higher in HAM (Figure 4 and Figure 5). In both years, water temperatures were higher in HAM from late May to September, reaching 15 °C approximately one month earlier. The maximal temperature measured in HAM exceeded 23 °C but remained below 20 °C in BP (Figure 4a). Despite a global similarity between POM concentrations observed in both sites each year (1–2 mg L−1), we observed a major peak from late August to late September in 2015 (Year 2), with values three times higher in HAM than in BP (Figure 4b). The period of maximum chlorophyll-a concentration (>2 µg L−1) ranged from mid-July to mid-October in both sites (Figure 4c), but values were 2–3 times higher in HAM than in BP. At both sites, planktonic communities were dominated by pico-eukaryotes (Figure 5a) and pico-cyanobacteria (Figure 5c). Overall, bacteria and eukaryotes (pico and nano) concentrations tended to be higher in the shallow lagoon than in the offshore site, with average abundances in HAM being 2–3 times (bacteria) and 4–10 times (eukaryotes) higher than those measured in BP. This spatial pattern was not observed for cyanobacteria in Year 3, where abundances (pico and nano) showed similar patterns at both sites. These trends were associated with interannual variations of planktonic concentrations in HAM. The overall average abundances in HAM decreased by two (pico-plankton) and three (nano-plankton) times between Year 2 and Year 3, while they tended to follow a similar pattern for all planktonic groups in BP.

Table 2.
Multivariate PERMANOVA results investigating environmental conditions between the lagoon (HAM) and offshore (BP) sites used for mussel growth in Year 2 and Year 3. Variables were temperature, particulate organic matter, chlorophyll-a, total heterotrophic bacteria, pico- and nano-eukaryotes, and pico- and nano-cyanobacteria.
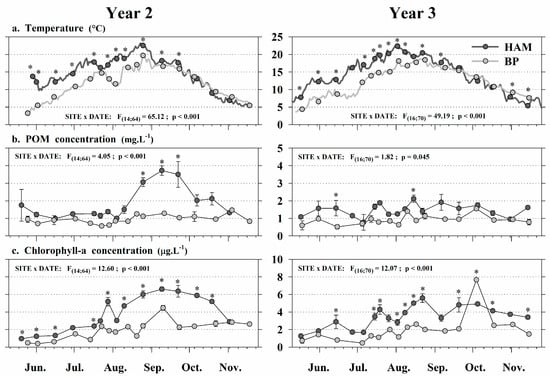
Figure 4.
Monitoring environmental conditions in the HAM (dark gray) and BP (light gray) grow-out sites in Year 2 and Year 3 (2015, 2016): (a) temperature; (b) particulate organic matter; (c) chlorophyll-a. Data are Mean ± SE; n = 3 samples. Significant results of two-way ANOVAs are presented. * = significant difference (p < 0.05) between grow-out sites.
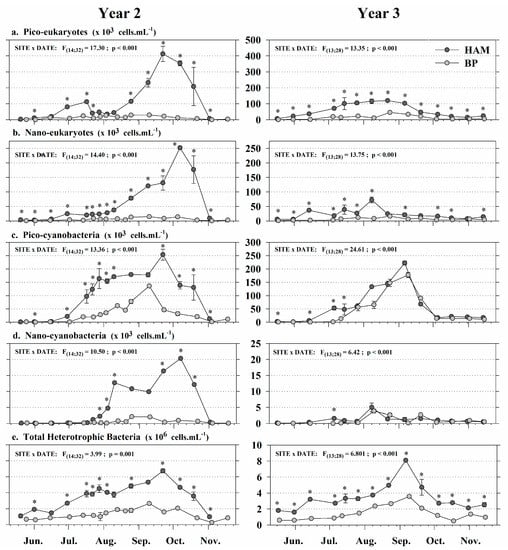
Figure 5.
Monitoring plankton component concentrations in the HAM (dark gray) and BP (light gray) grow-out sites, in Year 2 and Year 3 (2015, 2016): (a) Pico-eukaryotes; (b) nano-eukaryotes; (c) pico-cyanobacteria; (d) nano-cyanobacteria; (e) total heterotrophic bacteria. Data are Mean ± SE; n = 2 samples. Significant results of two-way ANOVAs are presented. * = significant difference (p < 0.05) between grow-out sites.

Table 3.
SIMPER analysis investigating the contribution of environmental and trophic parameters measured in the lagoon (HAM) and offshore (BP) sites used for mussel growth in Year 2 and Year 3.
4. Discussion
We hypothesized that the variable performance of mussel stocks previously described by Myrand and Gaudreault [16] could have been rewritten by the management strategy used for the last 25 years. Using a novel indicator based on net incomes, this potential mitigation was assessed on commercial productivity rather than only on survival and growth measures obtained in cages. Until now, mussel culture at the microgeographic scale of the Îles-de-la-Madeleine Archipelago (85 km long) has been done with BHA spat transferred to all grow-out sites. We expected that successive massive transfers, year after year, would homogenize the microgeographic performance differences that have been observed [17,18,19,20,21,22]. However, our results clearly show that the commercial performance of spat exhibited significant differences among stocks (characterized by origin, year and season of spat recovery) and grow-out sites, with systematic stock and site interactions for all commercial parameters measured on sleeved mussels. Thus, the microgeographic differences in commercial performance has been maintained, with interannual performance variability. The better performance of BHA stocks—with larger sized spat than those from any other site—has been maintained for two decades. Once sleeved, BHA spat showed high productivity levels, corroborating its commercial efficiency for local mussel growers, such as demonstrated in the 1990s [16].
4.1. Spat Supply Performance
Strong variations in spat performance between years and origins highlight the high spatiotemporal variability and corroborate previous observations in different sites of this archipelago [14,23,33,34,42,51]. The different patterns in spat recovery observed between seasons (fall and spring) revealed that collector overwintering impacts stock performance (spat density and % of spat >15 mm). This result agrees with the self-thinning theory, suggesting a negative relationship between density and biomass to self-regulate the intraspecific competition for food and space [52,53,54,55]. The number of individuals on collectors probably decreases through mussel fall-off during overwintering [56], thus improving conditions for the remaining mussels through increased food availability that in turn stimulates growth and increases the percentage of mussels exceeding 15 mm. In a self-thinning modelling study [57], mussel densities on collectors were constant at first and followed by a sharp decrease after 310 days. We observed a similar trend, with a decrease in spat density coupled with an increase in size between spat sleeved in the fall (i.e., 100–120 days after settlement) and spat sleeved in spring (i.e., 300–340 days after settlement). Furthermore, control stocks (BHA-Fall) exhibited the lowest spat density but the highest percentage of spat exceeding 15 mm. These results are in accordance with a general negative relationship between mussel density and size, as demonstrated in the western Gulf of St. Lawrence by Lachance-Bernard et al. [56]. Nevertheless, some stocks did not follow this trend: stocks from HAM and those from BP in Year 2 did not reveal a drastic decrease in spat density but an increase in size was noted. Unfortunately, the loss of collectors for these stocks in Year 1 means that we only have a single year’s observation of this phenomenon with both spat stocks from HAM and BP. Moreover, the lack of coupling between density decrease and size increase could be related to the presence of late or secondary settlement on the collectors in late summer [57,58]. This visible “second set” of spat was discarded in fall with the sleeving size criteria (>6 mm), but it was large enough to be sleeved in spring. Bourque and Myrand [14] suggested that second set spat could enhance intraspecific competition, resulting in partial fall-off of spat exceeding 15 mm and reducing the contrast between seasons induced by self-thinning.
Long lines in the offshore site (BP) are set up just above the potential thermocline, a site also characterized by higher turbulence [59]. As an experimental study on giant scallops (Placopecten magellanicus, G. 1791) recruitment [60] demonstrated an interaction effect of thermocline and turbulence on the settlement success, we think the depth in BP may have impacted the recruitment success of mussels on collectors. Several field studies confirmed that blue mussel settlement on artificial collectors is influenced by the presence of a thermocline and depth [13,61,62]. However, we have no indication that collectors’ depth is an important driving factor for spat supply differentiation between lagoon and offshore sites in this area. We suggest that settlement and recruitment success differentiation between lagoon and offshore sites could be mostly related to seawater temperature and the differential temporal patterns inducing spawning delay in BP (see Appendix A for variations in gametogenesis in BHA mussels raised at both grow-out sites) and consequently the first occurrence of spat on collectors (approximately 3–4 weeks) and their size in the fall (Figure 2b). However, further study is needed to clearly identify the importance level of each factor on recruitment success of blue mussels in this offshore site.
The sock:collector ratio is a commercial indicator taking into account both spat density and size to determine the volume of commercial-sized spat that can be sleeved; this technique has been recommended in several studies conducted in eastern Canada [37,38,39,63,64]. In this context, the sock:collector ratio is a standardized way to compare the performance of different spat supplies. Mussel stock efficiency is determined by the level of interseasonal, interannual, and intersite variability. We observed that control stocks (BHA-Fall) performed well for sleeving, but not when spat were collected in spring. With equivalent sock:collector ratios as BHA-Fall, GE-Fall and BP-Spring appeared to be two good alternatives for spat supply, but the two stocks from HAM (HAM-2014-Fall and HAM-2015-Spring) outperformed all the other stocks tested (ratio >9:1) and represented promising strategies for sleeving.
4.2. Environment and Stock Performance
Collection of juvenile wild mussels depends on biotic (e.g., synchronized with food availability and food quality, unsynchronized with predators) and abiotic (e.g., environmental conditions, collector structure) factors [14,42,43] that act on spawning, larval supply, settlement, and spat performance on collectors [23,59]. According to Cyr et al. [33,34], the first occurrence of settled spat on collectors was observed in late June in BHA, in early July in HAM and GE, and between late July/early August in BP. Thus, since all collectors were retrieved simultaneously, BP mussels had a shorter period to grow on collectors. Bourque and Myrand [14] suggested that spat collected in the fall at the offshore site (BP) should not be sleeved before the following spring, due to later spawning events. Our results support this recommendation: in fall, we observed only 25% to 35% of spat (>15 mm) in BP compared to >50% the following spring. In addition to a longer period of growth for spat from lagoons, shallow sites are characterized by higher food availability, since all trophic markers showed higher values in the lagoon than at the offshore site (Figure 4 and Figure 5). HAM lagoon is characterized by a high level of quality trophic resources, stimulating increased accumulations of energetic reserves [65]. Increased energy reserves favor gonadal development and lead to early massive spawning events, these being stimulated in periods of summer temperature increase [24,66].
Different studies have suggested that spat recruitment success may be affected by the presence of competitor or predator species [66,67,68]. Hydrozoans and filamentous algae (Cladophora sp. and Enteromorpha sp.), which are favorable habitats for the first settlement of mussel larvae [69,70], are problematic on suspended collectors: they are related to massive mussel fall-off when spat grow [14,23,35,71]. To limit the impact of fouling and predation by benthic species like sea stars (Asterias sp.), all collectors underwent brining operations at the same time to limit potential variable impacts. However, another predatory species affected this study—the abundant sea ducks (Somateria mollissima, Clangula hyemalis and Aythya marila) in the GE lagoon. Diving ducks are a widespread problem for mussel farming in eastern Canada; they forage on both spat and adult mussels [72,73,74,75], with a preference for shallow waters and small-sized mussels [74,75,76,77]. From our observations, we believe ducks were responsible for the low densities on GE collectors retrieved in spring. The deeper mussel aquaculture practices in BP could offer a higher protection against avian predators comparatively to mussel farming in the lagoon. Generally, sea ducks prefer shallow water due to potential energetic gain by diving shallower, but Guillemette et al. [76] suggested prey selection is rather related to their preferences (size and nutritional values of species). Tardive spawning of mussels in BP increase their nutritional value during the summer, as gametes are rich in polyunsaturated fatty acids [78]. Thus, mussels with higher nutritional quality in BP could be stimulating for avian predation, as some molluscivore ducks seem able to discriminate quality of blue mussels [74,75]. Furthermore, sea ducks can dive down to 50 m depth [73] and even if the avian predators’ activity could be less recurrent in offshore than in the lagoons, such pressure remains a stake for mussel industry in offshore waters.
The performance of the BHA spat supply could probably be linked to the restricted configuration of this lagoon (3 km2, mean depth of 2–3 m), which leads to earlier, faster, and higher warming of the water column that stimulates earlier spawning events [23,33,34]. In addition, several studies have revealed specific metabolic and genetic characteristics of BHA mussels that provide better resistance to stress as well as higher growth and survival rates [18,19,20,21,22,38,39]. However, these advantages did not systematically improve the commercial performance of this stock.
4.3. Commercial Performance in Different Grow-out Sites
Our results clearly demonstrate that all mussel stocks sleeved and cultured in the BP offshore site had higher commercial performance after a two year production cycle, as shown by the higher net incomes (Figure 3). Specifically, the quantity of commercial-sized mussels was three times higher in BP than in HAM. The commercial mass of mussels harvested per meter of sock was on average twice as high in BP, thus the mean net incomes were three times higher at the offshore site. Our results agree with studies by Bourque and Myrand [14] and Seguin-Heine et al. [15], which were conducted at the same sites with mussels spat from BHA. The authors reported that mussel shell weight was lower and meat yield higher in BP compared to HAM. Similarly, in another study in Notre Dame Bay (Newfoundland, Canada), Gallardi et al. [12] observed higher shell weight in cultivated mussels suspended in shallow water (5 m) than in deep water (15 m). We suggest that specific conditions in the lagoon and offshore sites could induce differential energetic allocation strategies that explain the higher commercial productivity in BP. As a reaction to environmental conditions and competition, mussels differentially allocate their energy to protective tissues including their shells and byssus filaments [79,80]. As already reported, cultured mussels suspended in offshore sites (BP) invest less energy in shell and byssus production than do mussels in lagoons [15], suggesting that more energy is available for soft tissue production. Contrary to the suggestion of Gallardi et al. [12], the lower energetic investment in mussel shells cultivated in BP was not related to a less turbulent environment: Seguin-Heine et al. [15] reported turbulence values < 1 J m−3 in HAM compared to 5 J m−3 in BP. In the same study [15], the authors suggested that lower investment in protective tissues, particularly in byssus production, could result in higher mussel fall-off in offshore waters and therefore lead to lower commercial productivity. Our results contradict this hypothesis. We demonstrated that the mass of all harvested commercial mussels was systematically higher for each stock cultivated in BP compared to HAM. In addition, different reproduction trends were observed in mussels maintained in BP and HAM (Appendix A). The significantly higher gamete volume fraction in BP and the earlier spawning events in lagoons highlighted the fact that mussels allocate more energy to gamete development when cultured in deep offshore waters. The concomitant periods for mussel commercial harvest and spawning in the lagoons could explain the less valuable productivity in HAM, illustrated by a lower mass of commercial mussels recovered per meter of sock. These observations agree with previous studies on suspension-cultured mussels [21,59], which showed that mussel growth is affected by early spawning in lagoon sites. Moreover, the large amount of energy invested in gamete production usually weakens mussels and can increase mortality [21,63]. Because they are in poor condition due to depleted reserves, mussels are more vulnerable to stressful environmental factors, especially temperature and food quality [19,21,22,66,67,81].
Temperature and food availability were higher in the shallow lagoon compared to the offshore site, agreeing with previous studies [14,15,16,21]. Temperature directly affects physiological rates and thus every aspect of the biology and ecology of mussels [67,82,83]. Although [84] established that the scope for growth remains stable between 10 °C and 20 °C, other studies demonstrated that temperatures greater than a 20 °C threshold are associated with stressful conditions for recruitment, metabolism, growth, and survival of M. edulis [21,85,86,87]. Cheney et al. [8] affirmed that the stable temperature of offshore waters results in lower stress and a faster growth. In our study, the performance in BP could be related to the water temperature, which never reached 20 °C. In HAM, this threshold was exceeded for 19 days in Year 2 and 26 days in Year 3.
The seston availability (POM and chlorophyll-a) was higher in HAM than in BP and varied throughout the season. Multivariate analyses demonstrated than all environmental and trophic parameters contributed to site differentiation, with total heterotrophic bacteria and POM concentrations having the highest contributions. Lagoons in this area have been described as oligotrophic, with heterotrophic planktonic communities dominating the mussel culture environment [41] and representing the main mussel carbon intakes [88]. In St. Peter’s Bay (southern Gulf of St. Lawrence), Sonier et al. [89] reported an averaged mussel retention efficiency of 20% for pico-phytoplankton, and 60% for nano-phytoplankton. However, our results suggest food availability is not the main parameter explaining mussel performance and productivity: HAM had higher pico- and nano-phytoplankton concentrations than BP, but commercial performance of mussels was lower in the lagoon site. According to Incze et al. [86], variations in food availability in thermally stressful conditions (>20 °C) could lower mussel performance. Thus, potential stressful temperatures in HAM could decrease the efficiency of food assimilation, hence affecting growth and commercial performance, but more studies are necessary to clearly demonstrate this phenomenon. Furthermore, we did not analyze the nutritive value or the energy inputs, which could be different depending on culture conditions [12,79].
4.4. Stock-site Productivity
In this study, we assessed the commercial productivity of nine stocks cultured at two sites with very different conditions. To our knowledge, the assessment of net incomes generated by different stock-site combinations and using the traditional techniques and criteria of mussel producers has never been investigated. Capital expenses were not included (buildings, boats, vehicles, working equipment, and land travel), nor were expenses related to licences, taxes, insurance, and quality analyses since they are too variable and depend on business governance. These need to be included by individual producers to obtain a true industrial estimate. However, our assessments of productivity and incomes for each scenario were standardized on spat supply from one long-line of collectors, so commercial performance is related to characteristics of stock origin, their biological particularities, density, size performance of the spat supply, grow-out site characteristics, size and mass at harvest time, and production costs.
From the 17 stock-site combinations, five scenarios generated incomes higher than $20,000, including four stocks cultivated in the BP offshore site (BHA-Year1-Fall, HAM-Year1-Fall, BP-Year2-Fall, HAM-Year2-Spring) while only one (HAM-Year1-Fall) showed this high net income in HAM lagoon. Another interesting but slightly less productive combination (net income of $13,000) was the BHA-Year1-Fall stock cultured in HAM. Our data showed nine combinations that were not commercially attractive, with two negative values. Globally, our results indicated that productivity and commercial performance were better when stocks were cultivated in the deep offshore waters than in the shallow lagoon. We hypothesise that the higher energetic investment in gamete production for mussels growing in the offshore site, revealed by a higher gamete volume fraction in BP and earlier spawning events in HAM, could be a factor favoring better commercial productivity in BP. The potentially stressful temperature in HAM could also be a factor limiting mussel culture performance in the lagoon. The best results were obtained with HAM-Year2-Spring grown in BP. The high sock:collector ratio of this stock produced a large number of socks that generated abundant harvests of commercial-sized mussels, leading to the highest commercial performance in this study ($40,278 ± 3414). The other stock from HAM (HAM-Year1-Fall) produced the highest sock:collector ratio, but the commercial performance in BP was lower and quite similar to HAM. Increased predation by sea stars on socks cultivated in BP in Year 2 and the less stressful thermal environment in HAM (shorter periods above 20 °C) in Year 3 could explain this pattern. Thus, our results suggest that mussel spat collected in HAM could constitute an alternative strategy for local aquaculture, mostly due to its high supply of commercial spat. In addition, our results showed that mussel culture at offshore sites was more efficient. The higher productivity associated with the less stressful thermal conditions in BP showed offshore mussel aquaculture to be a highly valuable strategy in this area.
BHA-Year1-Fall cultivated in BP was the second-best scenario for generating high income ($29,170 ± 5038). This stock is characterized by a greater amount of large spat for sleeving and a higher mass of commercial sized mussels. However, spat collected in BHA during fall (control) showed strong interannual variability, with commercial performance fluctuating by two to three times in the two grow-out sites. Moreover, this stock was the only one cultivated both years, confirming the importance of interannual variability affecting both spat collection and on-site mussel cultivation. Interannual variability of spat collection has been well documented in the Îles-de-la-Madeleine [33,34,42], specifically in BHA. Specific years like 2004, 2006, 2010, and 2019 were disastrous for commercial supply in BHA lagoon, affecting dramatically further production [23]. Our results clearly highlight the better performances of spat supply in BHA, but also the potential important interannual commercial performance. Thus, we suggest that interannual stability in mussel production in this area could be based on the use of different scenarios, until five, generating important incomes.
Author Contributions
Conceptualization, C.C., N.T. and R.T.; methodology, E.G., C.C., J.-F.L., F.B., N.T. and R.T.; software, E.G.; validation, E.G., N.T. and R.T.; formal analysis, E.G.; investigation, E.G., C.C. and N.T.; resources, C.C., N.T. and R.T.; data curation, E.G. and N.T.; writing—original draft preparation, E.G.; writing—review and editing, E.G., F.B., N.T. and R.T.; visualization, E.G.; supervision, C.C., J.-F.L., F.B., N.T. and R.T.; project administration, C.C. and N.T.; funding acquisition, C.C., N.T. and R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by by the research fund Innovamer from the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec, the Mitacs accélération program from MITACS (#IT09688), by Ressources Aquatiques Québec Research Network (Fonds de Recherche du Québec-Nature et Technologies, #2014-RS-171172), and by an NSERC-Discovery Grant from the Natural Sciences and Engineering Research Council of Canada awarded to RT (#299100).
Acknowledgments
We address special thanks to the technical staff of MERINOV for their indispensable implication in fieldwork and help in laboratories: Francine Aucoin, Pascale Chevarie, Thierry Marcoux, Stéphanie Arnold, Michelle Langford, Claude Poirier, Denis Boudreau, Jules Arseneau, François Gallien, and Yvon Chevarie. We are indebted to Michel Fournier (Les moules de Culture des Îles Inc.), Carlo Eloquin (Grande-Entrée Aquaculture Inc.), Christian Vigneau (La Moule du Large Inc.) and Sylvain Vigneau (Cultimer Inc.) who kindly let us use their long-lines and/or provided us with experimental seed stocks. We would like to thank Claude Belzile (ISMER, UQAR) for flow cytometer analysis. We also grateful to Laure Devine for her suggestions and comments on the text. Finally, we thank the anonymous reviewers for their comments and suggestions to improve an earlier version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Reproductive Conditions in Grow-out Sites
In fall 2002, mussel spat was collected in BHA following the procedure described in the main text and suspended for 12 months at the two grow-out sites (lagoon HAM, open sea BP). Different trends in environmental conditions were observed at the two grow-out sites, with higher temperature, seston, and chlorophyll-a concentrations in HAM than in BP. Once harvested, 300 adult mussels were placed in Vexar cages to monitor the reproductive conditions at each grow-out site from late-May to late-September 2004. Every two weeks, triplicate batches of 15 mussels were randomly sampled at each site and stored on ice. At the laboratory, mussels were sectioned following the sagittal plane. One half of the mussel was used for sex determination under microscope and the second half for histological analyses. Within each triplicate sample, a section of mantle from four females was fixed in a 1G4F solution (1% glutaraldehyde; 4% formaldehyde) for 24 h and then transferred to Davidson’s fixative for storage [21]. The tissue was embedded in paraffin wax and four thick sections (5 mm) from the mantle were cut with hematoxylin and eosin. Reproductive condition was assessed from three fields per section of each mussel using stereological analysis [90]. The proportion of gametes per field (hereafter, the gamete volume fraction, GVF) was measured using a compound microscope at a magnification of 400× coupled to an image capture kit CoolSNAP-Pro cf Digital Kit TM 4.1 (Meyer Instruments Inc., Houston, TX, USA), as described by Pernet et al. [91]. We performed a two way ANOVA to compare variations in gametogenesis information (GVF) according to the grow-out site (two fixed levels) and the sampling date (nine fixed levels).
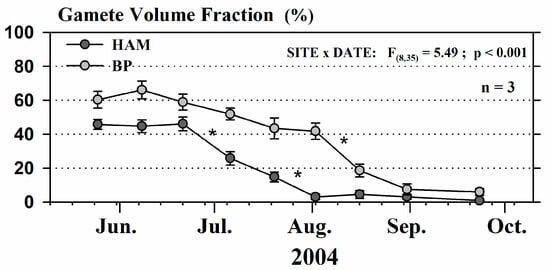
Figure A1.
Variation of the gamete volume fraction (GVF) measured on mussels from BHA and grown in HAM (dark gray) and BP (light gray), in 2004. Data are Mean ± SE; n = 3 samples. Significant results of a two-way ANOVA are presented. * = significant difference (p < 0.05) between two successive dates within each grow-out site.
Interaction effect of grow-out sites and sampling dates was observed on GVF (Figure A1): values were higher in the BP offshore site (maximum value of 66 ± 5%) throughout the spawning seasons (until late September) compared to the HAM lagoon site (maximum value of 46 ± 4%). A significant decrease in GVF suggests spawning events, i.e., oocytes release by mature females. In HAM, the spawning period ranged from mid-June to early August while it occurred later in BP and continued throughout August.
References
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; p. 227. [Google Scholar]
- Statistics Canada. Agriculture Division—Commodities Section—Aquaculture Statistics 2015; Catalogue n° 23-222-X; Statistics Canada: Ottawa, ON, Canada, 2016; p. 29. [Google Scholar]
- Perez Camacho, A.; Gonzalez, R.; Fuentes, J. Mussel culture in Galicia (N.W. Spain). Aquaculture 1991, 92, 263–278. [Google Scholar] [CrossRef]
- Weitzman, J.; Steeves, L.; Bradford, J.; Filgueira, R. Chapter 11—Far-field and near-field effects of marine aquaculture. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Elsevier Academic Press: London, UK, 2019; pp. 197–220. [Google Scholar] [CrossRef]
- Drapeau, A.; Comeau, L.A.; Landry, T.; Stryhn, H.; Davidson, J. Association between longline setup and mussel productivity in Prince Edward Island, Canada. Aquaculture 2006, 261, 879–889. [Google Scholar] [CrossRef]
- Filgueira, R.; Guyondet, T.; Comeau, L.A.; Tremblay, R. Bivalve aquaculture-environment interactions in the context of climate change. Glob. Chang. Biol. 2016, 22, 3901–3913. [Google Scholar] [CrossRef] [PubMed]
- Hickman, R.W. Mussel cultivation. In The Mussel Mytilus: Ecology, Physiology, Genetics and Culture; Gosling, E., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1992; pp. 465–510. [Google Scholar]
- Cheney, D.; Langan, R.; Heasman, K.; Friedman, B.; Davis, J. Shellfish culture in the open ocean: Lessons learned for offshore expansion. Mar. Technol. Soc. J. 2010, 44, 55–67. [Google Scholar] [CrossRef]
- Langan, R. Mussel culture, open ocean innovations. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer Science Business Media: New York, NY, USA, 2013; pp. 1929–1939. [Google Scholar] [CrossRef]
- Steeves, L.E.; Filgueira, R.; Guyondet, T.; Chassé, J.; Comeau, L. Past, present, and future: Performance of two bivalve species under changing environmental conditions. Front. Mar. Sci. 2018, 5, 184. [Google Scholar] [CrossRef]
- McKindsey, C.; Thetmeyer, H.; Landry, T.; Silvert, W. Review of recent carrying capacity models for bivalve culture and recommendations for research and management. Aquaculture 2006, 261, 451–462. [Google Scholar] [CrossRef]
- Gallardi, D.; Mills, T.; Donnet, S.; Parrish, C.C.; Murray, H.M. Condition and biochemical profile of blue mussels (Mytilus edulis L.) cultured at different depths in a cold water coastal environment. J. Sea Res. 2017, 126, 37–45. [Google Scholar] [CrossRef]
- Mizuta, D.; Wikfors, G. Depth selection and in situ validation for offshore mussel aquaculture in northeast United States federal waters. J. Mar. Sci. Eng. 2019, 7, 293. [Google Scholar] [CrossRef]
- Bourque, F.; Myrand, B. Potentiel de production mytilicole en milieu ouvert aux Îles-de-la-Madeleine. Merinov, Research and development report, MERINOV, Gaspé; 2014; n° 14-09. 34. [Google Scholar]
- Séguin-Heine, M.O.; Lachance, A.A.; Genard, B.; Myrand, B.; Pellerin, C.; Marcotte, I.; Tremblay, R. Impact of open sea habitat on byssus attachment of suspension-cultured blue mussels (Mytilus edulis). Aquaculture 2014, 426, 189–196. [Google Scholar] [CrossRef]
- Myrand, B.; Gaudreault, J. Summer mortality of blue mussels (Mytilus edulis Linneaus, 1758) in the Magdalen Islands (Southern Gulf of St. Lawrence, Canada). J. Shellfish Res. 1995, 14, 395–404. [Google Scholar]
- Tremblay, R.; Myrand, B.; Guderley, H. Temporal variation of lysosomal capacities in relation to susceptibility of mussels, Mytilus edulis, to summer mortality. Mar. Biol. 1998, 132, 641–649. [Google Scholar] [CrossRef]
- Tremblay, R.; Myrand, B.; Sévigny, J.M. Genetic characterization of wild and suspension-cultured blue mussels (Mytilus edulis Linneaus, 1758) in the Magdalen Islands (southern Gulf of St. Lawrence, Canada). J. Shellfish Res. 1998, 17, 1191–1202. [Google Scholar]
- Tremblay, R.; Myrand, B.; Guderley, H. Thermal sensitivity of organismal and mitochondrial oxygen consumption in relation to the susceptibility of blue mussels, Mytilus edulis (L.), to summer mortality. J. Shellfish Res. 1998, 17, 141–152. [Google Scholar]
- Tremblay, R.; Myrand, B.; Sévigny, J.M.; Blier, P.; Guderley, H. Bioenergetic and genetic parameters in relation to susceptibility of blue mussels, Mytilus edulis (L.) to summer mortality. J. Exp. Mar. Biol. Ecol. 1998, 221, 27–58. [Google Scholar] [CrossRef]
- Myrand, B.; Guderley, H.; Himmelman, J.H. Reproduction and summer mortality of blue mussels Mytilus edulis in the Magdalen Islands, southern Gulf of St. Lawrence. Mar. Ecol. Prog. Ser. 2000, 197, 193–207. [Google Scholar] [CrossRef]
- Myrand, B.; Tremblay, R.; Sévigny, J.M. Selection against blue mussels (Mytilus edulis L.) homozygotes under various stressful conditions. J. Hered. 2002, 93, 238–248. [Google Scholar] [CrossRef]
- Bourque, F.; Myrand, B.; Toupoint, N.; Tremblay, R. Facteurs responsables du succès de l’approvisionnement en naissain de moules de qualité dans le bassin du Havre Aubert aux Îles-de-la-Madeleine. Merinov, Research and development report, MERINOV, Gaspé; 2014; n° 14-13. 33. [Google Scholar]
- Hawkins, A.J.S.; Bayne, B.L. Physiology interrelations and the regulation of production. In The Mussel Mytilus: Ecology, Physiology, Genetics and Culture; Gosling, E., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1992; pp. 171–212. [Google Scholar]
- Schotanus, J.; Capelle, J.J.; Leuchter, L.; van de Koppel, J.; Bouma, T.J. Mussel seed is highly plastic to settling conditions: The influence of waves versus tidal emergence. Mar. Ecol. Prog. Ser. 2019, 624, 77–87. [Google Scholar] [CrossRef]
- Penney, R.W.; Hart, M.J.; Templeman, N.D. Genotype-dependent survival, growth, and production in cultured blue mussels, Mytilus spp.: Results of a reciprocal seed transfer experiment. J. Shellfish Res. 2006, 25, 515–525. [Google Scholar] [CrossRef]
- Mallet, A.L.; Carver, C.E.A.; Coffen, S.S.; Freeman, K.R. Winter growth of the blue mussel Mytilus edulis: Importance of stock and site. J. Exp. Mar. Biol. Ecol. 1987, 108, 217–228. [Google Scholar] [CrossRef]
- Mallet, A.L.; Carver, C.E.A.; Coffen, S.S.; Freeman, K.R. Mortality variations in natural populations of the blue mussel, Mytilus edulis. Can. J. Fish. Aquat. Sci. 1987, 44, 1589–1594. [Google Scholar] [CrossRef]
- Mallet, A.L.; Carver, C.E.A. Growth, mortality, and secondary production in natural populations of the blue mussel, Mytilus edulis. Can. J. Fish. Aquar. Sci. 1989, 46, 1154–1159. [Google Scholar] [CrossRef]
- Sanford, E.; Kelly, M.W. Local adaptation in marine invertebrates. Ann. Rev. Mar. Sci. 2011, 3, 509–535. [Google Scholar] [CrossRef] [PubMed]
- Blanquart, F.; Kaltz, O.; Nuismer, S.; Gandon, S. A practical guide to measuring local adaptation. Ecol. Lett. 2013, 16, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, P.S.; Chassé, J.; Nicot, P.; Caverhill, C.; Gilbert, D.; Pettigrew, B.; Lefaivre, D.; Brickman, D.; Devine, L.; Lafleur, C. Physical oceanographic conditions in the Gulf of St. Lawrence in 2014. DFO. Can. Sci. Advis. Sec. Res. Doc. 2015, 32, 87. [Google Scholar]
- Cyr, C.; Pedneault, E.; Lionard, M. Programme de monitoring en soutien à l’industrie mytilicole du Québec en 2014–2015. Merinov, Research and development report, MERINOV, Gaspé; 2015; n° 15-05. 23. [Google Scholar]
- Cyr, C.; Lionard, M.; Pedneault, E.; Toupoint, N. Programme de monitoring en soutien à l’industrie mytilicole du Québec en 2015–2016. Merinov, Research and development report, MERINOV, Gaspé; 2016; n° 16-03. 16. [Google Scholar]
- Bourque, F.; Myrand, B. Essais de stratégies pour contrer l’effet négatif des algues sur la collecte de moules au bassin du Havre-Aubert. MAPAQ, Research and development report, DIT, Gaspé; 2007; n° 157. 13. [Google Scholar]
- Bourque, F.; Myrand, B. Traitement des collecteurs de moules à la saumure pour contrer la prédation par les étoiles de mer. MAPAQ, Research and development report, DIT, Gaspé; 2007; n° 160. 20. [Google Scholar]
- Sénéchal, J.; Grant, J.; Archambault, M.C. Experimental manipulation of suspended culture socks: Growth and behaviour of juvenile mussels (Mytilus spp.). J. Shellfish Res. 2008, 27, 811–826. [Google Scholar] [CrossRef]
- Myrand, B.; Tremblay, R.; Sévigny, J.M. Impact of suspension culture using mesh sleeves on genetic characteristics of Mytilus edulis L. in Canada. Aquaculture 2009, 291, 147–153. [Google Scholar] [CrossRef]
- Myrand, B.; Tremblay, R.; Sévigny, J.M. Decreases in multi-locus heterozygosity in suspension-cultured mussels (Mytilus edulis) through loss of the more heterozygous individuals. Aquaculture 2009, 295, 188–194. [Google Scholar] [CrossRef]
- Laplante, J.-F.; Bourque, F. Intérêt économique d’un boudinage hâtif aux Îles-de-la-Madeleine. Merinov, Research and development report, MERINOV, Gaspé; 2014; n° 14-05. 8. [Google Scholar]
- Trottet, A.; Roy, S.; Tamigneaux, E.; Lovejoy, C. Importance of heterotrophic planktonic communities in a mussel culture environment: The Grande Entrée lagoon, Magdalen Islands (Québec, Canada). Mar. Biol. 2007, 151, 377–392. [Google Scholar] [CrossRef]
- Toupoint, N.; Solomon-Gilmore, L.; Bourque, F.; Myrand, B.; Pernet, F.; Olivier, F.; Tremblay, R. Match/mismatch between the Mytilus edulis larval supply and seston quality: Effect on recruitment. Ecology 2012, 93, 1922–1934. [Google Scholar] [CrossRef] [PubMed]
- Toupoint, N.; Mohit, V.; Linossier, I.; Bourgougnon, N.; Myrand, B.; Lovejoy, C.; Olivier, F.; Tremblay, R. Effect of the biofilm age on Mytilus edulis settlement. Biofouling 2012, 29, 985–1001. [Google Scholar] [CrossRef] [PubMed]
- Aminot, A.; Chaussepied, M. Manuel des Analyses Chimiques en Milieu Marin; Centre National Pour l’Exploitation des Océans CNEXO BNDO: Brest, France, 1983; p. 395. [Google Scholar]
- Belzile, C.; Brugel, S.; Nozais, C.; Gratton, Y.; Demers, S. Variations of the abundance and nucleic acid content of heterotrophic bacteria in Beaufort Shelf waters during winter and spring. J. Mar. Syst. 2008, 74, 946–956. [Google Scholar] [CrossRef]
- Tremblay, G.; Belzile, C.; Gosselin, M.; Poulin, M.; Roy, S.; Tremblay, J.E. Late summer phytoplankton distribution along a 3500 km transect in Canadian Arctic waters: Strong numerical dominance by picoeukaryotes. Aquat. Microb. Ecol. 2009, 54, 55–70. [Google Scholar] [CrossRef]
- R Core Development Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org (accessed on 12 February 2019).
- Sokal, R.R.; Rohlf, J.F. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W.H. Freeman and Company: San Francisco, CA, USA, 1995; p. 859. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2015; p. 300. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008; p. 214. [Google Scholar]
- Bourque, F.; Myrand, B. Boudinage estival en lagune aux Îles-de-la-Madeleine. MAPAQ, Research and development report, DIT, Gaspé; 2010; n°189. 15. [Google Scholar]
- Hughes, R.N.; Griffiths, C.L. Self-thinning in barnacles and mussels: The geometry of packing. Am. Nat. 1988, 132, 484–491. [Google Scholar] [CrossRef]
- Fréchette, M.; Bergeron, P.; Gagnon, P. On the use of self-thinning relationships in stocking experiments. Aquaculture 1996, 145, 91–112. [Google Scholar] [CrossRef]
- Alunno-Bruscia, M.; Petraitis, P.S.; Bourget, E.; Fréchette, M. Body size-density relationship for Mytilus edulis in an experimental food-regulated situation. Oikos 2000, 90, 28–42. [Google Scholar] [CrossRef]
- Guiñez, R. A review on self-thinning in mussels. Rev. Biol. Mar. Oceanogr. 2005, 40, 1–6. [Google Scholar] [CrossRef]
- Lachance-Bernard, M.; Himmelman, J.H.; Daigle, G.; Fréchette, M. Biomass density relationships and self-thinning of blue mussels (Mytilus spp.) reared on self-regulated long lines. Aquaculture 2010, 308, 34–43. [Google Scholar] [CrossRef]
- Fréchette, M. Self-thinning, biodeposit production, and organic matter input to the bottom in mussel suspension culture. J. Sea Res. 2012, 67, 10–20. [Google Scholar] [CrossRef]
- Newell, C.R.; Short, F.; Hoven, H.; Healey, L.; Panchang, V.; Cheng, G. The dispersal dynamics of juvenile plantigrade mussels (Mytilus edulis L.) from eelgrass (Zostera marina) meadows in Maine, USA. J. Exp. Mar. Biol. Ecol. 2010, 394, 45–52. [Google Scholar] [CrossRef]
- Lachance, A.A.; Myrand, B.; Tremblay, R.; Koutitonsky, V.; Carrington, E. Biotic and abiotic factors influencing attachment strength of blue mussels Mytilus edulis in suspended culture. Aquat. Biol. 2008, 2, 119–129. [Google Scholar] [CrossRef]
- Pearce, C.M.; Gallager, S.M.; Manuel, J.L.; Manning, D.A.; O’Dor, R.K.; Bourget, E. Effect of thermoclines and turbulence on depth of larval settlement and spat recruitment of the giant scallop Placopecten magellanicus in 9.5m deep laboratory mesocosms. Mar. Ecol. Prog. Ser. 1998, 165, 195–215. [Google Scholar] [CrossRef]
- Kenchington, E.; Freeman, K.R.; Vercaemer, B.; MacDonald, B. Comparative settlement depths of Mytilus edulis C. Linnaeus, 1758 and M. trossulus Gould, 1850: II. Field observations. J. Shellfish Res. 2002, 21, 67–74. [Google Scholar]
- Aghzar, A.; Talbaoui, M.; Benajiba, M.H.; Presa, P. Influence of depth and diameter of rope collectors on settlement density of Mytilus galloprovincialis spat in Baie de M’diq (Alboran Sea). Mar. Freshw. Behav. Physiol. 2012, 45, 51–61. [Google Scholar] [CrossRef]
- Mallet, A.L.; Myrand, B. The culture of the blue mussel in Atlantic Canada. In Cold-Water Aquaculture in Atlantic Canada; Boghen, A.D., Ed.; Canadian Institute for Research on Regional Development: Moncton, NB, Canada, 1995; pp. 255–296. [Google Scholar]
- Lauzon-Guay, J.S.; Dionne, M.; Barbeau, M.A.; Hamilton, D.J. Effects of seed size and density on growth, tissue-to-shell ratio and survival of cultivated mussels (Mytilus edulis) in Prince Edward Island, Canada. Aquaculture 2005, 250, 652–665. [Google Scholar] [CrossRef]
- Gilmore-Solomon, L.; Tremblay, R.; Toupoint, N.; Myrand, B. Aquaculture Marine Biology - Influence of Seston Quality on Physiological Condition of Mytilus Edulis in Oligotrophic Lagoon; MedCrave Group: Edmond, OK, USA, 2016; p. 21. [Google Scholar]
- Seed, R.; Suchanek, T.H. Population and community ecology of Mytilus. In The Mussel Mytilus: Ecology, Physiology, Genetics and Culture; Gosling, E., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1992; pp. 87–169. [Google Scholar]
- Bayne, B.L. The biology of mussel larvae. In Marine Mussels: Their Ecology and Physiology; Bayne, B.L., Ed.; Cambridge University Press: Cambridge, UK, 1976; pp. 81–120. [Google Scholar]
- Lesser, M.P.; Shumway, S.E.; Cucci, T.; Smith, J. Impact of fouling organisms on mussel rope culture: Interspecific competition for food among suspension-feeding invertebrates. J. Exp. Mar. Biol. Ecol. 1992, 165, 91–102. [Google Scholar] [CrossRef]
- Dobretsov, S.; Wahl, M. Recruitment preferences of blue mussel spat (Mytilus edulis) for different substrata and microhabitats in the White Sea (Russia). Hydrobiologia 2001, 445, 27–35. [Google Scholar] [CrossRef]
- Yang, J.L.; Satuito, C.G.; Bao, W.Y. Larval settlement and metamorphosis of the mussel Mytilus galloprovincialis on different macroalgae. Mar. Biol. 2007, 152, 1121–1132. [Google Scholar] [CrossRef]
- Sharp, G.J.; MacNair, N.; Campbell, E.; Butters, A.; Ramsay, A.; Semple, R. Fouling of mussel (Mytilus edulis) collectors by algal mats, dynamics, impacts and symptomatic treatment in P.E.I. Canada. Sci. Asia 2006, 32, 87–97. [Google Scholar] [CrossRef]
- Dionne, M.; Lauzon-Guay, J.S.; Hamilton, D.J.; Barbeau, M.A. Protective socking material for cultivated mussels: A potential non-disruptive deterrent to reduce losses to diving ducks. Aquacult. Int. 2006, 14, 595–613. [Google Scholar] [CrossRef]
- Varennes, E.; Hanssen, S.A.; Bonardelli, J.; Guillemette, M. Sea duck predation in mussel farms: The best nets for excluding common eiders safely and efficiently. Aquacult. Environ. Interact. 2013, 4, 31–39. [Google Scholar] [CrossRef]
- Varennes, E.; Hanssen, S.A.; Bonardelli, J.; Guillemette, M. A large molluscivore bird (Common Eider, Somateria mollissima) is able to discriminate quality of blue mussels (Mytilus edulis) based on size and provenance. Can. J. Zool. 2015, 93, 655–663. [Google Scholar] [CrossRef]
- Varennes, E.; Hanssen, S.A.; Bonardelli, J.; Guillemette, M. Mussel quality of preferred prey improves digestion in a molluscivore bird. Can. J. Zool. 2015, 93, 783–789. [Google Scholar] [CrossRef]
- Guillemette, M.; Reed, A.; Himmelman, J.H. Availability and consumption of food by common eiders wintering in the Gulf of St. Lawrence: Evidence of prey depletion. Can. J. Zool. 1996, 74, 32–38. [Google Scholar] [CrossRef]
- Hamilton, D.J.; Nudds, T.D.; Neate, J. Size-selective predation of blue mussels (Mytilus edulis) by common eiders (Somateria mollissima) under controlled field conditions. Auk 1999, 116, 403–416. [Google Scholar] [CrossRef]
- Miller, M.R.; Pearce, L.; Bettjeman, B.I. Detailed distribution of lipids in greenshell™ mussel (Perna canaliculus). Nutrients 2014, 6, 1454–1474. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, S.C.; Fox, S.P.; Ross, A.H.; James, M.R.; Schiel, D.R. Growth of cultured mussels (Perna canaliculus Gmelin 1791) at a deep-water chlorophyll maximum layer. Aquac. Res. 2004, 35, 1253–1260. [Google Scholar] [CrossRef]
- Babarro, J.M.F.; Abad, M.J. Co-existence of two mytilid species in a heterogeneous environment: Mmortality, growth and strength of shell and byssus attachment. Mar. Ecol. Prog. Ser. 2013, 476, 115–128. [Google Scholar] [CrossRef]
- Bayne, B.L. Reproduction in bivalve molluscs under environmental stress. In Physiological Ecology of Estuarine Organisms; Vernberg, F.J., Ed.; University of South Carolina Press: Columbia, NY, USA, 1975; pp. 259–277. [Google Scholar]
- Zippay, M.L.; Helmuth, B. Effects of temperature change on mussel, Mytilus. Integr. Zool. 2012, 7, 312–327. [Google Scholar] [CrossRef]
- Gosling, E. Marine Bivalve Molluscs, 2nd ed.; Wiley-Blackwell Publishing: West Sussex, UK, 2015; p. 524. [Google Scholar] [CrossRef]
- Bayne, B.L.; Widdows, J.; Thompson, R.J. Physiological integrations. In Marine Mussels: Their Ecology and Physiology; Bayne, B.L., Ed.; Cambridge University Press: Cambridge, UK, 1976; pp. 261–299. [Google Scholar]
- Widdows, J. The effects of temperature on the metabolism and activity of Mytilus edulis. Neth. J. Sea. Res. 1973, 7, 387–398. [Google Scholar] [CrossRef]
- Incze, L.S.; Lutz, R.A.; Watling, L. Relationships between effects of environmental temperature and seston on growth and mortality of Mytilus edulis in a temperate northern estuary. Mar. Biol. 1980, 53, 147–156. [Google Scholar] [CrossRef]
- Rayssac, N.; Pernet, F.; Lacasse, O.; Tremblay, R. Temperature effect on survival, growth, and triacylglycerol content during the early ontogeny of Mytilus edulis and M. trossulus. Mar. Ecol. Prog. Ser. 2010, 417, 183–191. [Google Scholar] [CrossRef]
- Trottet, A.; Roy, S.; Tamigneaux, E.; Lovejoy, C.; Tremblay, R. Impact of suspended mussels (Mytilus edulis L.) on plankton communities in a Madgalen Islands lagoon (Québec, Canada): A mesocosm approach. J. Exp. Mar. Biol. Ecol. 2008, 365, 103–115. [Google Scholar] [CrossRef]
- Sonier, R.; Filgueira, R.; Guyondet, T.; Tremblay, R.; Olivier, F.; Meziane, T.; Starr, M.; Leblanc, A.R.; Comeau, L.A. Picophytoplankton contribution to Mytilus edulis growth in an intensive culture environment. Mar. Biol. 2016, 163, 73. [Google Scholar] [CrossRef]
- Lowe, M.N.; Moore, M.N.; Bayne, B.L. Aspect of gametogenesis in the marine mussel Mytilus edulis L. J. Mar. Biol. Assoc. UK 1982, 62, 133–145. [Google Scholar] [CrossRef]
- Pernet, F.; Tremblay, R.; Bourget, E. Biochemical indicator of sea scallop (Placopecten magellanicus) quality based on lipid class composition. Part I: Broodstock conditioning and young larval performance. J. Shellfish Res. 2003, 22, 365–375. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).