Preliminary Numerical Study on Exhaust Emission Characteristics of Particulate Matters and Nitrogen Oxide in a Marine Engine for Marine Diesel Oil and Dimethyl Ether Fuel
Abstract
1. Introduction
2. Materials and Research Methods
2.1. Experiment Methods
2.2. Numerical Analysis Method
3. Results and Discussion
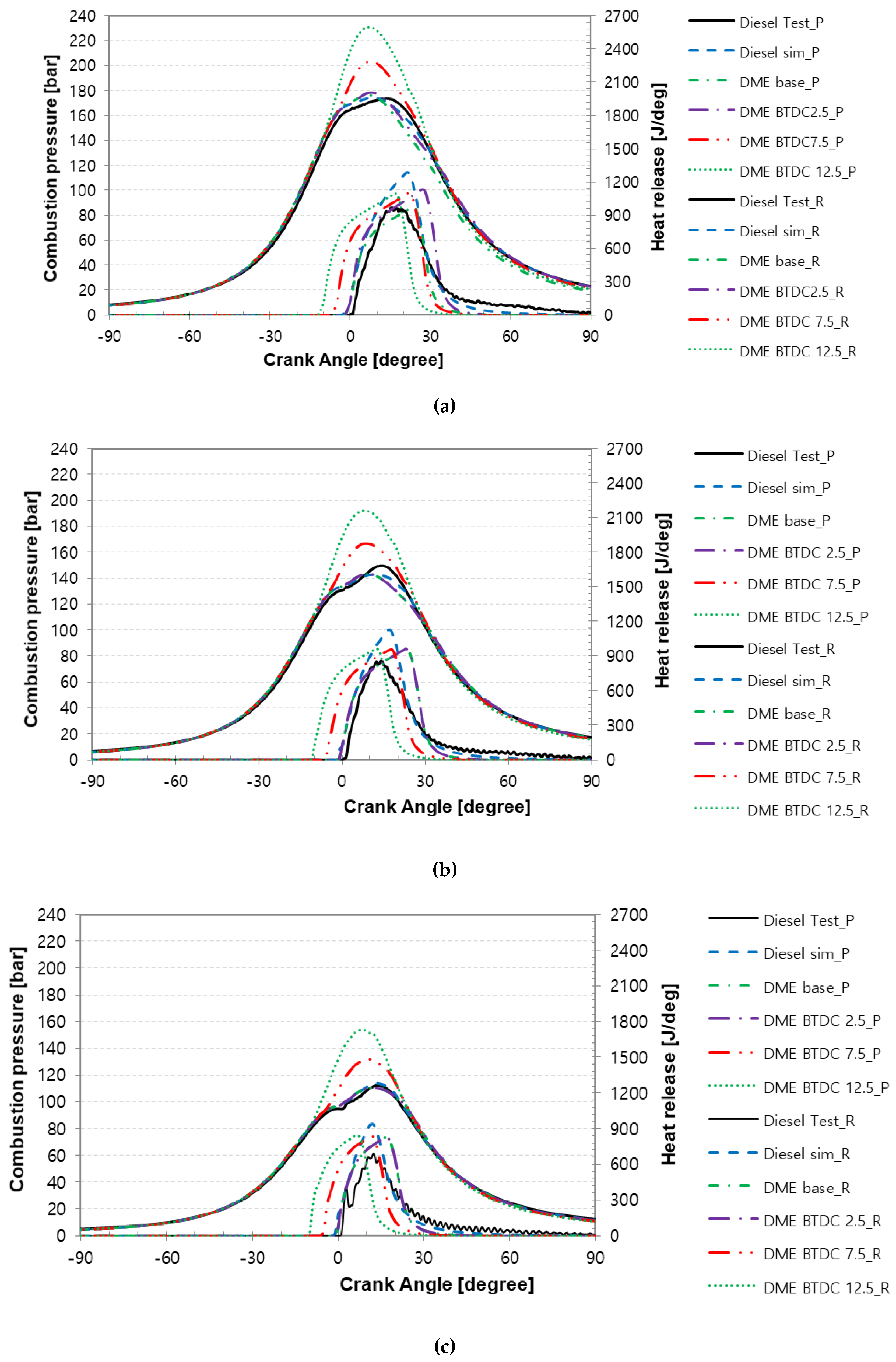
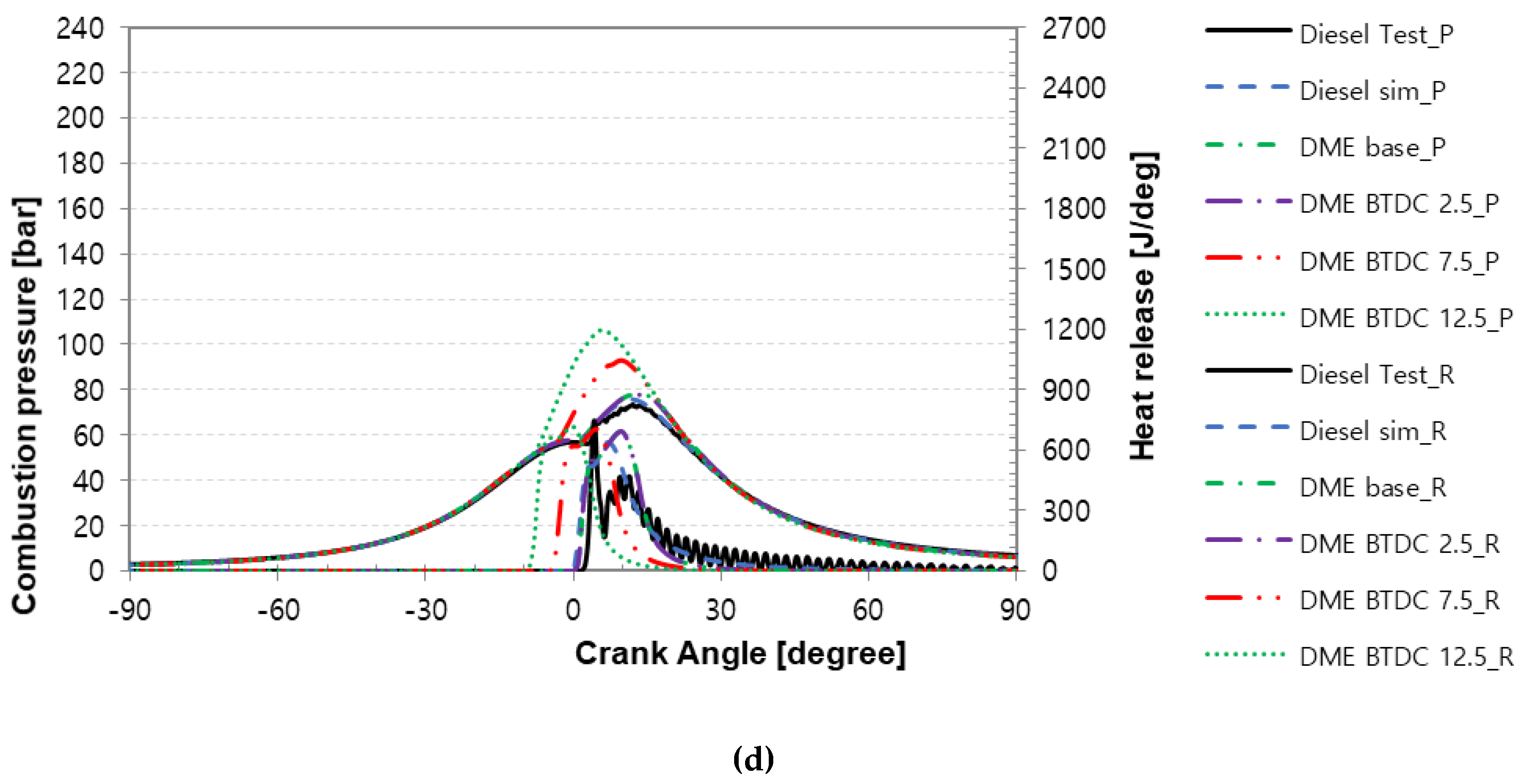
3.1. Characteristics of Combustion Pressure and Rate of Heat Release versus the Compression Ratio of the Turbocharger Nozzle Ring
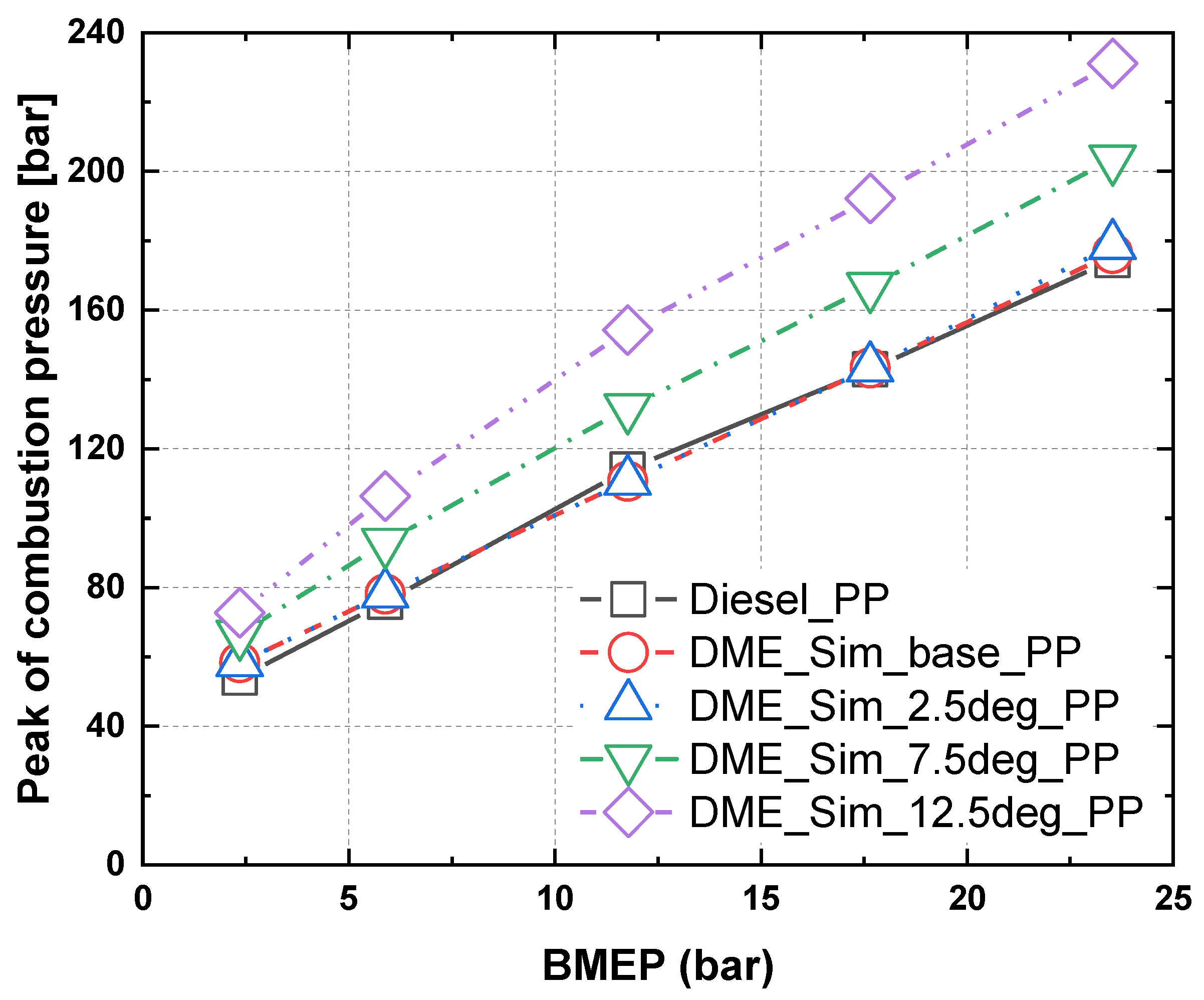
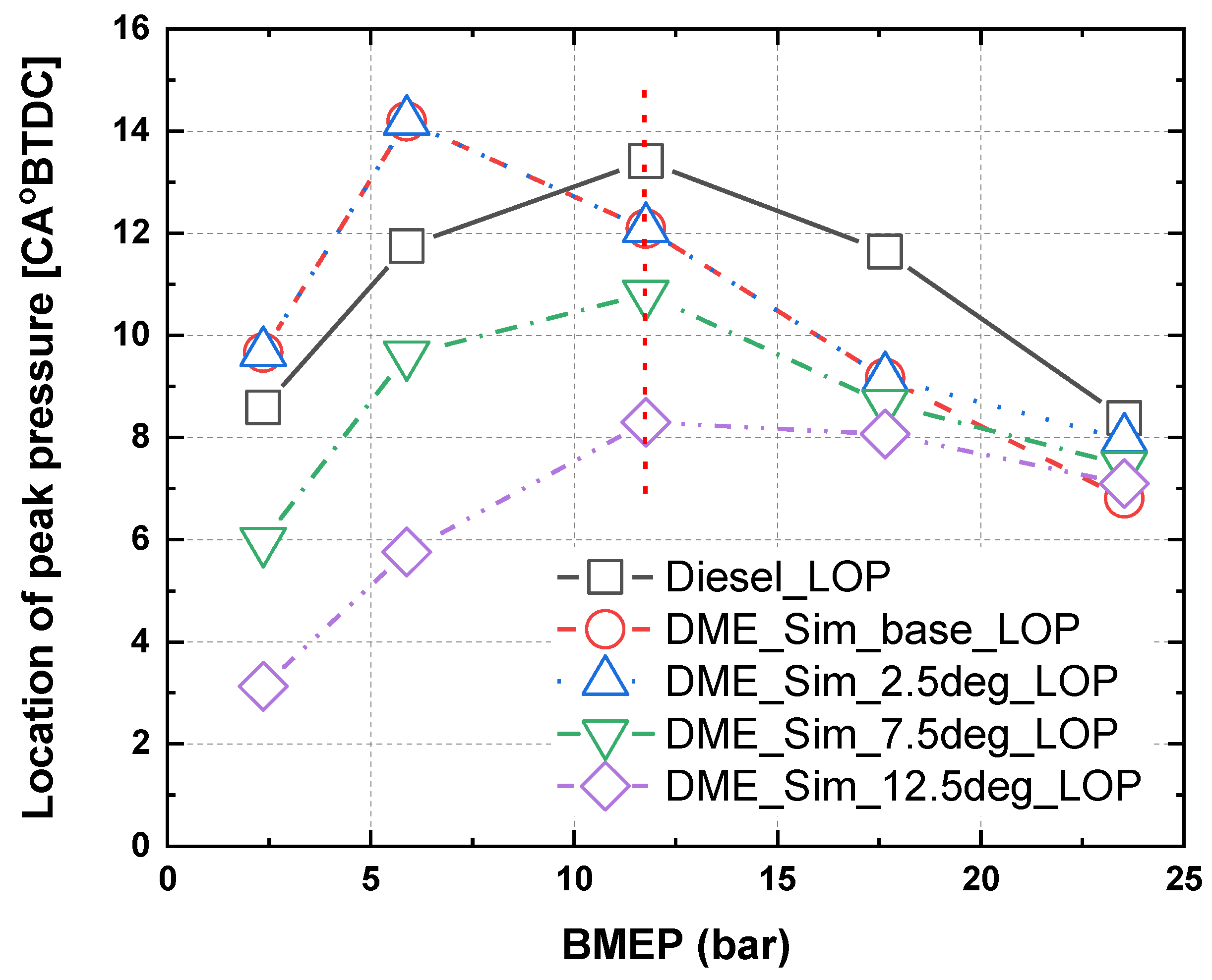
3.2. Characteristics of Peak Combustion Pressure in Accordance with Injection Timing for MDO and DME Fuel
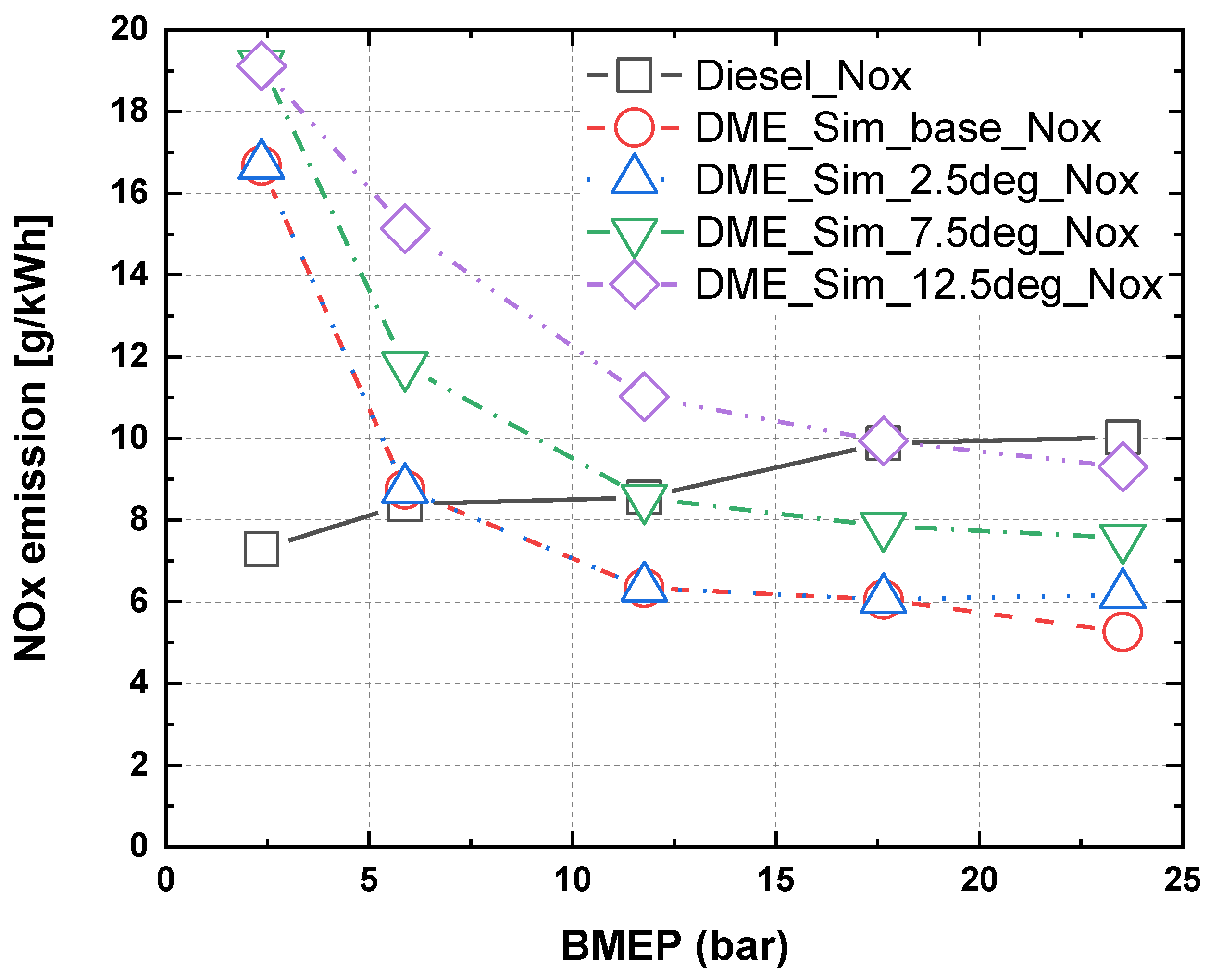
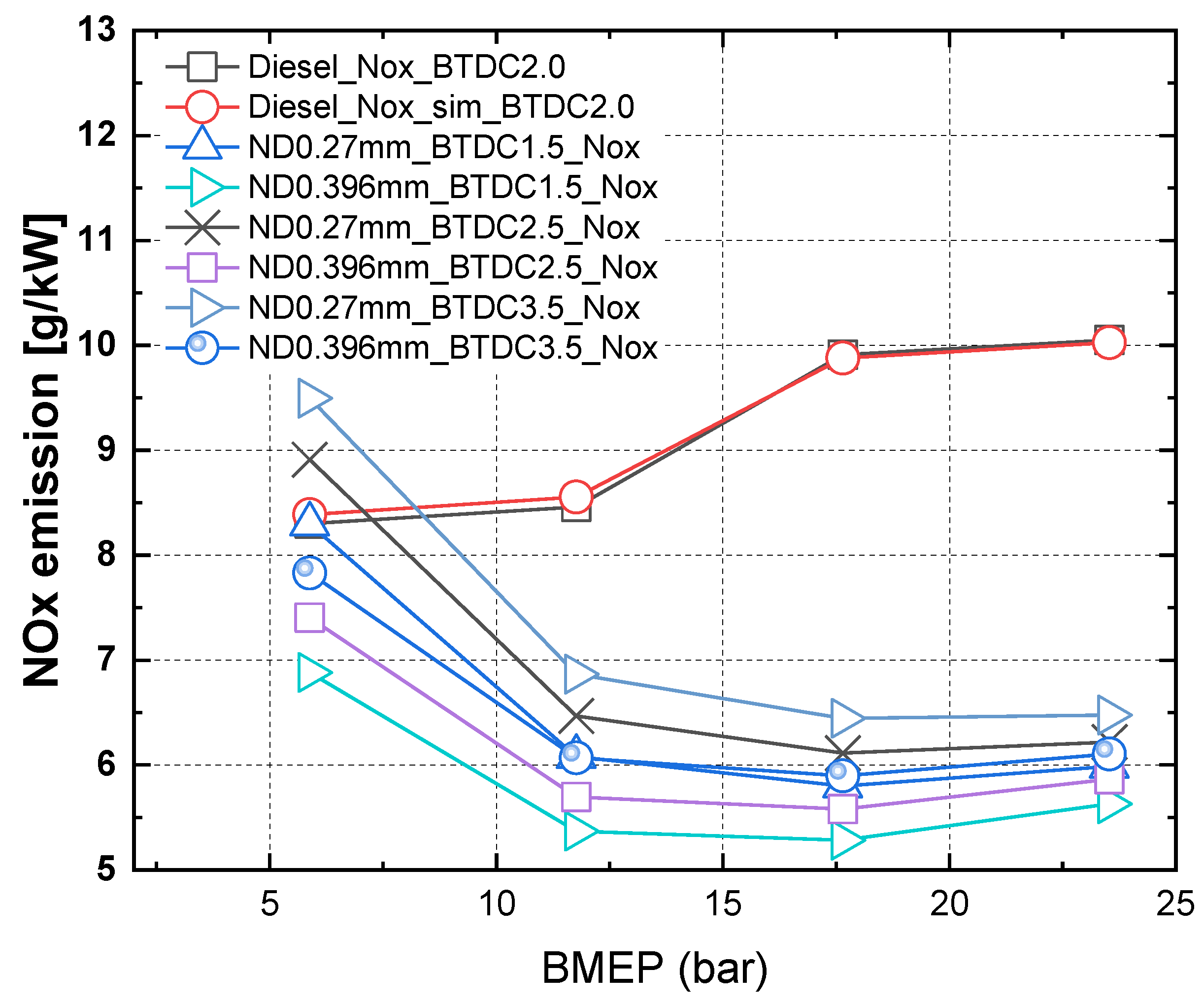
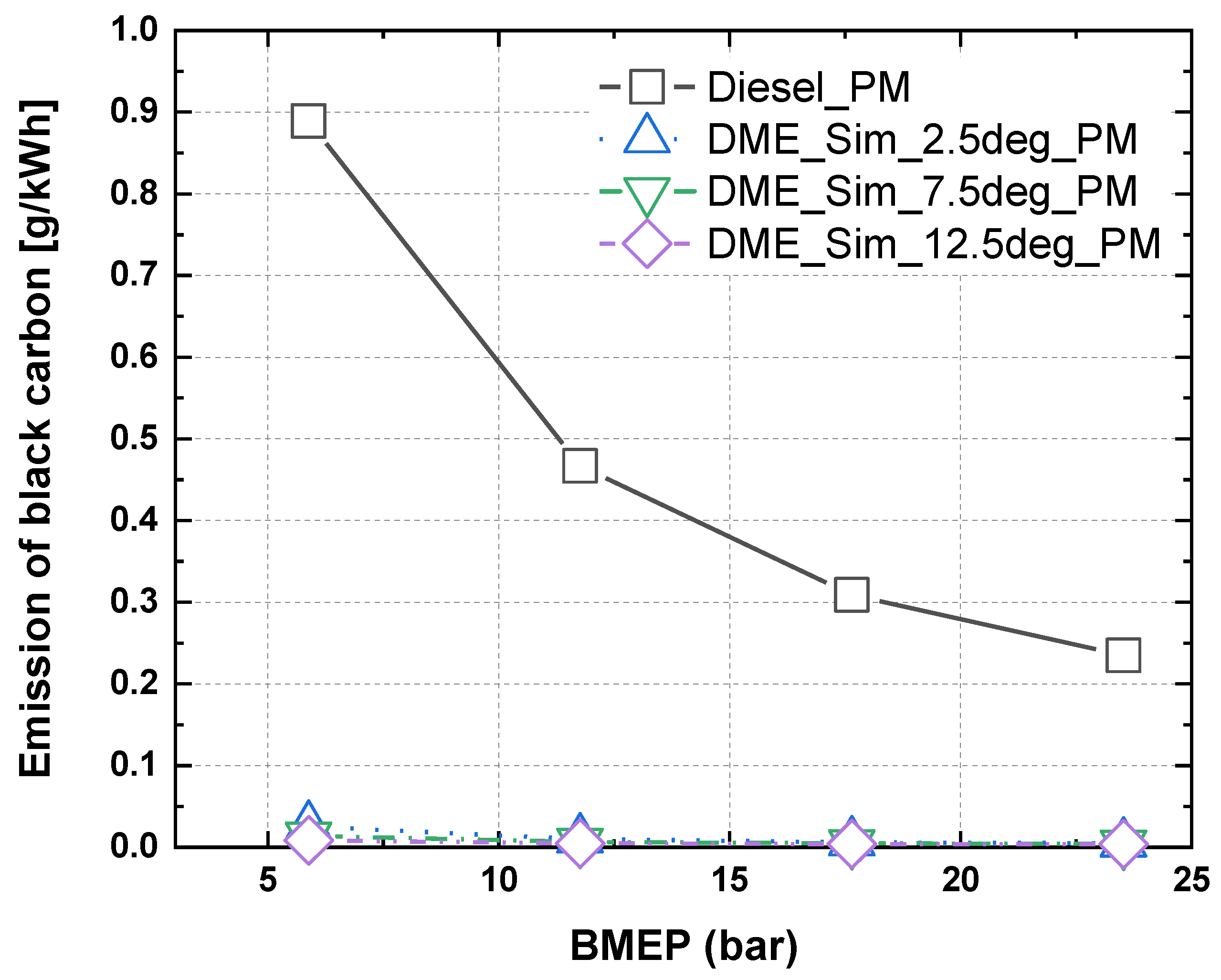
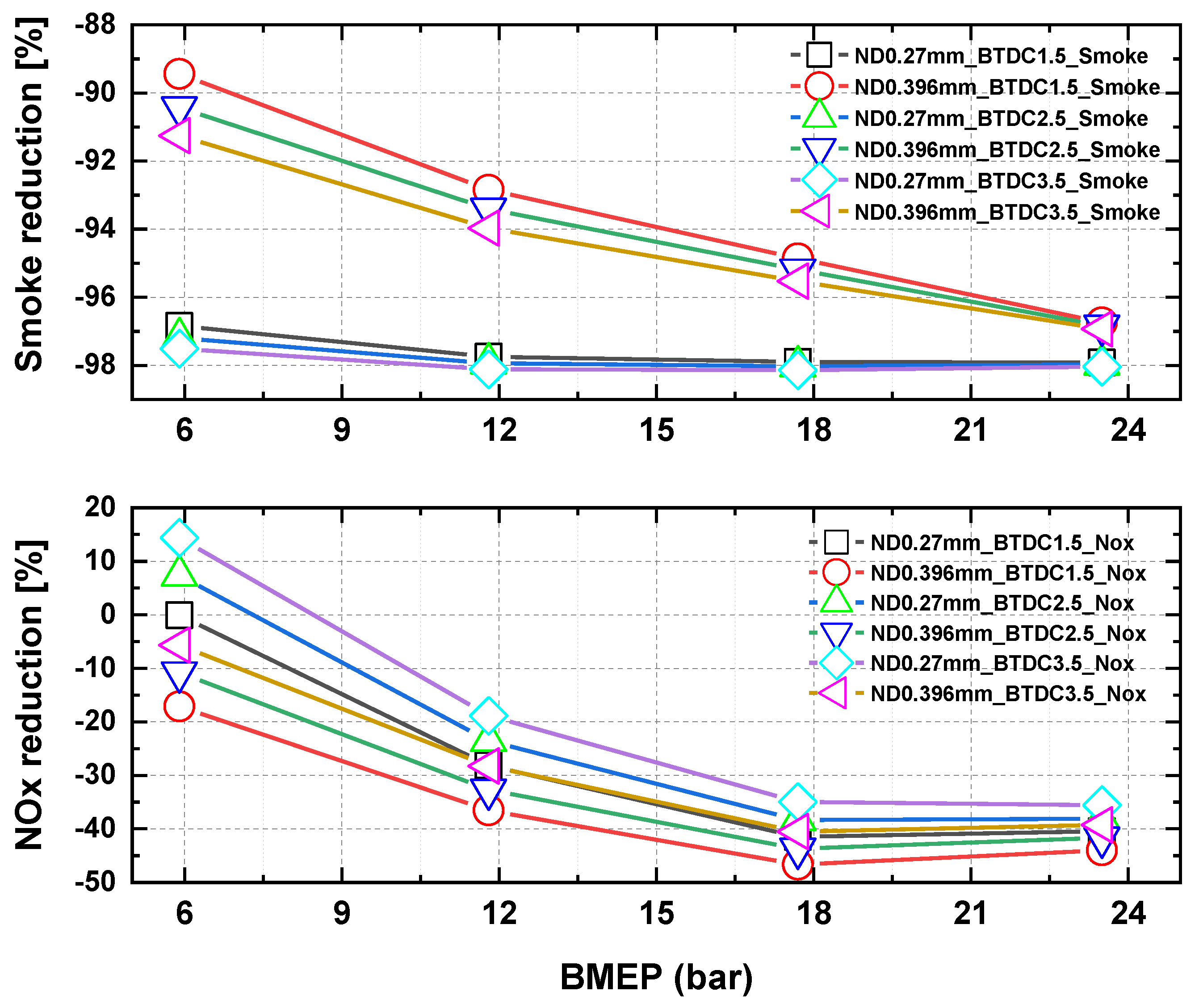
3.3. Characteristics of NOx and PM in Accordance with Injection Timing and Nozzle Hole Diameter for MDO and DME Fuel
4. Conclusions
- As a result of the combustion and heat release rate characteristics of MDO and DME fuels according to the injection timing, as the injection timing advanced, the combustion start point occurred earlier and post-combustion was faster.
- When MDO and DME fuels were injected at the same time point, the heat release rate of DME increased more gradually than that of MDO. The diffusion combustion phase of DME fuel was more significant than that of MDO fuel. It seems that the higher cetane number and low autoignition temperature of DME fuel cause a faster start of combustion and promote a faster premixed combustion phase, and the flame created in premixed combustion and excellent evaporation characteristics of DME fuel lead to diffusion combustion.
- Comparing the experimental and numerical analysis results of MDO and DME fuels at the same injection time, the nitrogen oxide emissions for DME fuel were higher than those of MDO when the BMEP was below 5 bar and the opposite was observed when the BMEP was above 5 bar. This is owing to the high cetane number and low autoignition temperature of oxygen-containing fuels such DME, which cause a rapid combustion start. Moreover, it is believed that the premixed combustion time point progressed faster.
- The results of nitrogen oxide and PM emission measurement for MDO fuel and nitrogen oxide and PM reduction rate assessment according to the injection timing and the change in the hole diameter of the nozzle with increasing BMEP for DME fuel indicated that, when the BMEP was 23.5 bar, the nitrogen oxide and PM reduction rates for DME fuel were 40% and 98%, respectively, compared with MDO fuel.
Author Contributions
Funding
Conflicts of Interest
References
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources 2006, 156, 497–511. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Ott, K.C.; Borup, R.L.; Greene, H.L. Role of acidity on the hydrolysis of dimethyl ether (DME) to methanol. Appl. Catal. B Environ. 2005, 61, 281–287. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Borup, R.L. Thermodynamic equilibrium calculations of hydrogen production from the combined processes of dimethyl ether steam reforming and partial oxidation. J. Power Sources 2006, 155, 340–352. [Google Scholar] [CrossRef]
- Yesilyurt, M.K.; Aydin, M. Experimental investigation on the performance, combustion and exhaust emission characteristics of a compression-ignition engine fueled with cottonseed oil biodiesel/diethyl ether/diesel fuel blends. Energy Convers. Manag. 2020, 205, 112355. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C.S. Applicability of dimethyl ether (DME) in a compression ignition engine as an alternative fuel. Energy Convers. Manag. 2014, 86, 848–863. [Google Scholar] [CrossRef]
- Rajak, U.; Verma, T.N. Effect of emission from ethylic biodiesel of edible and non-edible vegetable oil, animal fats, waste oil and alcohol in CI engine. Energy Convers. Manag. 2018, 166, 704–718. [Google Scholar] [CrossRef]
- Nour, M.; Attia, A.M.; Nada, S.A. Combustion, performance and emission analysis of diesel engine fuelled by higher alcohols (butanol, octanol and heptanol)/diesel blends. Energy Convers. Manag. 2019, 185, 313–329. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Michael, J.V.; Wagner, A.F.; Dawes, R.; Jasper, A.W.; Harding, L.B.; Georgievskii, Y.; Klippenstein, S.J. Roaming radicals in the thermal decomposition of dimethyl ether: Experiment and theory. Combust. Flame 2011, 158, 618–632. [Google Scholar] [CrossRef]
- Boehman, A.L. Developments in production and utilization of dimethyl ether for fuel applications. Fuel Process. Technol. 2008, 89, 1243. [Google Scholar] [CrossRef]
- Desai, S.; Sankaran, R.; Im, H.G. Auto-ignite deflagration speed of methane (CH4) blended dimethyl-ether (DME)/air mixtures at stratified conditions. Combust. Flame 2020, 211, 377–391. [Google Scholar] [CrossRef]
- Loganathan, S.; Martin, M.L.J.; Nagalingam, B.; Prabhu, L. Heat release rate and performance simulation of DME fuelled diesel engine using oxygenate correction factor and load correction factor in double Wiebe function. Energy 2018, 150, 77–91. [Google Scholar] [CrossRef]
- Benajes, J.; Novella, R.; Pastor, J.M.; Hernández-López, A.; Kokjohn, S. Computational optimization of a combustion system for a stoichiometric DME fueled compression ignition engine. Fuel 2018, 223, 20–31. [Google Scholar] [CrossRef]
- Lamani, V.T.; Yadav, A.K.; Narayanappa, K.G. Influence of low-temperature combustion and dimethyl ether-diesel blends on performance, combustion, and emission characteristics of common rail diesel engine: A CFD study. Environ. Sci. Pollut. Res. 2017, 24, 15500–15509. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Cao, E.; Tan, Q.; Wei, L. Effect of alternative fuels on the combustion characteristics and emission products from diesel engines: A review. Renew. Sustain. Energy Rev. 2017, 71, 523–534. [Google Scholar] [CrossRef]
- Park, W.; Park, S.; Reitz, R.D.; Kurtz, E. The effect of oxygenated fuel properties on diesel spray combustion and soot formation. Combust. Flame 2017, 180, 276–283. [Google Scholar] [CrossRef]
- Arcoumanis, C.; Bae, C.; Crookes, R.; Kinoshita, E. The potential of di-methyl ether (DME) as an alternative fuel for compression-ignition engines: A review. Fuel 2008, 87, 1014–1030. [Google Scholar] [CrossRef]
- Cipolat, D. The Effect of fuel characteristics on the fuel injection process in a CI engine fuelled on diesel and DME. SAE Tech. Paper 2007, 2007-24-0119. [Google Scholar] [CrossRef]
- Cipolat, D.; Bhana, N. Fuelling of a compression ignition engine on ethanol with DME as ignition promoter: Effect of injector configuration. Fuel Process. Technol. 2009, 90, 1107–1113. [Google Scholar] [CrossRef]
- Selvan, V.A.M.; Anand, R.B.; Udayakumar, M. Combustion characteristics of diesohol using biodiesel as an additive in a direct injection compression ignition engine under various compression ratios. Energy Fuels 2009, 23, 5413–5422. [Google Scholar] [CrossRef]
- Chen, H.; He, J.; Hua, H. Investigation on combustion and emission performance of a common rail diesel engine fueled with diesel/biodiesel/PODE blends. Appl. Therm. Eng. 2018, 31, 43–55. [Google Scholar] [CrossRef]
- Iannuzzi, S.E.; Barro, C.; Boulouchos, K.; Burger, J. POMDME-diesel blends: Evaluation of performance and exhaust emissions in a single cylinder heavy-duty diesel engine. Fuel 2017, 203, 57–67. [Google Scholar] [CrossRef]
- Bhide, S.; Morris, D.; Leroux, J.; Wain, K.S.; Perez, J.M.; Boehman, A.L. Characterization of the viscosity of blends of dimethyl ether with various fuels and additives. Energy Fuels 2003, 17, 1126–1132. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. High quality biodiesel and its diesel engine application: A review. Renew. Sustain. Energy Rev. 2010, 14, 1999–2008. [Google Scholar] [CrossRef]
- Rashedul, H.K.; Masjuki, H.H.; Kalam, M.A.; Ashraful, A.M.; Rahman, S.A.; Shahir, S.A. The effect of additives on properties, performance and emission of biodiesel fuelled compression ignition engine. Energy Convers. Manag. 2014, 88, 348–364. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. Factors affecting biodiesel engine performance and exhaust emissions—Part II: Experimental study. Energy 2014, 2, 17–34. [Google Scholar] [CrossRef]
- Puzun, A.; Wanchen, S.; Guoliang, L.; Manzhi, T.; Chunjie, L.; Shibao, C. Characteristics of particle size distributions about emissions in a common-rail diesel engine with biodiesel blends. Procedia Environ. Sci. 2011, 11, 1371–1378. [Google Scholar] [CrossRef][Green Version]
- Kapus, P.; Ofner, H. Development of fuel injection equipment and combustion system for DI diesels operated on dimethyl ether. SAE Paper 950062 SAE Trans. J. Fuel Lubr. 1995, 104, 54–59. [Google Scholar] [CrossRef]
- Hou, J.; Qiao, X.; Zhen, W.; Liu, W.; Huang, Z. Characterization of knocking combustion in HCCI DME engine using wavelet packet transform. Appl. Energy 2010, 87, 1239–1246. [Google Scholar] [CrossRef]
- Junjun, Z.; Xinqi, Q.; Wang, Z.; Guan, B.; Huang, Z. Experimental investigation of low-temperature combustion (LTC) in an engine fueled with dimethyl ether (DME). Energy Fuels 2009, 23, 170–174. [Google Scholar] [CrossRef]
- Kim, H.J.; Su, H.P.; Lee, K.S.; Lee, C.S. A study of spray strategies on improvement of engine performance and emissions reduction characteristics in a DME fueled diesel engine. Energy 2011, 36, 1802–1813. [Google Scholar] [CrossRef]
- Savadkouhi, L.; Jazayeri, S.A.; Shahangian, N.; Tavakoli, J. Performance and combustion characteristics of OM314 diesel engine fueled with DME: A theoretical and experimental analysis. J. Eng. Gas Turbines Power 2010, 132, 092801. [Google Scholar] [CrossRef]
- Gill, D.; Ofner, H.; Sturman, E.; Carpnter, J.; Wolverton, M.A. Production feasible DME technology for direct injection CI engines. SAE Paper 2001, 2001-01-2015. [Google Scholar] [CrossRef]
- Zhang, G.D.; Liu, H.; Xia, X.X.; Yang, Q.L. Study on the injection process of a directinjection diesel engine fueled with dimethyl ether. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2004, 218, 1341–1347. [Google Scholar] [CrossRef]
- Yu, J.; Bae, C. Dimethyl ether (DME) spray characteristics in a common-rail fuel injection system. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2003, 217, 1135–1144. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C.S. Combustion performance and emission reduction characteristics of automotive DME engine system. Prog. Energy Combust. Sci. 2013, 39, 147–168. [Google Scholar] [CrossRef]
- Wu, J.H.; Huang, Z.; Qiao, X.Q.; Lu, J.; Zhang, L.; Zhang, J. Study on combustion and emission characteristics of a turbocharged engine fuelled with dimethyl ether. Trans. CSICE 2006, 2, 79–85. [Google Scholar] [CrossRef]
- Song, R.; Ke, L.; Yang, F.; Liu, S. Performance and emission characteristics of DME engine with high ratio of EGR. Energy Fuels 2009, 23, 5460–5466. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Li, D.; Lei, X.; Liu, S. Combustion and emission characteristics of a DME (dimethyl ether)-diesel dual fuel premixed charge compression ignition engine with EGR (exhaust gas recirculation). Energy 2014, 72, 608–617. [Google Scholar] [CrossRef]
- Yang, S.; Lee, C. Exhaust Gas Characteristics According to the Injection Conditions in Diesel and DME Engines. Appl. Sci. 2019, 9, 647. [Google Scholar] [CrossRef]
- Curran, H.J.; Fischer, S.L.; Dryer, F.L. The Reaction Kinetics of Dimethyl Ether. II: Low-Temperature Oxidation in Flow Reactors. Int. J. Chem. Kinet. 2000, 32, 741–789. [Google Scholar] [CrossRef]
- Teng, H.; McCandless, J.C.; Schneyer, B. Thermodynamic Properties of Dimethyl Ether—An Alternative Fuel for Compression-Ignition Engines; SAE Paper 2004-01-0093; SAE International: Warrendale, PA, USA, 2004. [Google Scholar]
- Mizusawa, K.; Katoh, K.; Hamaguchi, S.; Hayashi, H.; Hocho, S. Development of Air Fuel Ratio Sensor for 1997 Model Year LEV Vehicle; SAE Technical Paper 970843; SAE International: Warrendale, PA, USA, 1997. [Google Scholar]
- Sorenson, S.C.; Glensvig, M.; Abata, D. Di-metyl ether in diesel fuel injection systems. SAE Paper 981159, SAE Trans. J. Fuel Lubr. 1998, 107, 438–449. [Google Scholar] [CrossRef]
- AVL BOOST Theory Reference. v2019 R1. Available online: https://www.avl.com/-/avl-boost-2019-r1 (accessed on 6 November 2019).
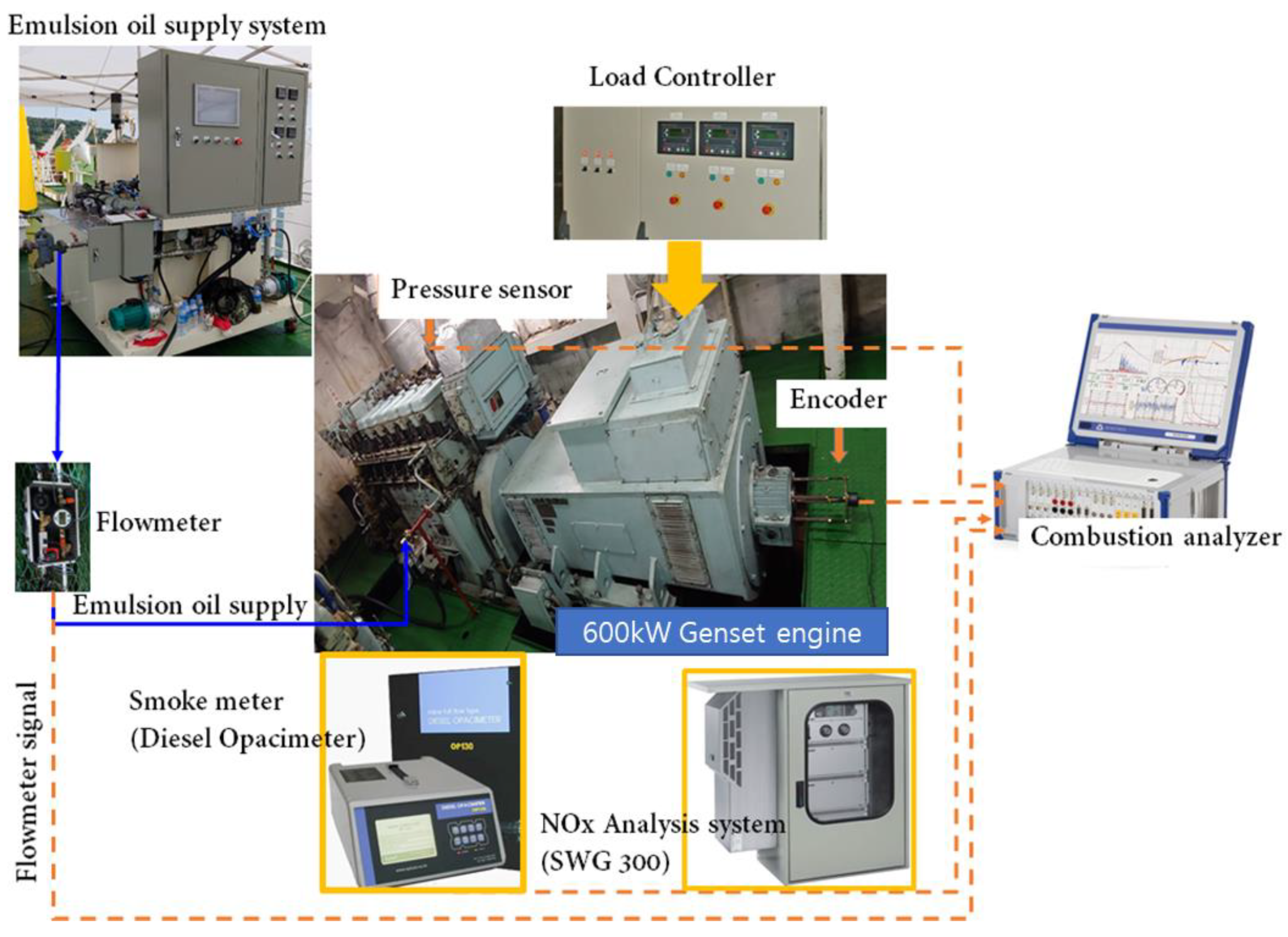
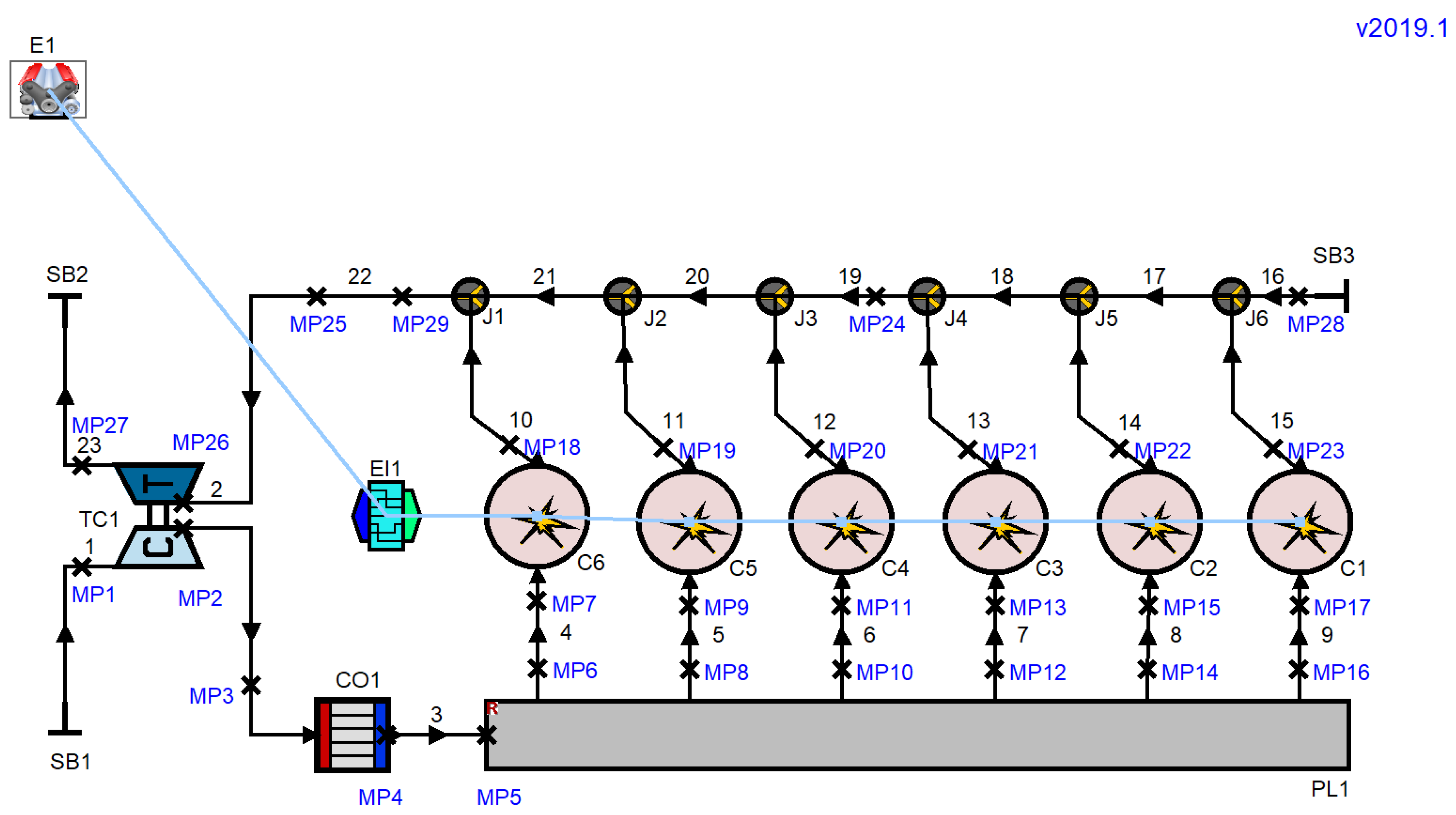

| Item | Specifications |
|---|---|
| Engine type | Four-stoke turbocharged DI marine generator engine |
| Number of cylinders Compression ratio | 6 15.9 |
| Bore × stroke (mm) | 165 × 265 |
| Displacement (cc) | 20,000 |
| Fuel injection system | Mechanical pumping system (Max. 1400 bar) |
| Engine’s maximum continuous rating (MCR) (kW/rpm) | 600 kW/900 rpm |
| Items | Specifications |
|---|---|
| Dynamometer | Load controller (in a marine ship) |
| Exhaust gas analyser | SWG 300 |
| Smoke meter | Diesel opacimeter (OP 130D) |
| Items | Specifications |
|---|---|
| Fuel | Marine diesel oil |
| Engine speed (rpm) | 900 |
| Load (kW) | 150, 300, 450, 600 |
| Properties (unit/condition) | Units | DME | Diesel fuel |
|---|---|---|---|
| Chemical structure | CH3–O–CH3 | − | |
| Molar mass | g/mol | 46 | 170 |
| Carbon content | mass% | 52.2 | 86 |
| Hydrogen content | mass% | 13 | 14 |
| Oxygen content | mass% | 34.8 | 0 |
| Carbon-to-hydrogen ratio | 0.337 | 0.516 | |
| Critical temperature | K | 400 | 708 |
| Critical pressure | MPa | 5.37 | 3.00a |
| Critical density | kg/m3 | 259 | − |
| Liquid density | kg/m3 | 667 | 831 |
| Relative gas density (air = 1) | 1.59 | − | |
| Cetane number | >55 | 40–50 | |
| Auto-ignition temperature | K | 508 | 523 |
| Stoichiometric air/fuel mass ratio | 9.0 | 14.6 | |
| Boiling point at 1 atm | K | 248.1 | 450–643 |
| Enthalpy of vaporisation | kJ/kg | 467.13 | 300 |
| Lower heating value | MJ/kg | 27.6 | 42.5 |
| Gaseous specific heat capacity | kJ/kg K | 2.99 | 1.7 |
| Ignition limits | vol% in air | 3.4/18.6 | 0.6/6.5 |
| Modulus of elasticity | N/m2 | 6.37E + 08 | 14.86E + 08 |
| Kinematic viscosity of liquid | cSt | <0.1 | 3 |
| Surface tension (at 298 K) | N/m | 0.012 | 0.027 |
| Vapour pressure (at 298 K) | kPa | 530 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Choi, I.; Oh, J.; Lee, C. Preliminary Numerical Study on Exhaust Emission Characteristics of Particulate Matters and Nitrogen Oxide in a Marine Engine for Marine Diesel Oil and Dimethyl Ether Fuel. J. Mar. Sci. Eng. 2020, 8, 316. https://doi.org/10.3390/jmse8050316
Park J, Choi I, Oh J, Lee C. Preliminary Numerical Study on Exhaust Emission Characteristics of Particulate Matters and Nitrogen Oxide in a Marine Engine for Marine Diesel Oil and Dimethyl Ether Fuel. Journal of Marine Science and Engineering. 2020; 8(5):316. https://doi.org/10.3390/jmse8050316
Chicago/Turabian StylePark, Jinkyu, Iksoo Choi, Jungmo Oh, and Changhee Lee. 2020. "Preliminary Numerical Study on Exhaust Emission Characteristics of Particulate Matters and Nitrogen Oxide in a Marine Engine for Marine Diesel Oil and Dimethyl Ether Fuel" Journal of Marine Science and Engineering 8, no. 5: 316. https://doi.org/10.3390/jmse8050316
APA StylePark, J., Choi, I., Oh, J., & Lee, C. (2020). Preliminary Numerical Study on Exhaust Emission Characteristics of Particulate Matters and Nitrogen Oxide in a Marine Engine for Marine Diesel Oil and Dimethyl Ether Fuel. Journal of Marine Science and Engineering, 8(5), 316. https://doi.org/10.3390/jmse8050316





