Biology and Environmental Preferences of Wahoo, Acanthocybium solandri (Cuvier, 1832), in the Western and Central Pacific Ocean (WCPO)
Abstract
:1. Introduction
2. Material and Methods
2.1. Environmental Observation
2.2. Data Analysis
2.2.1. Size Class
2.2.2. Sex Ratio
2.2.3. Length at Sexual Maturity
2.2.4. Spawning Season
2.2.5. Diet Analysis
2.2.6. The CPUEs (Number of Catch per 1000 Hooks) in Different Ranges of Various Environmental Variables
3. Results
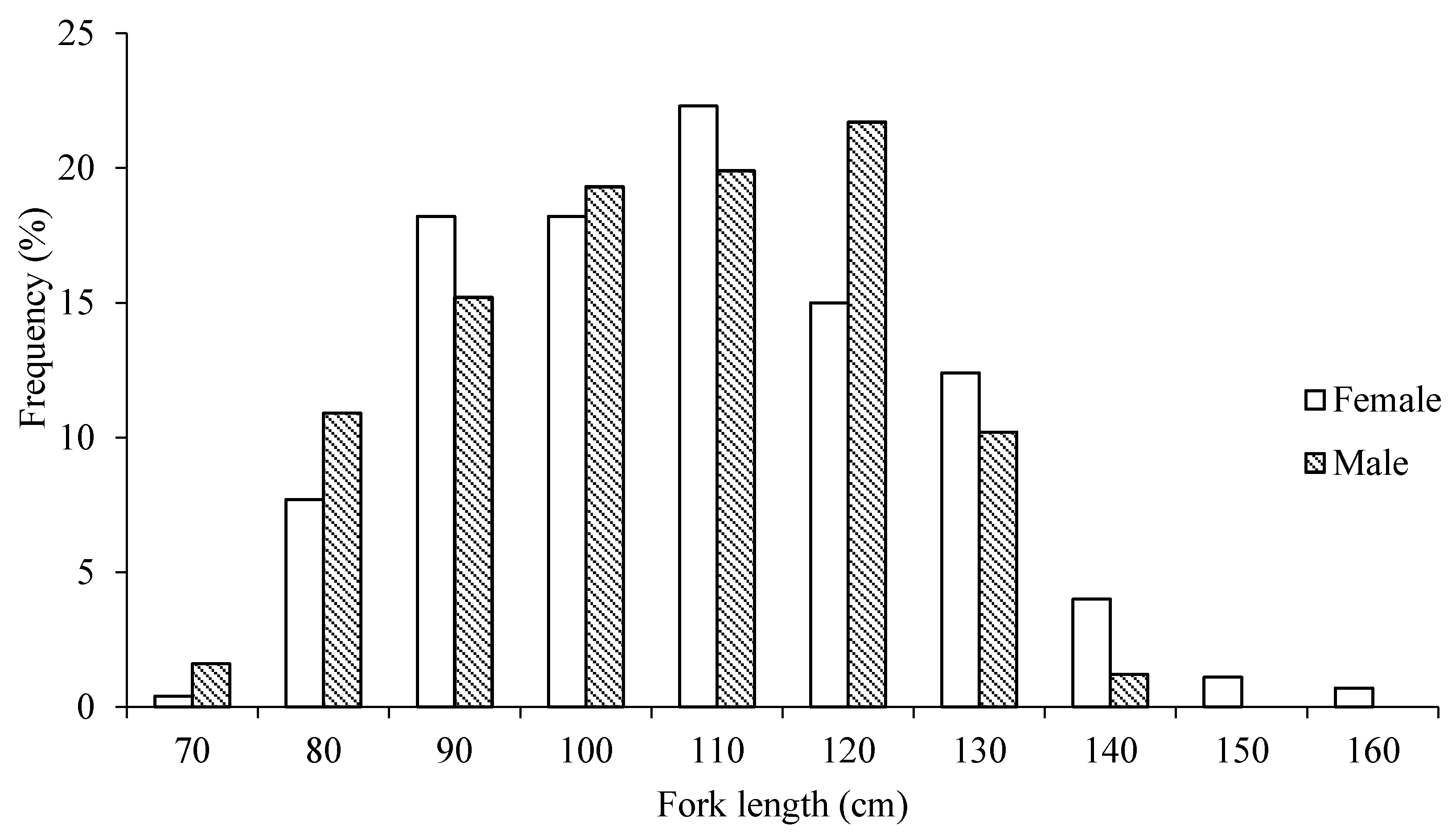
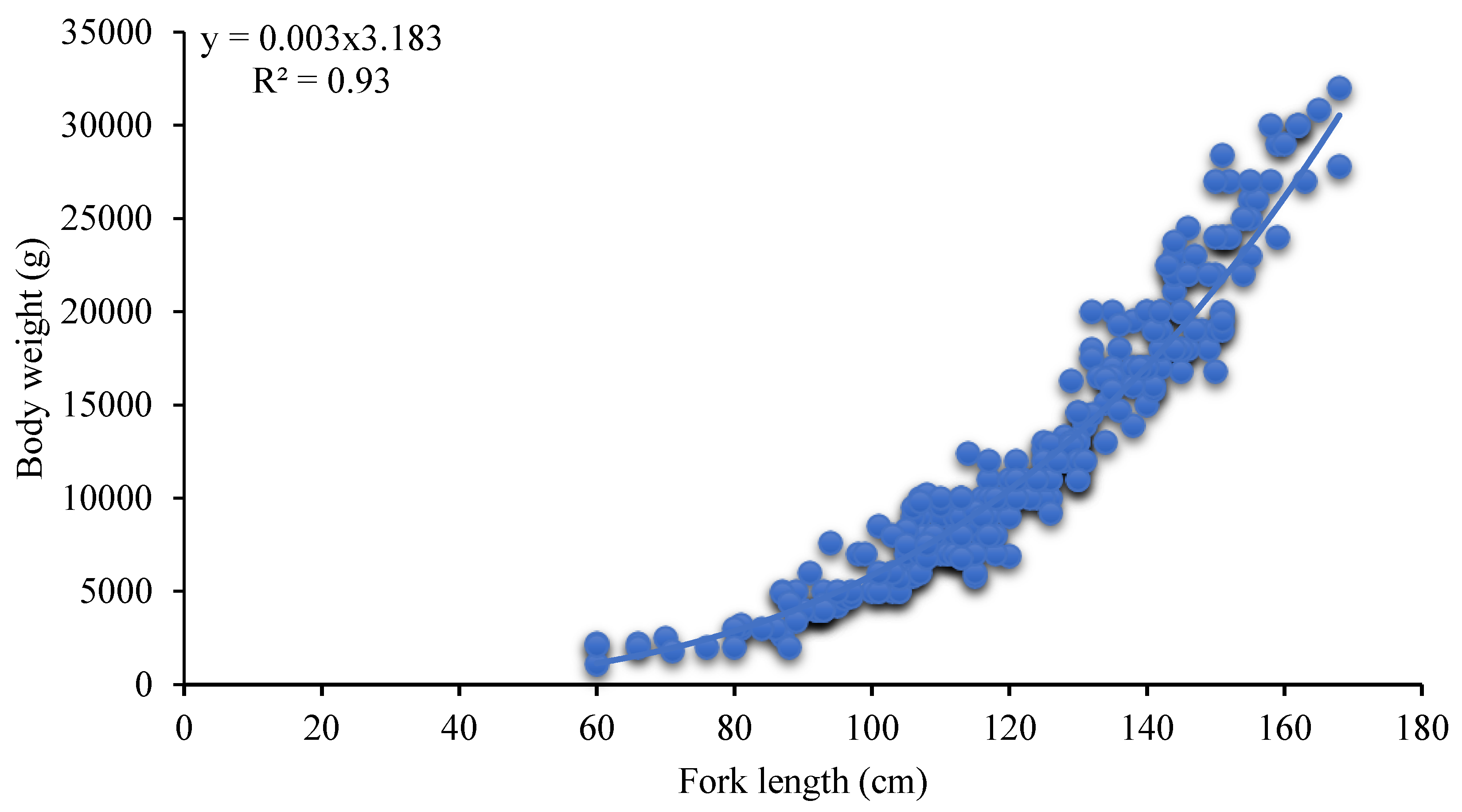
3.1. Fork Length Composition and the Relationship with Body Weight
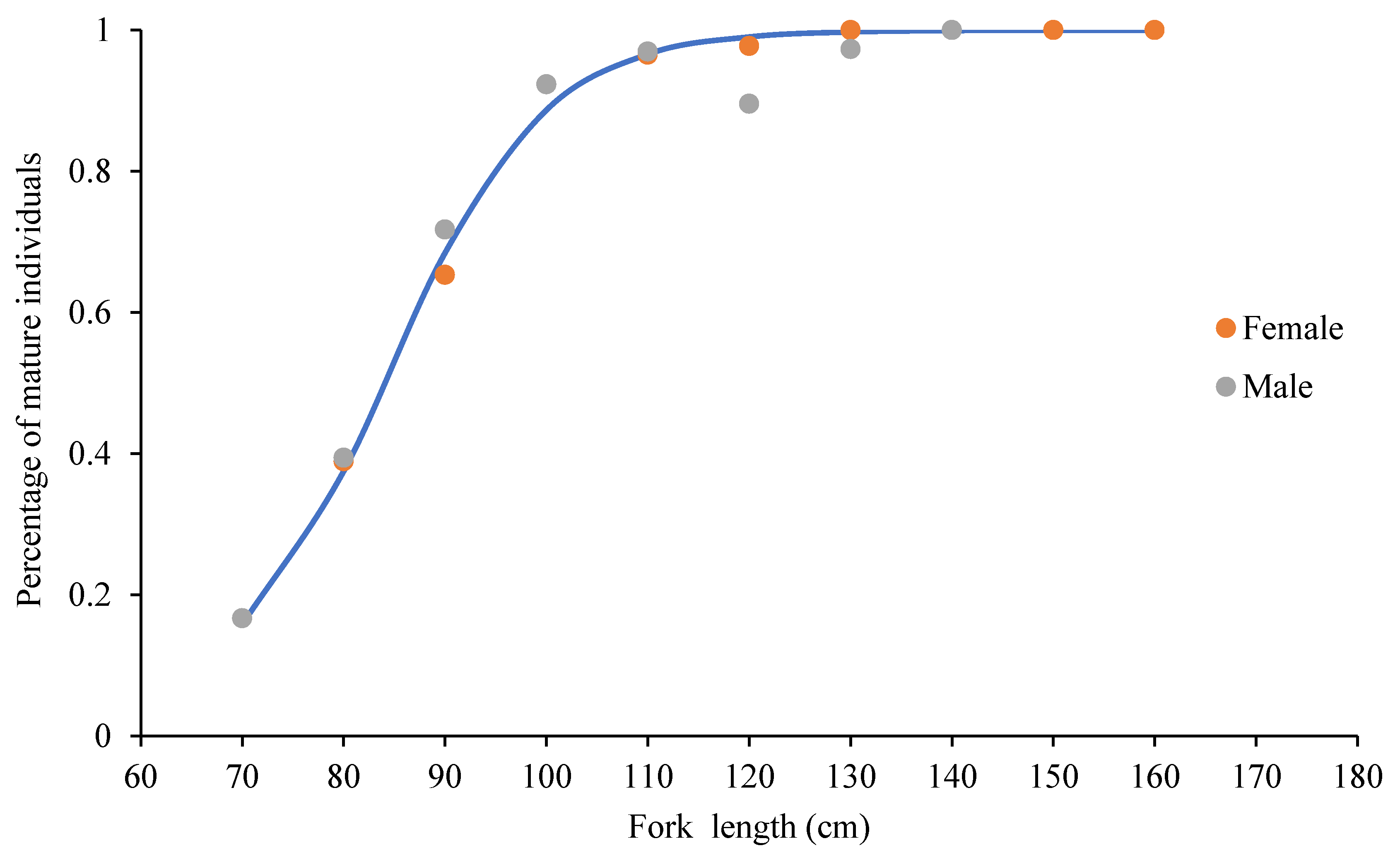
3.2. Sex Ratio and Fork Length at 50% Sexual Maturity
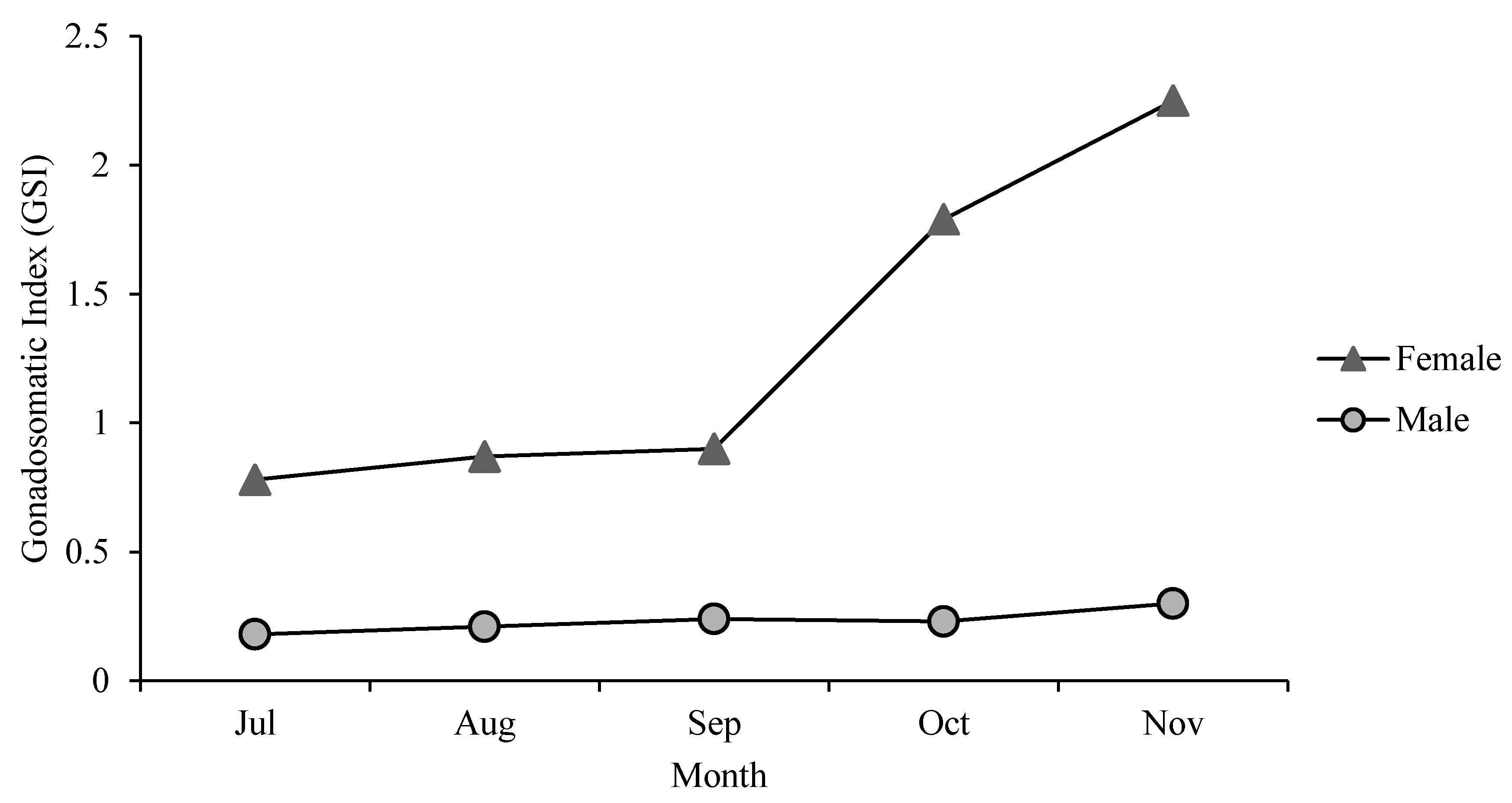
3.3. Gonadosomatic Index (GSI)
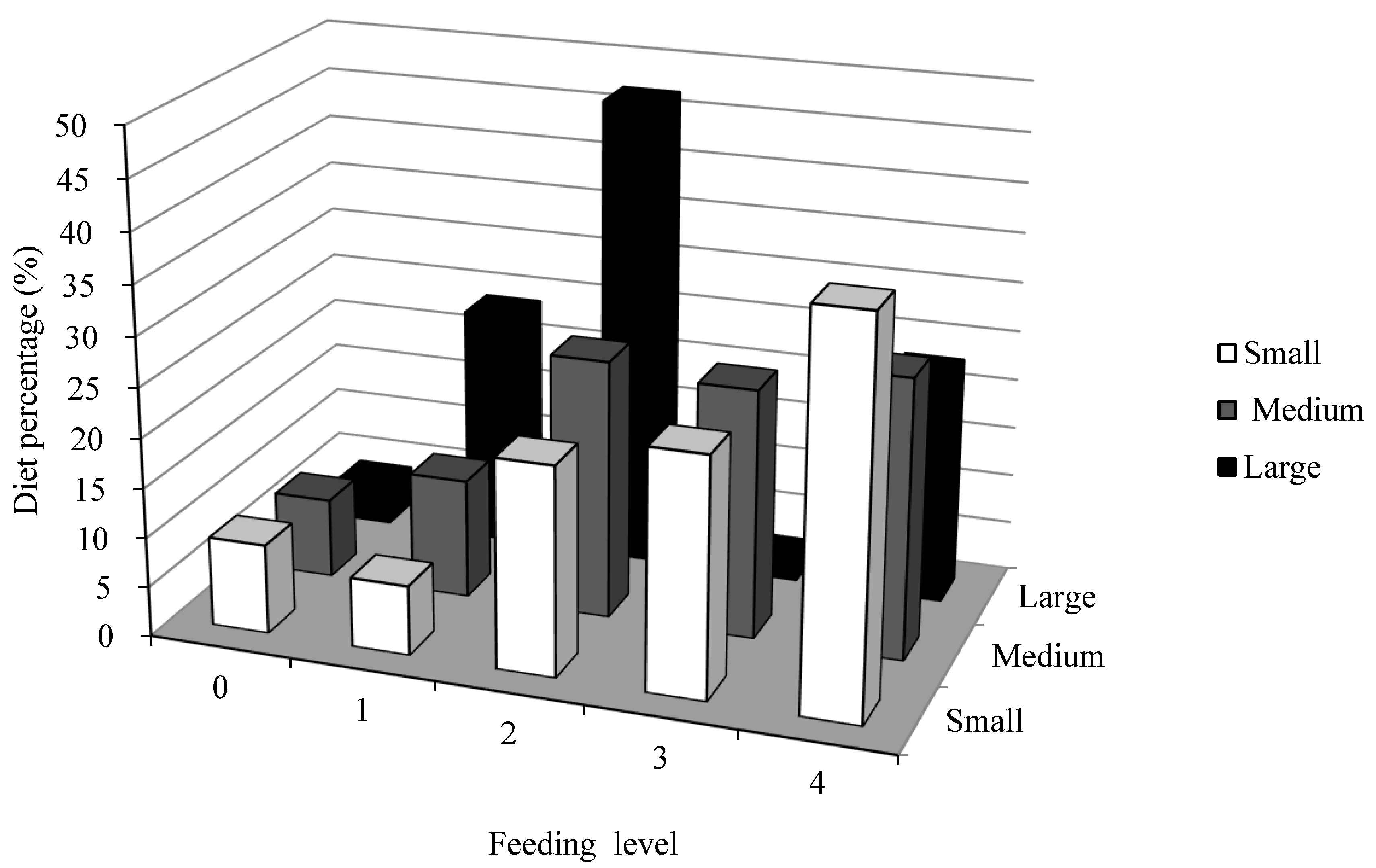
3.4. Feeding Habits
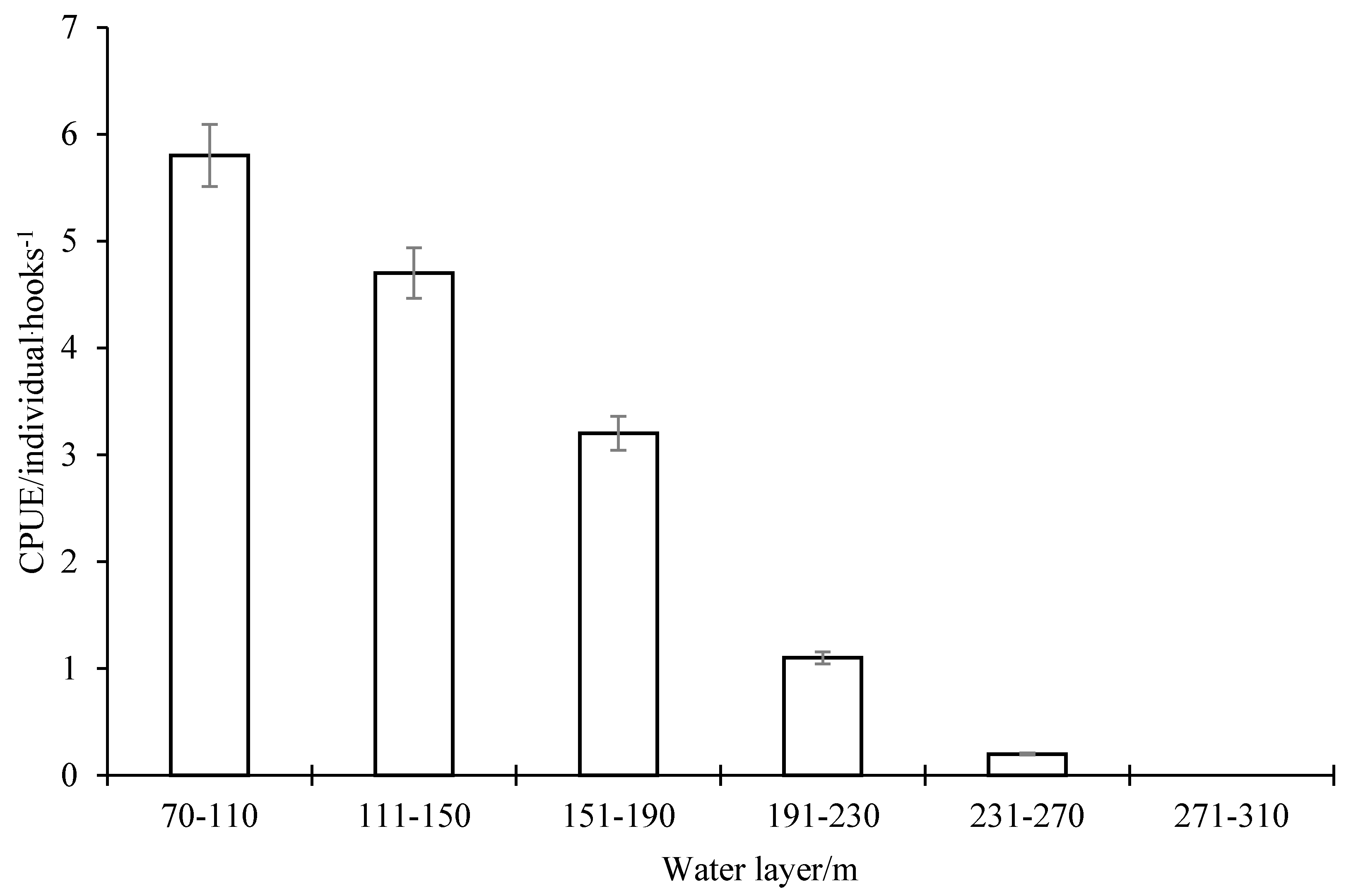
3.5. The Optimum Water Layer and Optimum Temperature of Wahoo
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hogarth, W.T. Life History Aspects of the Wahoo Acanthocybium solandri (Cuvier and Valenciennes) from the Coast of North Carolina. Ph.D. Thesis, North Carolina State University, Raleigh, North Carolina, 1976. [Google Scholar]
- McBride, R.S.; RichardsonA, A.K.; Maki, K.L. Age, growth, and mortality of wahoo, Acanthocybium solandri, from the Atlantic coast of Florida and the Bahamas. Mar. Freshw. Res. 2008, 59, 799–807. [Google Scholar] [CrossRef]
- Zischke, M.T.; Griffiths, S.P. Per-recruit stock assessment of wahoo (Acanthocybium solandri) in the southwest Pacific Ocean. Fish. Bull. 2015, 113. [Google Scholar] [CrossRef]
- Zischke, M.T.; Griffiths, S.P.; Tibbetts, I.R. Rapid growth of wahoo (Acanthocybium solandri) in the Coral Sea, based on length-at-age estimates using annual and daily increments on sagittal otoliths. ICES J. Mar. Sci. J. Cons. 2013, 70, 1128–1139. [Google Scholar] [CrossRef] [Green Version]
- Zischke, M.T.; Farley, J.H.; Griffiths, S.P.; Tibbetts, I.R. Reproductive biology of wahoo, Acanthocybium solandri, off eastern Australia. Rev. Fish Biol. Fish. 2013. [Google Scholar] [CrossRef]
- Oxenford, H.A.; Murray, P.A.; Luckhurst, B.E. The biology of wahoo (Acanthocybium solandri) in the western central Atlantic. Gulf Caribb. Res. 2003, 15, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.M. Estimation of Life History Parameters, Biological Reference Points, and Associated Uncertainties for Wahoo (Acanthocybium solandri) in the Waters off Eastern Taiwan. Master’s Thesis, National Taiwan University, Taiwan, 2008. [Google Scholar]
- Theisen, T.C.; Bowen, B.W.; Lanier, W.; Baldwin, J.D. High connectivity on a global scale in the pelagic wahoo, Acanthocybium solandri (tuna family Scombridae). Mol. Ecol. 2008, 17, 4233–4247. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, C.A.; Aalbers, S.A.; Ortega-Garcia, S.; Wegner, N.C.; Bernal, D. Depth distribution and temperature preferences of wahoo (Acanthocybium solandri) off Baja California Sur, Mexico. Mar. Biol. 2011, 158, 917–926. [Google Scholar] [CrossRef]
- Zischke, M.T. A review of the biology, stock structure, fisheries and status of wahoo (Acanthocybium solandri), with reference to the Pacific Ocean. Fish. Res. 2012, 119–120, 13–22. [Google Scholar] [CrossRef]
- Brown-Peterson, N.J.; Franks, J.S.; Burke, A.M. Preliminary observations on the reproductive biology of wahoo, Acanthocybium solandri, from the northern Gulf of Mexico and Bimini, Bahamas. Proc. Gulf Caribb. Fish. Inst. 2000, 51, 414–427. [Google Scholar]
- Jenkins, K.L.M.; McBride, R.S. Reproductive biology of wahoo, Acanthocybium solandri, from the Atlantic coast of Florida and the Bahamas. Mar. Fresh. Res. 2009, 60, 893–897. [Google Scholar] [CrossRef]
- Viana, D.; Branco, I.; Fernandes, C.; Fischer, A.; Carvalho, F.; Travassos, P.; Hazin, F. Reproductive biology of the wahoo, Acanthocybium solandri (Teleostei: Scombridae) in the Saint Peter and Saint Paul Archipelago, Brazil. Int. J. Plant Anim. Sci. 2013, 1, 049–057. [Google Scholar]
- James, S.F.; Eric, R.H.; James, R.B. Diet of Wahoo, Acanthocybium solandri, from the Northcentral Gulf of Mexico. In Proceedings of the 60th Gulf and Caribbean Fisheries Institute, Punta Cana, Dominican Republic, 2008; pp. 353–361. [Google Scholar]
- Collette, B.B.; Nauen, C.E. FAO Species Catalogue. Vol 2. Scombrids of the World. An Annotated and Illustrated Catalogue of Tunas, Mackerels, Bonitos and Related Species Known to Date. FAO Fish. Synop. 1983, 125, 137. [Google Scholar]
- Hamano, T.; Matsura, S. Sex ratio of the Japanese mantis shrimp in Hakata Bay. Nippon Suisan Gakkaishi 1987, 53, 2279. [Google Scholar] [CrossRef] [Green Version]
- Beardsley, G.L., Jr. Age, Growth and Reproduction of the Dolphin, Coryphaena hippurus, in the Straits of Florida. Copeia 1967, 441–451. [Google Scholar] [CrossRef]
- Chen, Y.; Paloheimo, J. Estimating fish length and age at 50% maturity using a logistic type model. Aquat. Sci. 1994, 56, 206–219. [Google Scholar] [CrossRef]
- Brown-Peterson, N.J.; Wyanski, D.M.; Saborido-Rey, F.; Macewicz, B.J.; Lowerre-Barbieri, S.K. A Standardized Terminology for Describing Reproductive Development in Fishes. Mar. Coast. Fish. Dyn. Man. Ecos. Sci. 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Hyslop, E.J. Stomach contents analysis, a review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef] [Green Version]
- Song, L.M.; Gao, P.F. Capture depth, water-temperature and salinity of bigeye tuna (Thunnus obesus) longlining in Maldives waters (in Chinese with English abstract). J. Fish. China 2006, 30, 335–340. [Google Scholar]
- Figuerola-Fernandez, M.; Pena-Alvarado, N.; Torres-Ruiz, W. Maturation and reproductive seasonality of the wahoo (Acanthocybium solandri), red-ear sardine (Harengula humeralis), false pilchard (Harengula clupeola), thread herring (Opisthonema oglinum), crevalle jack (Caranx hippos), horse-eye jack (Caranx latus), blue runner (Caranx crysos), and great barracuda (Sphyraena barracuda) in Puerto Rico. In Aspects of the Reproductive Biology of Recreationally Important Fish Species in Puerto Rico; Velez-Arocho, J., Diaz-Velazquez, E., Garcia-Perez, M., Berrios, J.M., Rosario-Jimenez, A., Eds.; US Department of Natural and Environmental Resources, Fish and Wildlife Bureau: Puerto Rico, USA, 2008; p. 134. [Google Scholar]
- Shimose, T.; Yokawa, K.; Satio, H.; Tachihara, K. Sexual difference in the migration pattern of blue marine, Makaira nigricans, related to spawning and feeding activities in the Western and Central North Pacific Ocean. Bull. Mar. Sci. 2012, 88, 231–249. [Google Scholar] [CrossRef]
- Luan, S.H.; Dai, X.J.; Tian, S.Q.; Li, W.W.; Fan, Y.C. Biology and environmental preferences of Acanthocybium solandri in the southeast Pacific Ocean. Mar. Fish. 2017, 1-0030-11, 23–30, (Chinese version). [Google Scholar]
- Iversen, E.S.; Yoshida, H.O. Notes on the biology of the wahoo in the Line Islands. Pac. Sci. 1957, 11, 370–379. [Google Scholar]
- Luckhurst, B.E.; Trott, T. Bermuda’s commercial line fishery for wahoo and dolphinfish: Landings, seasonality and catch per unit effort trends. Proc. Gulf Caribb. Fish. Inst. 2000, 51, 404–413. [Google Scholar]
- Allain, V. Diet of mahi-mahi, wahoo and lancetfish in the western and central Pacific. In Proceedings of the 16th Meeting of the Standing Committee on Tuna and Billfish, SCTB16, Mooloolaba, Queensland, Australia, 9–16 July 2003. [Google Scholar]
- Nakano, H.; Okazaki, M.; Okamoto, H. Analysis of catch depth by species for tuna longline fishery based on catch by branchlines. Nat. Res. Inst. Bull. Far Seas Fish. 1997, 24, 43–62. [Google Scholar]
- Beverly, S.; Curran, D.; Musyl, M.; Molonya, B. Effects of eliminating shallow hooks from tuna longline sets on target and non-target species in the Hawaii-based pelagic tuna fishery. Fish. Res. 2009, 96, 281–288. [Google Scholar] [CrossRef]
- Theisen, T.C.; Baldwin, J.D. Movements and depth/temperature distribution of the ectothermic Scombrid, Acanthocybium solandri (wahoo), in the western North Atlantic. Mar. Biol. 2012, 159, 2249–2258. [Google Scholar] [CrossRef]
- Schaefer, K.M.; Fuller, D.W. Movements, behavior and habitat selection of bigeye tuna (Thunnus obesus) in the eastern equatorial Pacific, ascertained through archival tags. Fish. Bull. 2002, 100, 765–788. [Google Scholar]
- Sepulveda, C.A.; Knight, A.; Nasby-Lucas, N.; Domeier, M.L. Fine-scale movements of the swordfish (Xiphias gladius) in the Southern California Bight. Fish. Oceanogr. 2010, 19, 279–289. [Google Scholar] [CrossRef]



| Environmental Variables | Starting Point | Final Point | Interval | Total Ranges |
|---|---|---|---|---|
| Depth | 75.6 m | 306.9 m | 40 m | 6 |
| Temperature | 23.9 °C | 19.8 °C | 1 °C | 6 |
| Ecological Group | Prey Items | %N | %F | Ecological Group | Prey Items | %N | %F |
|---|---|---|---|---|---|---|---|
| Fishes | Trichiurus lepturus | 14 | 24.49 | Konosirus punctatus | 1.25 | 0.68 | |
| Monacanthus chinensis | 6.14 | 11.22 | Alepisaurus brevirostris | 1.25 | 1.02 | ||
| Scomber | 5.09 | 6.46 | Lethrinidae | 1.25 | 1.02 | ||
| Tylosurus crocodiles | 3.56 | 6.12 | Psenopsis anomala | 1.16 | 0.85 | ||
| Assrtger anzac | 2.60 | 4.08 | Neoceratiidae | 1.11 | 0.51 | ||
| Drepane punctata | 2.56 | 3.57 | Taractichthys steindachneri | 1.07 | 0.34 | ||
| Mene maculata | 2.42 | 2.72 | Acanthocybium solandri | 1.07 | 0.34 | ||
| Kentrocapros aculeatus | 2.25 | 3.91 | Harpadon nehereus | 1.07 | 0.34 | ||
| Drosera capensis | 2.03 | 2.55 | Fistularia petimba lacépède | 1.07 | 0.51 | ||
| Cypselurus agoo | 1.90 | 3.06 | Lepidocybium flavobrunneum | 1.03 | 0.34 | ||
| Ostracion | 1.73 | 1.87 | Syngnathidae | 1.03 | 0.34 | ||
| Brama brama | 1.68 | 2.38 | Megalaspis cordyla | 1.03 | 0.34 | ||
| Stromateidae | 1.64 | 2.72 | Xiphias gladius | 1.03 | 0.34 | ||
| Lagocephalus lagocephalus | 1.60 | 1.7 | Mola mola | 1.03 | 0.34 | ||
| Gempylus serpens | 1.60 | 1.53 | Oxymonacanthus longirostris | 0.98 | 0.17 | ||
| Engraulidae | 1.42 | 0.85 | Priacanthus tayenus | 0.98 | 0.17 | ||
| Alepisaurus ferox | 1.42 | 1.7 | Lampris guttatus | 0.98 | 0.17 | ||
| Thunnus alalunga | 1.42 | 1.19 | Amphilophus citrinellus | 0.98 | 0.17 | ||
| Kaiwarinus equula | 1.33 | 1.02 | Sphyraena barracuda | 0.98 | 0.17 | ||
| Katsuwonus pelamis | 1.29 | 1.36 | Crustacea | Shrimp | 0.17 | 0.34 | |
| Aracana aurita aracanasp | 1.25 | 1.02 | Cephalopoda | Sepiidae | 13.32 | 32.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Tian, S.; Kindong, R.; Dai, X. Biology and Environmental Preferences of Wahoo, Acanthocybium solandri (Cuvier, 1832), in the Western and Central Pacific Ocean (WCPO). J. Mar. Sci. Eng. 2020, 8, 184. https://doi.org/10.3390/jmse8030184
Gao C, Tian S, Kindong R, Dai X. Biology and Environmental Preferences of Wahoo, Acanthocybium solandri (Cuvier, 1832), in the Western and Central Pacific Ocean (WCPO). Journal of Marine Science and Engineering. 2020; 8(3):184. https://doi.org/10.3390/jmse8030184
Chicago/Turabian StyleGao, Chunxia, Siquan Tian, Richard Kindong, and Xiaojie Dai. 2020. "Biology and Environmental Preferences of Wahoo, Acanthocybium solandri (Cuvier, 1832), in the Western and Central Pacific Ocean (WCPO)" Journal of Marine Science and Engineering 8, no. 3: 184. https://doi.org/10.3390/jmse8030184
APA StyleGao, C., Tian, S., Kindong, R., & Dai, X. (2020). Biology and Environmental Preferences of Wahoo, Acanthocybium solandri (Cuvier, 1832), in the Western and Central Pacific Ocean (WCPO). Journal of Marine Science and Engineering, 8(3), 184. https://doi.org/10.3390/jmse8030184






