A Preliminary Study on an Alternative Ship Propulsion System Fueled by Ammonia: Environmental and Economic Assessments
Abstract
1. Introduction
- NH3 is a cost-efficient alternative in terms of fuel price, and it has already existing infrastructure (approximately 10.6–30.2 times cheaper than H2) [9].
- NH3′s volumetric hydrogen content is significantly greater than that of H2 (about 1.7 times more than liquid-H2).
- Transportation and storage technologies for NH3 already exist and they are available today (annually, more than 18 M t of NH3 is traded internationally) [13].
- NH3 has acute toxicity with strong smell and is easy to detect, and the safety measures are commonly practiced.
- A carbon capture system (CCS) from an NH3 production plant is a feasible option [5].
2. Target Ship
3. Proposed Systems
3.1. Description of Proposed Systems
- Case 1. The conventional HFO-based M/E propulsion system.
- Case 2. The proposed NH3-based M/E propulsion system.
- Case 3. The proposed NH3-based electric propulsion system powered by generators.
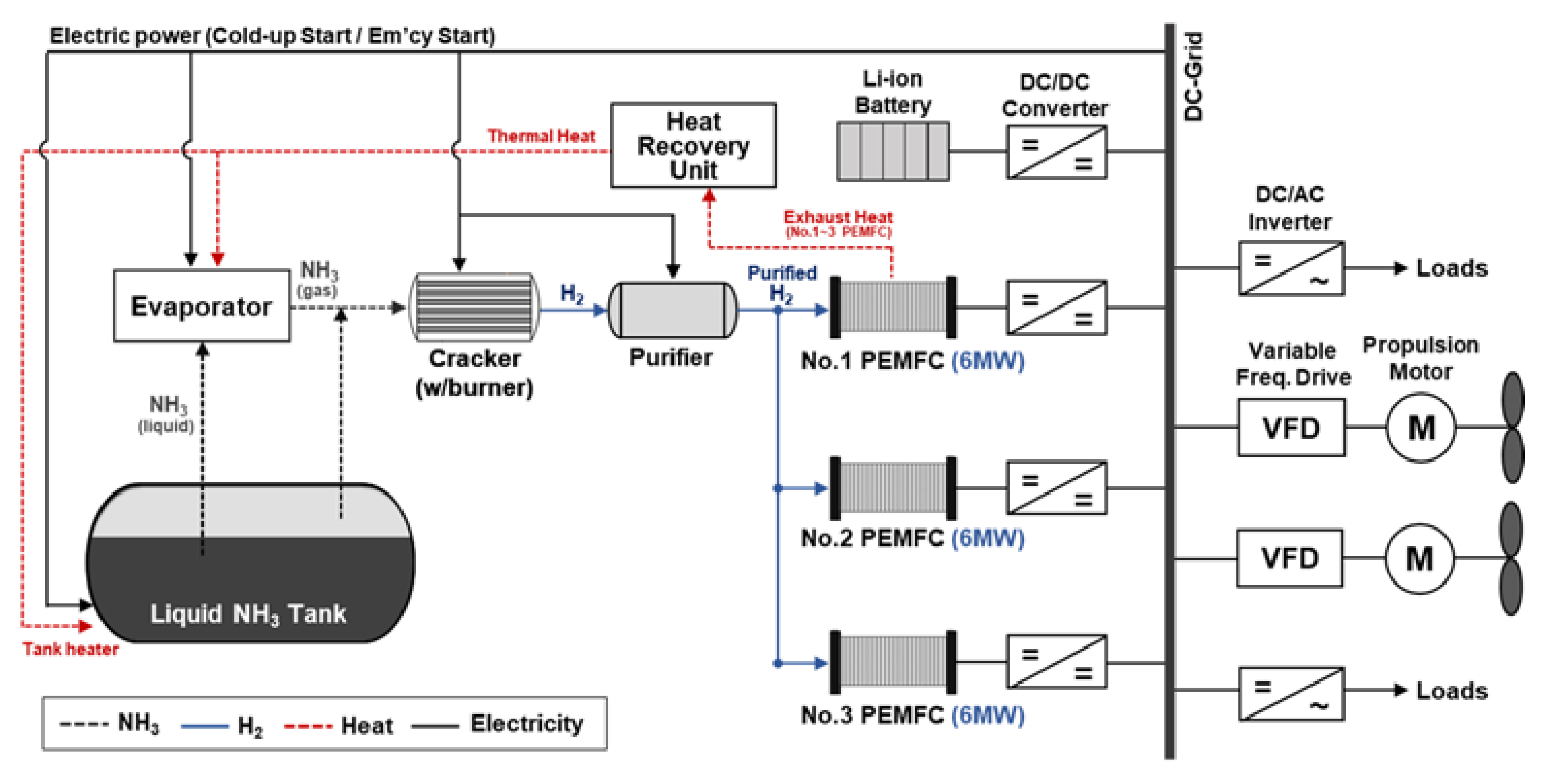
- Case 4. The proposed NH3-based electric propulsion system powered by PEMFC.
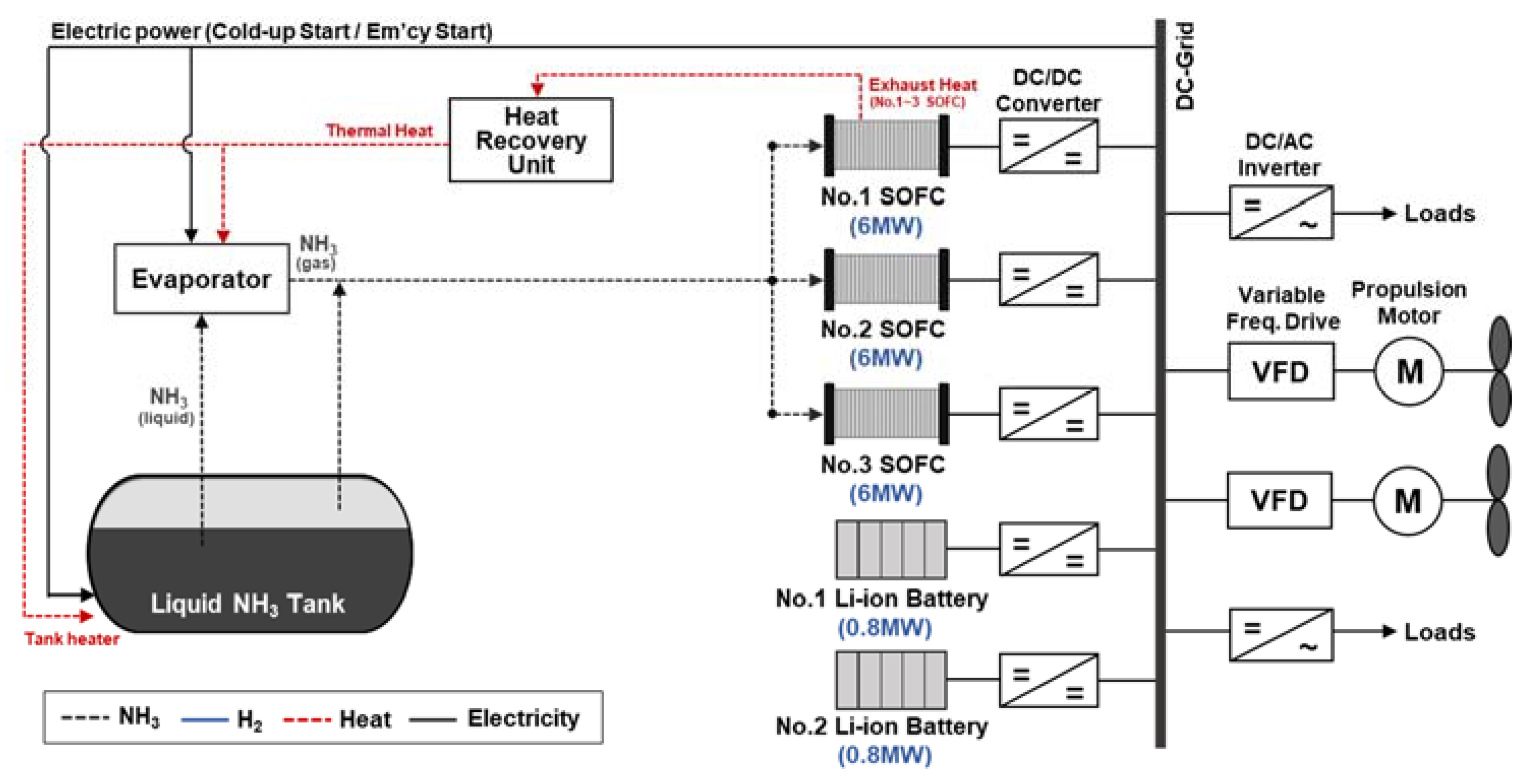
- Case 5. The proposed NH3-based electric propulsion system powered by SOFC.
3.2. Main Equipment & System
3.2.1. Ammonia Engine
3.2.2. PEMFC
3.2.3. SOFC
3.2.4. ESS
3.2.5. Cracking
3.2.6. BOG and Vaporizing
3.3. Fuel Consumption
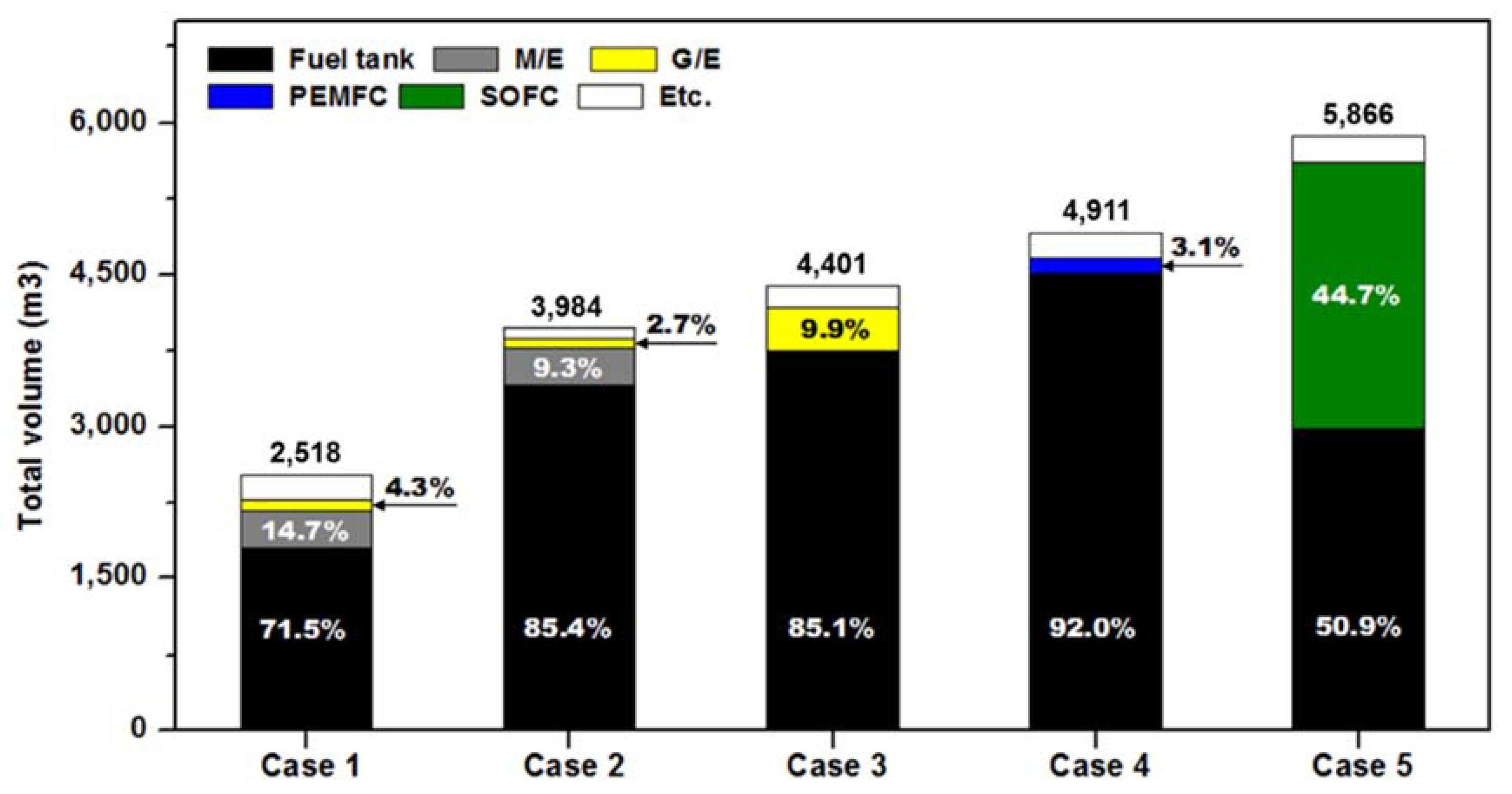
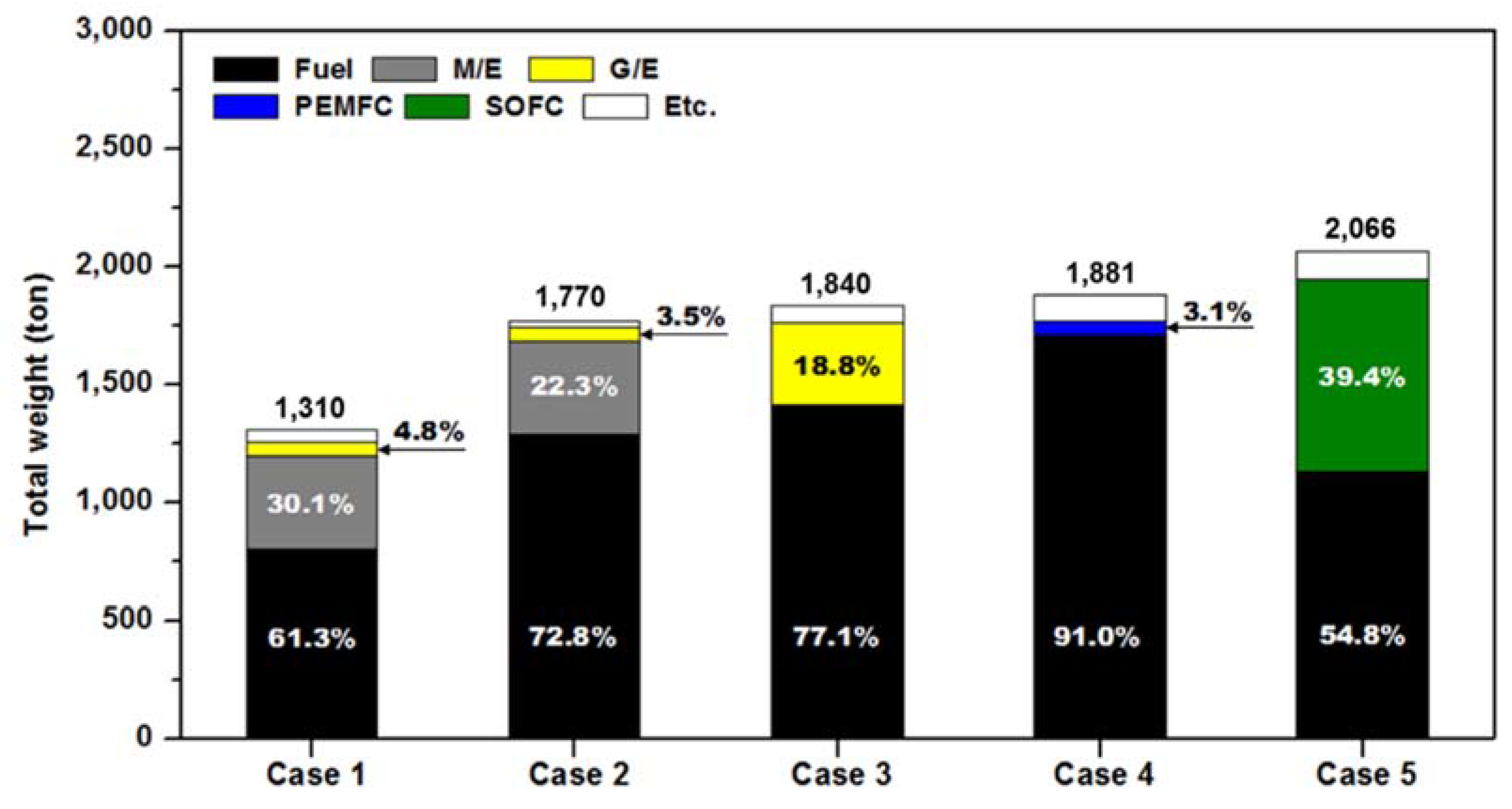
3.4. Volume and Weight
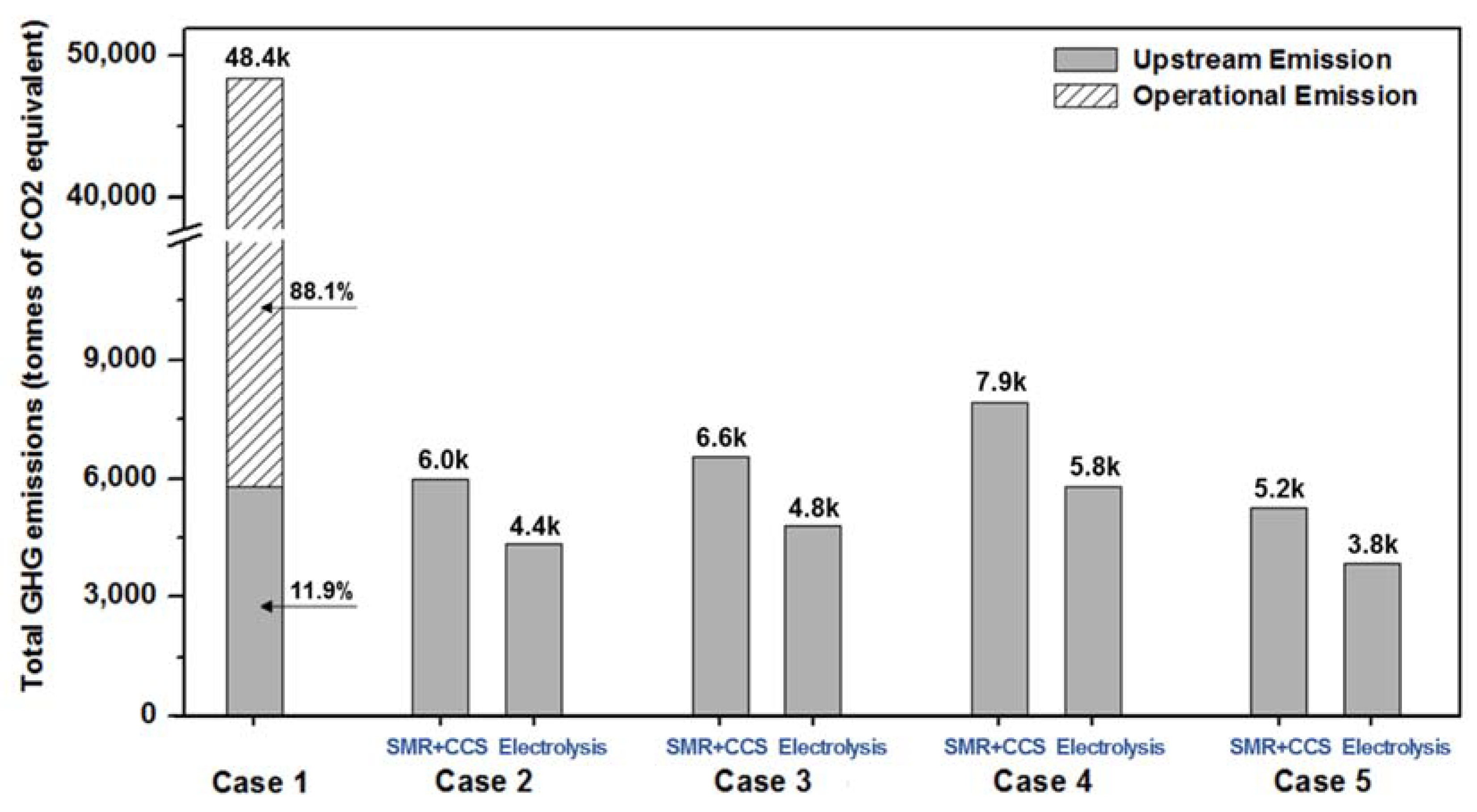
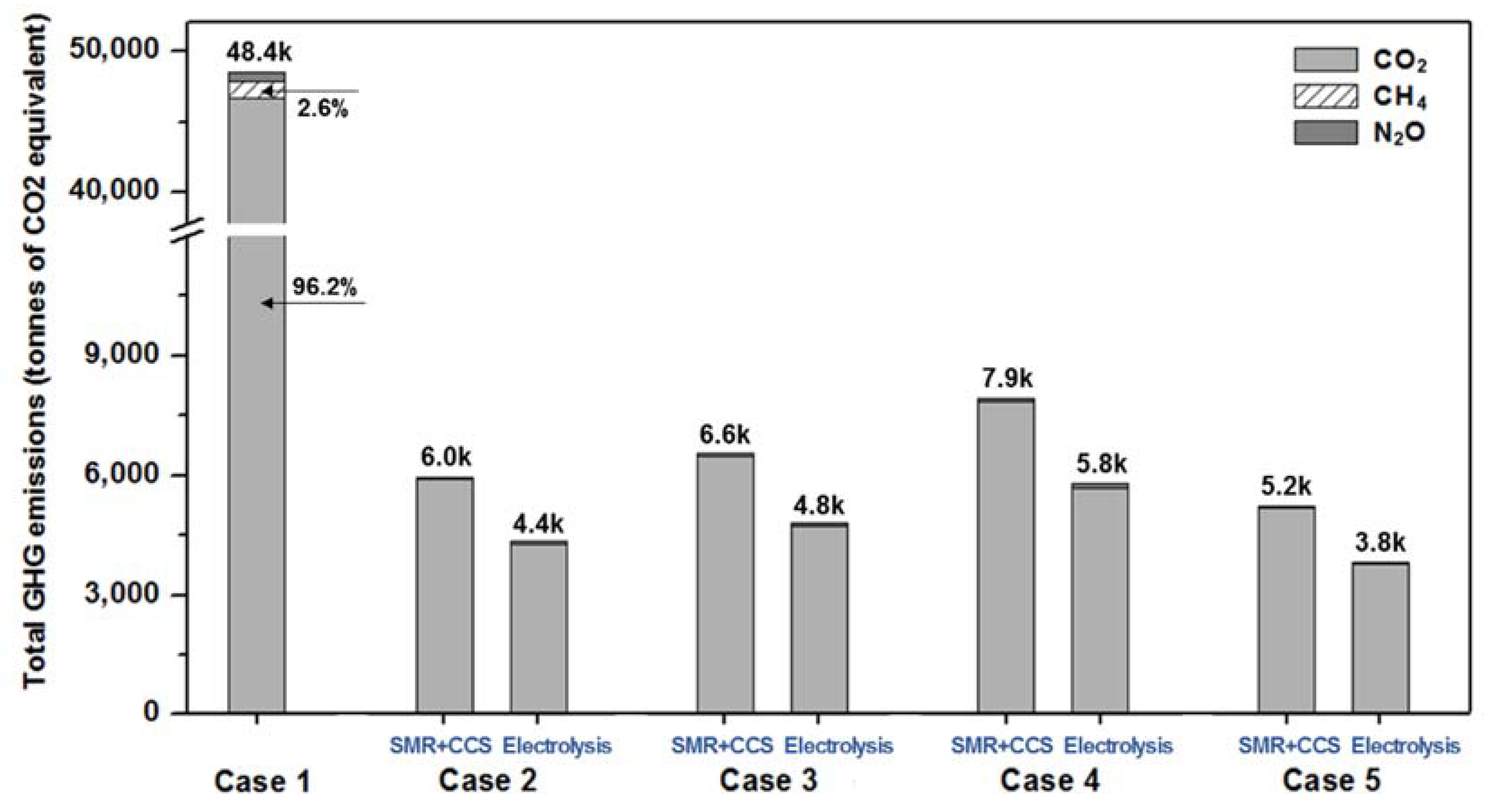
4. Environmental Analysis
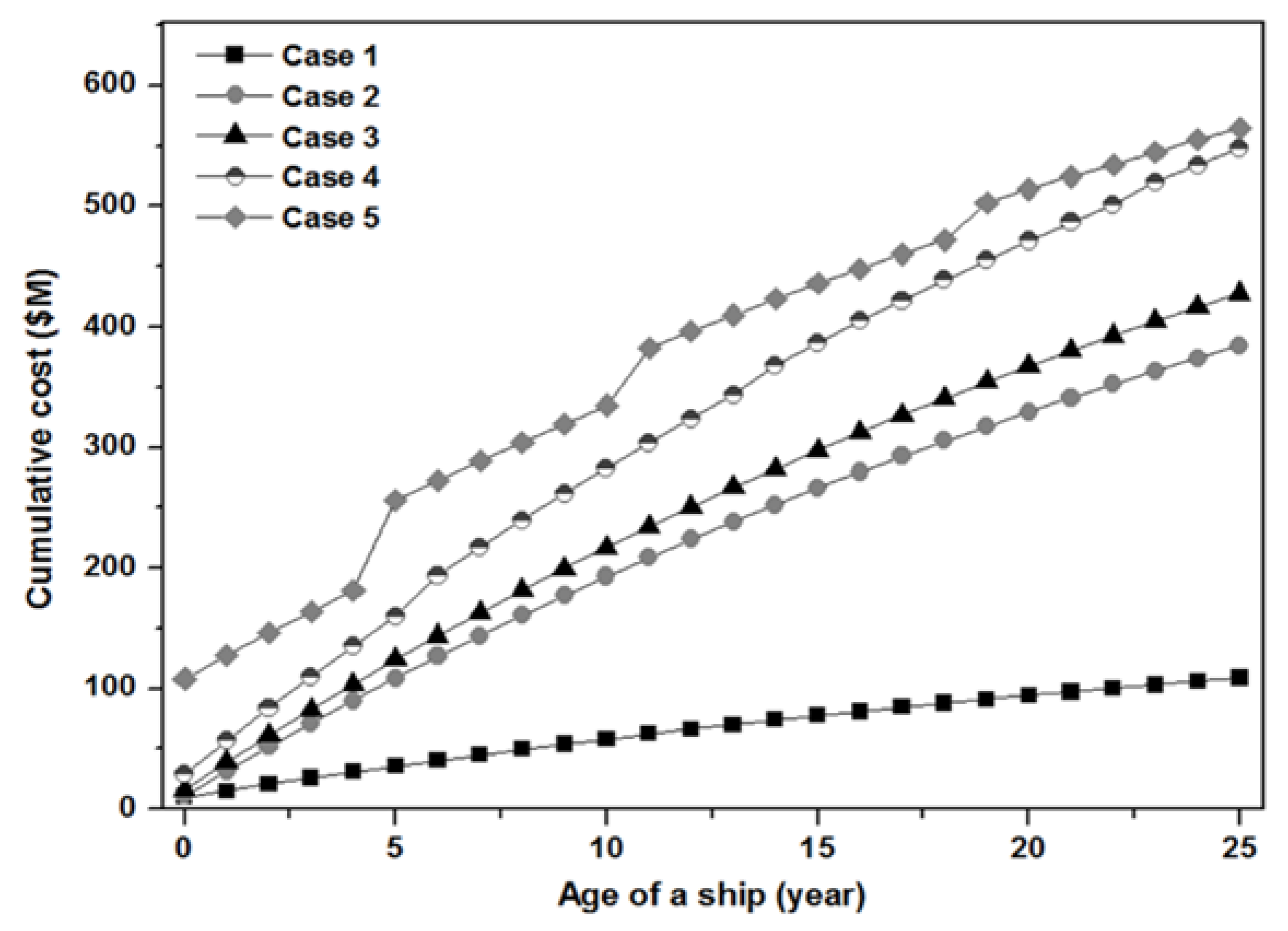
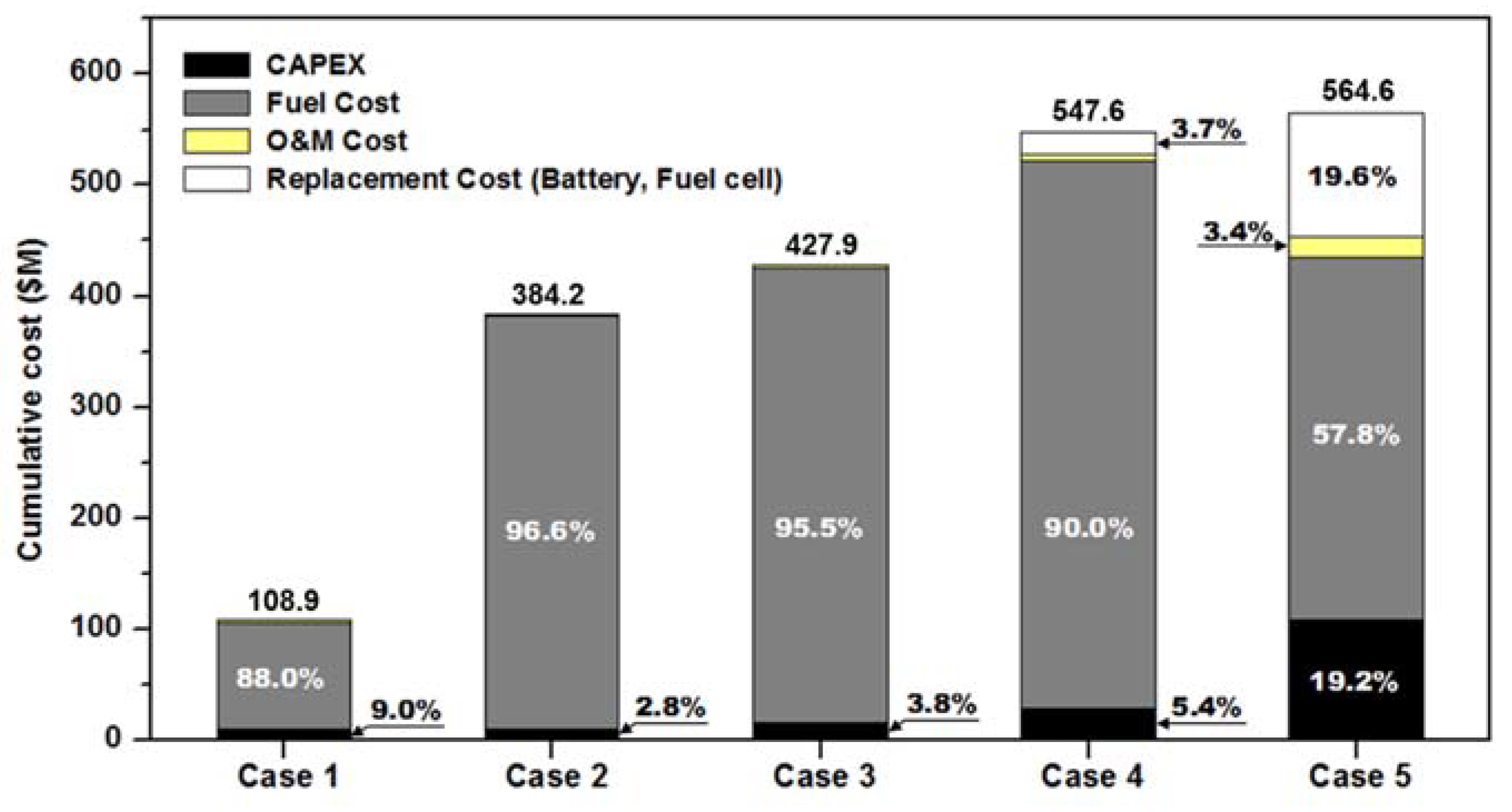
5. Economic Analysis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Maritime Organization (IMO). Greenhouse Gas Emissions. Available online: http://www.imo.org/en/OurWork/Environment/PollutionPrevention/AirPollution/Pages/GHG-Emissions.aspx (accessed on 30 November 2019).
- The International Transport Forum (ITF). Decarbonising Maritime Transport Pathways to Zero-Carbon Shipping by 2035; OECD: Paris, France, 2018. [Google Scholar]
- Ash, N.; Scarbrough, T. Sailing on Solar—Could Green Ammonia Decarbonise International Shipping; Environmental Defense Fund Europe (EDF Europe): London, UK, 2019. [Google Scholar]
- Lewis, J. Fuels Without Carbon - Prospects and the Pathway Forward for Zero-Carbon Hydrogen and Ammonia Fuels; Clean Air Task Force: Boston, MA, USA, 2018. [Google Scholar]
- International Energy Agency (IEA). The Future of Hydrogen—Seizing Today’s Opportunities; International Energy Agency (IEA): Paris, France, 2019. [Google Scholar]
- Van Biert, L.; Godjevac, M.; Visser, K.; Aravind, P.V. A Review of Fuel Cell Systems for Maritime Applications. J. Power Sources 2016, 327, 345–364. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for Power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.; Kunkuma, A.; Okafor, E.C. Science and Technology of Ammonia Combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- NCE Maritime CleanTech. Norwegian Future Value Chains for Liquid Hydrogen; NCE Maritime CleanTech: Stord, Norway, 2019. [Google Scholar]
- International Maritime Organization (IMO). 2014 Guidelines on the Method of the Attained Energy Efficiency Design Index (EEDI) for New Ships; as Amended (MEPC.1/Circ.866); IMO: London, UK, 2017. [Google Scholar]
- Misra, S.C. Design Principles of Ships and Marine Structures, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781482254464. [Google Scholar]
- Wright, A.A.; Wilson, T.S. Flashpoint of Marine Distillate Oil Fuels; Lloyd’s Register: London, UK, 2012. [Google Scholar]
- Muraki, S. R&D on Hydrogen Energy Carriers toward Low Carbon Society; Cross-ministerial Strategic Innovation Promotion Program (SIP): Tokyo, Japan, 2018.
- The Crown (Department for Transport). Clean Maritime Plan; The Crown: London, UK, 2019. [Google Scholar]
- Lloyd’s Register (LR) & University Maritime Advisory Services (UMAS). Zero-Emission Vessels 2030—How Do We Get There; LR: London, UK; UMAS: London, UK, 2017. [Google Scholar]
- De Vries, N. Safe and Effective Application of Ammonia as a Marine Fuel. Master’s Thesis, Delft University of Technology (TU Delft), Delft, The Netherlands, 2019. [Google Scholar]
- Shiozawa, B. CO2 Free Ammonia as CO2 Free Fuel and Hydrogen Carrier—Achievements of SIP “Energy Carriers”; Cross-Ministerial Strategic Innovation Promotion Program (SIP): Tokyo, Japan, 2019.
- Hellenic Shipping News. Sowing ZEEDS (Zero Emission Energy Distribution at Sea) of a New Era. Available online: https://www.hellenicshippingnews.com/sowing-zeeds-zero-emissioni-energy-distribution-at-sea-of-a-new-era/ (accessed on 13 August 2019).
- Global Maritime Forum. How Renewables Can Solve Shipping’s Need for Climate Friendly Fuel. Available online: https://www.globalmaritimeforum.org/news/how-renewables-can-solve-shippings-need-for-climate-friendly-fuel/ (accessed on 13 October 2019).
- NH3 Fuel Association. Introduction to NH3 Fuel. Available online: https://nh3fuelassociation.org/introduction/ (accessed on 31 October 2019).
- NH3 Fuel Association. The AmVeh—An Ammonia Fueled Car from South Korea. Available online: https://nh3fuelassociation.org/2013/06/20/the-amveh-an-ammonia-fueled-car-from-south-korea/ (accessed on 3 October 2019).
- NH3 Fuel Association. Ammonia Fueled Sports Car: Marangoni Toyota GT86 Eco Explorer. Available online: https://nh3fuelassociation.org/2013/04/25/ammonia-fuel-marangoni-eco-explorer/ (accessed on 28 September 2019).
- Ship Insight. Ammonia—Another New Fuel for the Future? Available online: https://shipinsight.com/articles/ammonia-another-new-fuel-for-the-future/ (accessed on 14 February 2020).
- The MotorShip. MAN Energy Solutions to Undertake Ammonia Study. Available online: https://www.motorship.com/news101/alternative-fuels/man-energy-solutions-to-undertake-ammonia-study (accessed on 19 September 2019).
- Alfa Laval. Press release. The Alfa Laval FCM LPG Booster System Excels with the New LPG-Fuelled Engine from MAN Energy Solutions; Alfa Laval: Lund, Sweden, 2019. [Google Scholar]
- Ammonia Energy Association. Maritime Ammonia Engines in Japan; Ammonia Shipbuilding in South Korea. Available online: https://www.ammoniaenergy.org/articles/maritime-ammonia-engines-in-japan-ammonia-shipbuilding-in-south-korea/ (accessed on 10 August 2019).
- The Maritime Executive. Offshore Vessel to Run on Ammonia-Powered Fuel Cell. Available online: https://www.maritime-executive.com/article/offshore-vessel-to-run-on-ammonia-powered-fuel-cell (accessed on 29 January 2020).
- Suda, T.; Uchida, M.; Ito, T.; Matsuo, T.; Fujimori, T. Technologies to Use Carbon Free Ammonia in Power Plant (IHI Corporation). In Proceedings of the Presented at NH3 Energy Implementation Conference, Pittsburgh, PA, USA, 1 November 2018. [Google Scholar]
- Hunter, H.M.A.; Makepeace, J.W.; Wood, T.J.; Mylius, O.S.; Kibble, M.G.; Nutter, J.B.; Jones, M.O.; David, W.I.F. Demonstrating Hydrogen Production from Ammonia Using Lithium Imide—Powering a Small Proton Exchange Membrane Fuel Cell. J. Power Sources 2016, 329, 138–147. [Google Scholar] [CrossRef]
- RenCat. Clean Hydrogen on Demand. Available online: https://www.rencat.net/ (accessed on 26 August 2019).
- Dolan, M. Delivering Clean Hydrogen Fuel from Ammonia Using Metal Membranes; The Commonwealth Scientific and Industrial Research Organization (CSIRO): Canberra, Australia, 1 November 2017.
- Olmer, N.; Comer, B.; Roy, B.; Mao, X.; Rutherford, D. Greenhouse Gas Emissions from Global Shipping, 2013–2015; ICCT (The International Council on Clean Transportation): Washington, DC, USA, 2017. [Google Scholar]
- MAN Diesel & Turbo. Costs and Benefits of LNG as Ship Fuel for Container Vessels; MAN Diesel & Turbo: Augsburg, Germany, 2012. [Google Scholar]
- MAN Energy Solutions. Propulsion of 2,200–3,000 TEU Container Vessels; MAN Energy Solutions: Augsburg, Germany, 2019. [Google Scholar]
- Damen Shipyards Group. CONTAINER FEEDER 2500—STANDARD; Damen Shipyards Group: Gorinchem, The Netherlands, 2017. [Google Scholar]
- Wärtsilä. WSD80 2400–2400 Teu Container Feeder; Wärtsilä: Helsinki, Finland, 2015. [Google Scholar]
- Comotti, M.; Frigo, S. Hydrogen Generation System for Ammonia–Hydrogen Fuelled Internal Combustion Engines. Int. J. Hydrog. Energy 2015, 40, 10673–10686. [Google Scholar] [CrossRef]
- Frigo, S.; Gentili, R. Analysis of the Behavior of a 4-Stroke Si Engine Fueled with Ammonia and Hydrogen. Int. J. Hydrog. Energy 2013, 38, 1607–1615. [Google Scholar] [CrossRef]
- Mørch, C.S.; Bjerre, A.; Gøttrup, M.P.; Sorenson, S.C.; Schramm, J. Ammonia/Hydrogen Mixtures in an SI-Engine: Engine Performance and Analysis of a Proposed Fuel System. Fuel 2011, 90, 854–864. [Google Scholar] [CrossRef]
- Frigo, S.; Gentili, R. A Hybrid Vehicle Powered by Ammonia and Hydrogen. In Proceedings of the 10th NH3 Fuel Conference, Sacramento, CA, USA, 22–25 September 2013. [Google Scholar]
- Pochet, M.; Truedsson, I.; Foucher, F.; Jeanmart, H.; Contino, F. Ammonia-Hydrogen Blends in Homogeneous-Charge Compression-Ignition. In Proceedings of the 13th International Conference on Engines & Vehicles, Capri, Italy, 10–14 September 2017. [Google Scholar] [CrossRef]
- Frigo, S.; Gentili, R.; Ricci, G.; Pozzana, G.; Comotti, M. Experimental Results Using Ammonia Plus Hydrogen in a S.I. Engine. In FISITA 2012 World Automotive Congress; Future Automotive Powertrains (I); Springer: Berlin/Heidelberg, Germany, 2013; Volume 3, pp. 65–76. [Google Scholar]
- Duynslaegher, C.; Jeanmart, H.; Vandooren, J. Use of Ammonia as a Fuel for SI Engine. In Proceedings of the European Combustion Meeting, Vienna, Austria, 14–17 April 2009. [Google Scholar]
- Hassan, S.Z.; Li, H.; Kamal, T.; Mumtaz, S.; Khan, L. Fuel Cell/Electrolyzer/Ultra-Capacitor Hybrid Power System: Focus on Integration, Power Control and Grid Synchronization. In Proceedings of the 13th International Bhurban Conference on Applied Sciences and Technology (IBCAST), Islamabad, Pakistan, 12–16 January 2016. [Google Scholar]
- Hyundai. All-New Hyundai NEXO—Technical Specifications. Available online: https://hyundai.news/eu/press-kits/all-new-hyundai-nexo-technical-specifications/ (accessed on 1 September 2019).
- Open Electrical. Battery Sizing. Available online: https://wiki.openelectrical.org/index.php?title=Battery_Sizing (accessed on 11 November 2019).
- Ogden, J. Review of Small Stationary Reformers for Hydrogen Production; International Energy Agency (IEA): Paris, France, 2002. [Google Scholar]
- Thomas, G.; Parks, G. Potential Roles of Ammonia in a Hydrogen Economy—A Study of Issues Related to the Use Ammonia for On-Board Vehicular Hydrogen Storage; U.S. Department of Energy: SW Washington, DC, USA, 2006.
- Giddey, S. Ammonia as a Renewable Energy Transportation Media. ACS Sustain. Chem. Eng. 2017, 10231–10239. [Google Scholar] [CrossRef]
- Kojima, Y.; Adachi, T. Ammonia As a Hydrogen Carrier for PEM Fuel Cells. In Proceedings of the 2018 AIChE Annual Meeting, Pittsburgh, PA, USA, 28 October–2 November 2018. [Google Scholar]
- Morgan, E.R. Techno-Economic Feasibility Study of Ammonia Plants Powered by Offshore Wind. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2013. [Google Scholar]
- Raucci, C. The Potential of Hydrogen to Fuel International Shipping. Ph.D. Thesis, University College London (UCL), London, UK, 2017. [Google Scholar]
- Wiesmann, A. Slow Steaming—A Viable Long-Term Option? Wärtsilä: Helsinki, Finland, 2010. [Google Scholar]
- Brake, M.C.T.; Kauffman, K.F.; Hulskotte, J. Sea Shipping Emissions 2017: Netherlands Continental Shelf, 12 Mile Zone and Port Areas; Maritime Research Institute Netherlands (MARIN): Wageningen, The Netherlands, 2019. [Google Scholar]
- PowerCell. Datasheet. PowerCell MS100 (50–100 kW PEM Fuel Cell System Prototype); PowerCell: Gothenburg, Sweden, 2018. [Google Scholar]
- Siemens. SINAVY PEM Fuel Cells––For Submarines (VRMS-B10018-00-7600); Siemens: Munich, Germany, 2016. [Google Scholar]
- Adam, A.; Fraga, E.S.; Brett, D.J.L. Options for Residential Building Services Design Using Fuel Cell Based Micro-CHP and the Potential for Heat Integration. Appl. Energy 2015, 138, 685–694. [Google Scholar] [CrossRef]
- Grzesiak, S. Alternative Propulsion Plants for Modern LNG Carriers. New Trends Prod. Eng. 2018, 1, 399–407. [Google Scholar] [CrossRef][Green Version]
- Wärtsilä. Wärtsilä Generating Sets. Available online: https://www.wartsila.com/marine/build/engines-and-generating-sets/generating-sets/wartsila-gensets (accessed on 30 September 2019).
- Energy, B. Energy Server 5—Technical Highlights (ES5-YA8AAN); Bloom Energy: San Jose, CA, USA, 2019. [Google Scholar]
- Fraunhofer Institute. High Power SiC DC/DC Converters. Available online: https://www.iisb.fraunhofer.de/en/press_media/brochures_flyers.html (accessed on 11 October 2019).
- Dynapower. DPS-500—500 kW DC-to-DC Converter. Available online: https://www.dynapower.com/products/energy-storage-solutions/dc-coupled-utility-scale-solar-plus-storage/dps-500/ (accessed on 26 September 2019).
- MAN. Diesel-Electric Propulsion Plants—A Brief Guideline How to Engineer a Diesel-Electric Propulsion System; MAN: Munich, Germany, 2012. [Google Scholar]
- GE Power Conversion. MV6 Medium Voltage Drive—Leading next Generation Technology; GE Power Conversion: Paris, France, 2017. [Google Scholar]
- Yaskawa Electric Corp. YASKAWA Marine Drive System; Yaskawa Electric Corp.: Fukuoka, Japan, 2019. [Google Scholar]
- Solar Power World. What are the Most Important Features to Look for in a String Inverter? Available online: https://www.cconline.com/2019/08/most-important-features-of-string-inverters/ (accessed on 11 October 2019).
- Soares, C.G.; Santos, T.A. Proceedings of MARTECH 2014, 2nd International Conference on Maritime Technology and Engineering. In Maritime Technology and Engineering; CRC Press/Balkema: Leiden, The Netherlands, 2015. [Google Scholar]
- DNV GL. DNV GL Updates NOx Tier III Compliance Guide—Choices and Challenges for on-Time Compliance; DNV GL: Oslo, Norway, 2017. [Google Scholar]
- Man Energy Solutions. Marine Engine Programme, 2nd ed.; Man Energy Solutions: Augsburg, Germany, 2019. [Google Scholar]
- Hydrogenics. Specification Sheet. In Hypm-HD 180 Power Module; Hydrogenics: Ontario, ON, Canada, 2016. [Google Scholar]
- Ballard Power Systems. Technical Brochure. In FCvelocity HD; Ballard Power Systems: Burnaby, BC, Canada, 2016. [Google Scholar]
- Mitsubishi Heavy Industries, Ltd. Cogeneration. In Proceedings of the Singapore Energy Efficiency Symposium, Singapore, 15–16 October 2019. [Google Scholar]
- Kobayashi, Y.; Tomida, K.; Nishiura, M.; Hiwatashi, K.; Kishizawa, H.; Takenobu, K. Development of Next-Generation Large-Scale SOFC toward Realization of a Hydrogen Society. Mitsubishi Heavy Ind. Tech. Rev. 2015, 52, 111–116. [Google Scholar]
- Minnehan, J.J.; Pratt, J.W. Practical Application Limits of Fuel Cells and Batteries for Zero Emission Vessels; SAND-2017-12665; Sandia National Laboratories: Livermore, CA, USA, 2017.
- Koyo Thermo Systems Co., Ltd. Ammonia Cracker for the Generation of Forming Gas. Available online: https://www.crystec.com/kllhyame.htm (accessed on 6 October 2019).
- ABB. Product Brochure. ABB Central Inverters—PVS800, 100 to 1000 kW; ABB: Zurich, Switzerland, 2014. [Google Scholar]
- ABB. Product Brochure. Solar Inverters—ABB Medium Voltage Compact Skid (PVS-175-MVCS); ABB: Zurich, Switzerland, 2019. [Google Scholar]
- ABB. Product Brochure. Medium Voltage AC Drive ACS 5000, 1.5 MW–36 MW, 6.0–6.9 kV; ABB: Zurich, Switzerland, 2013. [Google Scholar]
- Brochure, H.E.T. Induction Motors Medium & High Voltage; Hyundai Electric: Seongnam-si, Korea, 2019. [Google Scholar]
- Shu, S. A Life Cycle Cost Analysis of Marine Scrubber Technologies. Master’s Thesis, University of Rostock, Rostock, Germany, 2013. [Google Scholar]
- Uniteam. Specification Sheet. In 20ft ISO Container Specification; Uniteam: Torslanda, Sweden, 2014. [Google Scholar]
- Box. Shipping Containers–20ft Storage Containers for Sale and Hire. Available online: https://www.mrbox.co.uk/shipping-containers/20ft-storage-containers-for-sale-and-hire/ (accessed on 1 October 2019).
- Frontier economics. Reducing the UK Maritime Sector’s Contribution to Climate Change and Air Pollution; Frontier Economics: London, UK, 2019. [Google Scholar]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Chapter 8–Anthropogenic and Natural Radiative Forcing. In 5th Assessment Report (AR5); Intergovernmental Panel for Climate Change (IPCC): Geneva, Switzerland, 2014. [Google Scholar]
- Judd, S. Watermaths: Process Fundamentals for the Design and Operation of Water and Wastewater Treatment Technologies; IWA Publishing: London, UK, 2019; p. 85. [Google Scholar]
- Saito, N. The Economic Analysis of Commercial Ships with Hydrogen Fuel Cell through Case Studies; World Maritime University: Malmȍ, Sweden, 2018. [Google Scholar]
- International Renewable Energy Agency (IRENA). Hydrogen from Renewable Power: Technology Outlook for the Energy Transition; IRENA: Abu Dhabi, UAE, 2018. [Google Scholar]
- Thomas, D. Cost Reduction Potential for Electrolyser Technology; Hydrogenics: Mississauga, ON, Canada, 2018. [Google Scholar]
- Can, S.S.; Difiglio, C.; Esmaeili, D.; Gülgün, M.A.; Öncel, Ç.; Yesilyurt, S. IICEC Energy and Climate Research Paper. In Using Natural Gas as an Environmentally Sustainable Power Source with Solid Oxide Fuel Cells; Sabanci University Istanbul International Center for Energy and Climate (IICEC): Istanbul, Turkey, 2018. [Google Scholar]
- DNV GL KEMA. Battery Energy Storage Study for the 2017 IRP (PacifiCorp); DNV GL KEMA: Arnhem, The Netherlands, 2016. [Google Scholar]
- Schroeder, R.; Francesco Gattiglio. E-Mobility Battery R&D Roadmap 2030—Battery Technology for Vehicle Applications; EUROBAT (Association of European Automotive and Industrial Battery Manufacturers): Brussels, Belgium, 2015. [Google Scholar]
- Energy Storage News. BloombergNEF: Bullish on Cost Reductions, in Line with Others on Global Battery Market Forecast. Available online: https://www.energy-storage.news/news/bloombergnef-bullish-on-cost-reductions-in-line-with-others-on-global-batte (accessed on 18 September 2019).
- Ship & Bunker. World Bunker Prices—Global 20 Ports Average. Available online: https://shipandbunker.com/prices/apac/sea/sg-sin-singapore (accessed on 20 December 2019).
- California Air Resources Board (CARB). Technology Assessment: Ocean-Going Vessels; CARB: Sacramento, CA, USA, 2018.
- TNO (Netherlands Organization for Applied Scientific Research). PROMINENT project. In D2.8/D2.9 Standardized Model and Cost/Benefit Assessment for Right-Size Engines and Hybrid Configurations; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Small Business Innovation Research (SBIR). 2kW Solid Oxide Fuel Cell (SOFC) Power System. Available online: https://www.sbir.gov/sbirsearch/detail/1605929 (accessed on 1 December 2019).
- Heinzmann, J. GE (General Electric) Energy Storage. In Proceedings of the Solar Power International, Las Vegas, NV, USA, 13–15 September 2016. [Google Scholar]
- Aquino, T.; Roling, M.; Baker, C.; Rowland, L. Battery Energy Storage Technology Assessment; Platte River Power Authority: Fort Collins, CO, USA, 2017. [Google Scholar]
- Viswanathan, V.; Kintner-Meyer, M.; Balducci, P.; Jin, C. National Assessment of Energy Storage for Grid Balancing and Arbitrage Phase II—Volume 2: Cost and Performance Characterization; Pacific Northwest National Laboratory (PNNL): Richland, WA, USA, 2013.
- Interreg Europe. Danube Transnational Program—Grendel. FACT SHEET N°2—Diesel-Electric Propulsion; Interreg Europe: Lille, France, 2019. [Google Scholar]
- CERAM & H+H Umwelt and Industrietechnik GmbH. SCR for Marine Applications; CERAM: Frauental, Austria; H+H Umwelt and Industrietechnik GmbH: Hargesheim, Germany, 2007. [Google Scholar]
- Vms. Ge Diesel Engines. Available online: https://vms.dk/products/ge-diesel-engines/ (accessed on 1 December 2019).
- MPC Container Ships. Scrubber Initiative and Waiver Request Investor Presentation; MPC Container Ships: Oslo, Norway, 2019. [Google Scholar]
- Den Boer, E.; Hoen, M. Scrubbers—An Economic and Ecological Assessment; CE Delft: Delft, The Netherlands, 2015. [Google Scholar]
- Karimpour, R. Scrubber; Is it the Option to Meet the Shipping Fuel Sulphur Cap on 2020? Available online: https://www.onthemosway.eu/18823-2/?cn-reloaded=1 (accessed on 19 November 2019).
- The Maritime Executive. With Ammonia, There’s No “Chicken or Egg” Dilemma. Available online: https://www.maritime-executive.com/article/with-ammonia-there-s-no-chicken-or-egg-dilemma (accessed on 15 February 2020).
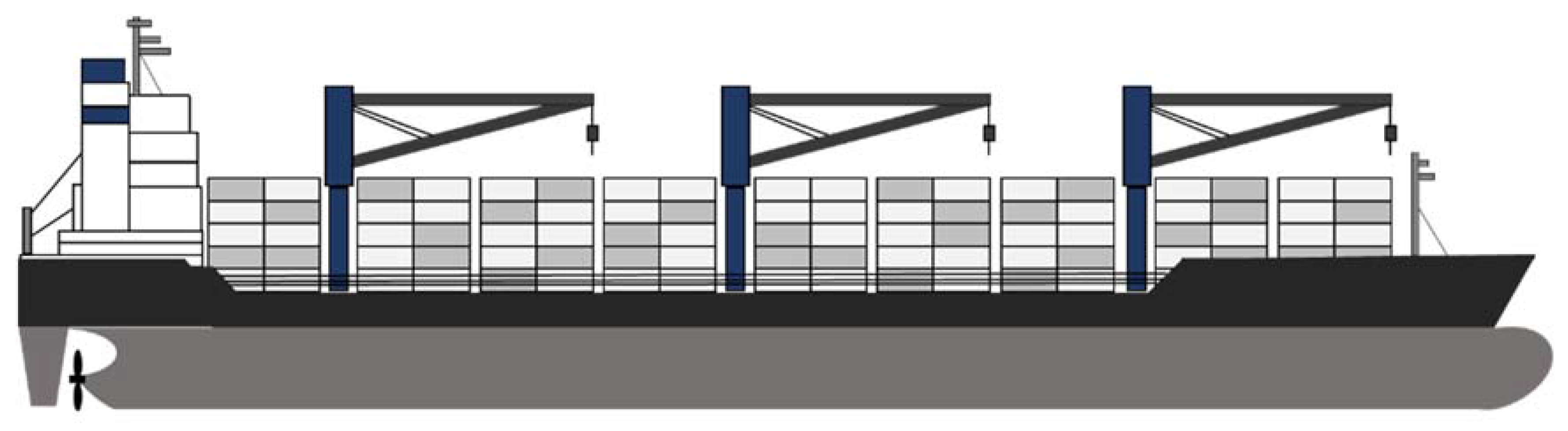
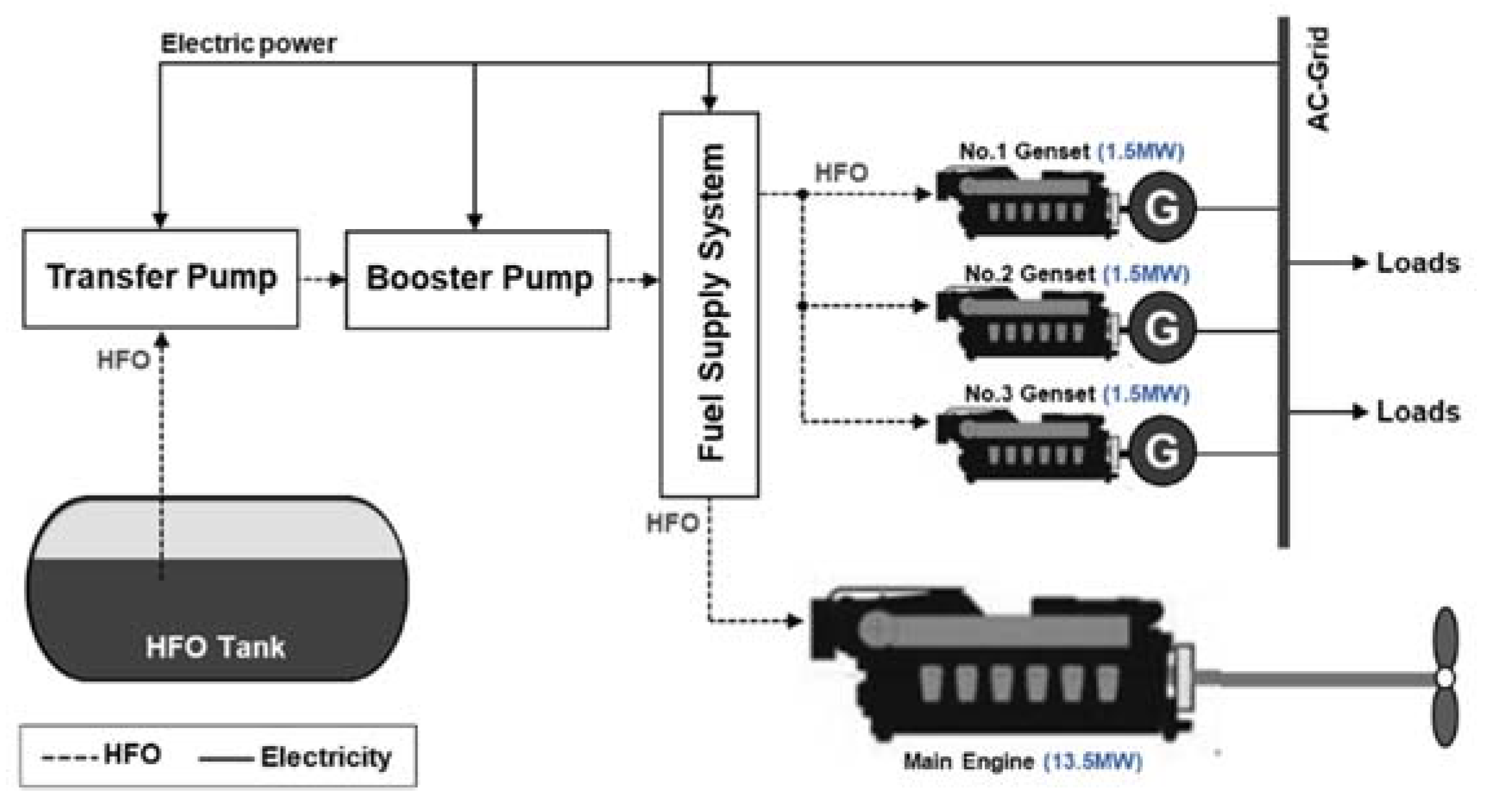
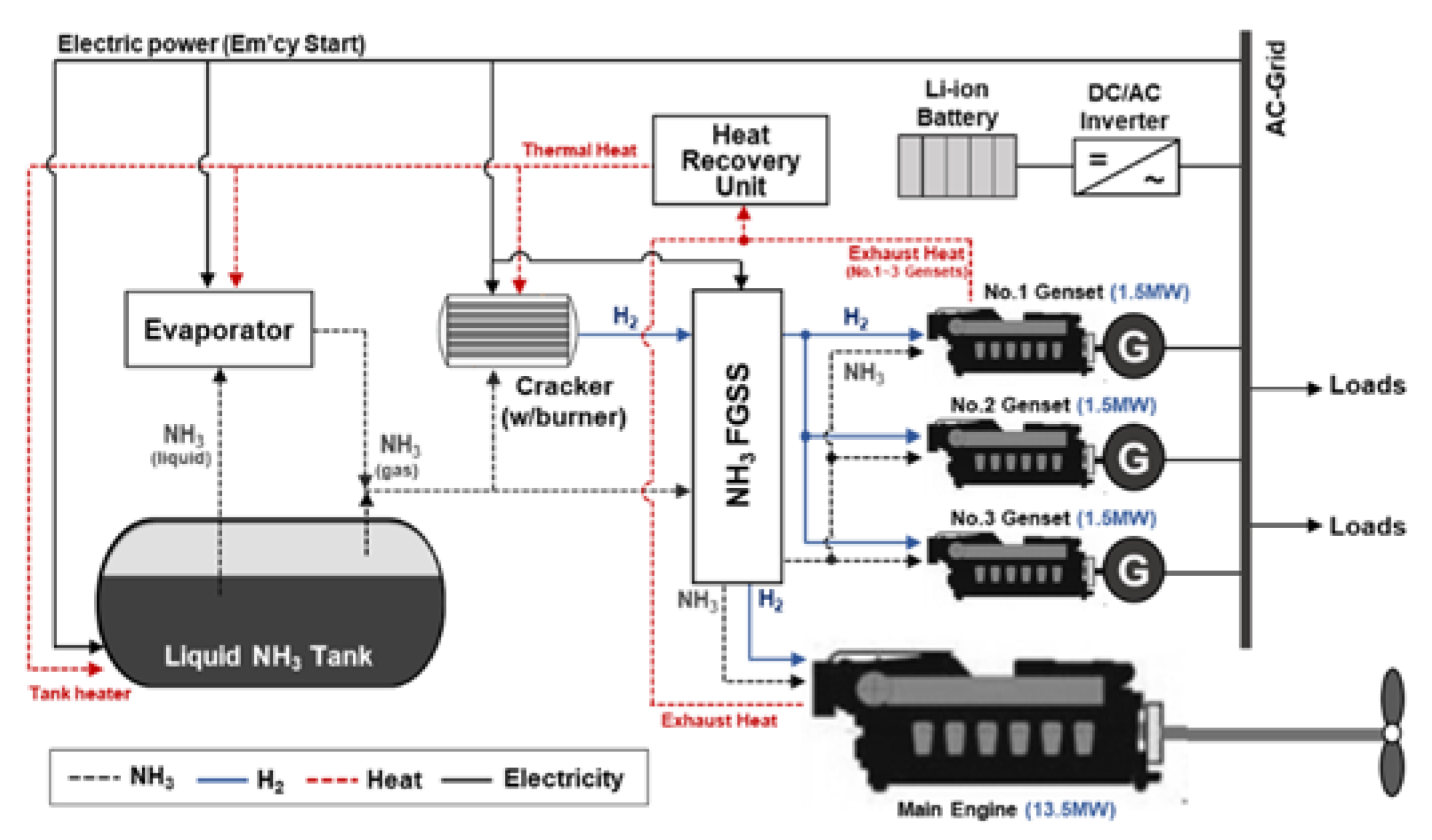
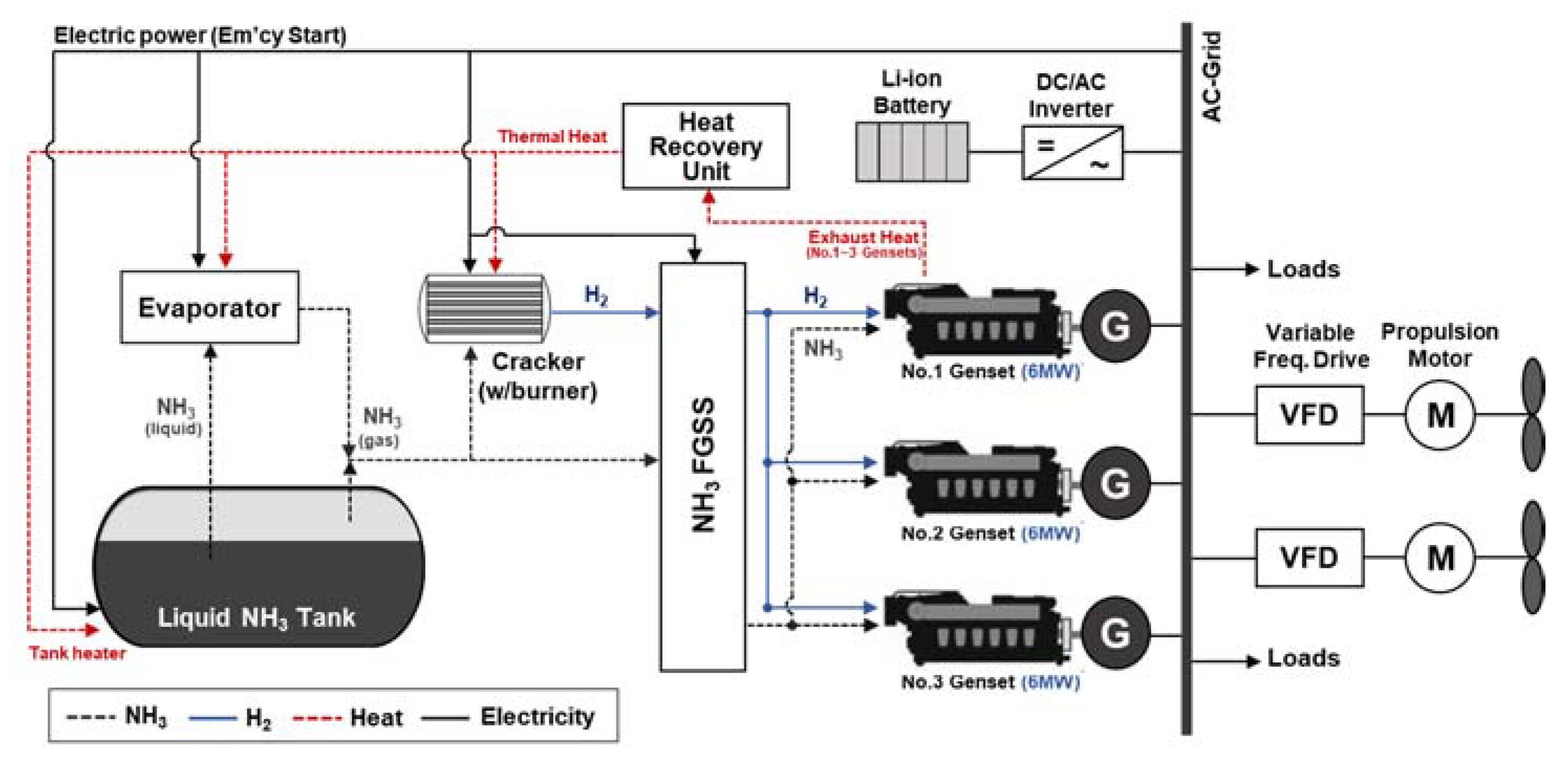
| Fuel Property | Unit | HFO | Compressed Hydrogen (350 bar) | Liquid Hydrogen | Liquid Ammonia | Reference |
|---|---|---|---|---|---|---|
| Low heating value | MJ/kg (kWh/kg) | 40.2 (11.17) | 120.00 (33.33) | 120.00 (33.33) | 18.6 (5.17) | [7,8,9,10] |
| Volumetric energy density | MJ/m3 (kWh/m3) | 39,564–42,036 (10,990–11,677) | 5040 (1400) | 8500 (2361) | 14,100 (3917) | [5,9,11] |
| Min. auto-ignition temperature | °C | 250 | 500–577 | 500–577 | 650–657 | [7,8,12] |
| Boiling temperature at 1atm | °C | N/A | N/A | −253 | −33.4 | [8,9] |
| Condensation pressure at 25 °C | atm | N/A | N/A | N/A | 9.90 | [8] |
| Hydrogen content | % by mass | N/A | 100.0 | 100.0 | 17.8 | [7,8] |
| Category | Specification |
|---|---|
| Length overall (LOA) | 195 m |
| Breadth | 32 m |
| Deadweight | 30,000 t |
| Container capacity | 2500 TEU (reefer: 500 TEU) |
| Ship speed | Max. 19.0 knots |
| M/E power output (MCR) | 1 × 13,500 kW |
| Diesel gensets (MCR) | 3 × 1500 kW |
| Fuel | HFO |
| Tank capacity | 1800 m3 |
| Main voltage | AC 440V |
| Propulsion Load Factor (%) | Propulsion Power (kW) | Electric Service Load (kW) | Reefer Container Load (kW) | Time Spent per Year (Hour) |
|---|---|---|---|---|
| 100 | 13,500 | 780 | 875 | 336 |
| 85 | 11,475 | 780 | 875 | 2016 |
| 65 | 8775 | 780 | 875 | 3024 |
| 50 | 6750 | 780 | 875 | 336 |
| 35 | 4725 | 780 | 875 | 336 |
| 25 | 3375 | 780 | 875 | 336 |
| 10 | 1350 | 780 | 875 | 236 |
| 5 | 675 | 1800 | 875 | 100 |
| 0 | - | 550 | 875 | 2040 |
| Fuel | Energy Density | Weight Ratio | Energy Ratio |
|---|---|---|---|
| NH3 | 18.6 MJ/kg (5.17 kWh/kg) | 95% | 74.7% |
| H2 | 120.0 MJ/kg (33.33 kWh/kg) | 5% | 25.3% |
| Case 1 & 2 | Case 3 | Case 4 | Case 5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Equipment | η (%) | Ref. | Equipment | η (%) | Ref. | Equipment | η (%) | Ref. | Equipment | η (%) | Ref. |
| M/E | 0.52 | [52,58] | Genset | 0.50 | [59] | Cracker | 0.80 | [49,50] | SOFC | 0.65 | [60] |
| Shafting | 0.99 | [58] | Alternator | 0.98 | [58] | H2 purifier | 0.90 | [50] | Converter | 0.98 | [61,62] |
| Genset | 0.50 | [59] | Switchboard | 0.98 | [63] | PEMFC 2 | 0.65 | [55,56] | Switchboard | 0.98 | [63] |
| Alternator | 0.98 | [58] | VFD 1 | 0.97 | [64,65] | Converter | 0.98 | [61,62] | Inverter | 0.98 | [66] |
| Switchboard | 0.98 | [63] | Prop. motor | 0.98 | [58] | Switchboard | 0.98 | [63] | VFD 1 | 0.97 | [64,65] |
| - | - | - | - | - | - | Inverter | 0.98 | [66] | Prop. motor | 0.98 | [58] |
| - | - | - | - | - | - | VFD 1 | 0.97 | [64,65] | - | - | - |
| - | - | - | - | - | - | Prop. motor | 0.98 | [58] | - | - | - |
| ηprop3 | 0.51 | - | ηprop3 | 0.46 | - | ηprop3 | 0.43 | - | ηprop3 | 0.59 | - |
| ηservice4 | 0.48 | - | ηservice4 | 0.48 | - | ηservice4 | 0.44 | - | ηservice4 | 0.61 | - |
| Load Factor (i) (%) | Main Engine/Genset | PEMFC | SOFC |
|---|---|---|---|
| 85 < i ≤ 100 | 1.02 | 1.25 | 1.14 |
| 65 < i ≤ 85 | 1.00 | 1.19 | 1.09 |
| 50 < i ≤ 65 | 1.01 | 1.12 | 1.06 |
| 35 < i ≤ 50 | 1.04 | 1.07 | 1.04 |
| 25 < i ≤ 35 | 1.09 | 1.03 | 1.00 |
| 10 < i ≤ 25 | 1.13 | 1.00 | 1.06 |
| i ≤ 10 | 1.21 | 1.67 | 1.43 |
| Item | Case 1 (HFO) | Case 2 (NH3) | Case 3 (NH3) | Case 4 (NH3) | Case 5 (NH3) |
|---|---|---|---|---|---|
| Total weight of fuel consumption (t/y) | 13,507.0 (1.00 base) | 25,505.7 (1.89 times) | 28,083.4 (2.08 times) | 33,889.0 (2.51 times) | 22,447.3 (1.66 times) |
| Total volume of fuel consumption (m3/y) | 14,289.0 (1.00 base) | 33,645.8 (2.35 times) | 37,046.1 (2.59 times) | 44,704.6 (3.13 times) | 29,611.4 (2.07 times) |
| Equipment | Capacity | Volume (m3) | Weight (t) | Reference |
|---|---|---|---|---|
| Main Engine | 13.5 MW | 370.7 | 394.0 | [69] |
| Genset | 1.5 MW | 35.9 | 20.8 | [59] |
| 6.0 MW | 145.3 | 115.0 | [59] | |
| PEMFC 1 | 6.0 MW | 50.9 2 | 19.5 | [70,71] |
| SOFC 1 | 6.0 MW | 874.9 2 | 271.5 | [60,72,73] |
| ESS 3 | 300 kWh | 4.2 | 4.0 | [74] |
| 550 kWh | 7.7 | 7.4 | [74] | |
| 1600 kWh | 33.3 2 | 21.4 | [74] | |
| Cracker 4 (Case 2,3) | - | 6.3 | 3.0 | [75] |
| Cracker 4 (Case 4) | - | 19.5 | 8.0 | [75] |
| DC/AC converter | 300 kW | 2.7 | 1.1 | [76] |
| DC/DC converter (ESS) | 600 kW | 4.0 | 1.8 | [76] |
| 1600 kW | 10.7 | 4.8 | [62] | |
| DC/DC converter (F/C) | 6.0 MW | 30.6 | 14.0 | [77] |
| VFD | 7.0 MW | 30.9 | 8.0 | [78] |
| Propulsion Motor | 6.7 MW | 28.0 | 17.8 | [79] |
| SCR 5 | 18 MW | 90.0 | 17.0 | [80] |
| Scrubber | 18 MW | 150.0 | 33.0 | [80] |
| Fuel | CO2 t/t Fuel | CH4 t/t Fuel | N2O t/t Fuel | |||
|---|---|---|---|---|---|---|
| Upstream | Operation | Upstream | Operation | Upstream | Operation | |
| HFO | 0.338 | 3.114 | 0.0032 | 0.0001 | - | 0.00016 |
| NH3 (SMR+CCS) | 0.231 | - | - | - | 0.00001 | - |
| NH3 (electrolysis) | 0.168 | - | - | - | 0.00001 | - |
| Item | Expected Lifetime | Increasing Rate of Lifetime for each Replacement | Decreasing Rate of Cost for each Replacement |
|---|---|---|---|
| PEMFC | 6 year [86] | 25% [87,88] | 42% [87,88] |
| SOFC | 5 year [89] | 25% [87,88] | 42% [87,88] |
| Li-ion battery | 10–12 year 1,2 [90] | 30% 2 [91] | 45% [92] |
| Equipment | CAPEX | OPEX | ||
|---|---|---|---|---|
| Equipment Cost | Reference | O&M Cost | Reference | |
| M/E (HFO) | $ 300/kW | [94] | $ 5.2/kW/y | [95] |
| Genset (HFO) | $ 350/kW | [95] | $ 5.2//kW/y | [95] |
| M/E (NH3) | $ 500/kW 1 | - | $ 5.2/kW/y 2 | - |
| Genset (NH3) | $ 550/kW 1 | - | $ 5.2/kW/y 2 | - |
| PEMFC | $ 1000/kW | [14] | 1% of CAPEX | [88] |
| SOFC | $ 5500/kW | [96] | 1% of CAPEX 2 | - |
| Li-ion Battery | $ 500/kW 2 | - | $ 0.5/kW/y | [97] |
| Cracker (Engine) 3 | $ 670,000 | [14] | 1% of CAPEX | [14] |
| Cracker (PEMFC) 3 | $ 2,690,000 | [14] | 1% of CAPEX | [14] |
| Converter | $ 200/kW | [98] | $ 2/kW/y | [99] |
| VFD | $ 200/kW | [98] | $ 2/kW/y | [99] |
| Propulsion Motor | $ 135/kW | [100] | 1% of CAPEX | [14] |
| SCR | $ 44/kW | [14,101] | 3% of CAPEX | [102] |
| Scrubber | $ 3,400,000 | [103,104] | 2% of CAPEX | [105] |
| NH3 Price | Cumulative Cost Comparison for Ship’s Life Time | ||||
|---|---|---|---|---|---|
| Case 1 (Base) | Case 2 (Times) | Case 3 (Times) | Case 4 (Times) | Case 5 (Times) | |
| $ 830/t | 1.0 | 3.5 | 3.9 | 5.0 | 5.2 |
| $ 730/t | 1.0 | 3.1 | 3.5 | 4.5 | 4.8 |
| $ 630/t | 1.0 | 2.7 | 3.0 | 3.9 | 4.5 |
| $ 530/t | 1.0 | 2.3 | 2.6 | 3.4 | 4.1 |
| $ 405/t | 1.0 | 1.8 | 2.0 | 2.7 | 3.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Roh, G.; Kim, W.; Chun, K. A Preliminary Study on an Alternative Ship Propulsion System Fueled by Ammonia: Environmental and Economic Assessments. J. Mar. Sci. Eng. 2020, 8, 183. https://doi.org/10.3390/jmse8030183
Kim K, Roh G, Kim W, Chun K. A Preliminary Study on an Alternative Ship Propulsion System Fueled by Ammonia: Environmental and Economic Assessments. Journal of Marine Science and Engineering. 2020; 8(3):183. https://doi.org/10.3390/jmse8030183
Chicago/Turabian StyleKim, Kyunghwa, Gilltae Roh, Wook Kim, and Kangwoo Chun. 2020. "A Preliminary Study on an Alternative Ship Propulsion System Fueled by Ammonia: Environmental and Economic Assessments" Journal of Marine Science and Engineering 8, no. 3: 183. https://doi.org/10.3390/jmse8030183
APA StyleKim, K., Roh, G., Kim, W., & Chun, K. (2020). A Preliminary Study on an Alternative Ship Propulsion System Fueled by Ammonia: Environmental and Economic Assessments. Journal of Marine Science and Engineering, 8(3), 183. https://doi.org/10.3390/jmse8030183





