A Simple Superposition Formulation to Predict the Underwater Electric Potential Signature of Naval Vessels
Abstract
1. Introduction
2. Numerical Setup
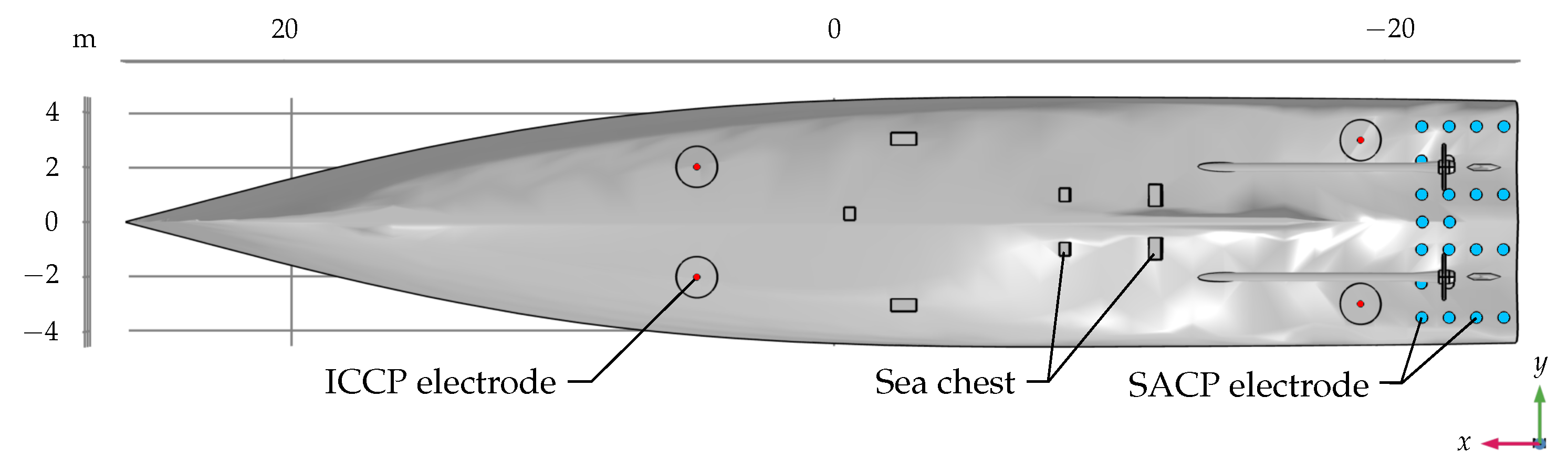
2.1. Ship Model
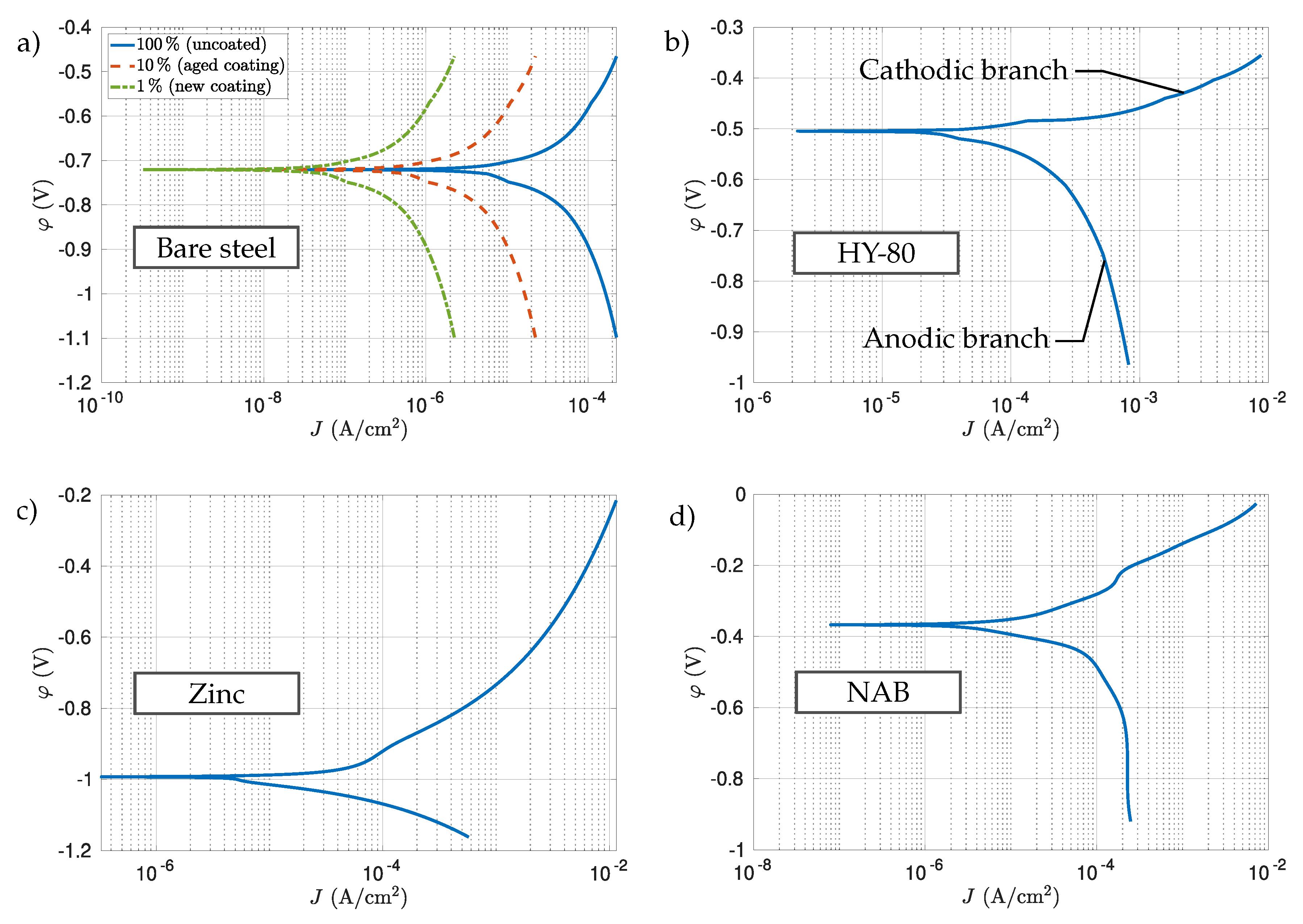
2.2. Governing Equations
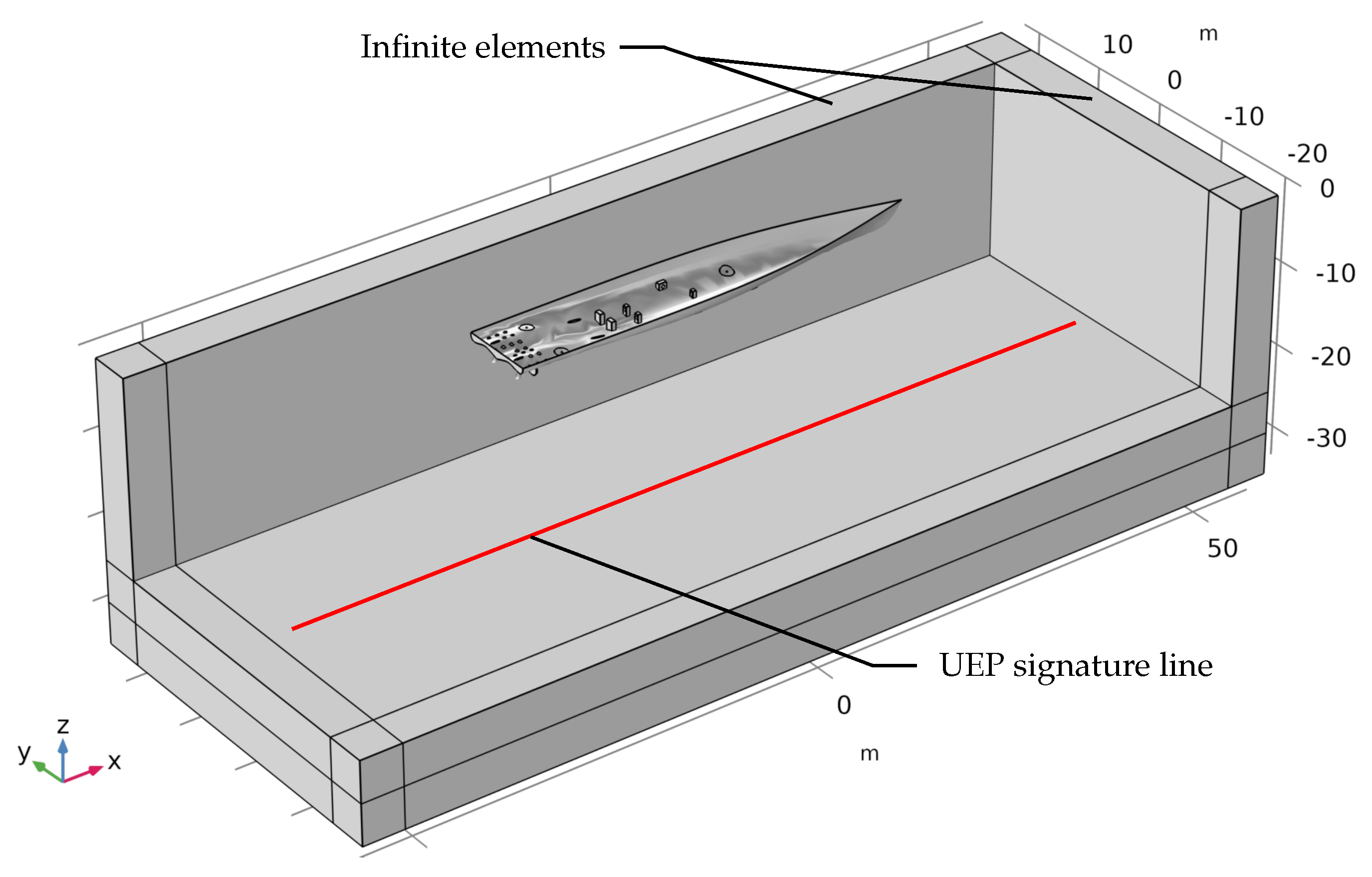
2.3. Computational Domain and Boundary Conditions
3. Results
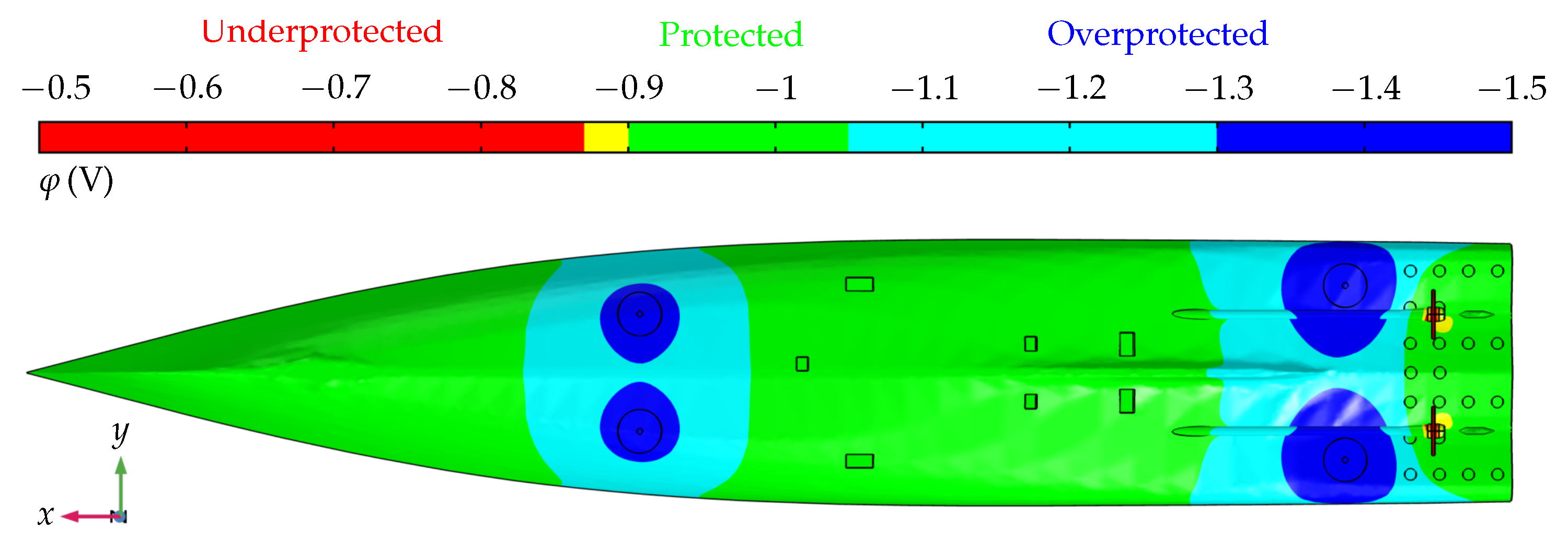
3.1. Reference Scenario
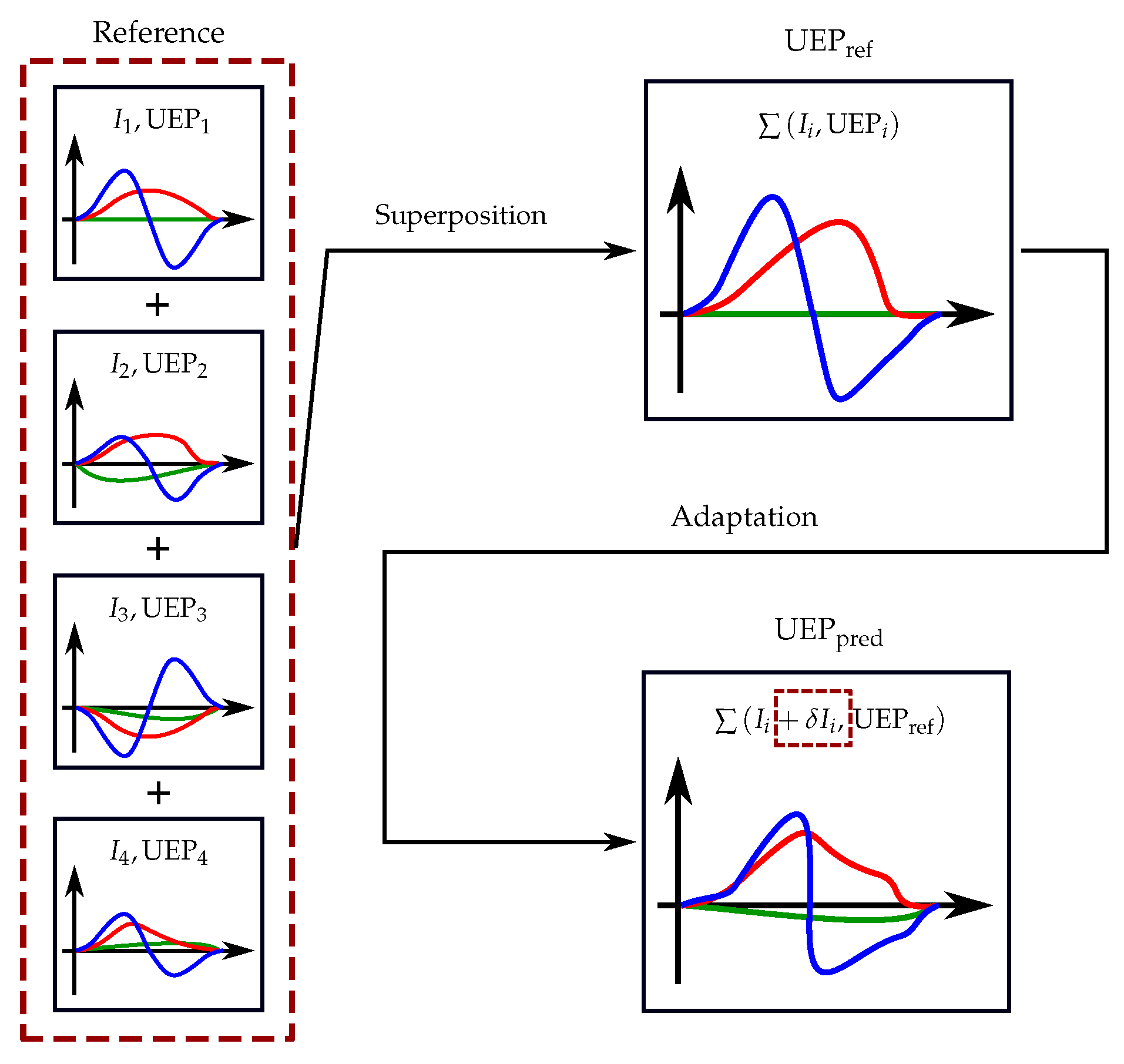
3.2. Superposition of UEP Signatures
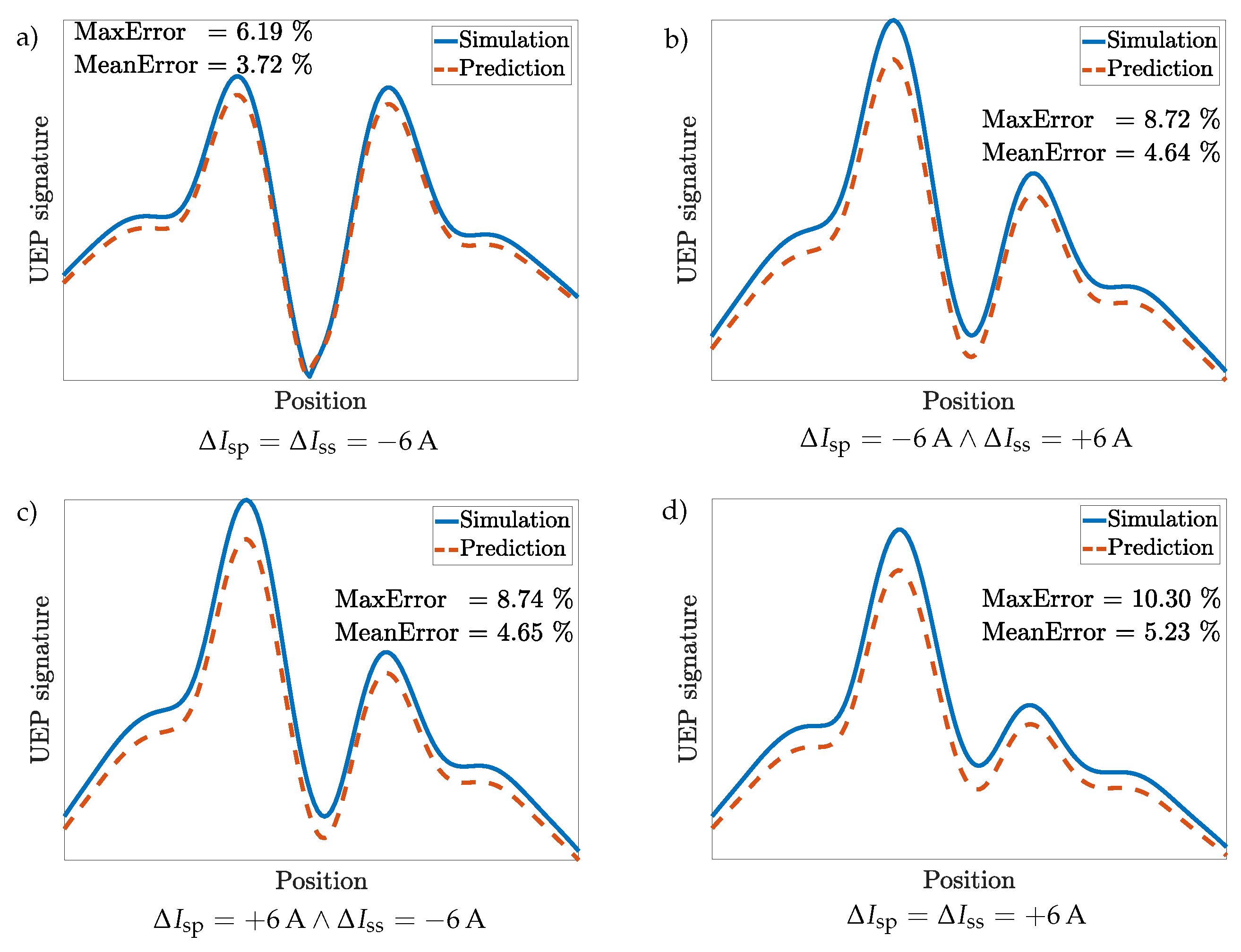
3.3. Scenario 1: HY-80 Steel Hull
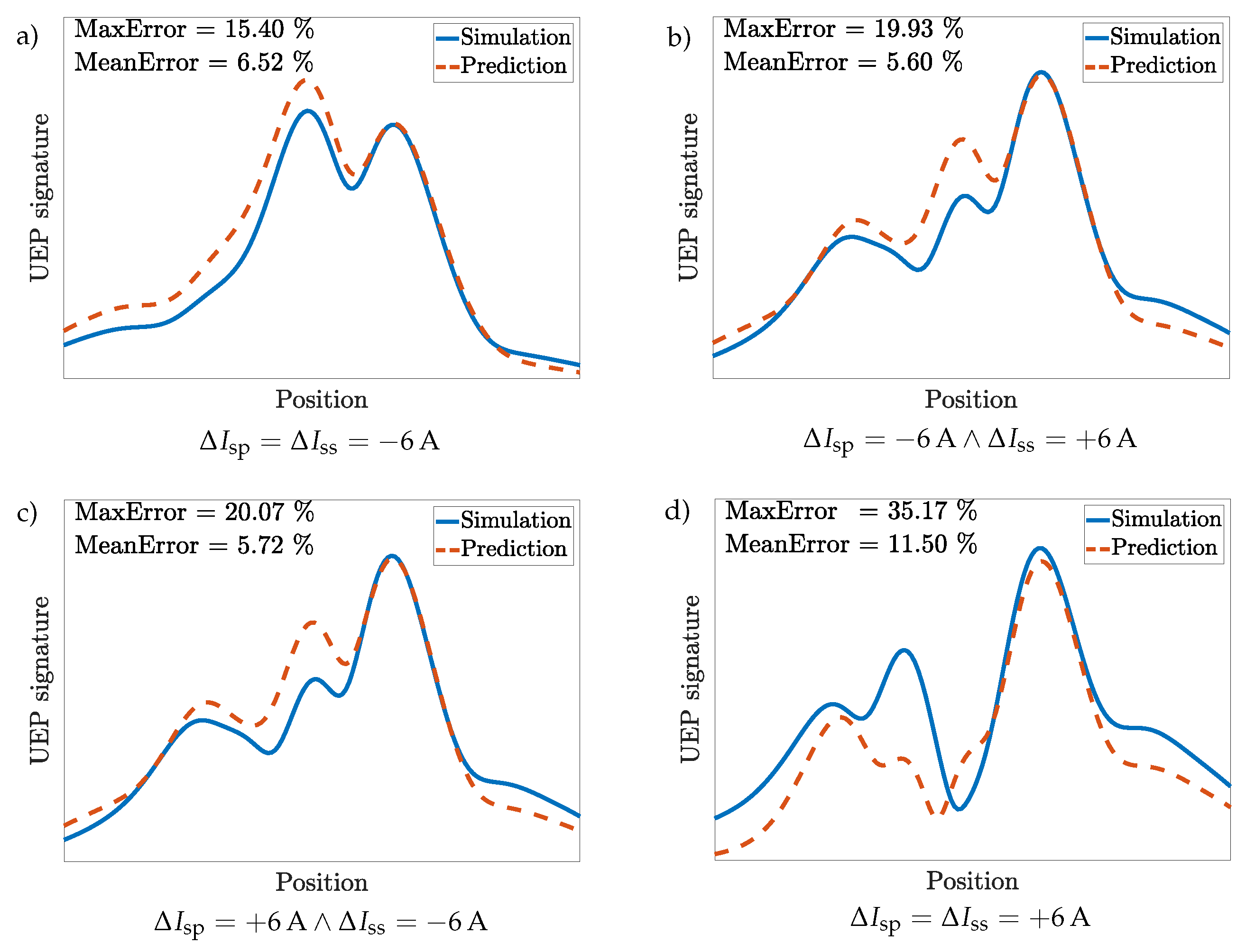
3.4. Scenario 2: Bare Steel Hull
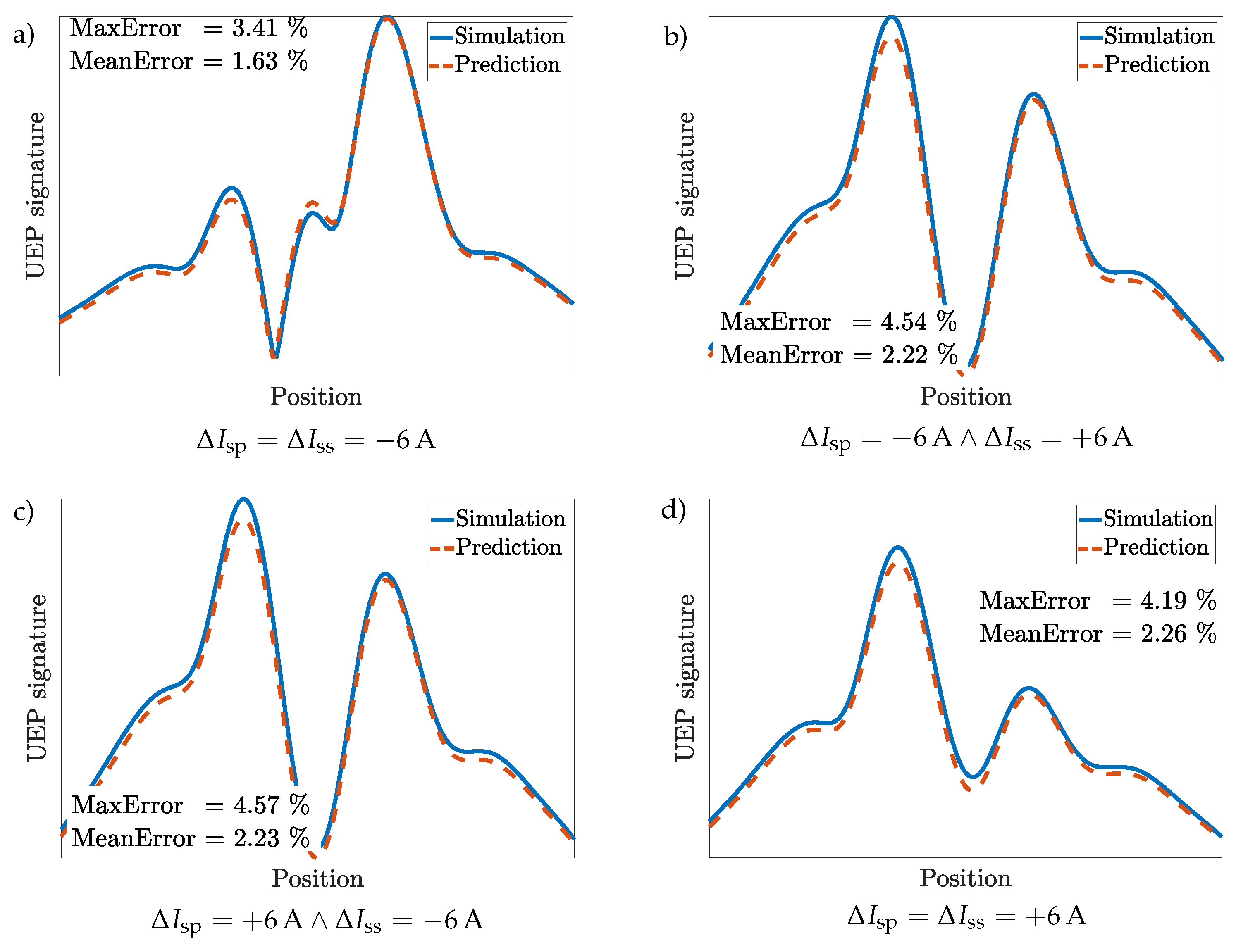
3.5. Scenario 3: Bare Steel with Coated Propellers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eberhart, R.; Kennedy, J. A new optimizer using particle swarm theory. In Proceedings of the 6th International Symposium on Micro Machine and Human Science, Nagoya, Japan, 4–6 October 1995; pp. 39–43. [Google Scholar]
- Peng, Y.; Cheng, J.; Jiang, R. Inversion of UEP signatures induced by ships based on PSO method. Def. Technol. 2019. [Google Scholar] [CrossRef]
- Schaefer, D.; Doose, J.; Pichlmaier, M.; Rennings, A.; Erni, D. Conversion of UEP signatures between different environmental conditions using shaft currents. IEEE J. Ocean. Eng. 2016, 41, 105–111. [Google Scholar]
- Schaefer, D. Vorhersage und Umrechnung korrosionsbedingter UEP-Signaturen von Wasserfahrzeugen. Ph.D. Thesis, University of Duisburg-Essen, Duisburg, Germany, 2015. [Google Scholar]
- Schaefer, D.; Thiel, C.; Doose, J.; Rennings, A.; Erni, D. Above water electric potential signatures of submerged naval vessels. J. Mar. Sci. Eng. 2019, 7, 53. [Google Scholar] [CrossRef]
- COMSOL Multiphysics. Finite Element Method Solver. Available online: https://www.comsol.de/ (accessed on 17 January 2020).
- Hack, H. Atlas of Polarization Diagrams for Naval Materials in Seawater; Technical report; Naval Surface Warfare Center: Bethesda, MD, USA, 1995. [Google Scholar]
- Carson, S.; Orazem, M. Time-dependent polarization behaviour of pipeline grade steel in low ionic strength environments. J. Appl. Electrochem. 1999, 29, 703–717. [Google Scholar] [CrossRef]
- Blackburn, J.M. Base Materials for Critical Applications: Requirements for lo Alloy Steel Plate, Forgings, Castings, Shapes, Bars, and Heads of HY-80/100/130 and HSLA-80/100; Techreport; NAVSEA: Washington, DC, USA, 2012. [Google Scholar]
- Datasheet: CuAl10Ni5Fe5-C, Deutsches Kupferinstitut; Technical report; Deutsches Kupferinstitut: Düsseldorf, Germany, 2005.
- Anodes, Corrosion Preventive, Zinc; Slab, Disc and Rod Shaped, Military Specification, MIL-A-18001J; Technical report; Military Specification, Naval Sea Systems Command, SEA 55Z3; Department of the Navy: Washington, DC, USA, 1983.
- Huber, T.; Wang, Y. Effect of propeller coating on cathodic protection current demand: Sea trial and modeling studies. CORROSION 2012, 68, 441–448. [Google Scholar] [CrossRef]
- Kathodischer Korrosionsschutz von Schiffen—Außenschutz durch Fremdstrom —VG 81 259 Teil 1–3; Technical report; Deutsches Institut für Normung (DIN): Berlin, Germany, 1994.
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiel, C.; Broecheler, C.; Ludwar, F.; Rennings, A.; Doose, J.; Erni, D. A Simple Superposition Formulation to Predict the Underwater Electric Potential Signature of Naval Vessels. J. Mar. Sci. Eng. 2020, 8, 105. https://doi.org/10.3390/jmse8020105
Thiel C, Broecheler C, Ludwar F, Rennings A, Doose J, Erni D. A Simple Superposition Formulation to Predict the Underwater Electric Potential Signature of Naval Vessels. Journal of Marine Science and Engineering. 2020; 8(2):105. https://doi.org/10.3390/jmse8020105
Chicago/Turabian StyleThiel, Christian, Claas Broecheler, Frank Ludwar, Andreas Rennings, Jens Doose, and Daniel Erni. 2020. "A Simple Superposition Formulation to Predict the Underwater Electric Potential Signature of Naval Vessels" Journal of Marine Science and Engineering 8, no. 2: 105. https://doi.org/10.3390/jmse8020105
APA StyleThiel, C., Broecheler, C., Ludwar, F., Rennings, A., Doose, J., & Erni, D. (2020). A Simple Superposition Formulation to Predict the Underwater Electric Potential Signature of Naval Vessels. Journal of Marine Science and Engineering, 8(2), 105. https://doi.org/10.3390/jmse8020105




