Small Jellyfish as a Supplementary Autumnal Food Source for Juvenile Chaetognaths in Sanya Bay, China
Abstract
1. Introduction
2. Materials and Methods
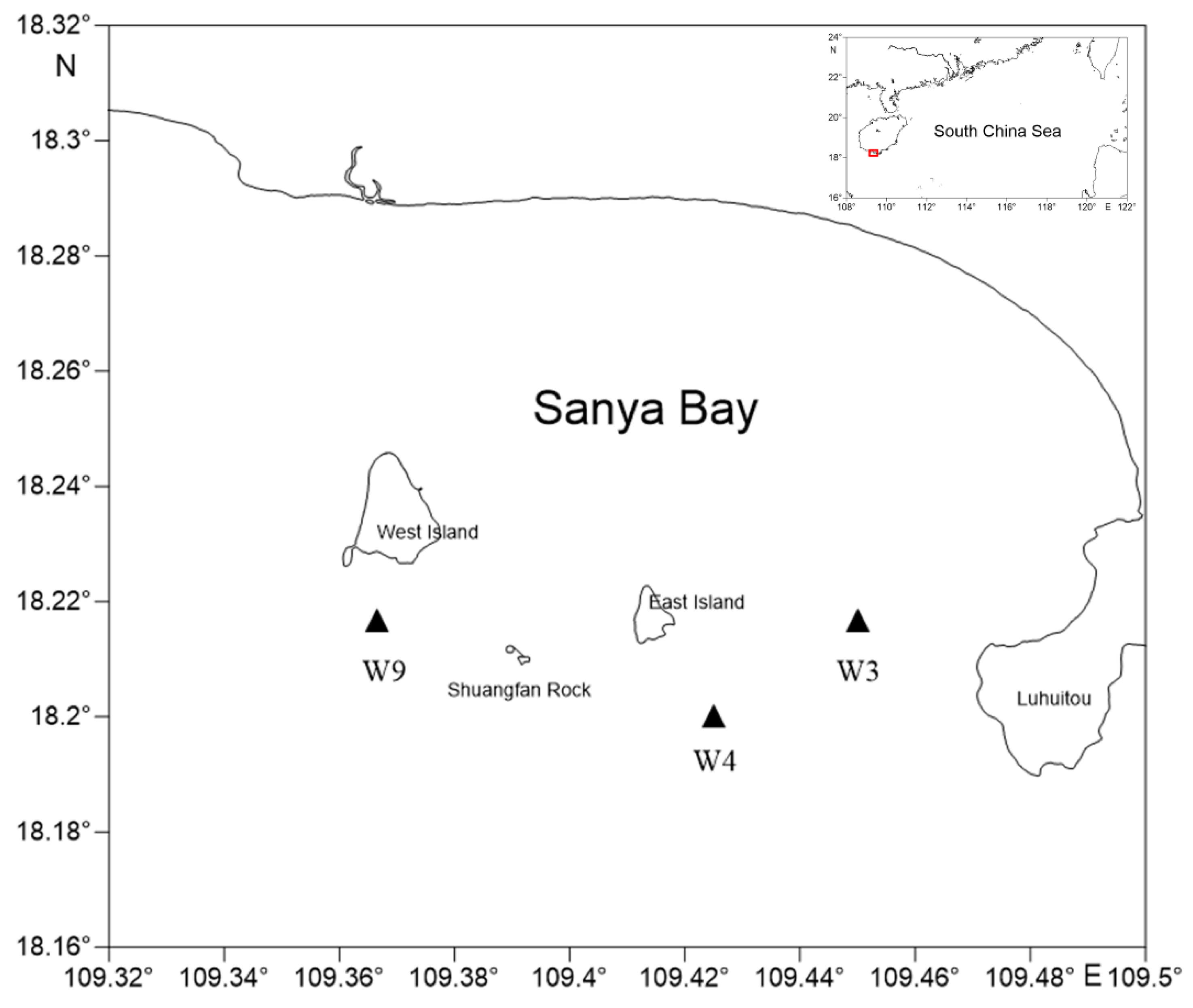
2.1. Sample Collection
2.2. Zooplankton Identification and Statistical Analysis
2.3. DNA Extraction of F. enflata Juveniles
2.4. Primers Design, Verification and PCR Protocol
2.5. Bioinformatic Analysis
3. Results
3.1. Environmental Parameters
3.2. Zooplankton Community
3.3. Diet Composition of F. enflata Juveniles in Different Seasons
4. Discussion
4.1. Prey Diversity
4.2. Small Jellyfish as Supplementary Food Sources for Juvenile Chaetognaths in Autumn
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
Appendix A
| July 2014 | October 2014 | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | RA | FO | Range | Mean ± SD | RA | FO | Range | |
| Total zooplankton | 148.64 ± 50.43 | 100.00% | 100.00% | 115.42−206.67 | 230.86 ± 147.9 | 100.00% | 100.00% | 96.88−389.57 |
| Cheatognatha | 9.24 ± 6.12 | 6.21% | 100.00% | 2.5−14.44 | 90.2 ± 56.17 | 39.07% | 100.00% | 33.75−146.09 |
| Flacciagitta enflata | 5.8 ± 4.83 | 3.90% | 100.00% | 1.67−11.11 | 83.95 ± 51.27 | 36.36% | 100.00% | 33.13−135.65 |
| Zonosagitta bedoti | 0.74 ± 1.28 | 0.50% | 33.33% | 0−2.22 | ||||
| Aidanosagitta delicala | 2.19 ± 2.11 | 1.47% | 100.00% | 0.83−4.62 | 6.25 ± 5.06 | 2.71% | 100.00% | 0.63−10.43 |
| Aidanosagitta johorensis | 0.51 ± 0.89 | 0.35% | 33.33% | 0−1.54 | ||||
| Mollusca | 1.65 ± 1.46 | 1.11% | 100.00% | 0.77−3.33 | 4.09 ± 5.79 | 1.77% | 100.00% | 0.63−10.77 |
| Creseis acicula | 1.75 ± 2.5 | 0.76% | 66.67% | 0−4.62 | ||||
| Creseis clava | 1.65 ± 1.46 | 1.11% | 100.00% | 0.77−3.33 | 0.8 ± 0.77 | 0.35% | 66.67% | 0−1.54 |
| Creseis virgula | 0.51 ± 0.89 | 0.22% | 33.33% | 0−1.54 | ||||
| Trochidae | 1.03 ± 1.78 | 0.45% | 33.33% | 0−3.08 | ||||
| Eumalacostraca | 1.42 ± 0.78 | 0.95% | 100.00% | 0.83−2.31 | 14.37 ± 18.44 | 6.22% | 100.00% | 3.08−35.65 |
| Lestrigonus macrophthalmus | 0.53 ± 0.46 | 0.36% | 66.67% | 0−0.83 | ||||
| Lucifer intermedius | 0.88 ± 0.79 | 0.59% | 66.67% | 0−1.54 | 13.33 ± 19.36 | 5.77% | 100.00% | 1.25−35.65 |
| Lucifer hanseni | 1.04 ± 1.8 | 0.45% | 33.33% | 0−3.13 | ||||
| Ostracoda | 0.37 ± 0.64 | 0.25% | 33.33% | 0−1.11 | ||||
| Euconchoecia aculeata | 0.37 ± 0.64 | 0.25% | 33.33% | 0−1.11 | ||||
| Copepoda | 77.27 ± 16.5 | 51.98% | 100.00% | 61.54−94.44 | 53.11 ± 36.21 | 23.01% | 100.00% | 18.13−90.43 |
| Canthocalanus pauper | 7 ± 2.18 | 4.71% | 100.00% | 4.62−8.89 | 7.12 ± 6.23 | 3.08% | 100.00% | 0.63−13.04 |
| Undinula valgaris | 1.48 ± 2.57 | 1.00% | 33.33% | 0−4.44 | ||||
| Subeucalanus subcrassus | 2.83 ± 1.48 | 1.90% | 100.00% | 1.54−4.44 | 13.67 ± 10.22 | 5.92% | 100.00% | 1.88−20 |
| Temora turbinata | 22.31 ± 4.95 | 15.01% | 100.00% | 16.92−26.67 | 1.45 ± 2.51 | 0.63% | 33.33% | 0−4.35 |
| Paracalanus parvus | 0.37 ± 0.64 | 0.25% | 33.33% | 0−1.11 | 0.58 ± 1 | 0.25% | 33.33% | 0−1.74 |
| Acrocalanus gibber | 1.03 ± 1.78 | 0.69% | 33.33% | 0−3.08 | 0.51 ± 0.89 | 0.22% | 33.33% | 0−1.54 |
| Acrocalanus gracilis | 0.77 ± 1.33 | 0.52% | 33.33% | 0−2.31 | ||||
| Centropages orsinii | 10.83 ± 6.98 | 7.28% | 100.00% | 6.67−18.89 | ||||
| Centropages forcatus | 0.26 ± 0.44 | 0.17% | 33.33% | 0−0.77 | 3.34 ± 3.49 | 1.45% | 66.67% | 0−6.96 |
| Candacia truncata | 2.92 ± 2.28 | 1.96% | 100.00% | 1.54−5.56 | ||||
| Labidocera euchaeta | 0.51 ± 0.89 | 0.35% | 33.33% | 0−1.54 | ||||
| Corycaeus speciosus | 0.26 ± 0.44 | 0.17% | 33.33% | 0−0.77 | ||||
| Corycaeus affinis | 0.53 ± 0.46 | 0.36% | 66.67% | 0−0.83 | ||||
| Tortanus gracilis | 9.49 ± 3.45 | 6.38% | 100.00% | 6.67−13.33 | 3.78 ± 2.83 | 1.64% | 100.00% | 0.63−6.09 |
| Oncaea venusta | 0.26 ± 0.44 | 0.17% | 33.33% | 0−0.77 | ||||
| Copilia mirabilis | 0.63 ± 0.57 | 0.42% | 66.67% | 0−1.11 | ||||
| Temora stylifera | 0.26 ± 0.44 | 0.17% | 33.33% | 0−0.77 | 0.29 ± 0.5 | 0.13% | 33.33% | 0−0.87 |
| Corycaeus andrewsi | 1.11 ± 1.92 | 0.75% | 33.33% | 0−3.33 | ||||
| candacia bradyi | 0.77 ± 1.33 | 0.52% | 33.33% | 0−2.31 | 0.8 ± 0.77 | 0.35% | 66.67% | 0−1.54 |
| calanus sinicas | 0.51 ± 0.89 | 0.35% | 33.33% | 0−1.54 | ||||
| Oncaea ornata | 0.28 ± 0.48 | 0.19% | 33.33% | 0−0.83 | ||||
| Acartia eryhraea | 12.88 ± 11.22 | 8.67% | 100.00% | 6.15−25.83 | 1.88 ± 3.25 | 0.81% | 33.33% | 0−5.63 |
| Temora discaudata | 0.51 ± 0.89 | 0.22% | 33.33% | 0−1.54 | ||||
| Labidocera sp. | 1.16 ± 2.01 | 0.50% | 33.33% | 0−3.48 | ||||
| Pontellopsis inflatodigitata | 0.21 ± 0.36 | 0.09% | 33.33% | 0−0.63 | ||||
| Subeucalanus pileatus | 0 ± 0 | 0.00% | 0.00% | 0−0 | ||||
| Subeucalanus crassus | 17.81 ± 13.98 | 7.71% | 100.00% | 8.75−33.91 | ||||
| Euphausiacea | 1.74 ± 3.01 | 0.75% | 33.33% | 0−5.22 | ||||
| Pseudeuphausia sinica | 1.74 ± 3.01 | 0.75% | 33.33% | 0−5.22 | ||||
| Cnidaria | 2.16 ± 1.03 | 1.45% | 100.00% | 0.83−3.33 | 7.95 ± 4.05 | 3.45% | 100.00% | 3.13−13.04 |
| Lensia subtiloides | 0.37 ± 0.64 | 0.25% | 33.33% | 0−1.11 | 3.81 ± 1.22 | 1.65% | 100.00% | 3.08−5.22 |
| Diphyes chamissonis | 0.65 ± 0.58 | 0.44% | 66.67% | 0−1.11 | 2.03 ± 3.51 | 0.88% | 33.33% | 0−6.09 |
| Aglaura hemistoma | 0.26 ± 0.44 | 0.17% | 33.33% | 0−0.77 | 0.29 ± 0.5 | 0.13% | 33.33% | 0−0.87 |
| Euphysora bigelowi | 0.37 ± 0.64 | 0.25% | 33.33% | 0−1.11 | 0.29 ± 0.5 | 0.13% | 33.33% | 0−0.87 |
| Halyractinia carnea | 0.26 ± 0.44 | 0.17% | 33.33% | 0−0.77 | ||||
| Nanomia bijuga | 0.26 ± 0.44 | 0.17% | 33.33% | 0−0.77 | 1.03 ± 1.78 | 0.45% | 33.33% | 0−3.08 |
| Vannucci aforbesii | 0.51 ± 0.89 | 0.22% | 33.33% | 0−1.54 | ||||
| Ctenophora | 0.74 ± 1.28 | 0.50% | 33.33% | 0−2.22 | 3.28 ± 2.85 | 1.42% | 66.67% | 0−5.22 |
| Pleurobrachia globosa | 0.74 ± 1.28 | 0.50% | 33.33% | 0−2.22 | ||||
| Ctenophores | 3.28 ± 2.85 | 1.42% | 66.67% | 0−5.22 | ||||
| Cladocera | 2.86 ± 0.87 | 1.92% | 100.00% | 2.22−3.85 | 0.87 ± 1.51 | 0.38% | 33.33% | 0−2.61 |
| Penilia avirostris | 2.58 ± 1.13 | 1.73% | 100.00% | 1.67−3.85 | 0.87 ± 1.51 | 0.38% | 33.33% | 0−2.61 |
| pseudevadne tergestina | 0.28 ± 0.48 | 0.19% | 33.33% | 0−0.83 | ||||
| Tunicata | 5.3 ± 6.63 | 3.57% | 100.00% | 0.77−12.92 | 1.67 ± 1.74 | 0.72% | 66.67% | 0−3.48 |
| Oikopleura rufescens | 2.73 ± 3.81 | 1.84% | 66.67% | 0−7.08 | ||||
| Oikopleura longicauda | 1.67 ± 2.89 | 1.12% | 33.33% | 0−5 | 1.67 ± 1.74 | 0.72% | 66.67% | 0−3.48 |
| Oikopleura dioica | 0.28 ± 0.48 | 0.19% | 33.33% | 0−0.83 | ||||
| Doliolum denticulatum | 0.26 ± 0.44 | 0.17% | 33.33% | 0−0.77 | ||||
| Doliolum gegenbauri | 0.37 ± 0.64 | 0.25% | 33.33% | 0−1.11 | ||||
| Planktonic larvae | 42.68 ± 29.32 | 28.71% | 100.00% | 16.67−74.44 | 53.58 ± 28.9 | 23.21% | 100.00% | 36.88−86.96 |
| Ophiopluteus larvae | 3.1 ± 4.66 | 2.08% | 66.67% | 0−8.46 | ||||
| Lucifer larvae | 17.59 ± 11.74 | 11.84% | 100.00% | 10−31.11 | 20.86 ± 15.13 | 9.04% | 100.00% | 7.69−37.39 |
| Polychaeta larvae | 1 ± 1.13 | 0.67% | 66.67% | 0−2.22 | 0.51 ± 0.89 | 0.22% | 33.33% | 0−1.54 |
| Macruran larvae | 13.5 ± 13.34 | 9.09% | 66.67% | 0−26.67 | 10.64 ± 9.95 | 4.61% | 100.00% | 2.5−21.74 |
| Brachyura larvae | 6.58 ± 5.85 | 4.43% | 100.00% | 3.08−13.33 | 15.21 ± 5.77 | 6.59% | 100.00% | 10.77−21.74 |
| Nauplius A | 0.53 ± 0.46 | 0.36% | 66.67% | 0−0.83 | ||||
| Nauplius B | 0.37 ± 0.64 | 0.25% | 33.33% | 0−1.11 | ||||
| Radiant larvae | 4.86 ± 3.87 | 2.11% | 100.00% | 1.88−9.23 | ||||
| Echinoplutes larvae | 0.21 ± 0.36 | 0.09% | 33.33% | 0−0.63 | ||||
| Fish larva | 1.2 ± 1.25 | 0.81% | 66.67% | 0−2.5 | 0.58 ± 1 | 0.25% | 33.33% | 0−1.74 |
| Fish egg | 3.76 ± 3.41 | 2.53% | 66.67% | 0−6.67 | 0.71 ± 0.64 | 0.31% | 66.67% | 0−1.25 |
Appendix B
| Clone ID | Best Hit Species | Best Hit ACC | E−Value | Similarity | Category | Percentage |
|---|---|---|---|---|---|---|
| W3−Sagen−Jul−j_diet | ||||||
| 1 | Cestum veneris | KJ754161.1 | 0 | 99% | Ctenophora | 5.56% |
| 2 | Pleurobrachia globosa | KJ859219.1 | 0 | 99% | Ctenophora | 2.78% |
| 3 | Temora turbinata | GU969211.1 | 0 | 98−99% | Copepoda | 55.56% |
| 4 | Temora turbinata | GU969211.1 | 0 | 95−96% | Copepoda | 11.11% |
| 5 | Temora turbinata | GU969211.1 | 0 | 90−92% | Copepoda | 5.56% |
| 6 | Canthocalanus pauper | GU969164.1 | 0 | 98−99% | Copepoda | 8.33% |
| 7 | Canthocalanus pauper | GU969164.1 | 0 | 91% | Copepoda | 2.78% |
| 8 | Canthocalanus pauper | GU969164.1 | 0 | 96% | Copepoda | 2.78% |
| 9 | Subeucalanus crassus | GU969168.1 | 0 | 96% | Copepoda | 2.78% |
| 10 | Ophiomusium cf. glabrum | KU519536.1 | 0 | 97% | Echinodermata | 2.78% |
| W4−Sagen−Jul−j_diet | ||||||
| 1 | Labidocera euchaeta | GU969153.1 | 0 | 96−98% | Copepoda | 6.45% |
| 2 | Labidocera euchaeta | GU969153.1 | 0 | 90% | Copepoda | 3.23% |
| 3 | Temora turbinata | GU969211.1 | 0 | 95−97% | Copepoda | 6.45% |
| 4 | Labidocera acuta | JQ280463.1 | 0 | 98% | Copepoda | 3.23% |
| 5 | Telepsavus spec. | AF448165.1 | 0 | 98% | Polychaeta | 3.23% |
| 6 | Spiochaetopterus bergensis | DQ209214.1 | 0 | 98% | Polychaeta | 3.23% |
| 7 | Chaetoceros debilis | AY229896.1 | 0 | 98−99% | Bacillariophyta | 79.97% |
| 8 | Chaetoceros debilis | AY229896.1 | 0 | 90% | Bacillariophyta | 3.23% |
| W9−Sagen−Jul−j_diet | ||||||
| 1 | Temora turbinata | GU969211.1 | 0 | 99%−100% | Copepoda | 46.81% |
| 2 | Temora turbinata | GU969211.1 | 0 | 95% | Copepoda | 2.13% |
| 3 | Candacia bispinosa | GU969213.1 | 0 | 99% | Copepoda | 6.38% |
| 4 | Canthocalanus pauper | GU969164.1 | 0 | 99% | Copepoda | 6.38% |
| 5 | Sulcanus conflictus | HM997064.1 | 0 | 99% | Copepoda | 2.13% |
| 6 | Tortanus gracilis | HM997065.1 | 0 | 97% | Copepoda | 2.13% |
| 7 | Cestum veneris | KJ754161.1 | 0 | 99% | Ctenophora | 12.77% |
| 8 | Pleurobrachia globosa | KJ859219.1 | 0 | 99% | Ctenophora | 4.26% |
| 9 | Ophiomusium cf. | KU519536.1 | 0 | 98% | Echinodermata | 12.77% |
| 10 | Chaetoceros sp. | FR865486.1 | 0 | 99% | Bacillariophyta | 4.26% |
| W3−Sagen−Oct−j_diet | ||||||
| 1 | Pleurobrachia globosa | KJ859219.1 | 0 | 98% | Ctenophora | 1.72% |
| 2 | Solmissus marshalli | AF358060.1 | 0 | 97−99% | Cnidaria | 53.45% |
| 3 | Sulculeolaria quadrivalvis | AY937329.1 | 0 | 97−99% | Cnidaria | 6.90% |
| 4 | Bougainvillia fulva | EU305490.1 | 0 | 98%% | Cnidaria | 1.72% |
| 5 | Subeucalanus crassus | GU969168.1 | 0 | 99% | Copepoda | 3.45% |
| 6 | Labidocera euchaeta | GU969153.1 | 0 | 92% | Copepoda | 1.72% |
| 7 | Paracalanus aculeatus | GU969180.1 | 0 | 99% | Copepoda | 1.72% |
| 8 | Candacia bispinosa | GU969213.1 | 0 | 99% | Copepoda | 1.72% |
| 9 | Tortanus gracilis | HM997065.1 | 0 | 99% | Copepoda | 1.72% |
| 10 | Phyllochaetopterus sp. | DQ209216.1 | 0 | 98−99% | Polychaeta | 12.07% |
| 11 | Gymnodinium mikimotoi | JF791035.1 | 0 | 97−98% | Dinophyceae | 5.17% |
| 12 | Karenia bidigitata | HM067002.1 | 0 | 90% | Dinophyceae | 1.72% |
| 13 | Karenia bidigitata | HM067002.1 | 0 | 95% | Dinophyceae | 1.72% |
| 14 | Karenia papilionacea | HM067005.1 | 0 | 95% | Dinophyceae | 1.72% |
| 15 | Amphidinium semilunatum | JQ179860.1 | 0 | 96% | Dinophyceae | 1.72% |
| 16 | Azadinium dexteroporum | KR362890.1 | 0 | 96% | Dinophyceae | 1.72% |
| W4−Sagen−Oct−a_diet | ||||||
| 1 | Pleurobrachia globosa | KJ859219.1 | 0 | 97−99% | Ctenophora | 23.53% |
| 2 | Pleurobrachia globosa | KJ859219.1 | 0 | 94% | Ctenophora | 1.96% |
| 3 | Diphyes dispar | AY937318.1 | 0 | 99−100% | Cnidaria | 15.69% |
| 4 | Solmissus marshalli | AF358060.1 | 0 | 98−99% | Cnidaria | 9.80% |
| 5 | Ectopleura obypa | KT722393.1 | 0 | 98% | Cnidaria | 1.96% |
| 6 | Euphysa aurata | EU876562.1 | 0 | 99% | Cnidaria | 1.96% |
| 7 | Lensia conoidea | AY937360.1 | 0 | 96% | Cnidaria | 1.96% |
| 8 | Muggiaea sp. | AF358073.1 | 0 | 96% | Cnidaria | 1.96% |
| 9 | Proboscidactyla flavicirrata | EU305500.1 | 0 | 99% | Cnidaria | 1.96% |
| 10 | Pachycerianthus sp. | AB859829.1 | 0 | 97% | Anthozoa | 1.96% |
| 11 | Subeucalanus crassus | GU969168.1 | 0 | 99% | Copepoda | 15.69% |
| 12 | Subeucalanus crassus | GU969168.1 | 0 | 92% | Copepoda | 1.96% |
| 13 | Paracalanus aculeatus | GU969180.1 | 0 | 97−99% | Copepoda | 7.84% |
| 14 | Candacia bispinosa | GU969213.1 | 0 | 97% | Copepoda | 1.96% |
| 15 | Labidocera euchaeta | GU969153.1 | 0 | 96% | Copepoda | 1.96% |
| 16 | Phyllochaetopterus sp. | DQ209216.1 | 0 | 99% | Polychaeta | 5.88% |
| 17 | Amphioplus cf. daleus | KU519529.1 | 0 | 98% | Echinodermata | 1.96% |
| W9−Sagen−Oct−a_diet | ||||||
| 1 | Bougainvillia fulva | EU305490.1 | 0 | 98−99% | Cnidaria | 97.83% |
| 2 | Amphioplus cf. daleus | KU519529.1 | 0 | 99% | Echinodermata | 2.17% |
Appendix C
| Category | Species | Summer | Autumn | ||
|---|---|---|---|---|---|
| Environment | Gut | Environment | Gut | ||
| Copepoda | Calanidae | √ | √ | √ | |
| Candaciidae | √ | √ | √ | √ | |
| Centropagidae | √ | √ | |||
| Paracalanidae | √ | √ | √ | ||
| Pontellidae | √ | √ | √ | √ | |
| Subeucalanidae | √ | √ | √ | √ | |
| Temoridae | √ | √ | √ | ||
| Tortanidae | √ | √ | √ | √ | |
| Sulcanidae | √ | ||||
| Anthozoa | Cerianthidae | √ | |||
| Cnidaria | Corymorphidae | √ | √ | √ | |
| Diphyidae | √ | √ | √ | ||
| Bougainvilliidae | √ | ||||
| Cuninidae | √ | ||||
| Proboscidactylidae | √ | ||||
| Tubulariidae | √ | ||||
| Ctenophora | Pleurobrachiidae | √ | √ | √ | |
| Cestidae | √ | ||||
| Echinodermata | Amphiuridae | √ | |||
| Ophiosphalmidae | √ | ||||
| Polychaeta | Chaetopteridae | √ | √ | ||
References
- Bone, Q.; Kapp, H.; Pierrot-Bults, A.C. The Biology of Chaetognaths; Oxford Universtity Press: New York, NY, USA, 1991. [Google Scholar]
- Kehayias, G.; Lykakis, J.; Fragopoulu, N. The diets of the chaetognaths Sagitta enflata, S. serratodentata atlantica and S. bipunctata at different seasons in Eastern Mediterranean coastal waters. ICES J. Mar. Sci. 1996, 53, 837–846. [Google Scholar] [CrossRef]
- Øresland, V. Diel feeding of the chaetognath Sagitta enflata in the Zanzibar Channel, western Indian Ocean. Mar. Ecol. Prog. Ser. 2000, 193, 117–123. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, Q.; Zhang, F.; Li, C.; Tao, Z.; Bi, H.; Fan, C.; Zhang, J.; Sun, S. Biomass and estimated production, and feeding pressure on zooplankton of chaetognaths in the Yellow Sea, China. Terr. Atmos. Ocean. Sci. 2020, 31, 61–75. [Google Scholar] [CrossRef]
- Kimmerer, W. Selective predation and its impact on prey of Sagitta enflata (Chaetognatha). Mar. Ecol. Prog. Ser. 1984, 15, 55–62. [Google Scholar] [CrossRef]
- Johnson, T.B.; Nakagami, M.; Ueno, Y.; Narimatsu, Y.; Suyama, S.; Kurita, Y.; Sugisaki, H.; Terazaki, M. Chaetognaths in the diet of Pacific saury (Cololabis saira) in the northwestern Pacific Ocean. Coast. Mar. Sci. 2008, 32, 39–47. [Google Scholar]
- Sun, S.; Huo, Y.Z.; Yang, B. Zooplankton functional groups on the continental shelf of the yellow sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 1006–1016. [Google Scholar] [CrossRef]
- Yamamura, O.; Honda, S.; Shida, O.; Hamatsu, T. Diets of walleye pollock Theragra chalcogramma in the Doto area, northern Japan: Ontogenetic and seasonal variations. Mar. Ecol. Prog. Ser. 2002, 238, 187–198. [Google Scholar] [CrossRef]
- Dilling, L.; Alldredge, A.L. Can chaetognath fecal pellets contribute significantly to carbon flux? Mar. Ecol. Prog. Ser. 1993, 92, 51–58. [Google Scholar] [CrossRef]
- Giesecke, R.; González, H.E.; Bathmann, U. The role of the chaetognath Sagitta gazellae in the vertical carbon flux of the Southern Ocean. Polar Biol. 2010, 33, 293–304. [Google Scholar] [CrossRef]
- Baier, C.T.; Purcell, J.E. Trophic interactions of chaetognaths, larval fish, and zooplankton in the South Atlantic Bight. Mar. Ecol. Prog. Ser. 1997, 146, 43–53. [Google Scholar] [CrossRef]
- Coston-Clements, L.; Waggett, R.J.; Tester, P.A. Chaetognaths of the United States South Atlantic Bight: Distribution, abundance and potential interactions with newly spawned larval fish. J. Exp. Mar. Biol. 2009, 373, 111–123. [Google Scholar] [CrossRef]
- Kehayias, G. Spatial and temporal abundance distribution of chaetognaths in eastern Mediterranean pelagic waters. Bull. Mar. Sci. 2004, 74, 253–270. [Google Scholar]
- Tönnesson, K.; Tiselius, P. Diet of the chaetognaths Sagitta setosa and S. elegans in relation to prey abundance and vertical distribution. Mar. Ecol. Prog. Ser. 2005, 289, 177–190. [Google Scholar] [CrossRef]
- Matsuda, S.; Taniguchi, A. Diel changes in vertical distribution and feeding conditions of the chaetognath Parasagitta elegans (Verill) in the Subarctic Pacific in Summer. J. Oceanogr. 2001, 57, 353–360. [Google Scholar] [CrossRef]
- Choe, N.; Deibel, D. Seasonal vertical distribution and population dynamics of the chaetognath Parasagitta elegans in the water column and hyperbenthic zone of Conception Bay, Newfoundland. Mar. Biol. 2000, 137, 847–856. [Google Scholar] [CrossRef]
- Giesecke, R.; González, H.E. Reproduction and feeding of Sagitta enflata in the Humboldt Current system off Chile. ICES J. Mar. Sci. 2008, 65, 361–370. [Google Scholar] [CrossRef]
- King, K.R. The life history and vertical distribution of the chaetognath, Sagitta elegans, in Dabob Bay, Washington. J. Plankton Res. 1979, 1, 153–167. [Google Scholar] [CrossRef]
- Sameoto, D. Annual life cycle and production of the chaetognath Sagitta elegans in Bedford Basin, Nova Scotia. J. Fish. Board Can. 1973, 30, 333–344. [Google Scholar] [CrossRef]
- Welch, H.E.; Siferd, T.D.; Bruecker, P. Population densities, growth, and respiration of the chaetognath Parasagitta elegans in the Canadian high Arctic. Can. J. Fish. Aquat. Sci. 1996, 53, 520–527. [Google Scholar] [CrossRef]
- Marazzo, A.; Machado, C.F.; Nogueira, C.S.R. Notes on feeding of chaetognatha in Guanabara Bay, Brazil. J. Plankton Res. 1997, 19, 819–828. [Google Scholar] [CrossRef]
- Nagasawa, S.; Marumo, R. Further studies on the feeding habits of Sagitta nagae alvariño in Suruga Bay, central Japan. J. Oceanogr. 1976, 32, 209–218. [Google Scholar] [CrossRef]
- Alvarez-Cadena, J.N. Feeding of the Chaetognath Sagitta elegans Verrill. Estuar. Coast. Shelf Sci. 1993, 36, 195–206. [Google Scholar] [CrossRef]
- Pearre, S. Feeding by chaetognatha: Energy balance and importance of various components of the diet of Sagitta elegans. Mar. Ecol. Prog. Ser. 1981, 5, 45–54. [Google Scholar] [CrossRef]
- Grigor, J.J.; Marais, A.E.; Falk-Petersen, S.; Varpe, Ø. Polar night ecology of a pelagic predator, the chaetognath Parasagitta elegans. Polar Biol. 2015, 38, 87–98. [Google Scholar] [CrossRef]
- Grigor, J.J.; Schmid, M.S.; Caouette, M.; Onge, V.S.; Brown, T.A.; Barthélémy, R.-M. Non-carnivorous feeding in Arctic chaetognaths. Prog. Oceanogr. 2020, 186. [Google Scholar] [CrossRef]
- Jeanpaul Casanova, R.B.; Michel, D.; Eric, F. Chaetognaths feed primarily on dissolved and fine particulate organic matter, not on prey: Implications for marine food webs. Hypotheses Life Sci. 2012, 2, 20–29. [Google Scholar]
- Vinogradov, M.E.; Tseitlin, V.B. Deep-sea pelagic domain (aspects of bioenergetics). Deep Sea Biol. 1983, 8, 123–166. [Google Scholar]
- Yoon, H.; Ko, A.R.; Kang, J.H.; Choi, J.K.; Ju, S.J. Diet of Chaetognaths Sagitta crassa and S. nagae in the Yellow Sea inferred from gut content and fatty acid analyses. Ocean Polar Res. 2016, 38, 35–46. [Google Scholar] [CrossRef]
- Kehayias, G. Quantitative aspects of feeding of chaetognaths in the eastern Mediterranean pelagic waters. J. Mar. Biol. Assoc. U.K. 2003, 83, 559–569. [Google Scholar] [CrossRef]
- Kehayias, G.; Ntakou, E. Abundance, vertical distribution and feeding of chaetognaths in the upper 50m layer of the eastern Aegean Sea. J. Nat. Hist. 2008, 42, 633–648. [Google Scholar] [CrossRef]
- Pearre, S. Ecological studies of three west-mediterranean chaetognaths. Investig. Pesq. 1974, 38, 325–369. [Google Scholar]
- Kehayias, G.; Michaloudi, E.; Koutrakis, E. Feeding and predation impact of chaetognaths in the north Aegean Sea (Strymonikos and Ierissos Gulfs). J. Mar. Biol. Assoc. U.K. 2005, 85, 1525–1532. [Google Scholar] [CrossRef]
- King, R.A.; Read, D.S.; Traugott, M.; Symondson, W.O.C. Molecular analysis of predation: A review of best practice for DNA-based approaches. Mol. Ecol. 2008, 17, 947–963. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Guo, Z.; Li, T.; Xu, C.; Huang, H.; Liu, S.; Lin, S. Molecular analysis of in situ diets of coral reef copepods: Evidence of terrestrial plant detritus as a food source in Sanya Bay, China. J. Plankton Res. 2015, 37, 363–371. [Google Scholar] [CrossRef]
- Lin, X.; Hu, S.; Sheng, L.; Hui, H. Unexpected prey of juvenile spotted scat (Scatophagus argus) near a wharf: The prevalence of fouling organisms in stomach contents. Ecol. Evol. 2018, 8, 8547–8554. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Lindeque, P.K.; Harris, R.P. Sagitta setosa predation on Calanus helgolandicus in the English Channel. J. Plankton Res. 2010, 32, 725–737. [Google Scholar] [CrossRef]
- Huang, L.M.; Tan, Y.H.; Song, X.Y.; Huang, X.P.; Wang, H.K.; Zhang, S.; Dong, J.; Chen, R. The status of the ecological environment and a proposed protection strategy in Sanya Bay, Hainan Island, China. Mar. Pollut. Bull. 2003, 47, 180. [Google Scholar] [CrossRef]
- Shi, X.; Wang, H.; Tan, Y.; Huang, L. Seasonal variation of zooplankton community structure and species composition in the Sanya Bay. Mar. Sci. Bull. 2007, 26, 42–49. [Google Scholar] [CrossRef]
- Yin, J.Q.; Zhang, G.X.; Tan, Y.H.; Huang, L.M.; Kai-Zhi, L.I. Species composition and quantitative distribution of zooplankton in Sanya Bay, Hainan Province, China. J. Trop. Oceanogr. 2004, 23, 1–9. [Google Scholar] [CrossRef]
- Li, K.Z.; Wu, X.J.; Tan, Y.H.; Hui, H.; Dong, J.D.; Huang, L.M. Spatial and temporal variability of copepod assemblages in Sanya Bay, northern South China Sea. Reg. Stud. Mar. Sci. 2016, 7, 168–176. [Google Scholar] [CrossRef]
- Guo, Z.L.; Liu, S.; Hu, S.M.; Li, T.; Huang, Y.S.; Liu, G.X.; Zhang, H.; Lin, S.J. Prevalent ciliate symbiosis on copepods: High genetic diversity and wide distribution detected using small subunit ribosomal RNA gene. PLoS ONE 2012, 7, e44847. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Guo, Z.; Li, T.; Carpenter, E.J.; Liu, S.; Lin, S. Detecting in situ copepod diet diversity using molecular technique: Development of a copepod/symbiotic ciliate-excluding eukaryote-inclusive PCR protocol. PLoS ONE 2014, 9, e103528. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.W.; Hong, H.X.; Zhang, S.M. Fauna Sinica; Science Press: Beijing, China, 2002; Volume 27. [Google Scholar]
- Kehayias, G.; Koutsikopoulos, C.; Fragopoulu, N.; Lykakis, J. A single maturity classification key for five common Mediterranean chaetognath species. J. Mar. Biol. Assoc. U.K. 1999, 79, 1137–1138. [Google Scholar] [CrossRef]
- Lie, A.A.Y.; Tse, P.; Wong, C.K. Diel vertical migration and feeding of three species of chaetognaths (Flaccisagitta enflata, Aidanosagitta delicata and Aidanosagitta neglecta) in two shallow, subtropical bays in Hong Kong. J. Plankton Res. 2012, 34, 670–684. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, S.J. Development of a cob-18S rRNA gene real-time PCR assay for quantifying Pfiesteria shumwayae in the natural environment. Appl. Environ. Microbiol. 2005, 71, 7053–7063. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Giesecke, R.; Gonzalez, H.E. Distribution and feeding of chaetognaths in the epipelagic zone of the Lazarev Sea (Antarctica) during austral summer. Polar Biol. 2012, 35, 689–703. [Google Scholar] [CrossRef]
- Øresland, V. Feeding of the chaetognaths Sagitta elegans and S. setosa at different seasons in Gullmarsfjorden, Sweden. Mar. Ecol. Prog. Ser. 1987, 39, 69–79. [Google Scholar] [CrossRef]
- Giesecke, R.; Gonzalez, H.E. Feeding of Sagitta enflata and vertical distribution of chaetognaths in relation to low oxygen concentrations. J. Plankton Res. 2004, 26, 475–486. [Google Scholar] [CrossRef]
- Sifford, P.J. A seasonal study of the diets of three sympatric chaetognaths. Investig. Pesq. 1976, 40, 1–16. [Google Scholar]
- Terazaki, M. Feeding of Carnivorous Zooplankton, Chaetognaths in the Pacific; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Pearre, S. Feeding by Chaetognatha: The relation of prey size to predator size in several species. Mar. Ecol. Prog. Ser. 1980, 3, 125–134. [Google Scholar] [CrossRef]
- Reeve, M.; Walter, M. Conditions of culture, food-size selection, and the effects of temperature and salinity on growth rate and generation time in Sagitta hispida Conant. J. Exp. Mar. Biol. Ecol. 1972, 9, 191–200. [Google Scholar] [CrossRef]
- Dong, D.; Li, X.Z.; Wang, H.F.; Zhang, B.L.; Kou, Q.; Gan, Z.B.; Xu, P. Macrobenthic community characters of coral reef at Sanya, Hainan. Mar. Sci. 2015, 39, 83–91. [Google Scholar] [CrossRef]
- Huang, L.M.; Zhang, S.; Wang, H.K.; Wen, W.; Zhang, Q. Ecological Environment and Bioresources for Sanya Bay, Hainan Island, China; Science Press: Beijing, China, 2007. [Google Scholar]
- Blanchard, G.F.; Paterson, D.M.; Stal, L.J.; Richard, P.; Galois, R.; Huet, V.; Kelly, J.; Honeywill, C.; de Brouwer, J.; Dyer, K.; et al. The effect of geomorphological structures on potential biostabilisation by microphytobenthos on intertidal mudflats. Cont. Shelf Res. 2000, 20, 1243–1256. [Google Scholar] [CrossRef]
- Hendler, G. Development of Amphioplus abditus (Verrill) (Echinodermata: Ophiuroidea): I. Larval Biology. Biol. Bull. 1977, 152, 51–63. [Google Scholar] [CrossRef]
- Nagasawa, S. The digestive efficiency of the chaetognath Sagitta crassa Tokioka, with observations on the feeding process. J. Exp. Mar. Biol. Ecol. 1985, 87, 271–282. [Google Scholar] [CrossRef]
- Reeve, M. Complete cycle of development of a pelagic chaetognath in culture. Nature 1970, 227, 381. [Google Scholar] [CrossRef]
- Schoener, A. Post-larval development of five deep-sea ophiuroids. Deep Sea Res. Oceanogr. Abstr. 1967, 14, 645–660. [Google Scholar] [CrossRef]
- Wyngaard, G.A.; Rasch, E.M. Patterns of genome size in the copepoda. Hydrobiologia 2000, 417, 43–56. [Google Scholar] [CrossRef]
- Moroz, L.L.; Kocot, K.M.; Citarella, M.R.; Dosung, S.; Norekian, T.P.; Povolotskaya, I.S.; Grigorenko, A.P.; Dailey, C.; Berezikov, E.; Buckley, K.M.; et al. The ctenophore genome and the evolutionary origins of neural systems. Nature 2014, 510, 109–114. [Google Scholar] [CrossRef]
- Kruse, S.; Hagen, W.; Bathmann, U. Feeding ecology and energetics of the Antarctic chaetognaths Eukrohnia hamata, E. bathypelagica and E. bathyantarctica. Mar. Biol. 2010, 157, 2289–2302. [Google Scholar] [CrossRef]
- Buskey, E.J.; Lenz, P.H.; Hartline, D.K. Escape behavior of planktonic copepods in response to hydrodynamic disturbances: High speed video analysis. Mar. Ecol. Prog. Ser. 2002, 235, 135–146. [Google Scholar] [CrossRef]
- Lee, K.; Bae, B.S.; Kim, I.O.; Yoon, W.D. Measurement of swimming speed of giant jellyfish Nemopilema nomurai using acoustics and visualization analysis. Fish. Sci. 2010, 76, 893–899. [Google Scholar] [CrossRef]
- Doyle, T.K.; Houghton, J.D.R.; Mcdevitt, R.; Davenport, J.; Hays, G.C. The energy density of jellyfish: Estimates from bomb-calorimetry and proximate-composition. J. Exp. Mar. Biol. 2007, 343, 239–252. [Google Scholar] [CrossRef]
- Liu, C.S.; Chen, S.Q.; Zhuang, Z.M.; Yan, J.P.; Liu, C.L.; Cui, H.T. Potential of utilizing jellyfish as food in culturing Pampus argenteus juveniles. Hydrobiologia 2015, 754, 189–200. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Sato, R.; Ishii, H.; Akiba, T.; Nogata, Y.; Tanaka, Y. Culture of phyllosomas of Ibacus novemdentatus (Decapoda: Scyllaridae) in a closed recirculating system using jellyfish as food. Aquaculture 2012, 330, 162–166. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.C.; Li, X.S. Phylogeny and biogeography of Chinese pomfret fishes (Pisces: Stromateidae). Studia Mar. Sin. 2002, 2002, 235–239. [Google Scholar]
- Miyajima, Y.; Masuda, R.; Kurihara, A.; Kamata, R.; Yamashita, Y.; Takeuchi, T. Juveniles of threadsail filefish, Stephanolepis cirrhifer, can survive and grow by feeding on moon jellyfish Aurelia aurita. Fish. Sci. 2011, 77, 41–48. [Google Scholar] [CrossRef]
- Boero, F.; Brotz, L.; Gibbons, M.J.; Piraino, S.; Zampardi, S. Explaining Ocean Warming: Causes, Scale, Effects and Consequences; IUCN: Gland, Switzerland, 2016; pp. 213–237. [Google Scholar]
- Marques, R.; Bouvier, C.; Darnaude, A.M.; Molinero, J.-C.; Przybyla, C.; Soriano, S.; Tomasini, J.-A.; Bonnet, D. Jellyfish as an alternative source of food for opportunistic fishes. J. Exp. Mar. Biol. Ecol. Evol. 2016, 485, 1–7. [Google Scholar] [CrossRef]
- Olesen, N.J. Clearance potential of jellyfish Aurelia aurita, and predation impact on zooplankton in a shallow cove. Mar. Ecol. Prog. Ser. 1995, 124, 63–72. [Google Scholar] [CrossRef]
- Fleming, N.E.; Harrod, C.; Newton, J.; Houghton, J.D. Not all jellyfish are equal: Isotopic evidence for inter-and intraspecific variation in jellyfish trophic ecology. PeerJ 2015, 3, e1110. [Google Scholar] [CrossRef] [PubMed]



| Station | Sampling Date | Temperature (°C) | Salinity (‰) | pH | Dissolved Oxygen (mg L−1) | Dissolved Organic Carbon (mg L−1) |
|---|---|---|---|---|---|---|
| W3 | 29 July | 27.64 | 34.98 | 8.13 | 6.37 | 4.17 |
| W4 | 29 July | 27.83 | 35.00 | 8.15 | 6.36 | 3.39 |
| W9 | 29 July | 27.86 | 34.89 | 8.14 | 6.25 | 3.96 |
| W3 | 26 October | 28.16 | 33.22 | 8.16 | 6.62 | 1.83 |
| W4 | 26 October | 28.04 | 33.17 | 8.16 | 6.56 | 1.77 |
| W9 | 26 October | 28.25 | 33.58 | 8.16 | 6.53 | 1.80 |
| Sample ID * | Taxa | Individuals/Clones | Simpson_D | Shannon_H | Chao1 |
|---|---|---|---|---|---|
| W3-Jul-J | 10 | 36 | 0.662 | 1.597 | 13.33 |
| W4-Jul-J | 8 | 31 | 0.4828 | 1.151 | 11.33 |
| W9-Jul-J | 10 | 47 | 0.7352 | 1.747 | 11 |
| W3-Oct-J | 16 | 58 | 0.6879 | 1.814 | 43.5 |
| W4-Oct-J | 17 | 51 | 0.872 | 2.364 | 72 |
| W9-Oct-J | 2 | 46 | 0.04253 | 0.1047 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Guo, M.; Li, T.; Huang, H.; Liu, S.; Hu, S. Small Jellyfish as a Supplementary Autumnal Food Source for Juvenile Chaetognaths in Sanya Bay, China. J. Mar. Sci. Eng. 2020, 8, 956. https://doi.org/10.3390/jmse8120956
Wang L, Guo M, Li T, Huang H, Liu S, Hu S. Small Jellyfish as a Supplementary Autumnal Food Source for Juvenile Chaetognaths in Sanya Bay, China. Journal of Marine Science and Engineering. 2020; 8(12):956. https://doi.org/10.3390/jmse8120956
Chicago/Turabian StyleWang, Lingli, Minglan Guo, Tao Li, Hui Huang, Sheng Liu, and Simin Hu. 2020. "Small Jellyfish as a Supplementary Autumnal Food Source for Juvenile Chaetognaths in Sanya Bay, China" Journal of Marine Science and Engineering 8, no. 12: 956. https://doi.org/10.3390/jmse8120956
APA StyleWang, L., Guo, M., Li, T., Huang, H., Liu, S., & Hu, S. (2020). Small Jellyfish as a Supplementary Autumnal Food Source for Juvenile Chaetognaths in Sanya Bay, China. Journal of Marine Science and Engineering, 8(12), 956. https://doi.org/10.3390/jmse8120956




