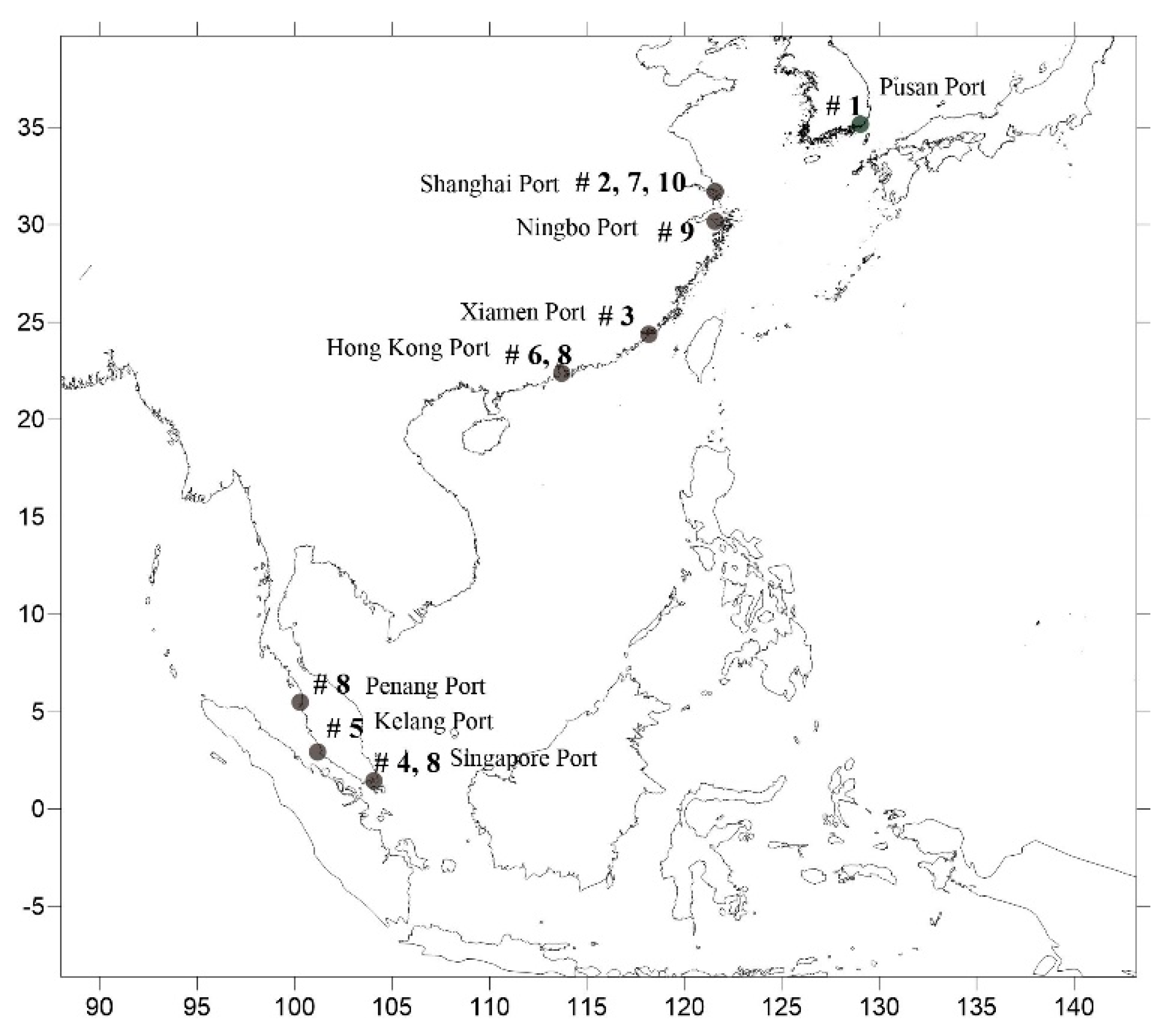
1. Introduction
Invasive aquatic species can disrupt native ecosystems of affected regions. Biofouling and ballast water are major mechanisms in the movement of exotic species among ocean ecosystems. Global ballast water discharged from vessels engaged in international trade has been estimated to exceed 3.1 billion tons per year, involving in excess of 7000 organisms being transferred among regions, potentially adversely affecting the environment, human health, and the economy in receiving environments [
1,
2,
3,
4,
5]. The International Maritime Organization (IMO) established the International Convention for the Control and Management of Ship’s Ballast Water and Sediments (BWMC) in 2004, which aimed to minimize the movement of harmful organisms and pathogens via ballast water [
6]. The BWMC came into force on 8 September 2017, and under Regulation B-3 (based on ship renewal surveys associated with the International Oil Pollution Prevention Certificate [
7]) it requires that most ships moving among international ports from that time to 8 September 2024 have installed a type-approved ballast water management system (BWMS).
For a BWMS to receive approval, land-based and shipboard ballast water tests must be conducted. The land-based test requires five successive tests involving freshwater, brackish water, and seawater under conditions in accordance with BWMS Code [
8]. However, aquatic environmental conditions in certain regions may be worse than the test water conditions specified in the BWMS Code. An experience-building phase (EBP) from 2018 to 2022 was approved by the Marine Environment Protection Committee (MEPC) to monitor implementation of the BWM Convention, with the aims of identifying those aspects of the convention’s implementation that are working well, and to shed light on issues that require further attention [
8,
9]. To this end, the EBP is structured in three stages: a data gathering stage, a data analysis stage, and a review stage. Another important goal of the EBP is to develop standardized sampling and analysis procedures.
Technologies used in BWMSs included electrolysis, ultraviolet irradiation, ozonation, filtration, and thermal treatment. A total of 45 BWMSs had received final approval from the IMO through November 2019. Among the approved BWMSs, the main treatment methods include electrolysis (22 BWMSs), chemical injection systems (13 BWMSs), UV systems (6 BWMSs), and ozone systems (4 BWMSs) [
10]. The BWMS implemented depends on the characteristics of the equipment and the installation conditions including vessel dimensions and ballast water flow rates of the ship. The main BWMSs installed on ships are UV and electrolysis devices. Of the 45 BWMS developed, 32 (71%) involve a filter unit in the pretreatment stage, designed to physically remove organisms ≥50 µm in size.
There are two BWMS approval test methods: the BWMS Code and the U.S Coast Guard Environmental Technology Verification (USCG ETV) protocol methods [
11,
12]. Although these involve slightly different test water conditions, the criteria for discharge of treated water are the same (
Table 1). The most significant difference between the test water conditions for the land-based test and the water from a specific port environment is the total suspended solids (TSS) (
Table 1). The test conditions for TSS in the USCG ETV protocol is 24 mg L
−1 in all test waters, while for the BWMS Code is TSS >50 mg L
−1 for fresh and brackish water, and 1 mg L
−1 for seawater. However, some ports worldwide have intense maritime traffic, and their waters may contain extremely high particle loads. For water having a high TSS concentration, filter clogging occurs even before backwashing, so it may be difficult to stably operate the BWMS [
13]. In addition, inorganic particles (e.g., sand) can impact UV transmittance (UV-T), cause light diffusion, and have an abrasive effect on equipment component surfaces (e.g., lamps), and the presence of organic particles can result in a reduction of the potential for oxidation of active substances, cause agglomeration, and may also result in shadowing (thereby diminishing UV-T) [
14].
The purpose of this study was to evaluate the performance of BWMSs in relation to the D-2 standard. The study was based on three international vessels equipped with the three types of BWMS widely used in vessels. In addition, we assessed problems that can occur during BWMS operation in certain environments, including high concentrations of TSS, and proposed some operational approaches that may eliminate or reduce the risk of introduction of invasive species. The study was intended to aid proper implementation of the BWMC, and thus protects environments from invasions by exotic aquatic species introduced through ballast water, which is the purpose of the Convention.
4. Discussion
The POC and DOC concentrations measured at the ports during this study were within the range of, or a little higher, than values for test waters for approval of BWMSs. However, the TSS concentration was higher than that for test waters in the land-based test, and this was particularly apparent in ports located near Shanghai (
Table 1 and
Table 4). Depending on the particle size distribution, density, and sediment quality, suspended solids can cause problems including filter clogging, interference with UV transmittance, and increased oxidation potential [
13,
14].
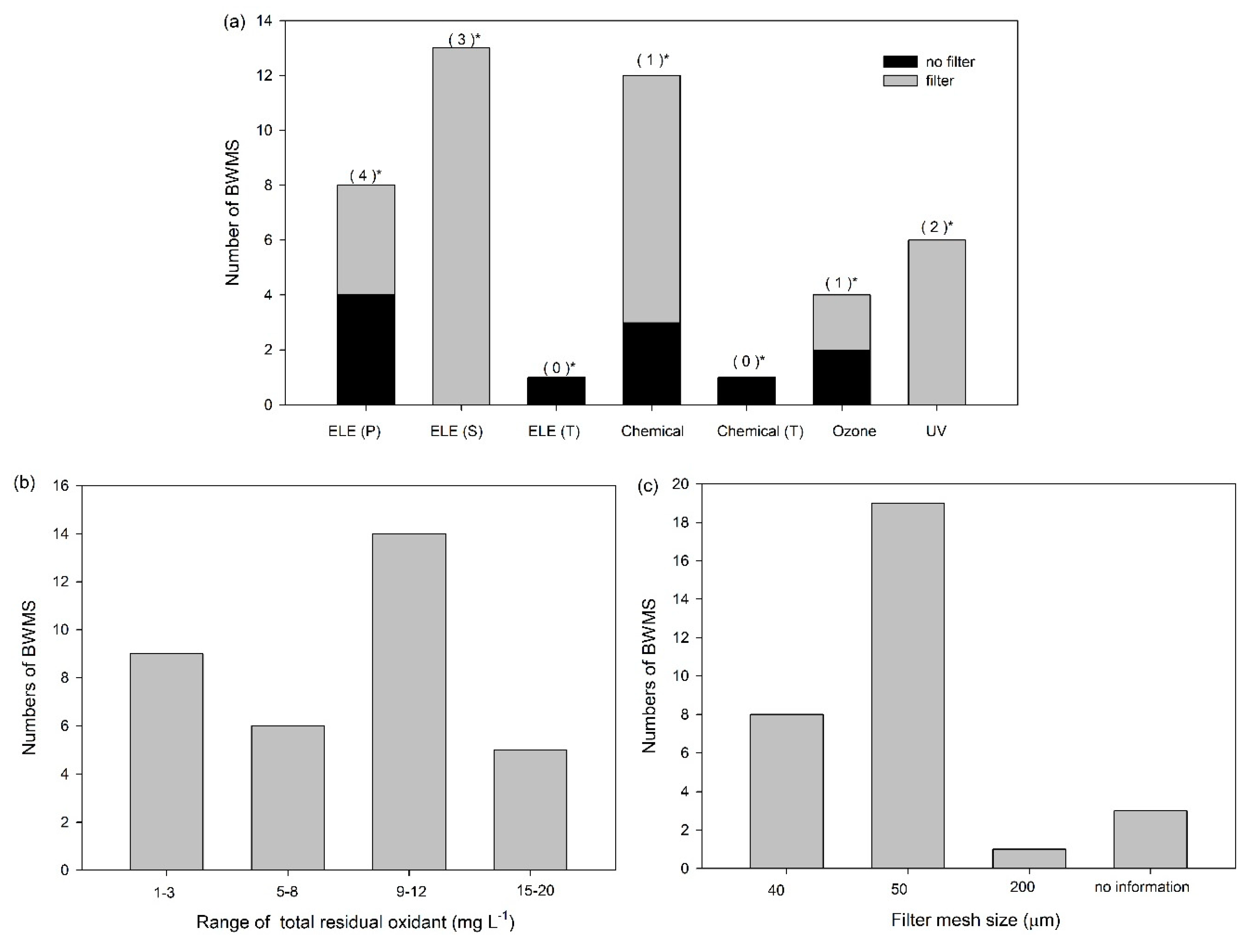
The filter units installed in BWMSs are primarily to remove organisms ≥50 μm in size, and not to remove suspended solids, so the filter sizes are usually 40–50 μm (
Figure 2). To enable normal BWMS operation, the equipment is designed for backflushing when the DP of the filter inlet and outlet increases. In a submission by China for the 70th MEPC, the concentration of suspended solids in Yangsan Port (Shanghai) was reported to be 230–1560 mg L
−1 throughout the year [
13]. In this study, the concentration of TSS in the uptake water of the port near Shanghai was 260–785 mg L
−1. At this level of TSS, filter equipment will largely be unable to operate near the Yangtze River. In cases where vessels cannot properly treat ballast water in ports having high suspended solids, those vessels could be permitted to take on ballast water without treatment, but exchange that ballast water on the high seas with BWMS-treated seawater. However, because of safety issues for vessels and crews associated with such ballast water exchange, no decision was made at the 70th MEPC [
18].
Milliman et al. [
19] and Shen [
20] suggested that approximately 40% of the suspended solids entering the estuary of the Yangtze River are deposited in the estuary. Wang et al. [
21] studied the major components of TSS in the estuary of the Yangtze River using a laser particle size analyzer. They found that suspended solids in the estuary comprised 16% clay, 42% silt, and 42% sand. It was also reported that the suspended particles in the estuary of the Yangtze River were larger than those in the estuary of the Huanghe River. This suggests that the suspended solids in Shanghai Port should more readily settle over time. This was confirmed in the pretreatment process for the TSS test in Shanghai Port, specifically that the TSS concentration in the 10th test decreased significantly during deballasting in Pusan Port. For this reason, filter clogging will not occur during deballasting. Therefore, for high turbidity areas such as Shanghai Port, ballasting could involve bypass of the filter with only UV operating, and then during deballasting the water can be treated by both filtration and UV treatment, as the BWMS should operate normally without filter clogging. This method may not remove 100% of organisms ≥50 μm in size, in accordance with the D-2 standard, but should remove most. However, it is very unusual for filtration to be used at deballasting, perhaps because the crew and manufacturer consider maintenance of the filter too difficult. Ship owners would probably be unhappy as this approach may result in additional sediment accumulation at the bottom the ballast tank. For this reason, most of the captains manage ballast tanks efficiency, and try not to do ballasting in Shanghai, China. However, if ballasting is only needed for an emergency situation in a port having high suspended solid concentrations, then this approach using only a filter unit in deballasting should be effective in preventing invasions by exotic aquatic species. The TRO generated in BWMSs having electrolytic equipment is designed to maintain a level sufficient to kill organisms during storage in ballast tanks, but UV equipment in BWMSs have no sterilizing effect during storage. To compensate for this, those BWMSs using UV equipment also perform UV treatment during deballasting. Among those BWMSs using filter devices, only one BWMS has been approved for application to both ballasting and deballasting. In addition, if a new filtration system is developed that can filter even in areas with high suspended solids, it may be an alternative to perform disinfection during ballasting.
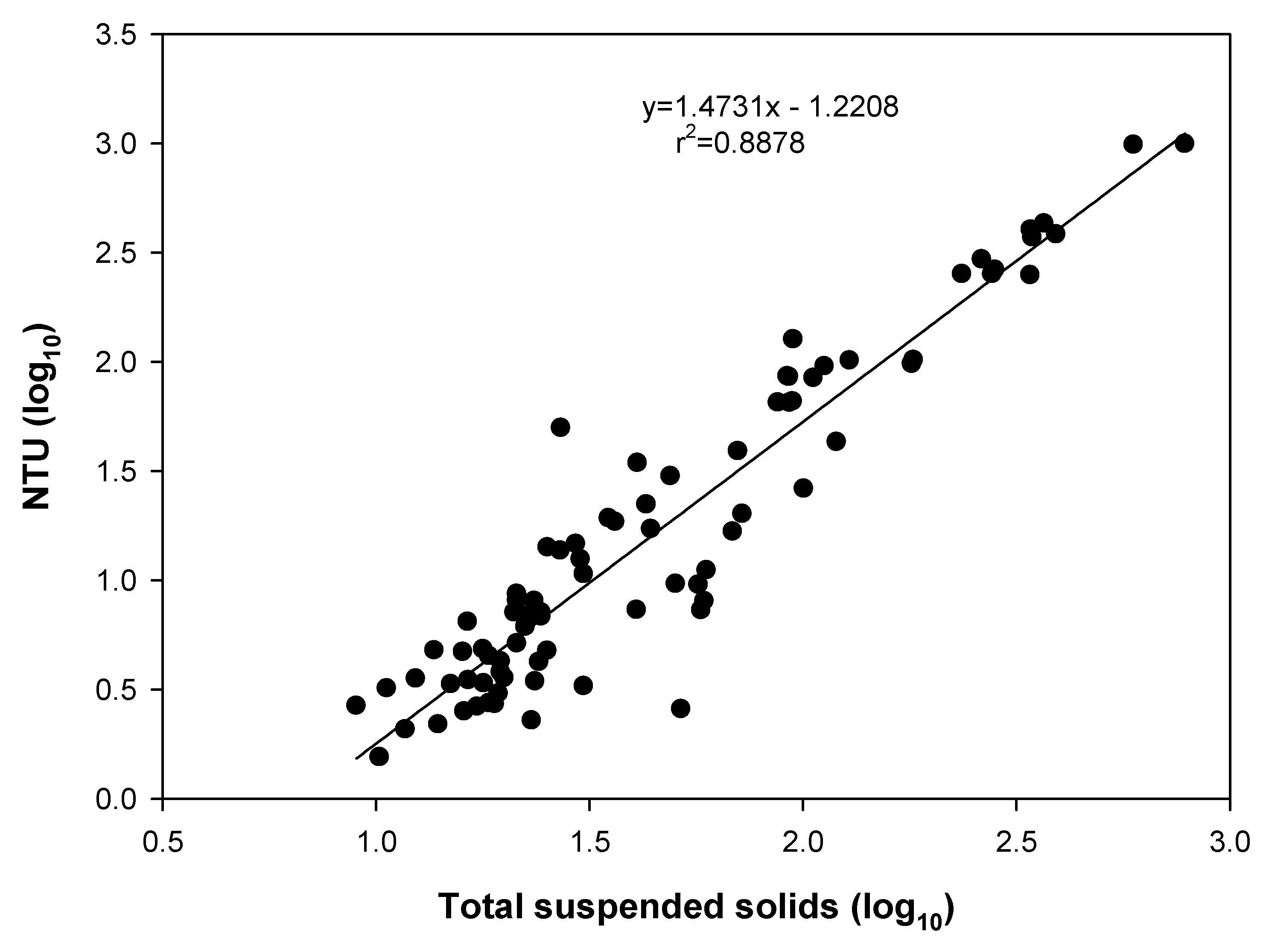
To apply filtration during deballasting it is necessary to know the concentration of TSS in the port water entering the vessel. In this study, the Pearson’s correlation coefficient (r
2) for NTU and TSS was 0.8878, indicating that the concentration of TSS can be indirectly measured using a turbidity meter (
Figure 3). In ports suspected of having a high TSS concentration, the crew could firstly measure the turbidity using sea-to-sea mode. If the measured turbidity value exceeds standard criteria, the BWMS could be programmed to operate as described above. In addition, in high turbidity conditions the UV transparency is lowered because of shadowing effects resulting from abrasive polishing of the surface of the UV lamp, and the accumulation of TSS components during ballasting, thereby reducing the UV intensity and reducing the BWMS efficiency [
14].
Electrolysis systems involving no filter may be preferred as they are easier to maintain and operate. However, electrolysis devices without filters (type B) can also have problems treating water from ports having high concentrations of suspended solids [
14]. Interference with sensor measurement can occur because of the introduction of suspended matter to the TRO sensor measurement area, which can cause failure of the BWMS operation through the TRO measurements. As a specific TRO is formed in the type B system from current and voltage, it is possible to predict the current and voltage values required to generate target TROs. The target TRO is set in the BWMS by adjusting the current voltage to achieve the target value. If operation of the BWMS is controlled by current and voltage when ballasting in ports having water containing high TSS concentrations, it may be possible to prevent shutdowns that may occur because of malfunction of the sensor caused by the suspended solids. To manage ballast water more stably when using this method, it is necessary to use a portable TRO meter to record whether the concentration formed is in the operating range, and to measure residual TRO during the period of ballast water storage.
In type B systems it is difficult to generate high TRO concentrations when the salinity is low, and an increase in the oxidation potential is necessary where the concentration of organic substances in suspended matter is high [
14]. In this study the type B system did not achieve the target TRO in the low salinity conditions of Shanghai Port (the average TRO was <2.0 mg L
−1 over approximately 10 min), so the BWMS was stopped. The portable TRO meter value was similar to the TRO measurement in the BWMS, so it was not a malfunction of the TRO sensor caused by suspended solids. This type of BWMS is equipped with a salinity meter, and when low salinity is detected in a port, the flow rate is automatically adjusted to achieve the allowable TRO concentration. As the type C system forms a target TRO using a salt water tank, there is no problem in operating this type of BWMS in a fresh water area, other than the disadvantage of having to fit an extra tank or ballast tank designated to store seawater on the vessel. In addition, all type C systems have a filter unit, making it difficult to operate these BWMSs when high TSS concentrations are involved.
For approval, land-based tests of BWMSs have to be conducted to determine holding times. According to the BWMS Code, in a land-based test the BWMS must operate successful in at least five consecutive test cycles at each salinity. For two test cycles, according to IMO guideline G9 [
22] the holding time should be five or more days, to assess the risk of disinfection byproducts generated in BWMSs that make use of active substances, and should be performed using the holding time specified by the manufacturer. Vessels are constantly entering and leaving ports that are sometimes in close proximity, so uptake and discharge of ballast water may occur within one day. In other cases, ballasted waters may be stored for at least 20 days before discharge, depending on the shipping route [
23,
24]. When the holding time is long, microbial pathogens <10 μm in size may increase in number because of the decomposition of organic matter, and as a result the water at discharge may not meet the D-2 standard. In this study, the microbial concentration in treated discharge water was higher than that in uptake water in the fifth test, which had the longest storage time (six days). As systems having the UV devices repeat UV sterilization at discharge, the problem of microbial growth during long-term storage may be less than that for systems using electrolysis devices. To solve this problem, BWMSs having electrolysis devices could be used to treat ballast water during discharge, with subsequent neutralization or UV treatment. In this case, since it is neutralized and discharged immediately after using the electrolysis device, the time may be too short to treat pathogenic microorganisms. As a result of the biological efficacy testing immediately after treatment in the land-based test for a type-approval test of BWMS, only pathogenic microorganisms were detected in 7 out of 30 test cycles, and most of the detected pathogenic microorganisms were less than 1 CFU 100 mL
−1. However, it takes about an hour to sample by neutralizing after continuous sampling, so the contact time of active substance (TRO) is longer than the method proposed in this paper. This proposed method should be conducted on land-based or on a shipboard test to evaluate whether it is effective in killing the microorganisms and is not hazardous to the environment. In the land-based test, a test should also be carried out as to how much TRO concentration is appropriate.
Another method to remove pathogenic organisms associated with long-term storage is to maintain the residual TRO concentration in the tank by applying partial ballasting to the ballast tank. In BWMSs fitted with electrolysis devices, TRO is formed during ballasting, but residual amounts remain during storage in the ballast tank, and this residual contributes to the killing of organisms. In the eighth trial of sequential ballasting, it was found that the residual TRO concentration in the ballast water tank was maintained, inferred because the amount of neutralizing agent that had to be injected increased by a factor of two over other periods during deballasting (
Table 3). However, future research into the formation of disinfection byproducts (DBPs) will be necessary, because in maintaining the residual TRO concentration and using electrolysis devices during deballasting it may be possible to form more disinfection byproducts than occurs using the approved operational method [
25]. An approach to compensating for the shortcomings of filter clogging in the presence of high suspended solid concentrations, the problem of TRO formation in fresh water areas and the rapid reduction of residual TRO by organic substances, is to treat ballast water using an in-tank method using TROs. However, using this approach it is difficult for the active substance to diffuse throughout the ballast water if the ballast tank in the hull contains complex bulkheads. Thus, in building new vessels it is important to design ballast tanks that take account of the diffusion of active materials.
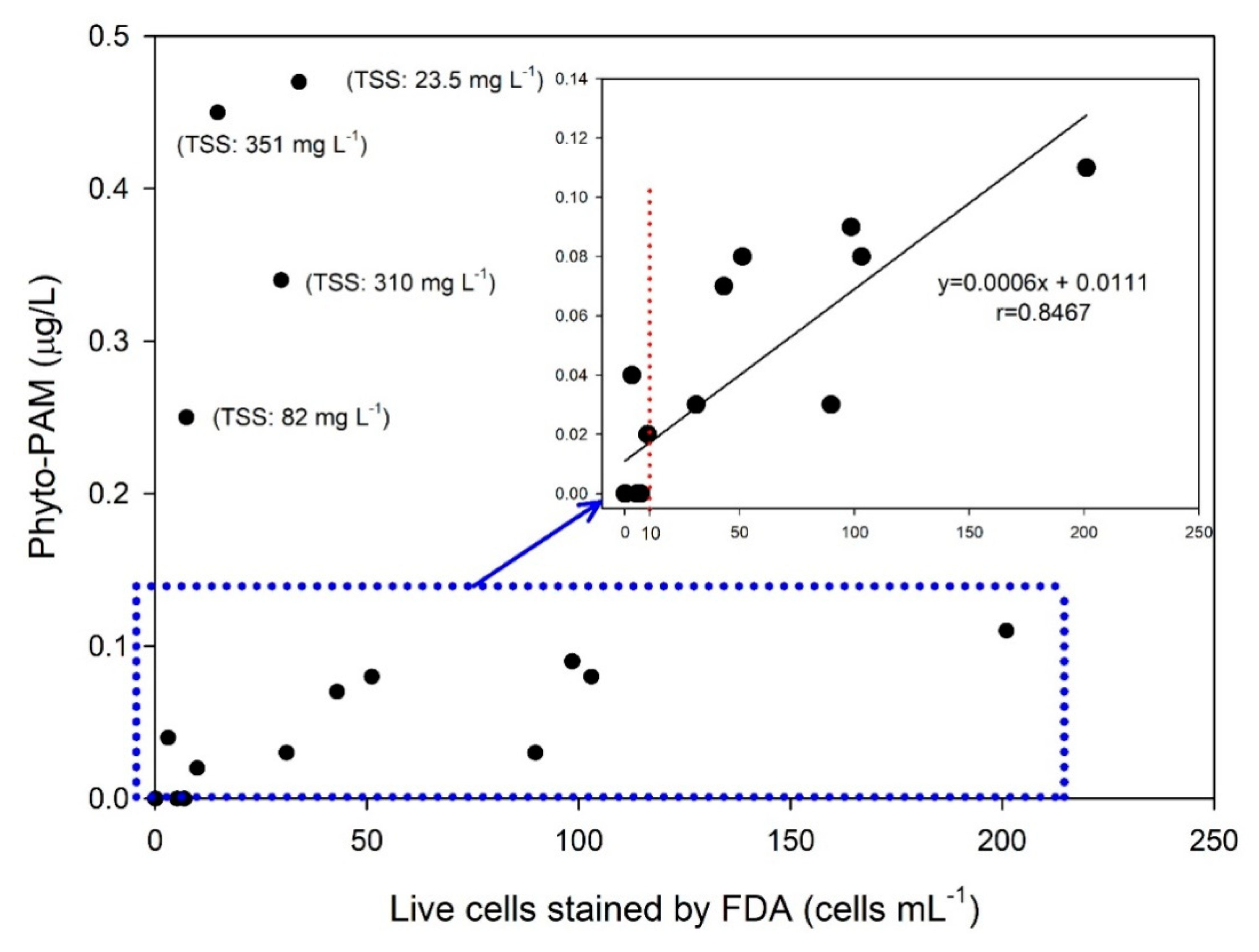
The IMO allows for use of an indicative analysis method that counts living organisms in the size range ≥10 to <50 µm in a device developed using the Pulse-Amplitude-Modulation (PAM) principle in a commissioning test that verifies performance after installation of a BWMS on a vessel [
26,
27]. The indicative analysis uses an instrument-based method for counting living organisms instead of methods involving direct counts using a fluorescent microscope or growth medium. The Phyto-PAM can analyze the chlorophyll of living organisms in the size range ≥10 to <50 μm within 10 min in treated discharge water, but not the number of these organisms. When the TSS concentration was high, an outlier of the Phyto-PAM value was often measured, indicating the possibility that the measured value might be disturbed by turbidity (
Figure 4). In addition, the D-2 criterion for treated discharge water is <10 organisms mL
−1, which increase the level of inaccuracy because this value is close to the detection limit (
Figure 4). Results reported by Gollasch and David [
26] and the document submitted by Singapore to the 75th MEPC suggest that indicative analysis can underestimate the true levels of these organisms [
27]. The Maritime and Port Authority of Singapore surveyed ballast water in 11 vessels operating BWMSs that entered the main port of Singapore between April and November 2019, and three of these did not meet the D-2 standard for treated discharge water [
26]. However, for shipboard tests in our study, conducted using six normal BWMSs and involving both ballasting and deballasting, the reliability of the approved BWMSs was confirmed because in all tests they satisfied the D-2 standards for the discharge of treated water.