Coral Resilience at Malauka`a Fringing Reef, Kāneʻohe Bay, Oʻahu after 18 years
Abstract
1. Introduction
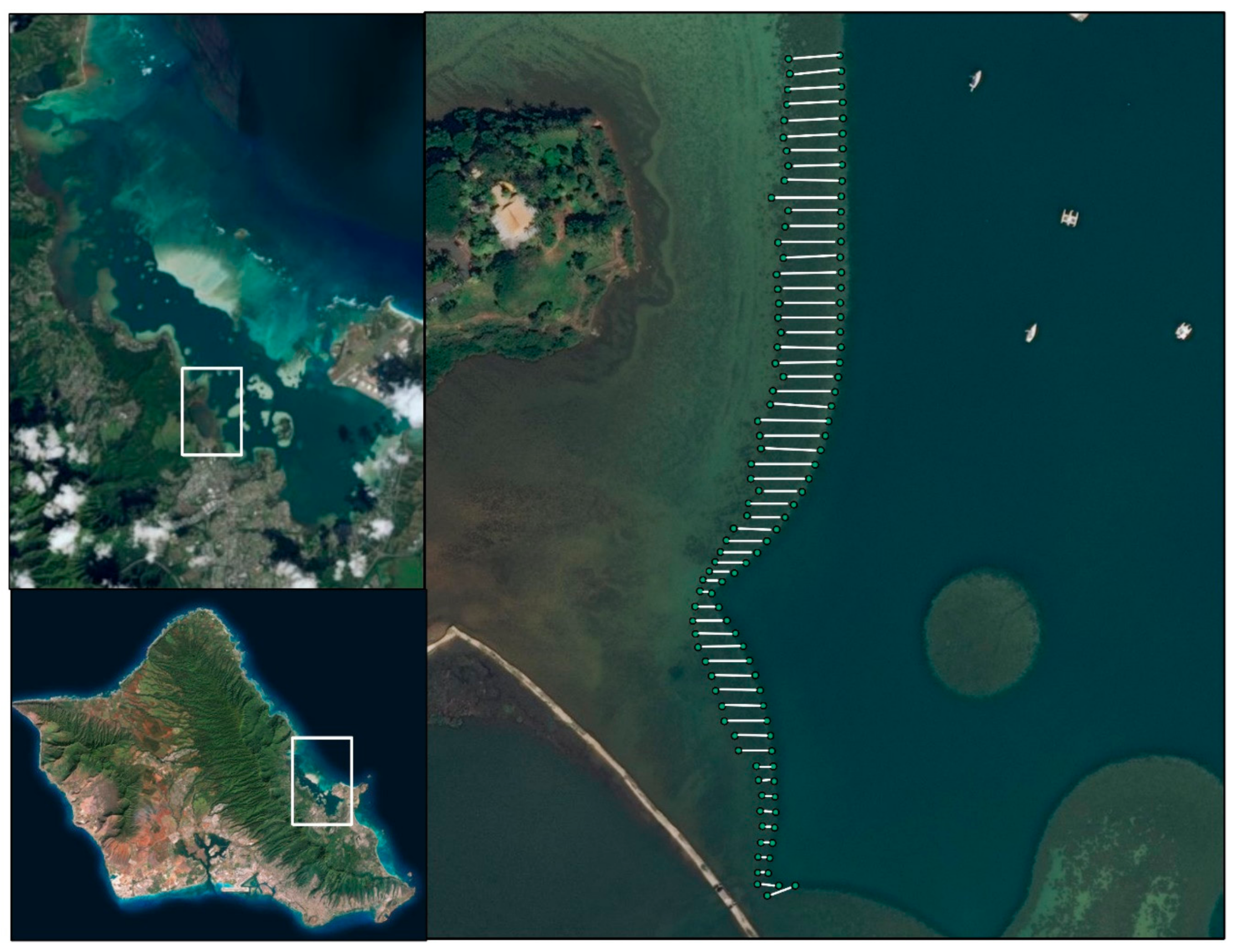
Kāneʻohe Bay, Hawai’i
2. Materials and Methods
2.1. Study Site: Kāneʻohe Bay, Hawaiʻi
2.2. Comparative Study Setup
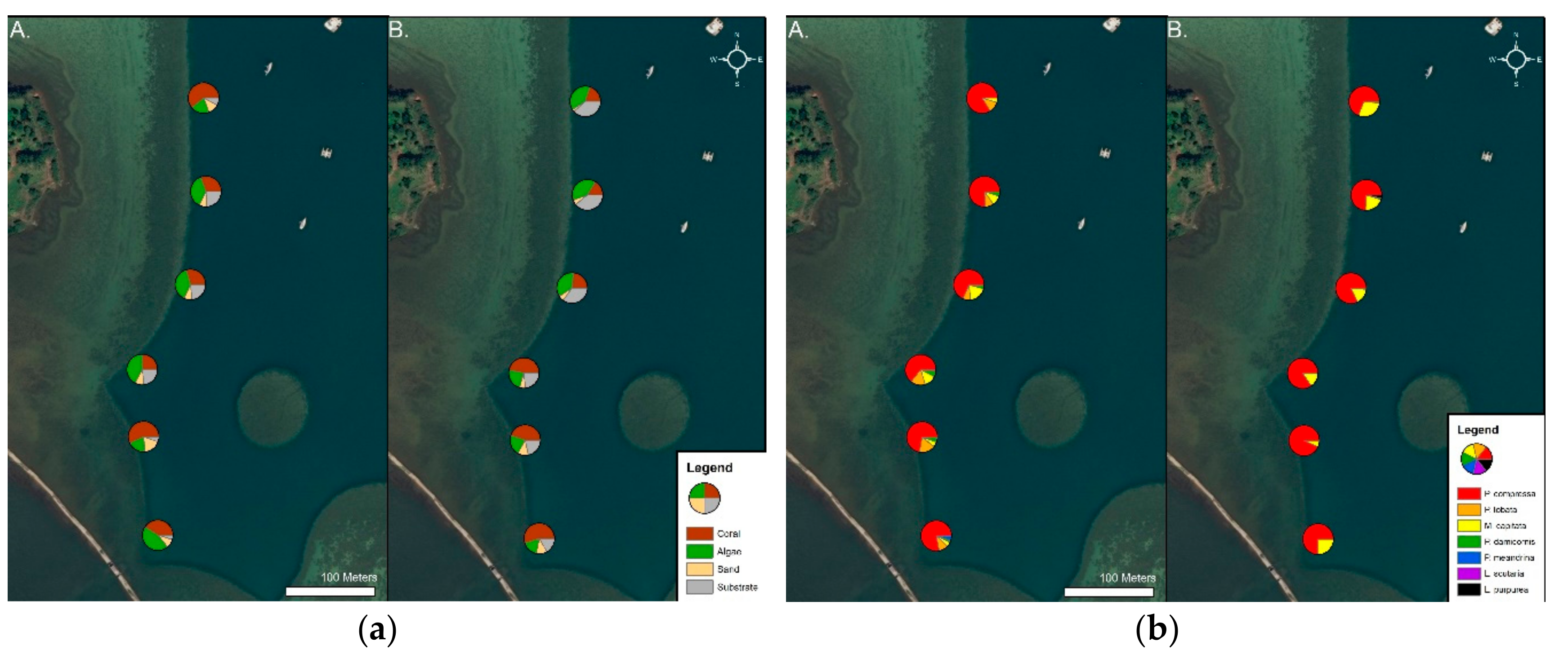
2.2.1. Benthic Survey
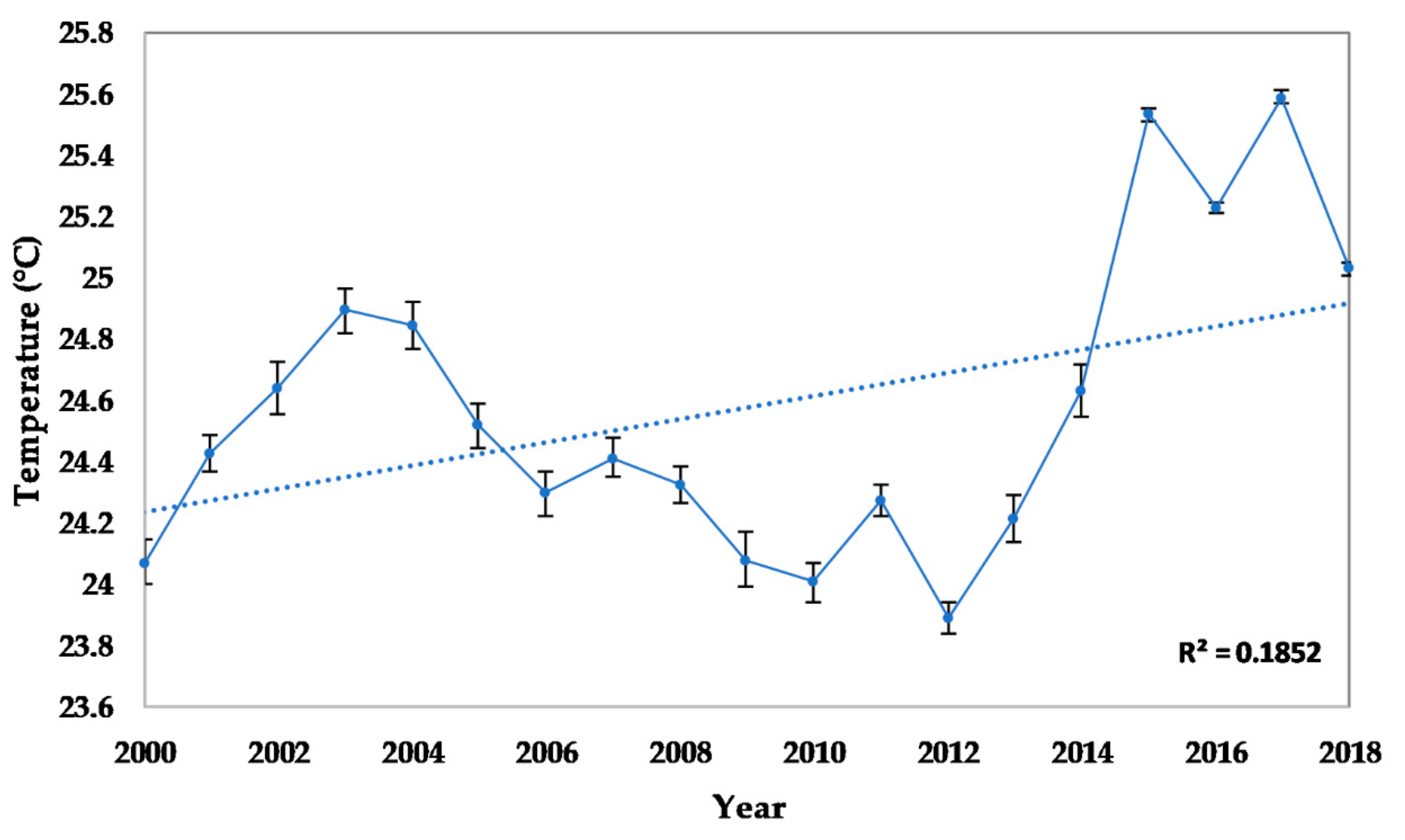
2.2.2. Seawater Temperature
2.2.3. Statistical Analysis
3. Results
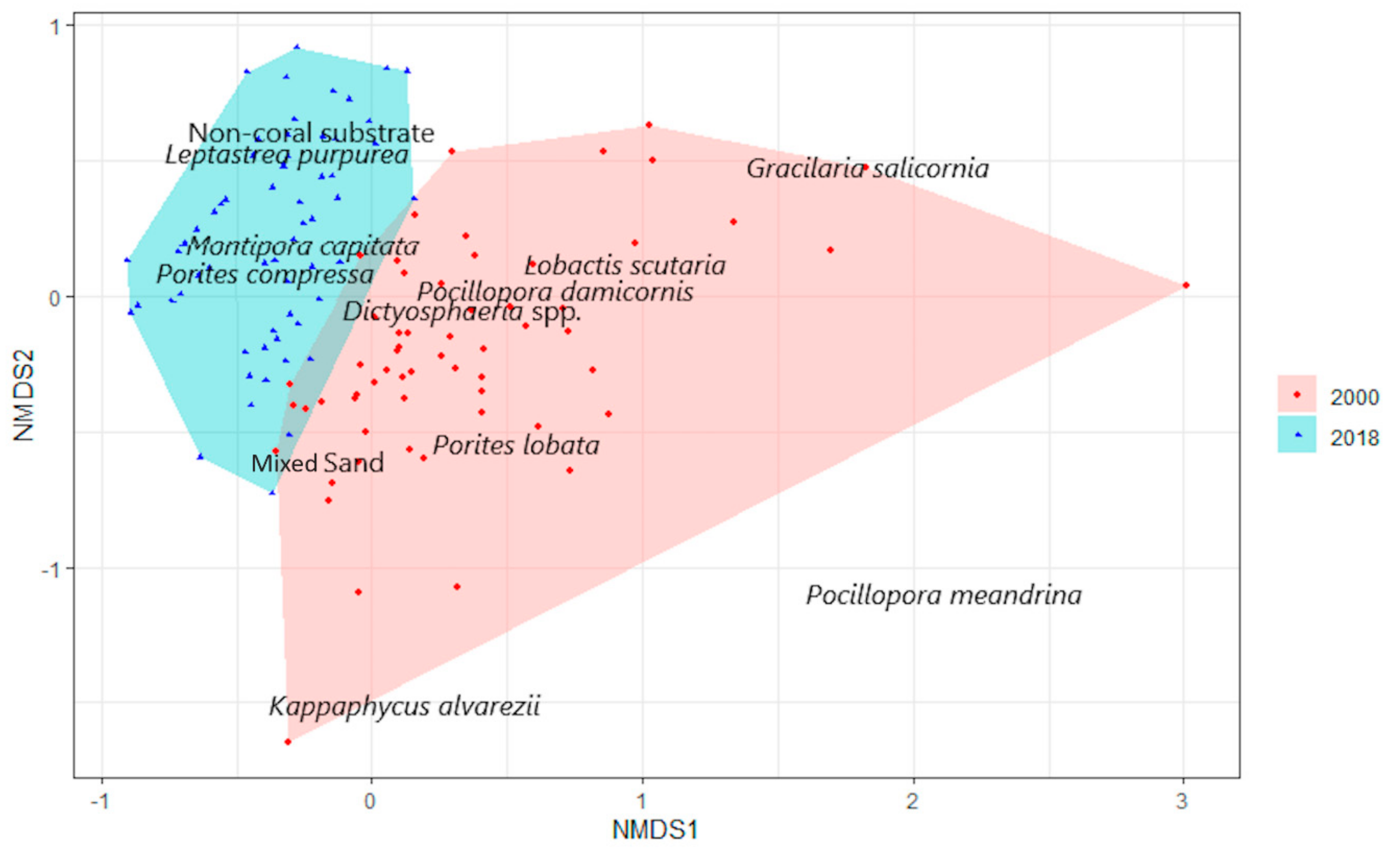
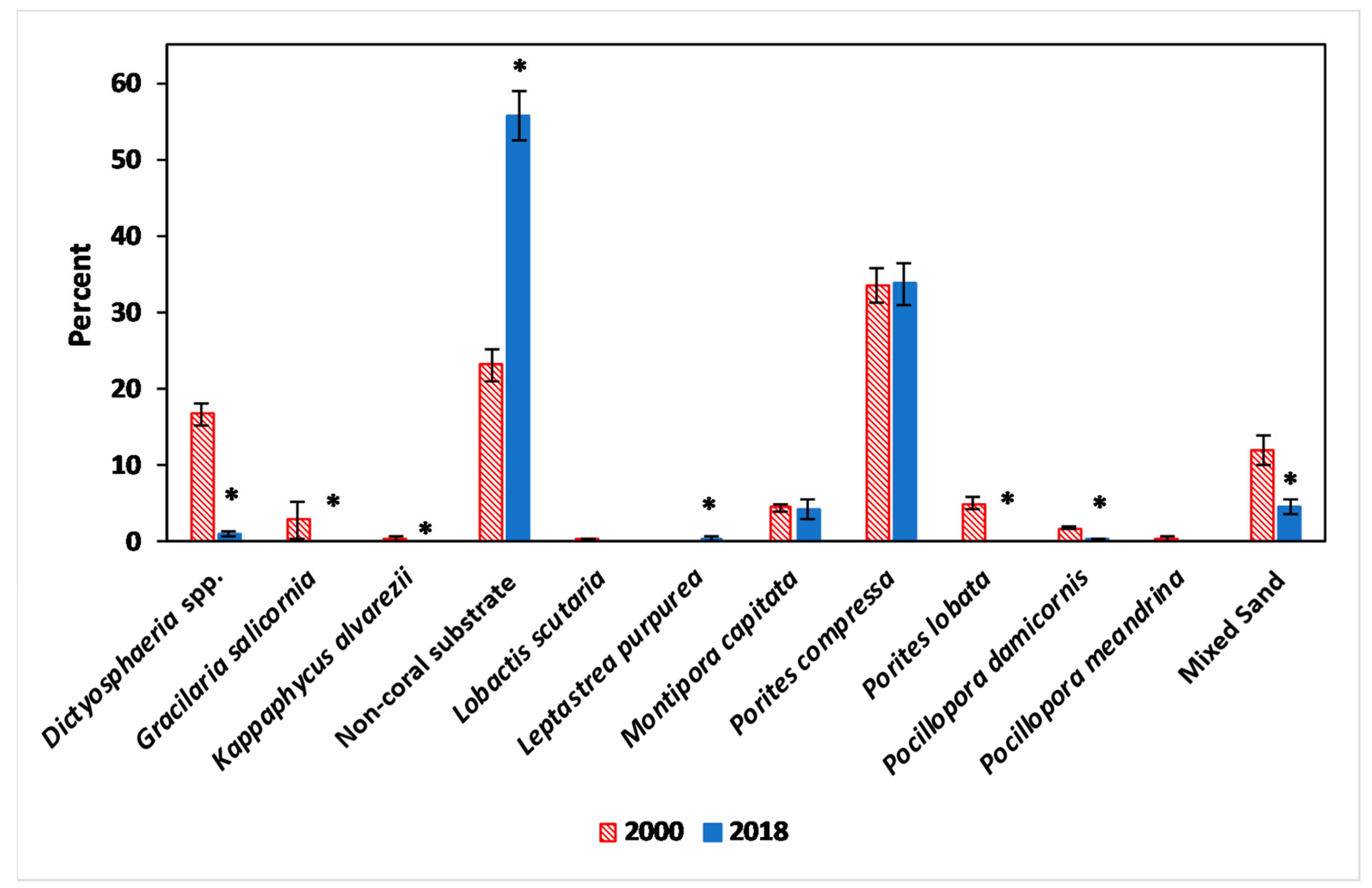
3.1. Benthic Survey
3.2. Statistical Analysis
3.2.1. Abiotic and Biotic Changes
3.2.2. Algae
3.2.3. Coral
4. Discussion
4.1. Abiotic and Biotic Changes
4.2. Algae
4.3. Corals
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Knowlton, N. Coral Reefs Under Rapid Climate Change and Ocean Acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Jokiel, P.L.; Coles, S.L. Response of Hawaiian and other Indo-Pacific Reef Corals to Elevated Temperature. Coral. Reefs 1990, 8, 155–162. [Google Scholar] [CrossRef]
- Baker, A.C.; Glynn, P.W.; Riegl, B. Climate Change and Coral Reef Bleaching: An Ecological Assessment of Long-term Impacts, Recovery Trends and Future Outlook. Estuar. Coast. Shelf Sci. 2008, 80, 435–471. [Google Scholar] [CrossRef]
- Graham, N.A.; Wilson, S.K.; Jennings, S.; Polunin, N.V.; Bijoux, J.P.; Robinson, J. Dynamic Fragility of Oceanic Coral Reef Ecosystems. Proc. Natl. Acad. Sci. USA 2006, 103, 8425–8429. [Google Scholar] [CrossRef] [PubMed]
- Moberg, F.; Folke, C. Ecological Goods and Services of Coral Reef Ecosystems. Ecol. Econ. 1999, 29, 215–233. [Google Scholar] [CrossRef]
- De’ath, G.; Fabricius, K.E.; Sweatman, H.; Puotinen, M. The 27–year Decline of Coral Cover on the Great Barrier Reef and its Causes. Proc. Natl. Acad. Sci. USA 2012, 109, 17995–17999. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C. Status of Coral Reefs of the World: 2000; Australian Institute of Marine Science: Queensland, Australia, 2000. [Google Scholar]
- Bruno, J.F.; Selig, E.R. Regional Decline of Coral Cover in the Indo-Pacific: Timing, Extent, and Subregional Comparisons. PLoS ONE 2007, 2, e711. [Google Scholar] [CrossRef]
- Burke, L.; Reytar, K.; Spalding, M.; Perry, A. Reefs at Risk; World Resources Institute: Washington, DC, USA, 2011; p. 124. [Google Scholar]
- Rodgers, K.S.; Jokiel, P.L.; Brown, E.K.; Hau, S.; Sparks, R. Over a Decade of Change in Spatial and Temporal Dynamics of Hawaiian Coral Reef Communities. Pac. Sci. 2015, 69, 1–13. [Google Scholar] [CrossRef]
- Bahr, K.D.; Rodgers, K.S.; Jokiel, P.L. Impact of Three Bleaching Events on the Reef Resiliency of Kāne ‘ohe Bay, Hawai ‘i. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Bridge, T.C.; Butler, L.R.; Byrne, M.; Cantin, N.E.; et al. Global Warming and Recurrent Mass Bleaching of Corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Coles, S.L.; Bahr, K.D.; Ku’ulei, S.R.; May, S.L.; McGowan, A.E.; Tsang, A.; Han, J.H.; Bumgarner, J. Evidence of Acclimatization or Adaptation in Hawaiian Corals to Higher Ocean Temperatures. PeerJ 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.N.; Morri, C.; Lasagna, R.; Montefalcone, M.; Gatti, G.; Parravicini, V.; Rovere, A. Resilience of the Marine Animal Forest: Lessons from Maldivian Coral Reefs After the Mass Mortality of 1998. In Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Rossi, S., Bramanti, L., Gori, A., Orejas, C., Eds.; Springer: Cham, Switzerland, 2017; pp. 1241–1269. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Hunter, C.L.; Taguchi, S.; Watarai, L. Ecological Impact of a Fresh-water “Reef Kill” in Kaneohe Bay, Oahu, Hawaii. Coral Reefs 1993, 12, 177–184. [Google Scholar] [CrossRef]
- Bahr, K.D.; Jokiel, P.L.; Toonen, R.J. The Unnatural History of Kāne ‘ohe Bay: Coral Reef Resilience in the Face of Centuries of Anthropogenic Impacts. PeerJ 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Stimson, J. Recovery of Coral Cover in Records Spanning 44 Yr for Reefs in Kāne‘ohe Bay, Oa‘hu, Hawai ‘i. Coral Reefs 2018, 37, 55–69. [Google Scholar] [CrossRef]
- Banner, A.H. Kaneohe Bay, Hawaii; Urban Pollution and a Coral Reefs Ecosystem. In Proceedings of the 2nd International Coral Reef Symposium, The Great Barrier Reef Province, Australia, 22 June–2 July 1974; Volume 2. [Google Scholar]
- Smith, S.V.; Kimmerer, W.J.; Laws, E.A.; Brock, R.E.; Walsh, T.W. Kaneohe Bay Sewage Diversion Experiment: Perspective, on Ecosystem Responses to Nutritional Perturbation. Pac. Sci. 1981, 35, 279–395. [Google Scholar]
- Stimson, J.; Larned, S.; Conklin, E. Effects of Herbivory, Nutrient Levels, and Introduced Algae on the Distribution and Abundance of the Invasive Macroalga Dictyosphaeria cavernosa in Kaneohe Bay, Hawaii. Coral Reefs 2001, 19, 343–357. [Google Scholar] [CrossRef]
- Hunter, C.L.; Evans, C.W. Coral Reefs in Kaneohe Bay, Hawaii: Two Centuries of Western Influence and Two Decades of Data. Bull. Mar. Sci. 1995, 57, 501–515. [Google Scholar]
- Jokiel, P.L.; Brown, E.K. Global Warming, Regional Trends and Inshore Environmental Conditions Influence Coral Bleaching in Hawaii. Glob. Chang. Biol. 2004, 10, 1627–1641. [Google Scholar] [CrossRef]
- Bahr, K.D.; Jokiel, P.L.; Rodgers, K.S. The 2014 Coral Bleaching and Freshwater Flood Events in Kāneʻohe Bay, Hawaiʻi. PeerJ 2015, 3. [Google Scholar] [CrossRef]
- Jury, C.P.; Toonen, R.J. Adaptive Responses and Local Stressor Mitigation Drive Coral Resilience in Warmer, More Acidic Oceans. Proc. R. Soc. Lond. B. Biol. Sci. 2019, 286. [Google Scholar] [CrossRef]
- Mühlig-Hofmann, A. The Distribution of Corals and Macroalgae in Relation to Freshwater and Sediment Discharge on a Fringing Reef in Kane’ohe Bay, O’ahu, Hawai’i. Master’s Thesis, University of Bremen, Bremen, Germany, 2001. [Google Scholar]
- Bremer, L.; Falinski, K.; Ching, C.; Wada, C.; Burnett, K.; Kukea-Shultz, K.; Reppun, N.; Chun, G.; Oleson, K.L.L.; Ticktin, T. Biocultural Restoration of Traditional Agriculture: Cultural, Environmental, and Economic Outcomes of Lo ‘i Kalo Restoration in He‘eia, O ‘ahu. Sustainability 2018, 10, 4502. [Google Scholar] [CrossRef]
- Möhlenkamp, P.; Beebe, C.; McManus, M.; Kawelo, A.; Kotubetey, K.; Lopez-Guzman, M.; Nelson, C.E.; Alegado, R. Kū Hou Kuapā: Cultural Restoration Improves Water Budget and Water Quality Dynamics in Heʻeia Fishpond. Sustainability 2019, 11, 161. [Google Scholar] [CrossRef]
- Hill, J.; Wilkinson, C. Methods for Ecological Monitoring of Coral Reefs; Australian Institute of Marine Science: Queensland, Australia, 2004; Volume 117, ISBN 0-642-32237-6. [Google Scholar]
- Jokiel, P.L.; Rodgers, K.S.; Brown, E.K.; Kenyon, J.C.; Aeby, G.; Smith, W.R.; Farrell, F. Comparison of Methods Used to Estimate Coral Cover in the Hawaiian Islands. PeerJ 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2015; Available online: http://www.rstudio.com/ (accessed on 1 May 2019).
- JMP®, version 13; SAS Institute Inc.: Cary, NC, USA, 1989–2019.
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2015. [Google Scholar]
- Jokiel, P.L.; Coles, S.L. Effects of Heated Effluent on Hermatypic Corals at Kahe Point, Oahu. Pac. Sci. 1974, 28, 1–18. [Google Scholar]
- Stimson, J.; Conklin, E.J. Potential Reversal of a Phase Shift: The Rapid Decrease in the Cover of the Invasive Green Macroalga Dictyosphaeria cavernosa Forsskål on Coral Reefs in Kāne ‘ohe Bay, Oahu, Hawai ‘i. Coral Reefs 2008, 27, 717–726. [Google Scholar] [CrossRef]
- Smith, J.E.; Hunter, C.L.; Conklin, E.J.; Most, R.; Sauvage, T.; Squair, C.; Smith, C.M. Ecology of the Invasive Red Alga Gracilaria salicornia (Rhodophyta) on O’ahu, Hawai’i. Pac. Sci. 2004, 58, 325–343. [Google Scholar] [CrossRef]
- Stimson, J.; Cunha, T.; Philippoff, J. Food Preferences and Related Behavior of the Browsing Sea Urchin Tripneustes gratilla (Linnaeus) and its Potential for use as a Biological Control Agent. Mar. Biol. 2007, 151, 1761–1772. [Google Scholar] [CrossRef]
- Neilson, B.J.; Wall, C.B.; Mancini, F.T.; Gewecke, C.A. Herbivore Biocontrol and Manual Removal Successfully Reduce Invasive Macroalgae on Coral Reefs. PeerJ 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Bahr, K.D.; Coffey, D.M.; Rodgers, K.S.; Balazs, G.H. Observations of a Rapid Decline in Invasive Macroalgal Cover Linked to Green Turtle Grazing in a Hawaiian Marine Reserve. Micronesica 2018, 7, 1–11. [Google Scholar]
- Russell, D.J. Ecology of the Imported Red Seaweed Eucheuma striatum Schmitz on Coconut Island, Oahu, Hawaii. Pac. Sci. 1983, 37, 87–107. [Google Scholar]
- Rodgers, K.S.; Cox, E.F. Rate of Spread of Introduced Rhodophytes Kappaphycus alvarezii, Kappaphycus striatum, and Gracilaria salicornia and Their Current Distribution in Kane’ohe Bay, O’ahu Hawai’i. Pac. Sci. 1999, 53, 232–241. [Google Scholar]
- Conklin, E.J.; Smith, J.E. Abundance and Spread of the Invasive Red Algae, Kappaphycus spp., in Kane’ohe Bay, Hawai’i and an Experimental Assessment of Management Options. Biol. Invasions 2005, 7, 1029–1039. [Google Scholar] [CrossRef]
- Price, N. Habitat Selection, Facilitation, and Biotic Settlement Cues Affect Distribution and Performance of Coral Recruits in French Polynesia. Oecologia 2010, 163, 747–758. [Google Scholar] [CrossRef]
- Carricart-Ganivet, J.P.; Cabanillas-Teran, N.; Cruz-Ortega, I.; Blanchon, P. Sensitivity of Calcification to Thermal Stress Varies Among Genera of Massive Reef-building Corals. PLoS ONE 2012, 7, e32859. [Google Scholar] [CrossRef] [PubMed]
- Grottoli, A.G. Variability of Stable Isotopes and Maximum Linear Extension in Reef-coral Skeletons at Kaneohe Bay, Hawaii. Mar. Biol. 1999, 135, 437–449. [Google Scholar] [CrossRef]
- Franklin, E.C.; Jokiel, P.L.; Donahue, M.J. Predictive Modeling of Coral Distribution and Abundance in the Hawaiian Islands. Mar. Ecol. Prog. Ser. 2013, 481, 121–132. [Google Scholar] [CrossRef]
- Franklin, E.C.; Jokiel, P.L.; Donahue, M.J. Data from: Predictive Modeling of Coral Distribution and Abundance in the Hawaiian Islands. Dryad Digit. Repos. 2013. [Google Scholar] [CrossRef]
- Forsman, Z.H.; Knapp, I.S.S.; Tisthammer, K.; Eaton, D.A.R.; Belcaid, M.; Toonen, R.J. Coral Hybridization or Phenotypic Variation? Genomic Data Reveal Gene Flow Between Porites lobata and P. Compressa. Mol. Phylogenet. Evol. 2017, 111, 132–148. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.; Conklin, E.J.; Minton, D.; Most, R.; Couch, C.S.; Williams, G.J.; Gove, J.M.; Dieter, T.; Schumacher, B.; Martinez, J.; et al. Relative Resilience Potential and Bleaching Severity in the West Hawai’i Habitat Focus Area in 2015; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Maragos, J.E. Reef Corals of Canton Atoll: II. Local distribution. In Phoenix Islands Report I. An. Environmental Survey of Canyon Atoll Lagoon 1973; Smith, S.V., Henderson, R.S., Eds.; Smithsonian Institute: Washington, DC, USA, 1978; Volume 221, pp. 73–97. [Google Scholar]
- Lacks, A. Reproductive Ecology and Distribution of the Scleractinian coral Fungia Scutaria in Kane’ohe bay, O’ahu, Hawai’i. Master’s Thesis, University of Hawaii, Manoa, HI, USA, 2000. [Google Scholar]
- Van Woesik, R.; Sakai, K.; Ganase, A.; Loya, Y. Revisiting the Winners and the Losers a Decade after Coral Bleaching. Mar. Ecol. Prog Ser. 2011, 434, 67–76. [Google Scholar] [CrossRef]
- Bahr, K.D.; Rodgers, K.S.; Jokiel, P.L. Relative Sensitivity of Five Hawaiian Coral Species to High Temperature Under High-pCO2 Conditions. Coral Reefs 2016, 35, 729–738. [Google Scholar] [CrossRef]
- Mayer, A.G. Is Death from High Temperature Due to the Accumulation of Acid in the Tissues? Am. J. Physiol. Leg. Content 1917, 44, 581–585. [Google Scholar] [CrossRef][Green Version]
- Mouillot, D.; Bellwood, D.R.; Baraloto, C.; Chave, J.; Galzin, R.; Harmelin-Vivien, M.; Kulbicki, M.; Lavergne, S.; Lavorel, S.; Paine, C.T.; et al. Rare Species Support Vulnerable Functions in High-diversity Ecosystems. PLoS Biol. 2013, 11, e1001569. [Google Scholar] [CrossRef] [PubMed]

| PERMANOVA | PERMDISP | |||||||
|---|---|---|---|---|---|---|---|---|
| Source | df | SS | MS | Pseudo-F | p-Value | Unique Perms | F | p-Value |
| Year | 1 | 25896 | 25896 | 5.3004 | 0.003 | 999 | 11.806 | 0.004 |
| Transect (Year) | 10 | 48975 | 4897.5 | 9.5632 | 0.001 | 998 | 9.8724 | 0.001 |
| Residuals | 108 | 55308 | 512.11 | |||||
| Total | 119 | 1.3004E+05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnhill, K.A.; Bahr, K.D. Coral Resilience at Malauka`a Fringing Reef, Kāneʻohe Bay, Oʻahu after 18 years. J. Mar. Sci. Eng. 2019, 7, 311. https://doi.org/10.3390/jmse7090311
Barnhill KA, Bahr KD. Coral Resilience at Malauka`a Fringing Reef, Kāneʻohe Bay, Oʻahu after 18 years. Journal of Marine Science and Engineering. 2019; 7(9):311. https://doi.org/10.3390/jmse7090311
Chicago/Turabian StyleBarnhill, Kelsey A., and Keisha D. Bahr. 2019. "Coral Resilience at Malauka`a Fringing Reef, Kāneʻohe Bay, Oʻahu after 18 years" Journal of Marine Science and Engineering 7, no. 9: 311. https://doi.org/10.3390/jmse7090311
APA StyleBarnhill, K. A., & Bahr, K. D. (2019). Coral Resilience at Malauka`a Fringing Reef, Kāneʻohe Bay, Oʻahu after 18 years. Journal of Marine Science and Engineering, 7(9), 311. https://doi.org/10.3390/jmse7090311






