Ultrasound Assisted Adsorptive Removal of Cr, Cu, Al, Ba, Zn, Ni, Mn, Co and Ti from Seawater Using Fe2O3-SiO2-PAN Nanocomposite: Equilibrium Kinetics
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Instrumentation
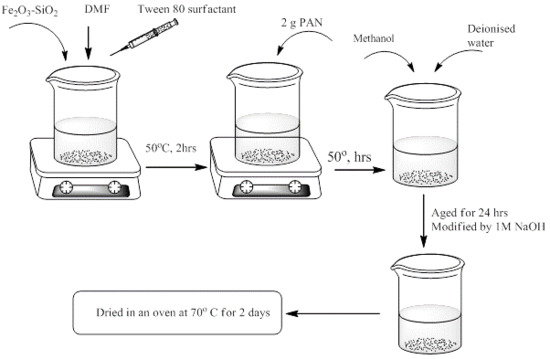
2.3. Synthesis of Fe2O3-SiO2 by Sol Gel Method
2.4. Preparation of Fe2O3-SiO2-PAN Nanocomposite
2.5. Optimization of the Adsorption Batch Method
2.6. Ultrasound Assisted Adsorptive Removal
2.7. Application to Real Samples
2.8. Data Analysis
3. Results
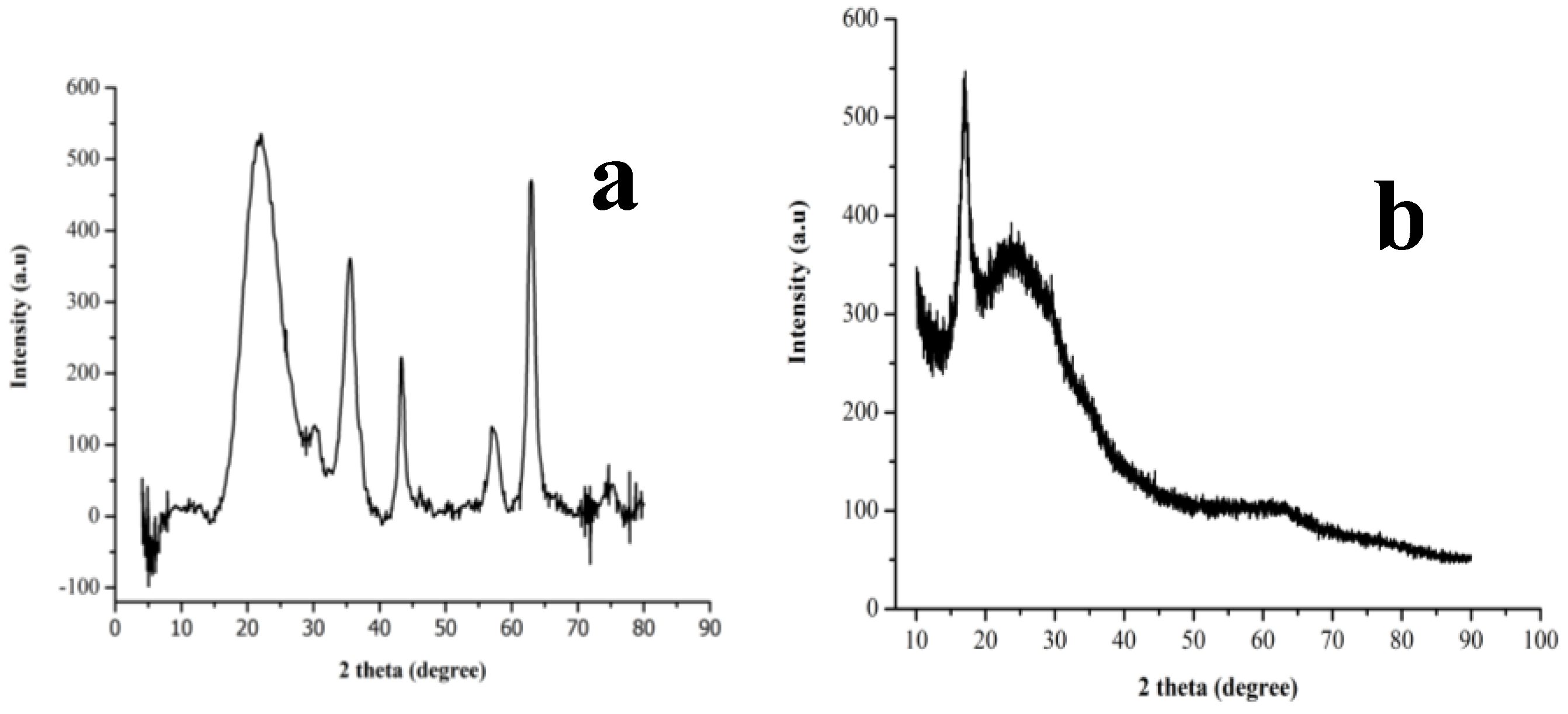
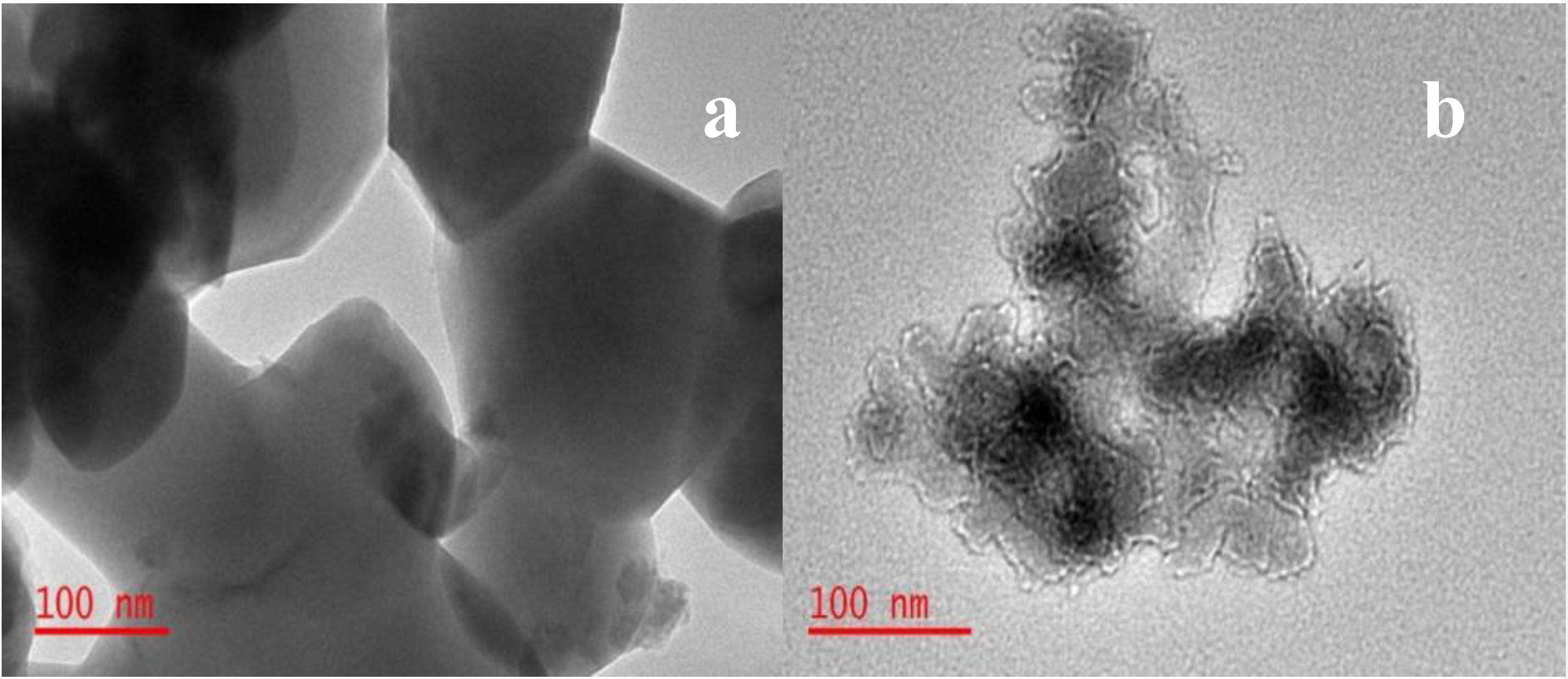
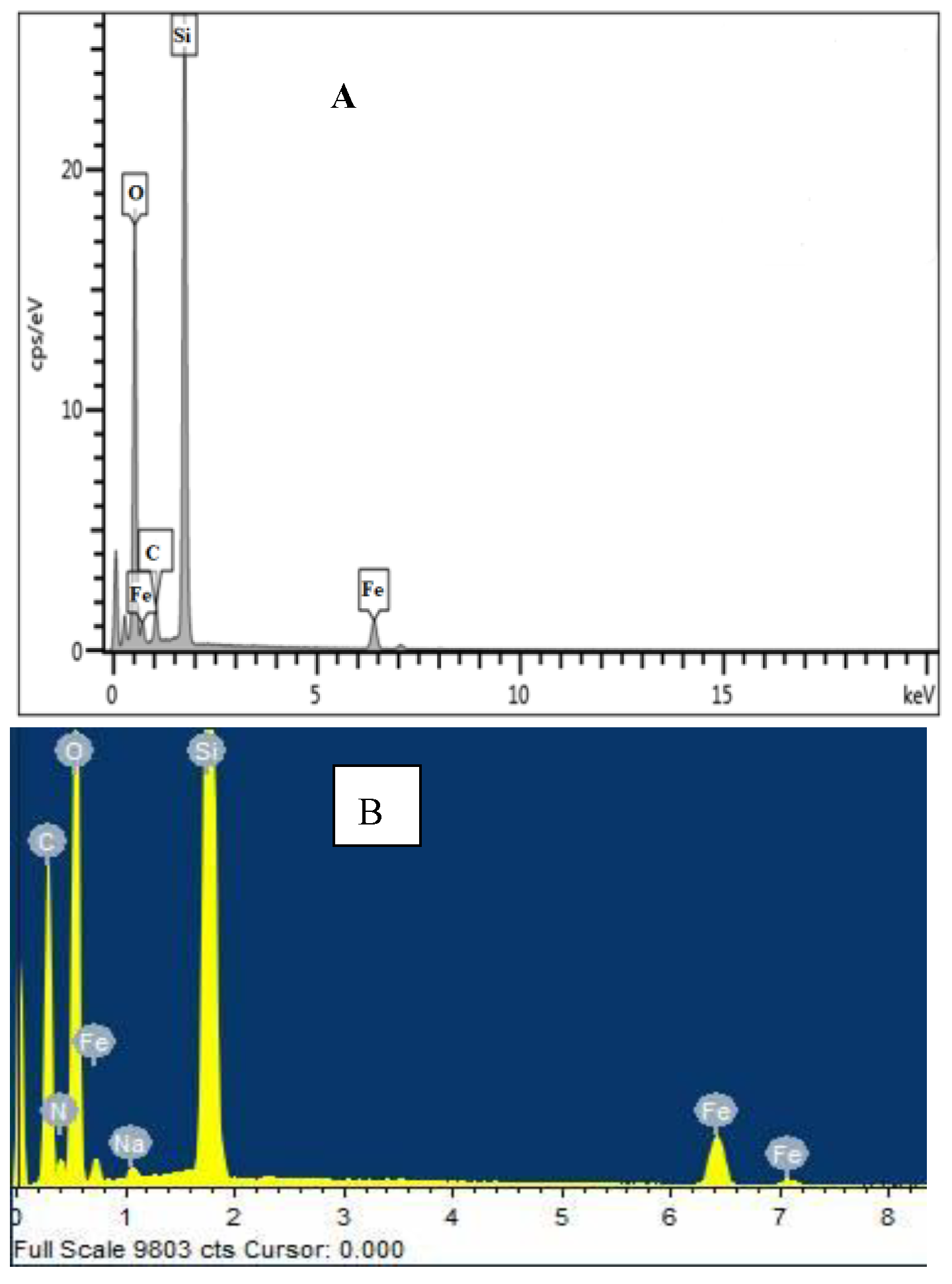
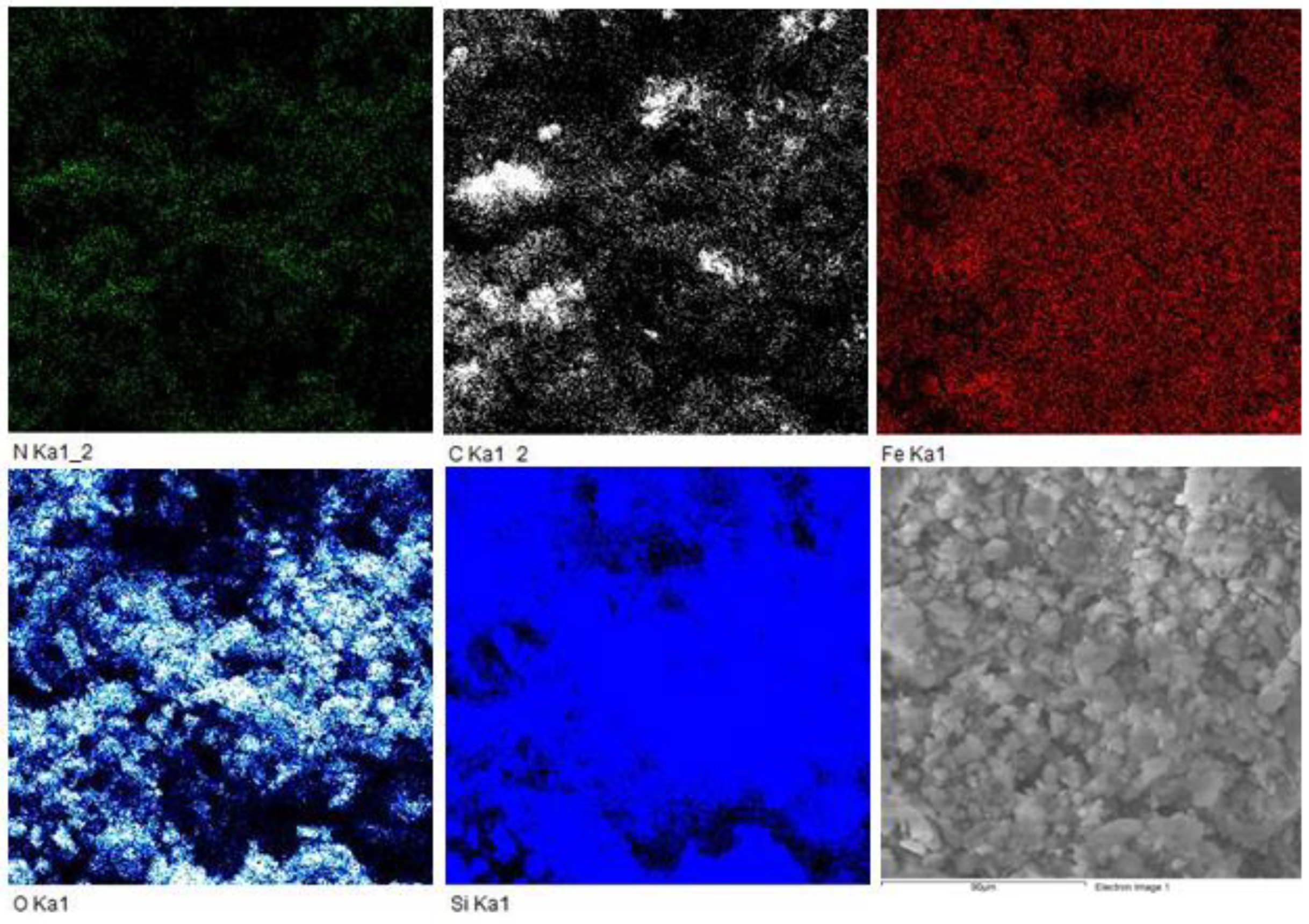
3.1. Surface Identification and Characterisation
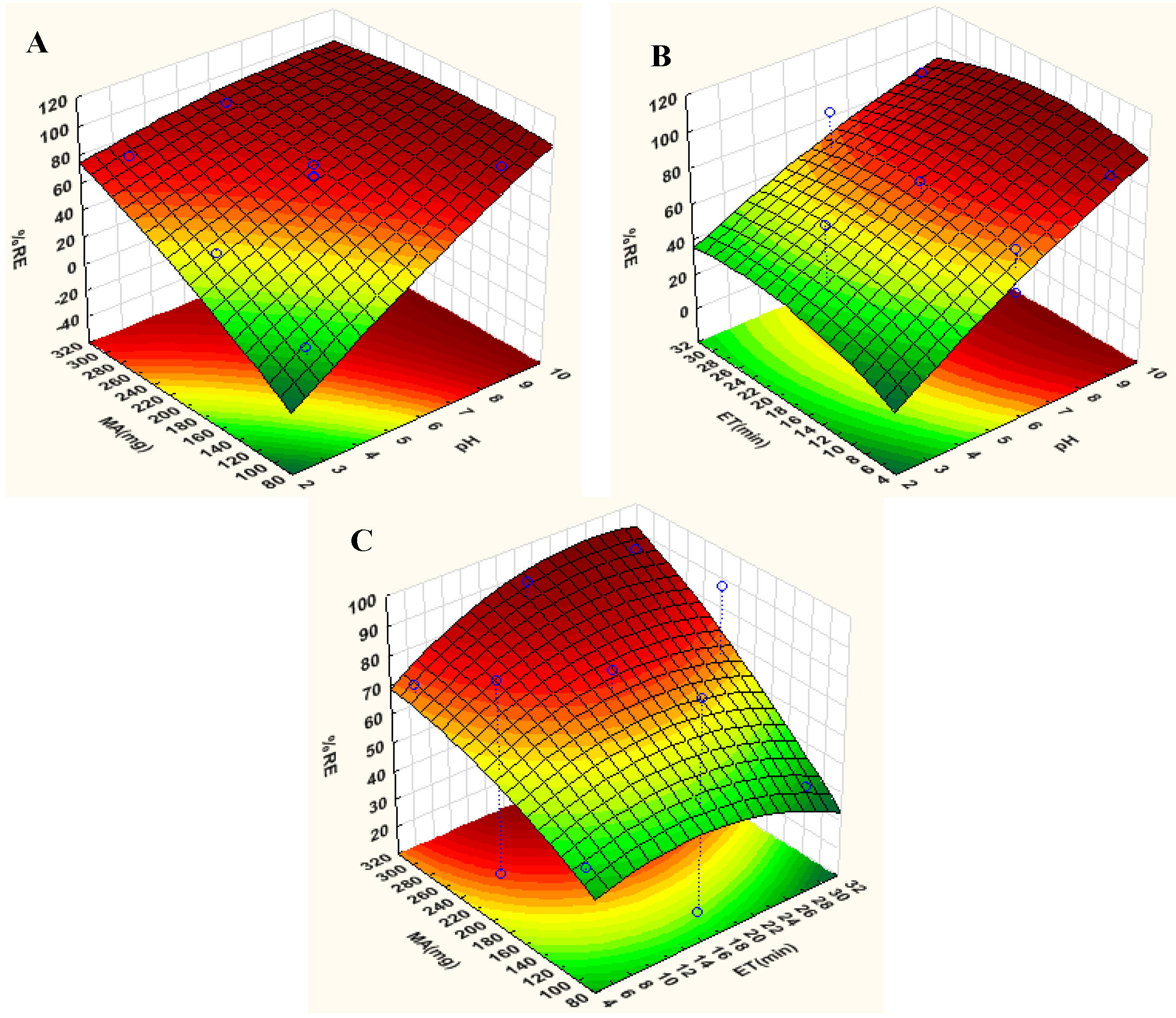
3.2. Optimization of the Adsorption Batch Method
3.3. Confirmatory Experiments and Adsorption Capacity
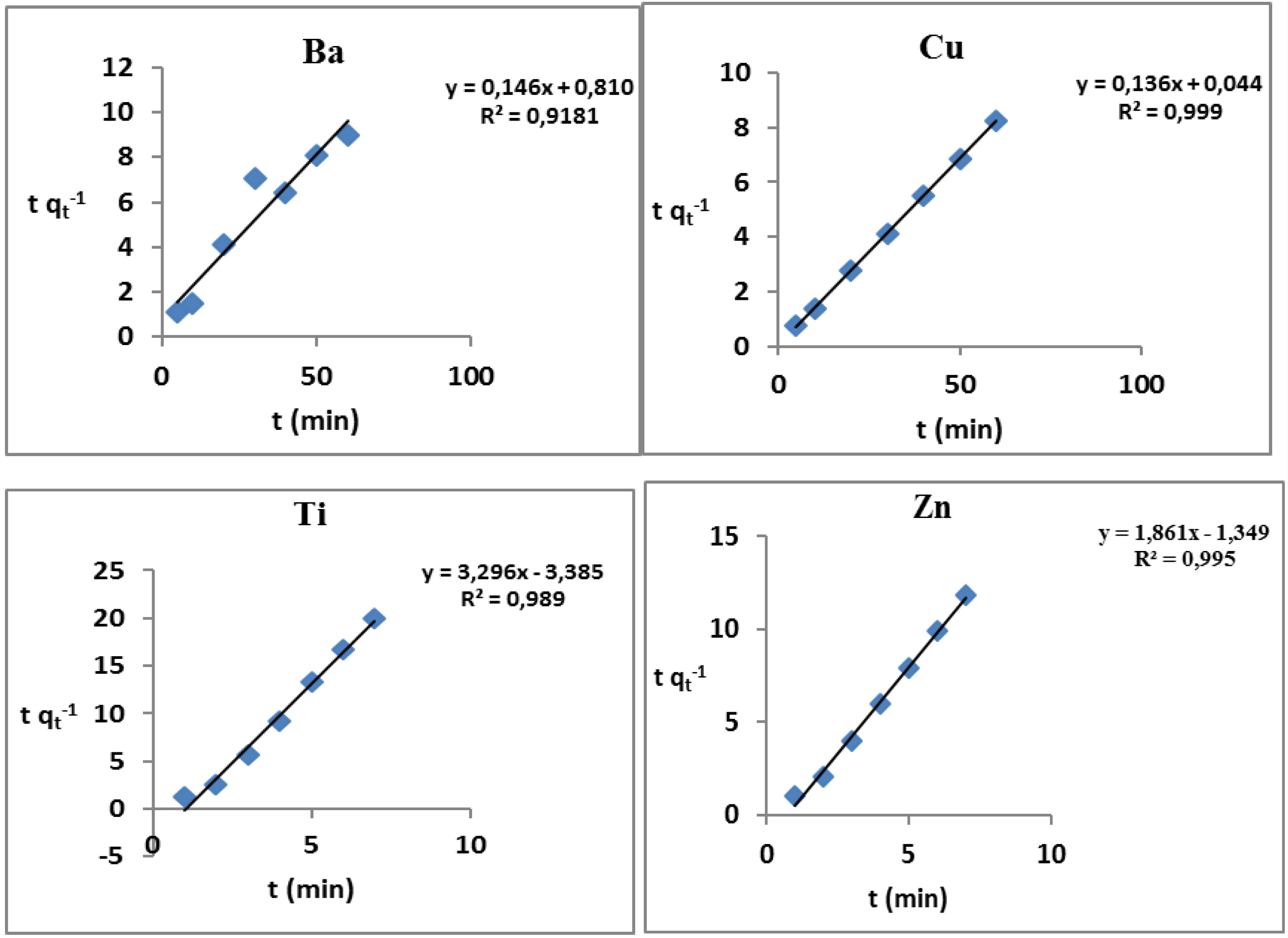
3.4. Adsorption Kinetics
3.5. Application of Fe2O3-SiO2-PAN in Real Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Gleick, P.H. Safe guarding our water making every drop count. Sci. Am. 2001, 284, 40–45. [Google Scholar] [CrossRef]
- Zuehlke, S.; Duennbier, U.; Heberer, T. Investigation of the behaviour and metabolism of pharmaceutical residues during purification of contaminated ground water used for drinking water supply. Chemosphere 2007, 69, 1673–1680. [Google Scholar] [CrossRef]
- Lyu, S.; Chen, W.; Zhang, W.; Fan, Y.; Jiao, W. Wastewater reclamation and reuse in China: opportunities and challenges. J. Environ. Sci. 2016, 39, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Bar-Zeev, E.; Berman-Frank, I.; Liberman, B.; Rahav, E.; Passow, U.; Berman, T. Transparent exopolymer particles: Potential agents for organic fouling and biofilm formation in desalination and water treatment plants. Desalin. Water Treat. 2009, 3, 136–142. [Google Scholar] [CrossRef]
- Ali, Z.; Malik, R.N.; Qadir, A. Heavy metals distribution and risk assessment in soils affected by tannery effluents. Chem. Ecol. 2013, 29, 676–692. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Hosamani, K.M.; Aminabhavi, T.M. Potential application of an electrodialysis pilot plant containing ion-exchange membranes in chromium removal. Desalination 2007, 217, 181–190. [Google Scholar] [CrossRef]
- Rodrigues, M.A.S.; Amado, F.D.R.; Xavier, J.L.N.; Streit, K.F.; Bernardes, A.M.; Ferreira, J.Z. Application of photoelectrochemical–electrodialysis treatment for the recovery and reuse of water from tannery effluents. J. Clean. Prod. 2008, 16, 605–611. [Google Scholar] [CrossRef]
- Ku, Y.; Jung, I.L. Photocatalytic reduction of Cr (VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Res. 2001, 35, 135–142. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Sohail, A.; Ali, S.I.; Khan, N.A.; Rao, R.A.K. Removal of chromium from wastewater by adsorption. J. Environ. Pollut. Control. 1999, 2, 27–31. [Google Scholar]
- Shaaban, S.; Yahya, H. Detailed analysis of reverse osmosis systems in hot climate conditions. Desalination 2017, 423, 41–51. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, J.; Zhang, W.; Wang, M.; Zhou, J. Kinetic studies of adsorption of Pb (II), Cr (III) and Cu (II) from aqueous solution by sawdust and modified peanut husk. J. Hazard. Mater. 2007, 141, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhu, L.; Xing, Y.; Wu, Y.; Gao, Y. Facile synthesis of Mn–Co oxide with a hierarchical porous structure for heavy metal removal. Mater. Lett. 2013, 108, 261–263. [Google Scholar] [CrossRef]
- Nyaba, L.; Matong, J.M.; Dimpe, K.M.; Nomngongo, P.N. Speciation of inorganic selenium in environmental samples after suspended dispersive solid phase microextraction combined with inductively coupled plasma spectrometric determination. Talanta 2016, 159, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Dimpe, K.M.; Nyaba, L.; Magoda, C.; Ngila, J.C.; Nomngongo, P.N. Synthesis, modification, characterization and application of AC@ Fe2O3@ MnO2 composite for ultrasound assisted dispersive solid phase microextraction of refractory metals in environmental samples. Chem. Eng. J. 2017, 308, 169–176. [Google Scholar] [CrossRef]
- Dimpe, K.M.; Ngila, J.C.; Nomngongo, P.N. Preparation and application of a tyre-based activated carbon solid phase extraction of heavy metals in wastewater samples. Phys. Chem. Earth Parts A/B/C 2018, 105, 161–169. [Google Scholar] [CrossRef]
- Zeng, L. A method for preparing silica-containing iron (III) oxide adsorbents for arsenic removal. Water Res. 2003, 37, 4351–4358. [Google Scholar] [CrossRef]
- Liu, B.; Wang, D.; Li, H.; Xu, Y.; Zhang, L. As (III) removal from aqueous solution using α-Fe2O3 impregnated chitosan beads with as (III) as imprinted ions. Desalination 2011, 272, 286–292. [Google Scholar] [CrossRef]
- Mishra, A.K. Nanomaterials for Water Remediation: Inorganic Oxide Materials; Smithers Rapra: Shrewsbury, UK, 2016; Volume 2. [Google Scholar]
- Ogoshi, T.; Chujo, Y. Synthesis of poly (vinylidene fluoride) (PVdF)/silica hybrids having interpenetrating polymer network structure by using crystallization between PVdF chains. Part A J. Polym. Sci. 2005, 43, 3543–3550. [Google Scholar] [CrossRef]
- Ko, J.; Lee, J.; Yoo, B.; Ryu, J.; Sohn, D. Capillarity-induced selective ex-situ synthesis of metal–organic framework inside mesoporous nanotubes. Micropor. Mesopor. Mater. 2016, 220, 16–20. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, H.; Wang, J.; Ling, Z.; Qiu, J. In-situ synthesis of chemically active ZIF coordinated with electrospun fibrous film for heavy metal removal with a high flux. Sep. Purif. Technol. 2017, 177, 257–262. [Google Scholar] [CrossRef]
- Lu, Q. Synthesis of PDMS-Metal Oxide Hybrid Nanocomposites Using an In-Situ Sol-Gel Route; Michigan Technological University: East Lansing, MI, USA, 2012. [Google Scholar]
- Feng, Y.; Wang, Y.; Wang, Y.; Zhang, X.F.; Yao, J. In-situ gelation of sodium alginate supported on melamine sponge for efficient removal of copper ions. J. Colloid Interf. Sci. 2018, 512, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, Z.; Abdolmaleki, A. Heavy metal remediation via poly (3, 4-ethylene dioxythiophene) deposition onto neat and sulfonated nonwoven poly (ether sulfone). J. Ind. Eng. Chem. 2017, 55, 164–172. [Google Scholar] [CrossRef]
- Park, H.S.; Kwak, S.H.; Mahardika, D.; Mameda, N.; Choo, K.H. Mixed metal oxide coated polymer beads for enhanced phosphorus removal from membrane bioreactor effluents. Chem. Eng. J. 2017, 319, 240–247. [Google Scholar] [CrossRef]
- Roosta, M.; Ghaedi, M.; Daneshfar, A.; Sahraei, R. Experimental design based response surface methodology optimization of ultrasonic assisted adsorption of safaranin O by tin sulfide nanoparticle loaded on activated carbon. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 122, 223–231. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Chiha, M.; Naffrechoux, E. Ultrasound-assisted removal of malachite green from aqueous solution by dead pine needles. Ultrason. Sonochem. 2008, 15, 799–807. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Hajati, S.; Goudarzi, A.; Bazrafshan, A.A. Simultaneous ultrasound-assisted ternary adsorption of dyes onto copper-doped zinc sulfide nanoparticles loaded on activated carbon: Optimization by response surface methodology. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 145, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Fernandes, J.P.; Carvalho, B.S.; Luchez, C.V.; Politi, M.J.; Brandt, C.A. Optimization of the ultrasound-assisted synthesis of allyl 1-naphthyl ether using response surface methodology. Ultrason. Sonochem. 2011, 18, 489–493. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Liu, Z.F. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef]
- Vojoudi, H.; Badiei, A.; Bahar, S.; Ziarani, G.M.; Faridbod, F.; Ganjali, M.R. A new nano-sorbent for fast and efficient removal of heavy metals from aqueous solutions based on modification of magnetic mesoporous silica nanospheres. J. Magn. Magn. Mater. 2017, 441, 193–203. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Bai, L.; Hu, H.; Fu, W.; Wan, J.; Cheng, X.; Zhuge, L.; Chen, Q. Synthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ions. J. Hazard. Mater. 2011, 195, 261–275. [Google Scholar] [CrossRef]
- Liu, J.; Ma, S.; Zang, L. Preparation and characterization of ammonium-functionalized silica nanoparticle as a new adsorbent to remove methyl orange from aqueous solution. Appl. Surf. Sci. 2013, 265, 393–398. [Google Scholar] [CrossRef]
- Ramutshatsha, D.; Ngila, J.C.; Ndungu, P.G.; Nomngongo, P.N. Simultaneous removal of Na, Ca, K and Mg from synthetic brine and seawater using Fe2O3-SiO2 mixed oxide nanostructures: Kinetics and isotherms studies. Desalin. Water Treat. 2018, 104, 206–216. [Google Scholar] [CrossRef]
- İnan, S.; Altaş, S. Preparation of zirconium–manganese oxide/polyacrylonitrile (Zr–Mn oxide/PAN) composite spheres and the investigation of Sr (II) sorption by experimental design. Chem. Eng. J. 2011, 168, 1263–1271. [Google Scholar] [CrossRef]
- Scharnagl, N.; Buschatz, H. Polyacrylonitrile (PAN) membranes for ultra-and microfiltration. Desalination 2001, 139, 191–198. [Google Scholar] [CrossRef]
- Almasian, A.; Najafi, F.; Maleknia, L. Mesoporous MgO/PPG hybrid nanofibers: Synthesis, optimization, characterization and heavy metal removal property. New J. Chem. 2018, 42, 2013–2029. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, H.; Yu, D.; Wu, C.; Zhang, Y.; White, C.J.B.; Zhu, L. Surface modification of electrospunpolyacrylonitrile nanofiber towards developing an affinity membrane for bromelain adsorption. Desalination 2010, 256, 141–147. [Google Scholar] [CrossRef]
- Panda, N.; Sahoo, H.; Mohapata, S. Decolourization of methyl orange using Fenton-like mesoporous Fe2O3–SiO2 composite. J. Hazard. Mater. 2011, 185, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, M.; Maity, A.; Srinivasu, V.V.; Onyango, M.S. Enhanced removal of Cr (VI) from aqueous solution using polypyrrole/Fe3O4 magnetic nanocomposite. J. Hazard. Mater. 2011, 190, 381–390. [Google Scholar] [CrossRef]
- Setshedi, K.Z.; Bhaumik, M.; Onyango, M.S.; Maity, A. High-performance towards Cr (VI) removal using multi-active sites of polypyrrole–graphene oxide nanocomposites: Batch and column studies. Chem. Eng. J. 2015, 262, 921–931. [Google Scholar] [CrossRef]
- Teo, H.T.; Siah, W.R.; Yuliati, L. Enhanced adsorption of acetylsalicylic acid over hydrothermally synthesized iron oxide-mesoporous silica MCM-41 composites. J. Taiwan Inst. Chem. Eng. 2016, 65, 591–598. [Google Scholar] [CrossRef]
- Munonde, T.S.; Maxakato, N.W.; Nomngongo, P.N. Preconcentration and speciation of chromium species using ICP-OES after ultrasound-assisted magnetic solid phase extraction with an amino-modified magnetic nanocomposite prepared from Fe3O4, MnO2 and Al2O3. Mikrochim. Acta 2017, 184, 1223–1232. [Google Scholar] [CrossRef]
- Naushad, M. Surfactant-assisted nano-composite cation exchanger: Development, characterization and applications for the removal of toxic Pb2+ from aqueous medium. Chem. Eng. J. 2014, 235, 100–108. [Google Scholar] [CrossRef]
- Lagergren, S. Zurtheorie der sogenannten adsorption gelosterstoffe, Kungligasvenskavetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Nomngongo, P.N.; Ngila, J.C.; Msagati, T.A.; Moodley, B. Kinetics and equilibrium studies for the removal of cobalt, manganese, and silver in ethanol using Dowex 50W-x8 cation exchange resin. Sep. Sci. Technol. 2014, 49, 1848–1859. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Equilibria and capacities for adsorption on carbon. J. Sanit. Eng. Div. 1964, 90, 79–108. [Google Scholar]
- Su, S.; Che, R.; Liu, Q.; Liu, J.; Zhang, H.; Li, R.; Wang, J. ZeoliticImidazolate Framework-67: A promising candidate for recovery of uranium (VI) from seawater. Colloids Surf. A Physicochem. Eng. Asp. 2018, 547, 73–80. [Google Scholar] [CrossRef]
- Hong, H.J.; Kim, B.G.; Ryu, J.; Park, I.S.; Chung, K.S.; Lee, S.M.; Ryu, T. Preparation of highly stable zeolite-alginate foam composite for strontium (90 Sr) removal from seawater and evaluation of Sr adsorption performance. J. Environ. Manag. 2018, 205, 192–200. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Ruiz, M.; Nogueras, M.; Sastre, A.M.; Guibal, E. Boron recovery from seawater with a new low-cost adsorbent material. Chem. Eng. J. 2014, 254, 463–471. [Google Scholar] [CrossRef]
- AlSuhaimi, A.O.; AlRadaddi, S.M.; Ali, A.K.A.S.; Shraim, A.M.; AlRadaddi, T.S. Silica-based chelating resin bearing dual 8-Hydroxyquinoline moieties and its applications for solid phase extraction of trace metals from seawater prior to their analysis by ICP-MS. Arab. J. Chem. 2017, 12, 360–369. [Google Scholar] [CrossRef]
- Trujillo, I.S.; Alonso, E.V.; de Torres, A.G.; Pavón, J.M.C. Development of a solid phase extraction method for the multielement determination of trace metals in natural waters including sea-water by FI-ICP-MS. Microchem. J. 2012, 101, 87–94. [Google Scholar] [CrossRef]
- Lo, S.F.; Wang, S.Y.; Tsai, M.J.; Lin, L.D. Adsorption capacity and removal efficiency of heavy metal ions by Moso and Ma bamboo activated carbons. Chem. Eng. Res. Des. 2012, 90, 1397–1406. [Google Scholar] [CrossRef]
- Athanasiadis, K.; Helmreich, B. Influence of chemical conditioning on the ion exchange capacity and on kinetic of zinc uptake by clinoptilolite. Water Res. 2005, 39, 1527–1532. [Google Scholar] [CrossRef]
- Argun, M.E. Use of clinoptilolite for the removal of nickel ions from water: Kinetics and thermodynamics. J. Hazard. Mater. 2008, 150, 587–595. [Google Scholar] [CrossRef]
- Vaseghi, Z.; Nematollahzadeh, A.; Tavakoli, O. Plant-mediated Cu/Cr/Ni nanoparticle formation strategy for simultaneously separation of the mixed ions from aqueous solution. J. Taiwan Inst. Chem. Eng. 2019, 96, 148–159. [Google Scholar] [CrossRef]
- Hegazi, H.A. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013, 9, 276–282. [Google Scholar] [CrossRef]
- Syukor, A.A.; Sulaiman, S.; Siddique, M.N.I.; Zularisam, A.W.; Said, M.I.M. Integration of phytogreen for heavy metal removal from wastewater. J. Clean. Prod. 2016, 112, 3124–3131. [Google Scholar] [CrossRef]


| Variables | Low Level (−) | Central Points (0) | High Level (+) |
|---|---|---|---|
| pH | 3.00 | 6.00 | 9.00 |
| Extraction time (ET) (min) | 5.00 | 17.5 | 30.0 |
| Mass of adsorbent (MA) (mg) | 100 | 200 | 300 |
| Materials | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| Fe2O3-SiO2 PAN | 253 32.0 | 0.96 0.26 | 14.4 35.4 |
| Fe2O3-SiO2-PAN | 158 | 0.53 | 22.1 |
| pH | ET | MA | %Re | |
|---|---|---|---|---|
| 1 | 3.00 | 5.00 | 200 | 25.5 |
| 2 | 9.00 | 5.00 | 200 | 91.7 |
| 3 | 3.00 | 30.0 | 200 | 43.5 |
| 4 | 9.00 | 30.0 | 200 | 92.6 |
| 5 | 3.00 | 17.5 | 100 | 14.1 |
| 6 | 9.00 | 17.5 | 100 | 87.4 |
| 7 | 3.00 | 17.5 | 300 | 76.6 |
| 8 | 9.00 | 17.5 | 300 | 92.4 |
| 9 | 6.00 | 5.00 | 100 | 48.0 |
| 10 | 6.00 | 30.0 | 100 | 40.6 |
| 11 | 6.00 | 5.00 | 300 | 72.2 |
| 12 | 6.00 | 30.0 | 300 | 89.2 |
| 13 | 6.00 | 17.5 | 200 | 51.1 |
| 14 | 6.00 | 17.5 | 200 | 79.3 |
| 15 | 6.00 | 17.5 | 200 | 70.8 |
| Pseudo-First Order | ||||
| Ions | qeexp | k1 (min−1) | qe (mg g−1) | R2 |
| Al3+ | 2.23 | 0.26 | 098 | 0.564 |
| Ba2+ | 6.60 | 0.15 | 1.20 | 0.606 |
| Cr3+ | 4.36 | 0.01 | 0.90 | 0.227 |
| Cu2+ | 7.20 | 0.02 | 3.40 | 0.389 |
| Co2+ | 6.65 | 0.23 | 1.30 | 0.899 |
| Mn2+ | 6.79 | 0.31 | 0.76 | 0.910 |
| Ti3+ | 3.00 | 0.05 | 1.40 | 0.874 |
| Ni2+ | 2.60 | 0.06 | 0.58 | 0.305 |
| Zn2+ | 5.06 | 0.01 | 0.72 | 0.824 |
| Pseudo-Second Order | ||||
| Ions | qe (mg g−1) | k2 (g mg−1 min−1) | t1/2 (min) | R2 |
| Al3+ | 2.16 | 1.00 | 0.48 | 0.999 |
| Ba2+ | 6.80 | 0.03 | 4.90 | 0.918 |
| Cr3+ | 4.34 | 7.50 | 0.03 | 0.999 |
| Cu2+ | 7.35 | 0.42 | 0.32 | 0.999 |
| Co2+ | 7.04 | 0.02 | 8.71 | 0.800 |
| Mn2+ | 3.22 | 0.02 | 0.31 | 0.611 |
| Ti3+ | 2.86 | 0.13 | 2.63 | 0.998 |
| Ni2+ | 2.51 | 0.18 | 2.17 | 0.994 |
| Zn2+ | 5.10 | 0.69 | 0.28 | 0.999 |
| Ions | kid (g/mg min1/2) | Qe (mg g−1) | R2 |
|---|---|---|---|
| Al | 0.05 | 2.07 | 0.987 |
| Ba | 1.13 | 1.16 | 0.832 |
| Cr | 0.03 | 4.43 | 0.753 |
| Cu | 0.32 | 6.85 | 0.515 |
| Co | 1.01 | 1.20 | 0.908 |
| Mn | 1.38 | 6.27 | 0.949 |
| Ti | 0.23 | 5.10 | 0.951 |
| Ni | 1.00 | 1.64 | 0.660 |
| Zn | 0.03 | 4.91 | 0.907 |
| Analytes | Initial Concentration (µg L−1) | Final Concentration (µg L−1) a | %RE |
|---|---|---|---|
| Al3+ | 203 | 2.79 | 98.6 |
| Ba2+ | 0.991 | 0.003 | 99.7 |
| Cr3+ | 0.270 | 0.002 | 99.2 |
| Cu2+ | 17.2 | 0.077 | 99.6 |
| Co2+ | 2.17 | 0.002 | 99.9 |
| Mn2+ | 1.49 | 0.002 | 99.9 |
| Ti3+ | 9.55 | 0.010 | 99.9 |
| Ni2+ | 65.3 | 1.36 | 98.0 |
| Zn2+ | 34.8 | 0.086 | 99.8 |
| Analytes | Adsorbents | Removal Efficiency (%) | Ref. |
|---|---|---|---|
| Pb (II), Cu (II), Cr (II), Cd (II) | Mabamboo activated carbon | 99.9, 100, 96.4, 98.2 | [57] |
| Zn (II) | Clinoptilolite | 100 | [58] |
| Ni (II) | Clinoptilolite | 93.6 | [59] |
| Cu (II), Cr (II), Ni (II) | Eryngium campestre | 98.9, 98.2, 93.4 | [60] |
| Fe, Pb, Cd, Cu, Ni | Fly ash | 86.8, 76.1, 73.5, 98.6, 96.0 | [61] |
| Cd, Cr, Mn, Cu, Ni, Pb, Zn, Fe | Aquatic plants | 61.1, 69.2, 68.0, 79.1, 74.9, 62.1, 63.0, 81.2 | [62] |
| Cr3+ Cu2+, Al3+, Ba2+, Zn2+, Ni2+, Mn2+, Co2+, Ti3+ | Fe2O3-SiO2-PAN | 99.2, 99.6, 98.6, 99.7, 99.8, 98.0, 99.9, 99.9, 99.9 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramutshatsha-Makhwedzha, D.; Ngila, J.C.; Ndungu, P.G.; Nomngongo, P.N. Ultrasound Assisted Adsorptive Removal of Cr, Cu, Al, Ba, Zn, Ni, Mn, Co and Ti from Seawater Using Fe2O3-SiO2-PAN Nanocomposite: Equilibrium Kinetics. J. Mar. Sci. Eng. 2019, 7, 133. https://doi.org/10.3390/jmse7050133
Ramutshatsha-Makhwedzha D, Ngila JC, Ndungu PG, Nomngongo PN. Ultrasound Assisted Adsorptive Removal of Cr, Cu, Al, Ba, Zn, Ni, Mn, Co and Ti from Seawater Using Fe2O3-SiO2-PAN Nanocomposite: Equilibrium Kinetics. Journal of Marine Science and Engineering. 2019; 7(5):133. https://doi.org/10.3390/jmse7050133
Chicago/Turabian StyleRamutshatsha-Makhwedzha, Denga, Jane Catherine Ngila, Patrick G. Ndungu, and Philiswa Nosizo Nomngongo. 2019. "Ultrasound Assisted Adsorptive Removal of Cr, Cu, Al, Ba, Zn, Ni, Mn, Co and Ti from Seawater Using Fe2O3-SiO2-PAN Nanocomposite: Equilibrium Kinetics" Journal of Marine Science and Engineering 7, no. 5: 133. https://doi.org/10.3390/jmse7050133
APA StyleRamutshatsha-Makhwedzha, D., Ngila, J. C., Ndungu, P. G., & Nomngongo, P. N. (2019). Ultrasound Assisted Adsorptive Removal of Cr, Cu, Al, Ba, Zn, Ni, Mn, Co and Ti from Seawater Using Fe2O3-SiO2-PAN Nanocomposite: Equilibrium Kinetics. Journal of Marine Science and Engineering, 7(5), 133. https://doi.org/10.3390/jmse7050133