Karenia brevis is a marine dinoflagellate which naturally produces a suite of neurotoxins known as brevetoxins. In humans, brevetoxins can cause respiratory distress following exposure to aerosols, gastrointestinal distress, and mild neurological symptoms following consumption of contaminated shellfish. In addition to potential human health effects, blooms of
K. brevis can cause significant economic and environmental impacts. Monitoring
K. brevis abundance is well established in Gulf of Mexico waters with blooms reported frequently in Florida and Texas, and more recently in the northern Gulf of Mexico [
1]. Routine monitoring efforts rely on light microscopy, and the characteristic cellular morphology is a cornerstone of
Karenia identification. Likewise, accurate quantification of cell abundance in the water column is essential for regulatory monitoring in coastal waters. For
K. brevis, a threshold of 5000 cells per liter has been recommended by the U.S. National Shellfish Sanitation Program [
2] to close shellfish harvest areas in U.S. waters. This guideline relies on the accurate identification and enumeration of
K. brevis cells and initiates monitoring of waters and shellfish meats for the presence of brevetoxins.
The morphology of the unarmored dinoflagellate
K. brevis has been described in detail elsewhere (e.g.,
Gymnodinium breve in [
3],
K. brevis in [
4]). A typical description of
K. brevis addresses the cell shape as dorso-ventrally flattened, ventrally concave, with a straight apical groove. Cells are wider than they are long (20–40 µm), with a round nucleus located in the posterior left quadrant, and consist of peripheral chloroplasts. Two flagella are located at the equatorial cingulum and descend approximately two times the length of the cell. The last characteristic defining
K. brevis is the sulcus that extends to the epicone. As with many phytoplankton species, other factors including geographic location, salinity preference, toxin profile, pigment profile, and even behavior (e.g., swimming behavior, bloom dynamics) can be key to accurate identification. The salinity optimum for
K. brevis growth and proliferation has been reported as 30–35 ppt; however, blooms have been detected in salinities as low as 5 ppt in northern Gulf of Mexico [
5]. In cultures,
Karenia spp. tolerate salinities between 17.5 to 45 ppt depending on the clone [
5]. In terms of photo-pigments,
K. brevis contains gyroxanthin, high levels of fucoxanthin, and other accessory pigments, rather than carotenoid peridinin, which is utilized by other dinoflagellates [
6]. The shape of the cell and the location and shape of the nucleus are the most distinctive characteristics of
Karenia spp. and are often the deciding attributes for
K. brevis identification. The unique shape of
K. brevis allows identification even at relatively low resolution, an advantageous characteristic for manual and automated counting (e.g., FlowCam particle analysis, Imaging flow cytobot). In addition, the majority of preservation techniques do not significantly alter the cell shape, which increases taxonomic confidence based solely on shape.
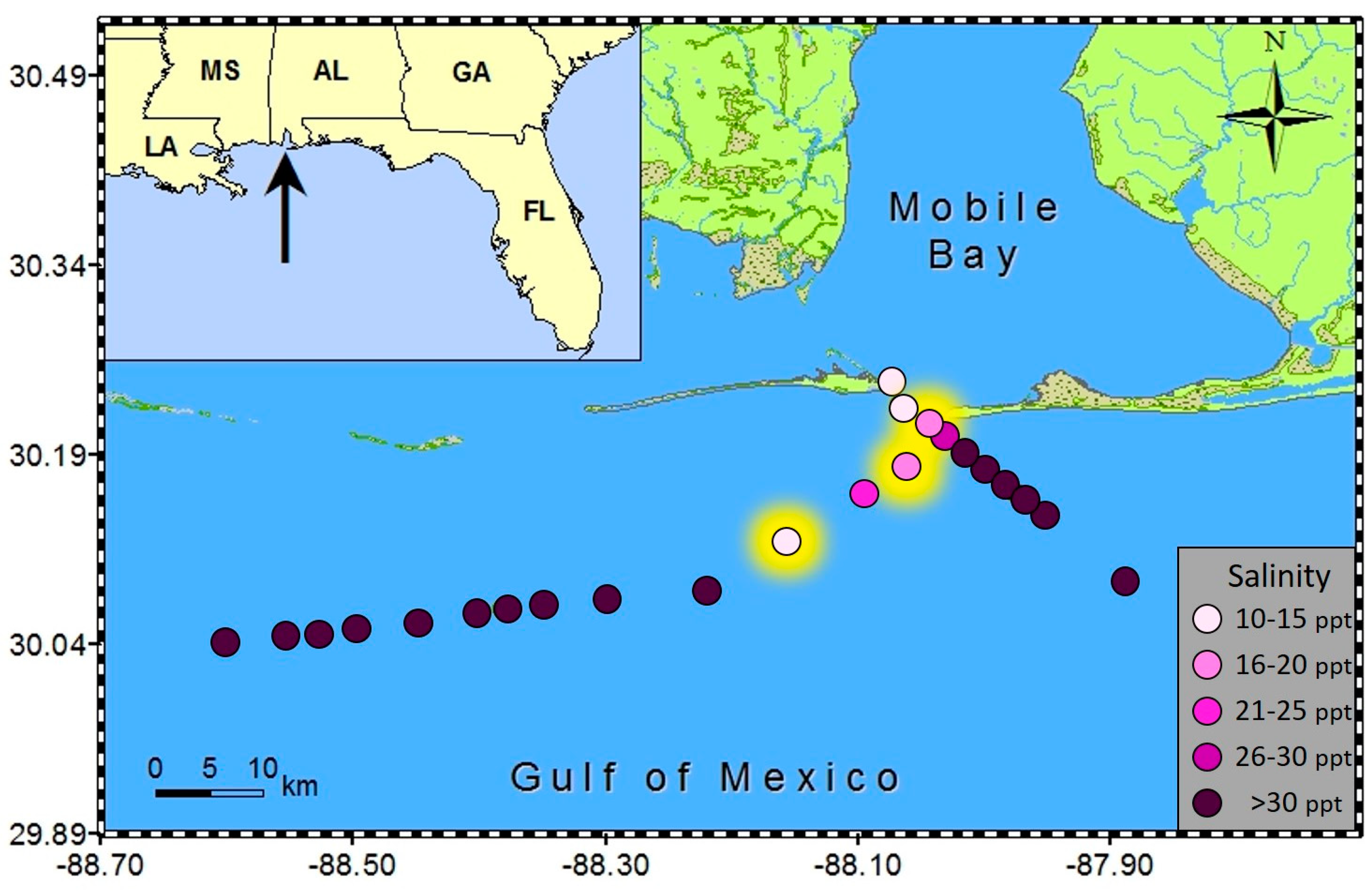
During blooms of
K. brevis in Alabama coastal waters (late fall 2015) we conducted several opportunistic cruises. During two of these offshore sampling events (11/30/15 and 12/1/15), we collected 23 surface water samples in transects southeast of Dauphin Island and westward parallel to the AL and MS coastline, respectively (
Figure 1). Subsamples were immediately preserved for phytoplankton identification in both Lugols and glutaraldehyde using standard techniques and remaining non-preserved whole water allowed preparation samples for subsequent brevetoxin analysis (particulate toxin levels from water filtered on GF/F (Whatman), dissolved toxin from filtrate, and total toxin from non-filtered seawater). Preserved and non-preserved cells were identified and enumerated by light microscopy (OM900 inverted light microscope at 400×, Meiji Techno America, CA, USA). During these sampling events, we identified not only
K. brevis and
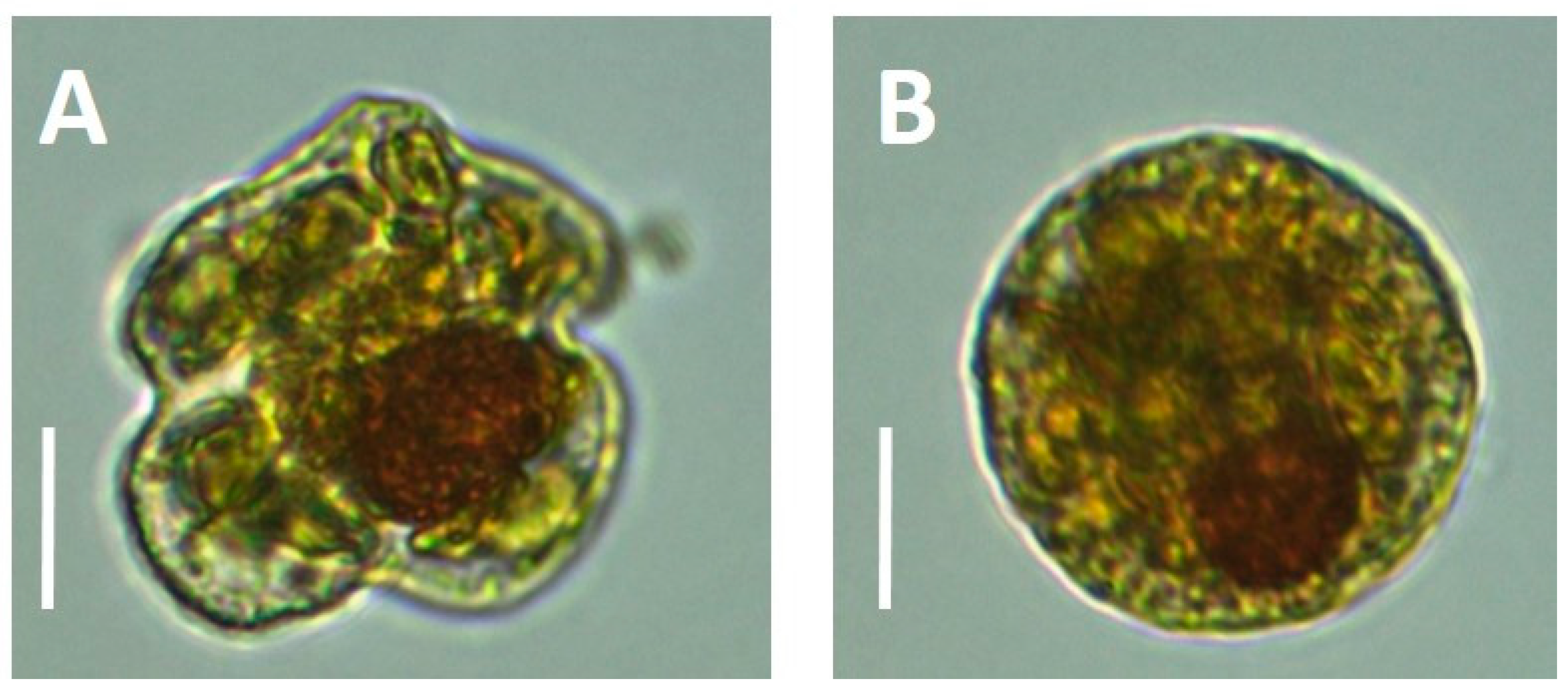
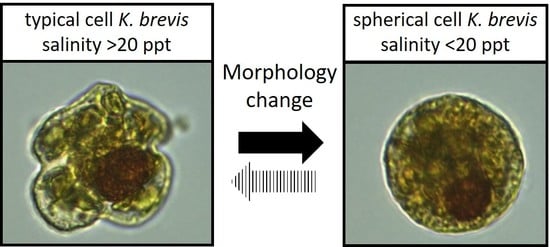
K. mikimotoi (a smaller species of equal length and width but with an oblong nucleus), but also encountered spherical cells of what we believe to be atypical
K.
brevis. These “balls” contained many characteristic identifiers of
K. brevis, including the production of brevetoxins. By microscopy, the cells were of similar volume, possessing a large visible, spherical nucleus, and chloroplasts that were similar in shape and color to regular
K. brevis cells found in adjacent samples (
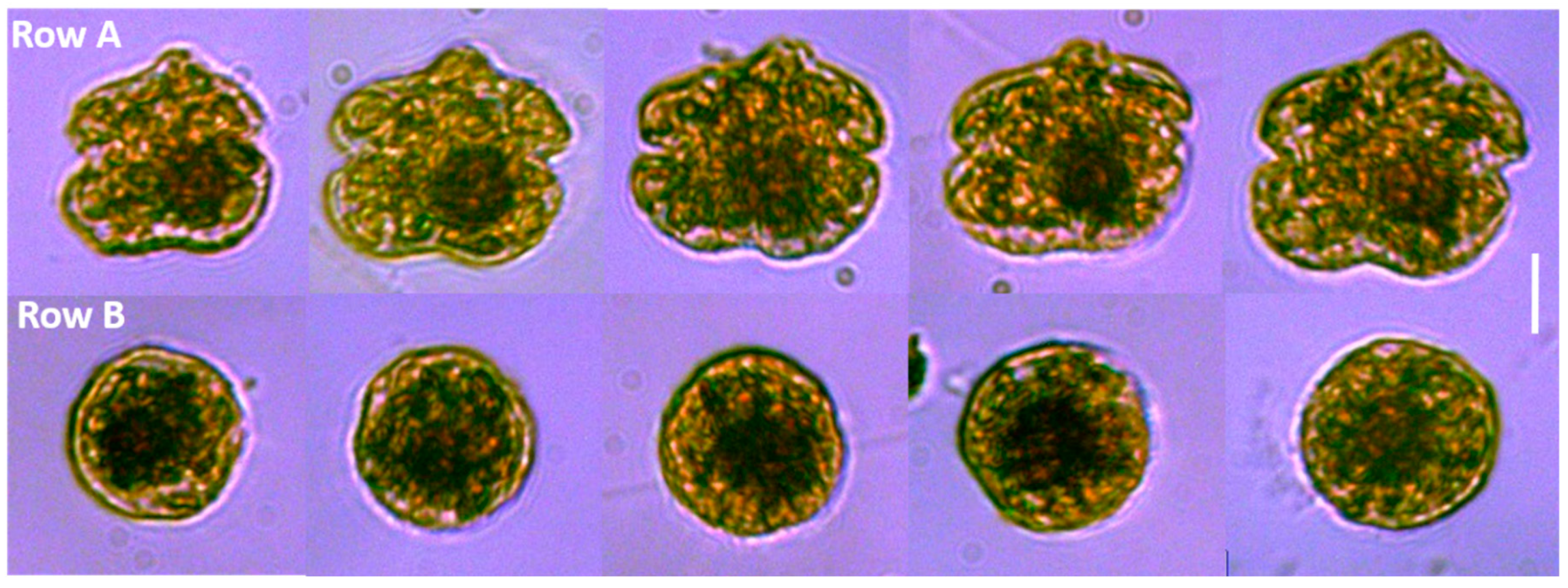
Figure 2). The typical
K. brevis cells exhibited higher variability in cell size with mean length of 24.6 ± 1.5 µm and mean width of 28.3 ± 2.9 µm (
n = 20). Spherical cells showed very consistent size with a mean width (diameter) of 22.4 ± 0.8 µm (
n = 20). We were not able to measure the volume of the cells accurately by light microscopy, so we were not able to statistically determine relationships with volume; however, the volume of the sphere and typical
K. brevis were similar (
Figure 3). Brevetoxin (PbTx) concentrations in the field collected samples (particulate, dissolved, total) were quantified using enzyme linked immunosorbent assay (ELISA; Abraxis, NJ, USA), a specific and highly sensitive method that cross reacts with several PbTX congeners (i.e., PbTX-3 100%; deoxyPbTX-2 133%; PbTX-5 127%; PbTX-2 102%; PbTX-9 83%; PbTX-6 13%; and PbTX-1 5%) but does not cross react with other shellfish toxins such as saxitoxins or domoic acid (Abraxis), or the functionally related ciguatoxins (also tested during this study). The analyses revealed that field samples containing the spherical cells contained PbTx’s, ranging from 3 to 7 ng/ml PbTx-3 equivalents. This was comparable to water samples containing cells of typical
K. brevis morphology but without spherical cells which had toxin concentrations ranging from 1 to 10 ng/mL PbTx-3 equivalents.
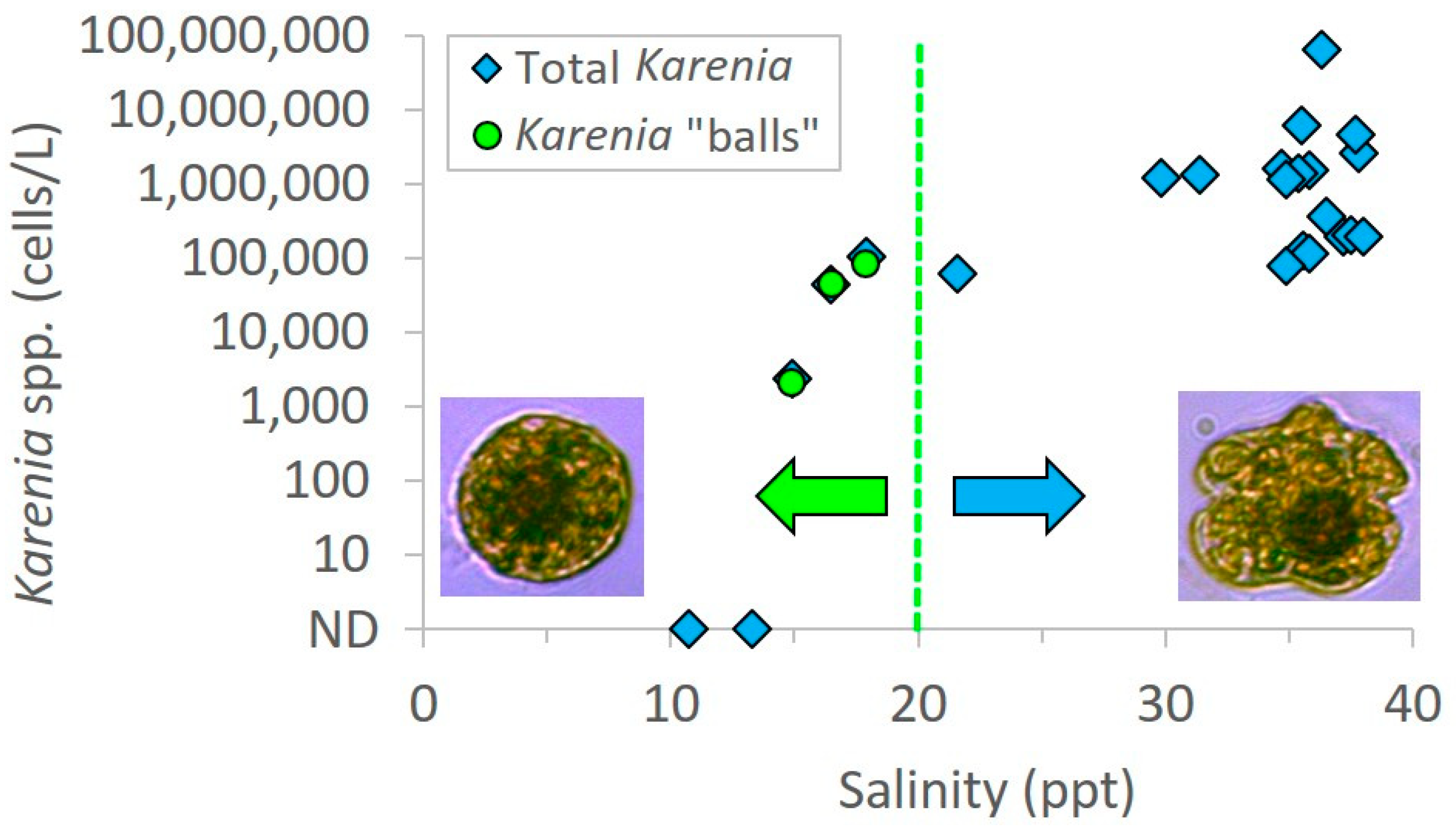
Salinity was below 20 ppt in all three samples in which spherical shaped cells were encountered. Specifically, the salinities were 16.5 ppt, 14.9 ppt, and 17.9 ppt, while surrounding samples in the same transects ranged from 21.6 to 38 ppt (
Figure 4). Patchy surface salinity and relatively rapid rates of change are common in coastal Alabama waters [
7]. There were two samples at very low salinities (10.7 and 13.3 ppt) that did not contain
Karenia “balls”; however, these samples were also lacking any regular
Karenia cells that would serve as a necessary precursor for “ball” formation (
Figure 4). These two water samples contained typical brackish species of phytoplankton. The most dominant dinoflagellate was
Prorocentrum minimum and
Heterocapsa rotundata; both species are associated with freshwater discharge and are typical in coastal waters. These dinoflagellate species are not known to produce brevetoxins which were supported by negative PbTX tests. Since water temperatures were consistent across sampling sites in the transects, and brevetoxins were confirmed in samples containing only the “balls”, we hypothesize that the spherical cells could be a rapid morphological response to low salinity stress of
K. brevis, which was blooming in the vicinity and confirmed in adjacent high salinity waters. This is supported by the lack of spheres in water samples collected in adjacent high salinity waters; however we acknowledge that other factors could also be involved in this response and should be evaluated in detail in future culture studies.
In the samples containing spheres, 90–100% of cells were in the sphere stage, with only a small portion of the cells maintaining the “traditional” K. brevis morphology. Importantly, when present, these “balls” were found in non-preserved, Lugol’s preserved, and glutaraldehyde preserved samples, indicating that the preservation method did not induce sphere formation from these field samples. Since brevetoxins were confirmed in samples containing only spheres (in total, particulate, and dissolved partitions), there were morphological similarities between typical K. brevis and spherical cells, and spheres were detected in waters adjacent to K. brevis blooms, we speculate that a rapid drop in salinity caused by freshwater plumes from the Mobile Bay estuary triggered a physiological response and Karenia cells entered an “emergency shut down” sphere stage, similar to first steps of dinoflagellate cyst development. For Karenia cells to go through this dramatic physiological change, significant re-organization of the cytoskeletal proteins would be involved. This brings two additional questions to this possible phenomenon, (1) what is the benefit to this response (e.g., increased resistance or survivability)? and (2) is this process reversible when cells return to optimal environmental conditions (i.e., akin to a temporary pellicle cyst)? Unfortunately, we are unable to answer these questions at this time, but future efforts with cultured isolates may enhance our understanding of these processes.
Sphere-shaped
K. brevis has been previously reported in culture experiments and were associated with aging cultures [
8], reproductive cells [
9], a cyst-like stage [
10], and with cell desiccation under the microscope [
10]. However, morphological changes identified in field samples have not been well documented, and may represent an important anomaly in
K. brevis bloom dynamics, toxin production by cells, or growth cycle. Experienced taxonomists have communicated seeing these effects following salinity drop below 25 ppt (e.g., Wolny, 2015 pers. comm.) but these observations have not been reported in the literature. Brown et al. [
5] highlighted that the salinity optima of
K. brevis is highly dependent on cultured clones, but in natural waters
K. brevis generally prefers salinities above 30 ppt. It is expected that increased osmotic pressure as a result of low salinity would cause the cell volume to increase, and several
Karenia toxicity studies have documented significant changes in cell volume as a response to a salinity drop. For example, Sunda et al. [
11] detected an initial increase in cell volume by 17% when
K. brevis cells acclimated at 35 ppt, and were then exposed to a salinity of 27 ppt, but a morphological change was not reported.
Cysts of
Karenia have been hypothesized by several investigators. Resting cysts have been identified in samples from sediment on the West Florida Shelf in 2010 [
12]. Temporary, thin wall cysts of
Karenia mikimotoi were recorded under nitrogen starvation in the laboratory [
13]. A true dormant dinoflagellate cyst has been characterized as having a very thick cell wall, lack of pigments/chloroplasts, and being packed with stored energy compounds that have no color (e.g., sugars and lipids) [
13]. The spherical cells that we have reported here were not consistent with this, containing a relatively thin cell wall and packed with chloroplasts (
Figure 2), which is more consistent with temporary cysts. Persson et al. [
9] reported on
K. brevis cells changing from a traditional shape to a sphere within one minute following cold shock from 20 °C to 15 °C. This was interpreted as pellicle cysts of late zygotes. Our samples of the
K. brevis bloom that was ongoing at the time of our sampling transects (as evidenced by our clear microscopic identification; see
Figure 2) were collected in cold winter waters of the northern Gulf of Mexico where surface water temperatures were approximately 13 °C ± 1 °C. The
Karenia cells blooming in these waters were therefore exposed to temperatures below their optima. However, all samples were collected within a similar temperature range (±1 °C) and no temperature gradients were observed. Samples containing “balls” were only observed in the low salinity patches during our sampling transects and were confirmed to produce PbTXs, hence our suggestion of the salinity hypothesis for ball formation of
K.brevis. It appears that
K. brevis can be morphologically very plastic, and a variety of environmental stressors may be able to trigger these gross cell changes [
9,
13]. More data is required to determine the exact mechanisms and environmental parameters required to induce these morphological effects in
K. brevis. We hope that the data and observations presented here may stimulate further investigations of this kind by phytoplankton ecologists.
The absence of a theca allows some morphological flexibility in all unarmored dinoflagellates, however sphere-shaped cells of
K. brevis are not expected during traditional monitoring.
K. brevis has a distinctive cytoskeleton structure with unique microtubule arrangement and a sterol rich membrane that contributes to cell flexibility [
14]. This unusual potential for flexibility is often overlooked since
K. brevis is known to be very fragile and can rupture easily with turbulence, resulting in the aerosolization of brevetoxins [
15]. To date, there have been twelve different
Karenia species described and many of these have been reported as brevetoxin producers [
4,
16], so accurate identification of cells and toxins is extremely important in the assessment of risk. In areas where toxin profiles of
Karenia have been established, intrageneric, and, or, intraspecific molecular probes (e.g., [
15]) may provide a useful companion technique for unambiguous identification, regardless of morphology. Molecular identification was attempted in isolated cells from preserved samples during this study, but samples were unfortunately too degraded to amplify. Additional studies using advanced methods are ongoing to sequence and clone these spherical cells. Interestingly, some raphidophytes (
Chattonella antigua, Chattonella marina, Heterosigma akashiwo, and Fibrocapsa japonica) also produce brevetoxin-like compounds [
17,
18], however none of these species were detected during this study. Since only dinoflagellates were present and only
Karenia spp. have been shown to produce PbTXs to date, confirmation of brevetoxins in field collected samples (as was conducted in this study) is also an important piece of confirmatory evidence for identification. Some unarmored dinoflagellates in order Brachidiniales have been studied because of possible phylogenetic connection to
Karenia but have not been shown to produce PbTXs [
18]. Two genera in this order (
Brachidinium and
Asterodinium) have been reported to have high morphological versatility with projected body extensions hypothesized as an adaptation to environmental conditions [
18]. Together with our observations, these studies support our hypothesis of morphological plasticity as a response to adverse conditions in some dinoflagellate species.
Morphological plasticity of K. brevis could make species identification in field samples considerably more difficult. While taxonomists may be able to correctly identify sphere-shaped cells as K. brevis by microscopy (particularly when coupled with specific brevetoxin analyses), many monitoring programs are time-intensive and thereby enhanced by junior scientists, volunteers, and citizen scientists who may not be aware of these possible modifications or have toxin detection methods readily available. With no clear records of these potential morphological changes currently in the literature, this could be easily overlooked. However, if the dinoflagellate balls described in this study are in fact a morph of K. brevis, this may result in a significant underestimation of bloom density. While many factors are involved in brevetoxin bioaccumulation in shellfish (e.g., time, temperature, salinity, flow, feeding rate), K. brevis density above 5000 cells per liter of seawater has been adopted as the current closure threshold for all U.S. waters. At this very low cell density and with only a small subsample for microscopic evaluation, a potential underestimate of K. brevis cells could cause delays in precautionary harvest closures.